Abstract
Hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) gene was identified because of its increased expression in 25% of human hepatocellular carcinoma. HIP/PAP protein, a C-type lectin, binds laminin, acts as an adhesion molecule for hepatocytes, and has also been described as an acute phase secretory protein during acute pancreatitis in humans and rats. We investigated HIP/PAP protein expression in patients with various liver diseases associated with ductular reaction. At the same time, we analyzed patients with hepatocellular carcinoma and cholangiocarcinoma, and tested HIP/PAP protein levels in sera to establish the pattern of secretion. Our data show that HIP/PAP expression was not restricted to hepatocellular carcinoma, but was also detected in cholangiocarcinoma cells as well as in reactive non-malignant bile ductules. In contrast, HIP/PAP protein expression was undetectable in normal mature hepatocytes, but some ductular cells localized at the interface of portal tracts with parenchyma were HIP/PAP immunoreactive in normal liver. Finally, we present evidence that HIP/PAP serum levels were increased in 21/28 (75%) patients with hepatocellular carcinoma, and in 25/51 (49%) patients with nonmalignant cirrhosis. Altogether, these results suggest that HIP/PAP protein may be implicated in hepatocytic and cholangiolar differentiation and proliferation.
Cell differentiation and deregulated cell proliferation during carcinogenesis are triggered by the expression of distinct genes. To characterize the genes expressed during liver carcinogenesis, we previously undertook differential screening of a human hepatocellular carcinoma (HCC) cDNA library using subtracted probes and identified the hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) encoding gene. HIP/PAP mRNA was indeed abundantly expressed in tumoral but not in nontumoral or normal livers. 1
HIP/PAP belongs to group VII of the C-type lectin family according to Drickamer’s classification, 2 because it contains only one carbohydrate recognition domain (CRD) linked to a signal peptide. In this group, three PAPs (PAP I or peptide 23, PAPs II and III) have been described for the rat, 3 whereas three reg/lithostatines have been identified in the mouse (I, II, and III). 4,5 One PAP gene (tentatively named HIP/PAP) and one reg/lithostatine have been described in humans. 1,6,7
Our earlier investigations had shown that HIP/PAP was expressed in normal subjects in the intestine (Paneth and neuroendocrine cells), and the pancreas (acinar pancreatic cells and islets of Langerhans), whereas in the liver, its expression was triggered in the event of primary liver cancer. 8 Moreover, HIP/PAP is a secretory protein that is rapidly overexpressed during the acute phase of pancreatitis. 9 In addition, rat peptide 23, or PAP I, is secreted by rat pituitary and uterine cells under the influence of growth hormone-releasing hormone and estradiol, respectively. 10,11 Motor neurons are the only adult mammalian neurons of the central nervous system that regenerate after injury. The gene corresponding to human HIP/PAP gene in the rat, called Reg-2 or rat PAP I, has recently been found to be expressed in regenerating motor neurons, and the protein exhibits mitogen-like activity in vitro on Schwann cells. 12 Thus, the same protein has been identified using several independent approaches and exhibits marked, tissue-specific expression.
In the exocrine pancreas, HIP/PAP expression pattern is consistent with that of an acute phase reactant; however, this does not account for its expression in the islets of Langerhans of the endocrine pancreas. In human liver, limited data are available concerning the implications of HIP/PAP expression. Our previous investigations in the liver, demonstrating that HIP/PAP was expressed in HCC, suggested its potential importance in human liver carcinogenesis. Furthermore, increased serum secretion has been demonstrated in patients with HCC. 13 However, we still do not know whether the liver cells involved in the biliary or hepatocyte differentiation express the HIP/PAP protein. During the early stages of chemical hepatocarcinogenesis in the rat, a cell population (the so-called oval cells) appears and is thought to derive from a stem cell compartment. 14,15 Oval cells have been the subject of thorough investigation and exhibit a capacity toward both biliary and hepatocyte cell lineages. 16-19 Oval cell proliferation has also been observed during hepatocarcinogenesis in woodchuck virus carriers 20 and in human livers from patients with different pathological conditions, including chronic infection by hepatitis B virus (HBV), 21 and ductular reaction induced by regeneration and cholestasis. 22 Ductular reaction has been characterized by the proliferation of reactive ductules, which are made up of intermediate hepatocyte-like and bile duct-like cells. 23,24 Thus, the analysis of human liver sections exhibiting such cell proliferation should be relevant in this context. We therefore investigated HIP/PAP expression in patients with a range of liver diseases associated with ductular reaction, as well as in those with HCC and cholangiocarcinomas. We determined the serum levels of HIP/PAP and correlated the rate of secretion to liver expression profiles.
Patients and Methods
Patients
Tissues
Liver samples from 35 patients with HCC were obtained at surgery, immediately frozen in liquid nitrogen, and stored at −80°C for RNA extraction. Liver sections and sera were also available for testing using immunohistochemistry and enzyme-linked immunosorbent assay (ELISA) techniques. Clinical data are shown in Table 1 ▶ .
Table 1.
Summary of Results
| Case no. | Age | Sex | HBV DNA | HCV RNA | Alcohol | Cirrhosis | Capsule | Vascular emboles | Dissemination to adjacent liver | Edmonson score | RT-PCR | Northern blot | IH | AFP ng/ml | HIP/PAP ng/ml |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | F | + | − | − | − | + | − | − | III | − | − | − | 10 | 22 |
| 2 | 55 | M | + | − | − | − | + | + | + | II | − | − | − | 10 | 21 |
| 3 | 73 | F | + | + | − | + | − | + | + | II | − | − | − | 5474 | 38.5 |
| 4 | 74 | M | − | − | − | − | + | + | + | II | − | − | − | 5 | 26 |
| 5 | 68 | M | + | − | − | + | + | u | − | II | − | − | − | 10 | 42 |
| 6 | 60 | M | u | u | − | − | − | + | + | III | − | − | − | 29,245 | u |
| 7 | 61 | M | + | + | + | + | + | + | + | III | − | + | ++ | 25 | 66.5 |
| 8 | 41 | M | + | + | − | + | − | u | + | II | + | − | + | 5200 | 60 |
| 9 | 36 | M | − | − | − | − | + | − | + | III | + | − | + | 2000 | 121.5 |
| 10 | 69 | M | u | u | − | + | + | u | − | II | + | − | ++ | 10 | u |
| 11 | 57 | M | − | − | + | + | + | + | + | III | + | − | − | 10 | 108 |
| 12 | 83 | M | + | − | − | − | + | + | + | II | + | − | − | 250 | 81 |
| 13 | 67 | F | + | + | − | + | − | + | + | III | + | − | − | 250 | 62.5 |
| 14 | 69 | M | + | − | − | + | − | + | + | III | + | − | − | 5 | u |
| 15 | 62 | M | + | − | + | + | + | + | + | II | + | − | − | 10 | 45.5 |
| 16 | 85 | M | + | − | − | − | + | − | + | II | + | − | − | 10 | 55 |
| 17 | 69 | M | + | + | + | + | + | + | + | III | + | − | − | 3450 | 22 |
| 18 | 59 | M | + | − | − | + | − | u | − | III | + | − | − | 2000 | 22 |
| 19 | 61 | M | + | − | + | + | + | − | + | III | + | − | − | 10 | 44.5 |
| 20 | 61 | M | u | u | − | + | − | u | + | II | + | − | − | 10 | u |
| 21 | 74 | F | + | + | − | + | + | + | − | II | + | − | − | 39 | u |
| 22 | 53 | M | + | − | + | + | + | u | − | II | + | − | − | 3 | 19 |
| 23 | 48 | M | + | − | − | + | + | − | − | u | + | − | u | 3500 | 34 |
| 24 | 38 | M | − | − | − | − | − | + | − | u | + | − | u | 4000 | 38 |
| 25 | 76 | M | + | + | − | + | + | u | − | u | + | − | u | 955 | u |
| 26 | 38 | M | + | − | − | + | + | + | − | u | + | − | u | 2400 | 110 |
| 27 | 52 | M | − | + | + | + | − | + | + | III | + | + | + | 10 | 1450 |
| 28 | 70 | M | + | + | − | + | + | + | + | II | + | + | + | 10 | 237 |
| 29 | 57 | M | + | − | − | − | + | + | + | II | + | + | ++ | 2 | 2803 |
| 30 | 58 | M | − | − | − | + | + | + | − | II | + | + | ++ | 7109 | 110 |
| 31 | 64 | M | + | + | + | + | − | + | − | II | + | + | +++ | u | 78 |
| 32 | 65 | M | − | + | − | + | + | − | − | II | + | + | +++ | 10 | 42 |
| 33 | 66 | M | − | + | − | + | + | u | − | III | + | + | u | u | u |
| 34 | 66 | M | + | + | − | − | + | − | − | II | + | + | u | 10 | 410 |
| 35 | 65 | M | + | + | − | + | − | + | + | III | u | − | − | 300 | 19 |
Other tissue samples were available only for immunohistochemical investigations. These included samples with cholangiocarcinoma (n = 20), as well as 20 cases of nonmalignant pathologies involving ductular reaction: liver cell regeneration following massive necrosis (n = 3), hepatitis C virus (HCV)-related cirrhosis (n = 4), primary sclerosing cholangitis (noncirrhotic stage; n = 3), primary biliary cirrhosis (n = 3; 2 cirrhotic, 1 noncirrhotic stages), extrahepatic subobstruction (noncirrhotic stage; n = 4) and focal nodular hyperplasia (n = 3). Three normal livers were also analyzed.
Each liver sample was divided into two parts. The larger part was either fixed in B5 fixative and used for routine light microscopic diagnosis or treated with 4% formalin and paraffin-embedded for immunohistochemistry. The other part was snap-frozen in liquid nitrogen-cooled isopentane, stored at −70°C, and used for immunohistochemistry tests.
Sera
Sera from 28/35 patients with HCC, 51 patients with cirrhosis without overt HCC, and 26 healthy donors used as normal controls were stored at −20°C for the HIP/PAP assay. This protocol was approved by the local Institutional Review Board at Necker Enfants-Malades Hospital (Paris).
Methods
RNA Extraction, Northern Blots, and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using Trizol Reagent (Life Technologies, Gaithersburg, MD). RNAs (20 μg) were separated on a denaturing formaldehyde gel and then transferred onto Hybond-C-extra nitrocellulose membranes (Amersham, Buckinghamshire, UK). Hybridizations were performed using random-primed human HIP/PAP. Hybridization and washing conditions were as previously described. 1
Total RNA (1 μg) was processed for RT-PCR (MMLV-RT and Taq DNA Polymerase, Life Technologies) using the sense 19bis (5′ATGCTGCCTCCCATGGCCCTG3′) and the antisense 101 (5′GCCCATGATGAGTTGCACACCAAACA3′) primers in positions 1–21 and 553–578 of the HIP/PAP cDNA. Human HIP/PAP cDNA (one-third of the reverse reaction) was heated to 94°C for 5 minutes and amplified for 40 cycles of 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute, and the final cycle was identical except for the 10-minute extension.
Western Blot Analysis
Human ileum and sera were analyzed using Western blot with pre-HIP antibodies diluted to 1:5000, as previously described. 8
Immunohistochemistry
Two immunohistochemical methods were used for immunohistochemistry, whether the tissue was frozen or not.
Formalin-fixed sections 3 μm thick were deparaffinized, hydrated, and boiled for 20 minutes in 10 mmol/L citrate buffer, pH 6.2, in a microwave oven. Endogenous peroxidase activity was quenched by incubation with 0.3% hydrogen peroxide. Trypsin predigestion for 5 minutes at 37°C, phosphate-buffered saline washing, and blocking for 10 minutes with normal goat serum (dilution 1:20) were performed before incubation for 30 minutes at room temperature with polyclonal pre-HIP or CRD antibodies (dilution 1:400). 8 After washing three times in phosphate-buffered saline, biotinylated-linked secondary antibody (DAKO, Trappes, France) was added for 30 minutes. The sections were then washed and incubated for 30 minutes with a complex of streptavidin/biotinylated peroxidase. Signals were visualized using 3-amino-9-ethylcarbazole (Sigma, St Louis, MO) and counterstained with Mayer’s hematoxylin. Finally, the slides were mounted in an aqueous solution with glycergel (DAKO).
Semiserial 5-μm cryostat sections were dried overnight at room temperature, fixed in acetone for 10 minutes, and stained using a three-step peroxidase-anti-peroxidase method (Dakopatts, Copenhagen, Denmark). Two different HIP/PAP rabbit total IgGs (CRD and pre-HIP, dilution 1:100) 8 were incubated overnight at 4°C. Cytokeratin 7 (Dakopatts, dilution 1:50), and OV6 (Drs. H. Dunsford and S. Sell, University of Texas, Austin, TX, dilution 1:200) antibodies were incubated for 30 minutes at room temperature. The reagents were diluted in phosphate-buffered saline, pH 7.2, containing 10% normal human serum, and incubated for 30 minutes at room temperature. After each incubation, the sections were washed three times with phosphate-buffered saline, pH 7.2, for 15 minutes. The reaction product was developed using 3-amino-9-ethylcarbazole and H2O2. As a control, semiserial sections were incubated with anti-HIP/PAP antisera, preabsorbed with an HIP/PAP recombinant protein used for immunization in the production of anti-HIP/PAP antisera. Additional controls involved omitting the primary antibody, or using irrelevant isotypic primary antibodies.
ELISA
Serum HIP/PAP levels were assayed using a sandwich ELISA test in accordance with manufacturer’s instructions (Dynabio, La Gaude, France) and as previously described. 13
Statistical Analysis
Distribution of data were described using the box and whiskers representation (Statview 5′ software). The Mann-Whitney U test was used to compare HIP/PAP concentrations in controls, patients with HCC, and patients with cirrhosis. Spearman’s rank correlation was used to correlate α-fetoprotein and HIP/PAP concentrations in patients with HCC. Comparisons of observed frequencies for HIP/PAP expression and classic HCC prognostic factors were calculated with the χ 2 test. P values <0.05 were considered statistically significant.
Results
HIP/PAP RNA and Protein Expression in HCC Tissues
Among 35 HCC patients analyzed using Northern blot, 34 and 29 were also studied using RT-PCR and immunohistochemistry, respectively (Table 1) ▶ . Northern blots showed an accumulation of HIP/PAP mRNAs in 9/35 HCC. However, RT-PCR detected HIP/PAP gene transcription in 27/34 (79%) tumor tissues (Figure 1) ▶ . In contrast, HIP/PAP mRNA was not detectable in two samples of nontumoral liver, although the corresponding tumoral tissue expressed the gene (data not shown). Finally, HIP/PAP protein was detected in 10/29 (34%) HCC tissues using immunohistochemistry (Table 2A) ▶ .
Figure 1.
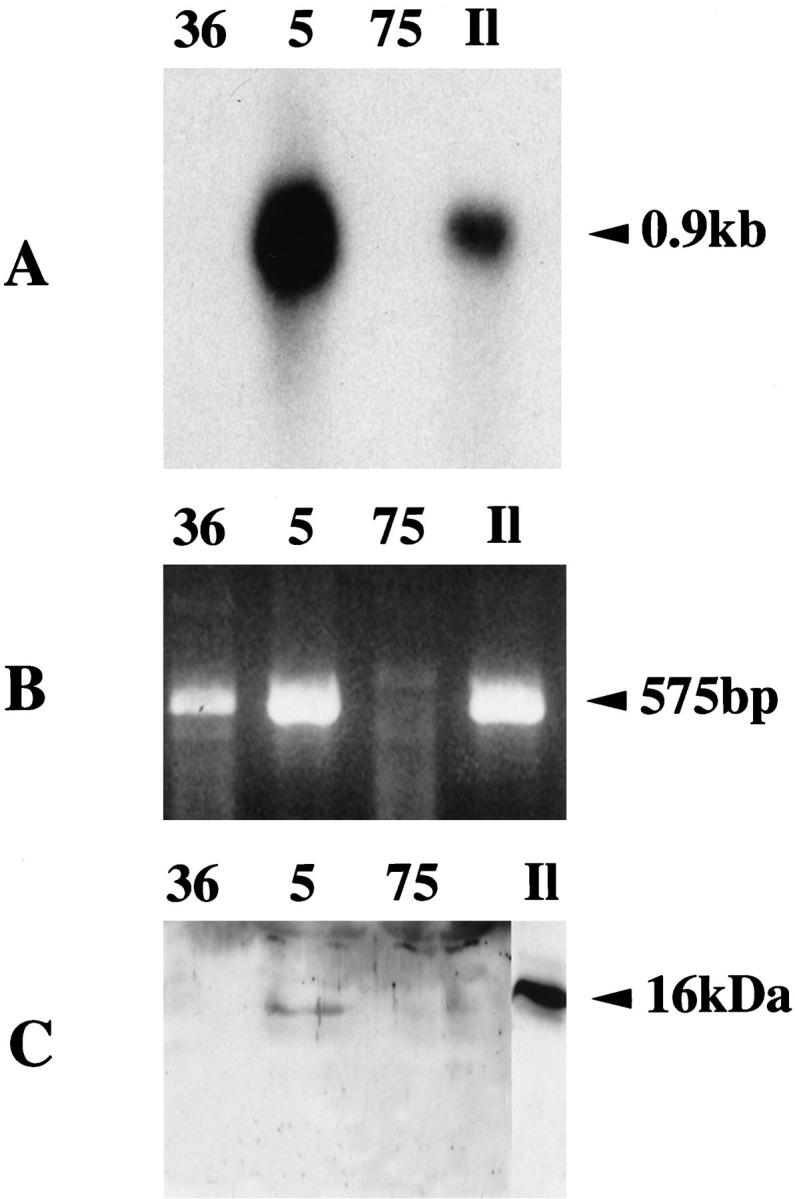
Northern blot 20 μg (A) and RT-PCR 1 μg (B) analysis of tumoral liver RNA from patients 36, 5, 75, and normal human ileum RNA (Il). Patient 36 was negative by Northern blot and positive by RT-PCR. Patient 5 was positive by Northern blot and RT-PCR. Patient 75 was negative by Northern blot and RT-PCR. Western blot analysis of serum (10 μl) from patients 36, 5, 75, and control normal human ileum tissue (20 μg) (Il) (C). HIP/PAP serum levels were 19, 237, and 42 ng/ml in patients 36, 5, and 75, respectively, as quantified using an ELISA test. Only patient 5 gave a detectable signal.
Table 2.
Prevalence of HIP/PAP Expression in HCC
| A Method | HCC-positive (n) | (%) |
|---|---|---|
| Northern blot | 9 /35 | 25% |
| RT-PCR | 27 /34 | 79% |
| Immunohistochemistry | 10 /29 | 34% |
| ELISA test | 21 /28 | 75% |
| B Immunohistochemistry | Northern blot-positive | RT-PCR- positive |
|---|---|---|
| Group I (n = 2) | 2 /2 | 2 /2 |
| Group II (n = 4) | 3 /4 | 3 /4 |
| Group III (n = 4) | 2 /4 | 4 /4 |
| Group IV (n = 19) | 0 /19 | 12 /19 |
Northern blot-positive HCC were also positive using RT-PCR (except for patient 7), and 7/9 tissues available for immunohistochemistry scored positive for HIP/PAP expression. Using immunohistochemical techniques, there was no staining in tumors which had proved negative with RT-PCR, except for patient 7. Among 19 tumors which were positive using RT-PCR and negative using Northern blot, 12, 2, and 1 tumors scored 0, 1%, and 5% of staining cells, respectively; 4 samples were not available for immunohistochemical analysis. Thus, a convergence could be observed in the results obtained with the three experimental approaches, further validating the specificity of the results. We also examined whether HIP/PAP might be expressed in nonmalignant liver proliferation. Using RT-PCR, we also observed that HIP/PAP was expressed in 1/1 hamartoma, 1/1 angioma, 4/5 focal nodular hyperplasia, and 1/1 regeneration nodes (data not shown).
We found no relationship between HIP/PAP gene expression and classic HCC prognostic factors (Table 3) ▶ , and there was no correlation between α-fetoprotein and HIP/PAP serum concentrations (Table 1) ▶ . Liver samples and sera from patients with cholangiocarcinomas were not available for these investigations.
Table 3.
Biological, Virological, and Histological Characteristics of HIP/PAP-positive and -negative HCC
| HIP/PAP-positive | HIP/PAP-negative | ||
|---|---|---|---|
| HBV | + | 5 | 19 |
| − | 4 | 4 | |
| u | 3 | ||
| HCV | + | 7 | 7 |
| − | 2 | 16 | |
| u | 3 | ||
| Alcohol | + | 3 | 5 |
| − | 6 | 21 | |
| Histology | cirrhosis | 7 | 18 |
| no cirrhosis | 2 | 8 | |
| Vascular | + | 6 | 14 |
| embolus | − | 2 | 5 |
| u* | 1 | 7 | |
| + | 7 | 17 | |
| Capsule | − | 2 | 9 |
| + | 4 | 16 | |
| Dissemination in | − | 5 | 10 |
| adjacent liver | II | 6 | 12 |
| Edmonson | III | 3 | 10 |
| Steiner grade | u* | 0 | 4 |
* unknown.
Immunohistochemical Analysis of HIP/PAP-Expressing Cells
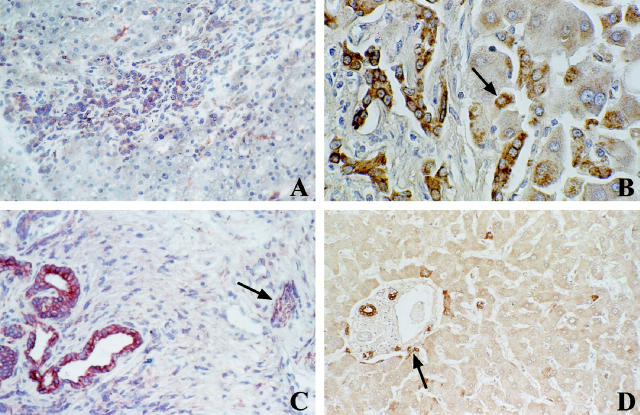
HIP/PAP protein was expressed in 10/29 (34%) HCC tissues, which were divided into four groups based on the signal intensity (Table 2B) ▶ . Group I exhibited strong labeling (more than 20% of staining cells; n = 2), group II a moderate labeling (5–20% of staining cells; n = 4) and group III a slight labeling (1–5% of staining cells; n = 4), whereas no signal was detected in group IV (n = 19). Positive cells were localized at the periphery of the carcinomatous nodules in 6/10 positive tumors. HIP/PAP immunostaining appeared as intracytoplasmic brown granules occupying most of the cytoplasm; in 6/10 and 2/10 HCC tissues, HIP/PAP was expressed in perinuclear bodies and at the plasmic membrane, respectively (Figure 2, A ▶ -C). Twenty cholangiocarcinomas, characterized by tubular structures and tumoral strands within a fibrous stroma, were also studied. HIP/PAP protein was expressed in 8/20 cholangiocarcinomas (40%). Signal intensity was virtually equivalent in these 8 positive tumors. Staining was cytoplasmic and diffuse in the majority of the tumor cells, but with varying intensity (Figure 2D) ▶ .
Figure 2.
Immunolocalization of HIP/PAP protein in HCC (A-C) and cholangiocarcinoma (D). A: Immunolocalization of HIP/PAP protein at the frontier between affected (left) and non-affected portions of the liver section (HCC no. 81, paraffin section, pre-HIP antibodies, original magnification, ×250). B: Intracytoplasmic brown granules occupying most of the cytoplasm, plus plasmic membrane labeling (HCC no. 82, paraffin section, pre-HIP antibodies, original magnification, ×250). C: Cytoplasmic granulous labeling plus perinuclear bodies (patient 93, paraffin section, pre-HIP antibodies, original magnification, ×400). D: Cholangiocarcinoma with a majority of tumor cells showing varying degrees of cytoplasmic immunoreactivity for HIP/PAP (frozen section, pre-HIP antibodies, original magnification, ×250).
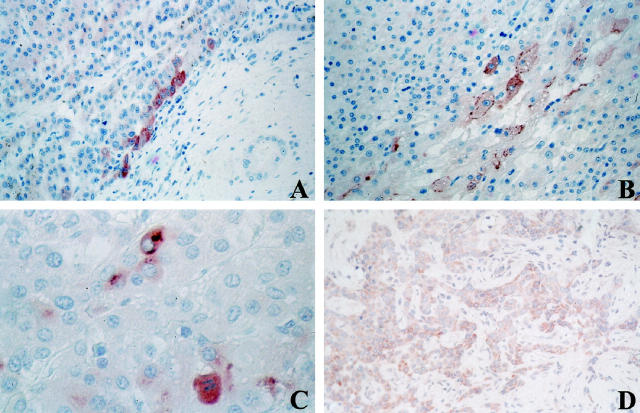
All of the nonmalignant tissues examined in this study were selected on the basis of the presence, to a variable extent, of ductular reaction. Specimens with massive necrosis showed widespread loss of parenchymal cells. In periportal areas, reactive ductules were a sign of regeneration. In chronic cholestatic conditions, small ductular structures at the portal tract-parenchyma interface were surrounded by polymorphonuclear leukocytes and periportal fibrosis of varying intensity. Ductular structures in regeneration after massive necrosis, as well as in cases of chronic cholestasis, were immunoreactive in the cytoplasm portion of their lining cells (Figure 3A) ▶ . Figure 3B ▶ shows HIP/PAP positive reactive ductules and hepatocyte-like intermediate cells (arrow) in a case of cirrhosis. In addition, nerves in portal tracts and portal tract equivalents (for focal nodular hyperplasia) were also immunoreactive, as shown in one case of chronic active hepatitis C (Figure 3C) ▶ .
Figure 3.
Immunolocalization of HIP/PAP protein in nonmalignant tissues. A: Chronic extrahepatic subobstruction. Reactive bile ductules at the edge of a portal tract are immunoreactive for HIP/PAP in their cytoplasm. (frozen section, pre-HIP antibodies, original magnification, ×250). B: Active cirrhosis. Reactive ductules and hepatocyte-like cells (arrow) are immunoreactive for HIP/PAP (paraffin section, CRD antibodies, original magnification, ×400). C: Chronic active hepatitis C. Larger portal tract showing immunoreactivity for HIP/PAP in ductular structures (left) and in nerve bundles (arrow) (frozen section, pre-HIP antibodies, original magnification, ×250). D: Portal tract in normal liver. Immunoreactivity in bile ducts and in some scarce ductular cells (arrow) with negative surrounding hepatocytes (paraffin section, CRD antibodies, original magnification, ×250).
In normal liver, we detected a cytoplasmic immunoreactivity in a few bile ducts and in some ductular cells, localized at the interface of portal tracts with parenchyma. In contrast, hepatocytes remained negative in all of the samples examined, including the nontumoral parts of livers with HCC and cholangiocarcinoma (Figure 3D) ▶ .
Whether HIP/PAP was really expressed in ductular cells, we performed immunohistochemistry test on semiserially cut cryostat sections of a portal tract in an extrahepatic subobstruction (Figure 4) ▶ . We observed that HIP/PAP, cytokeratin 7 (bile duct type cytokeratin) and OV6 (marker of oval cells) were expressed in ductular cells localized in the portal tracts.
Figure 4.
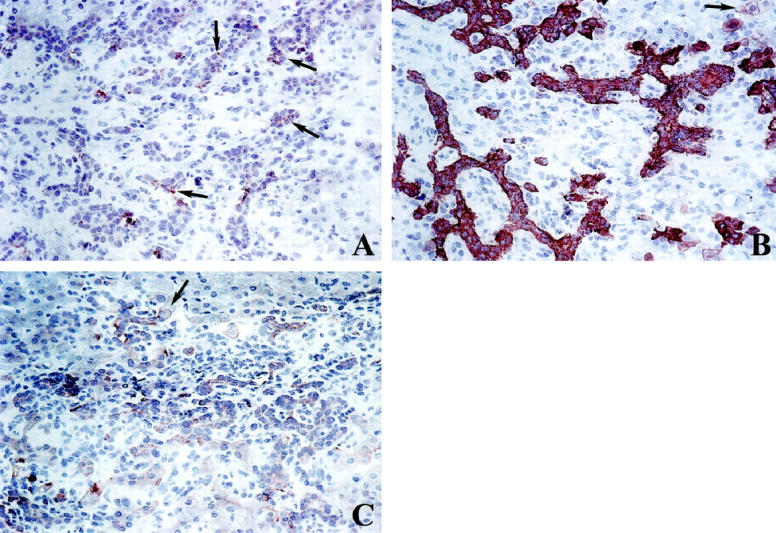
Semiserially cut cryostat sections of a portal tract in an extrahepatic subobstruction, immunostained (A) for HIP/PAP in a subpopulation of ductular cells with varying intensity (arrows), original magnification, ×250, (B) for cytokeratin 7 (bile duct type cytokeratin) in reactive ductules with a strong positive labeling. Some periportal hepatocyte-like intermediate cells are also immunoreactive (arrow), original magnification, ×250, (C) for OV6. The same portal tract shows immunoreactivity for OV-6 in reactive ductules and in hepatocyte-like intermediate cells (arrow), original magnification, ×250.
HIP/PAP Serum Levels
Sera HIP/PAP levels were compared in three groups of subjects (ng/ml): normal controls (healthy donors, n = 26), HBV, HCV, or alcoholic patients with liver cirrhosis (n = 51), and patients with HCC (n = 28). Serum samples from patients with cholangiocarcinoma were not available. HIP/PAP levels did not result in a normal distribution of data, so we used a nonparametric test to establish the comparisons. HIP/PAP levels were significantly higher in HCC patients (median 50.25 ng/ml; range 19–2803 ng/ml) compared to healthy donors (21 ng/ml; 7–55 ng/ml; P < 0.0001), and to patients with cirrhosis (28 ng/ml; 11.5–424 ng/ml; P = 0.0076) (Figure 5A) ▶ . Based on normal controls, we estimated the normal serum value at 27 ng/ml, which agreed with the results obtained by Dusetti et al. 13 HIP/PAP protein levels were indeed elevated in the sera of 21/28 (75%) patients with HCC and 25/51 (49%) cirrhotic patients (Table 2A) ▶ . Seventeen of 20 RT-PCR-positive patients showed elevated HIP/PAP values in the ELISA test; furthermore, 3 of 7 patients whose liver tissues showed a marked accumulation of HIP/PAP RNA, detectable by Northern blot, had serum values which reached more than 60, 10, and 3 times the threshold of normal values, respectively. Overall, these data showed that the rate of HIP/PAP secretion was directly related to its hepatic expression.
Figure 5.
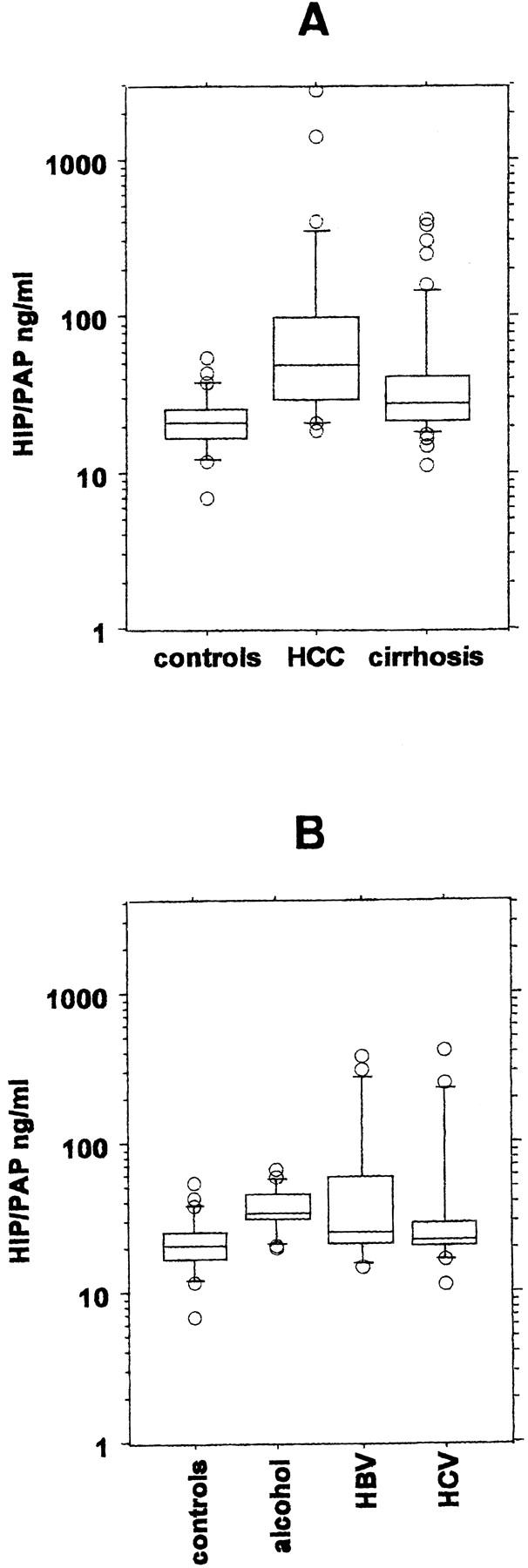
Serum HIP/PAP levels. Each box plot comprises five horizontal lines displaying the 10th, 25th, 50th, 75th, and 90th percentiles of a variable. All values for the variable above the 90th percentile and below the 10th percentile are plotted separately, so that the box plots are valuable in highlighting any outliers. A: Serum HIP/PAP concentrations were determined in controls (n = 26), in patients with HCC (n = 28), and in patients with cirrhosis without overt HCC (n = 51). B: Serum HIP/PAP concentrations were determined in controls (n = 26) and in cirrhotic patients taking into account the etiology of the cirrhosis (alcohol n = 17, HBV = 17, and HCV = 16). One cirrhotic patient with primitive sclerosing cholangitis (HIP/PAP = 111 ng/ml) was excluded.
Patients without HCC but suffering from cirrhosis also differed significantly from controls (P = 0.0011). Taking into account the etiology of cirrhosis (alcohol, HBV, and HCV), the analysis showed that there was no significant difference between alcoholic and non-alcoholic (HBV, HCV) cirrhotic patients (Figure 5B) ▶ . It is worth noting that there was, however, a highly significant difference between alcoholic patients and controls (P = 0.0006). Whether the frequent increase of HIP/PAP levels in alcohol-related cirrhotic patients might reflect an impact of alcohol on ductular reaction remains still elusive.
Discussion
This study demonstrates that HIP/PAP expression is not restricted to HCC in human liver. In nonmalignant liver, HIP/PAP is expressed in reactive ductules, a subset of cells located in the vicinity of the portal tracts, the proliferation of which is triggered during the so-called ductular reaction. 23,24 In contrast, HIP/PAP expression is undetectable in mature hepatocytes, although it is expressed in the tumoral cells of both HCC and cholangiocarcinoma.
The nature of the cellular response to liver injury depends largely on the location and degree of liver loss. After a two-thirds partial hepatectomy in the rat, or limited hepatotoxic injury in humans, the restoration of liver mass is accomplished through the proliferation of mature hepatocytes. However, the cellular response to severe liver injury, such as that induced by chemicals 24 and, possibly, by chronic viral infections, 20,21 may involve reserve liver stem cells (the so-called oval cells), which have so far resisted definite characterization. 25,26 In humans, the proliferative response to many types of liver injury is indeed characterized by an increase in bile duct-like structures and is termed a ductular reaction. Recent evidence has suggested that reactive ductules in humans may contain immature cells with a capacity for bipotential differentiation. 27 During chemically induced carcinogenesis in the rat 14-16 and mouse, 28 oval cells have been studied extensively and their dual capacities for biliary and hepatocyte differentiation have been demonstrated. In these models of liver carcinogenesis, oval cells are very likely to give rise to both HCC and cholangiocarcinoma. Furan-induced biliary hyperplasia and cholangiofibrosis are associated with the metaplastic appearance of cells with the morphology of hepatocytes (ductular hepatocytes) during the early stages of rat cholangiocarcinogenesis. 29 Our present results show that HIP/PAP-positive cells express biliary cell markers. It remains to be elucidated, however, whether such HIP/PAP-positive cells could differentiate in both biliary and hepatocytic lineages.
In humans, such reactive bile ductules exhibit neuroendocrine features (chromogranin A and neural cell adhesion molecule immunoreactivity). 30 It has been suggested that these molecules, produced in dense-cored secretory granules, may play a role in the growth and/or differentiation of liver cells through autocrine and/or paracrine pathways. Interestingly, we now present evidence that HIP/PAP is expressed in reactive bile ductules and in certain nerve bundles located in the portal tracts, and so may participate in this process. Three characteristics of HIP/PAP protein support this hypothesis. First, HIP/PAP protein is stored in zymogen secretion granules of exocrine pancreas acinar cells and in the endocrine pancreas. 8 Second, HIP/PAP is expressed in intestinal neuroendocrine and Paneth cells showing neuroendocrine marker and immunoreactivity for synaptophysin 8 (F. Carnot, personal data). Indeed, HIP/PAP is also expressed in regenerating motor neurons and could act as a mitogenic factor for Schwann cells. 12 We have also recently shown that HIP/PAP is expressed in nervous system of the postimplantation mouse embryo at day 14.5, before its expression in intestine and pancreas at day 16.5 31 Third, HIP/PAP interacts with both hepatocytes and extracellular matrix proteins and has been proposed as an adhesive molecule. 8 Taken together, these results suggest that HIP/PAP may constitute a further marker of liver cells which express a complex pattern of neuroendocrine and neural cell adhesion molecules, and may be activated in response to certain liver regeneration stimuli as well as during liver carcinogenesis.
This study also confirms the existence of abundant intrahepatic HIP/PAP gene expression in patients with HCC. Northern blot-based analysis showed an accumulation of HIP/PAP RNA in 9/35 subjects with HCC (25%), a figure which agrees with previous results (7/29). 1 The positive frequency of HIP/PAP expression was, however, significantly increased when other more sensitive experimental approaches were used; indeed, using RT-PCR, HIP/PAP RNA was detected in 28/35 (79%) liver tumors. Furthermore, HIP/PAP protein was expressed in 11/32 (34%) tumors and secreted into the serum of 21/28 (75%) patients with HCC. Thus, a convergence could be seen in the results obtained with the three experimental approaches, further validating the translation of the mRNA amplified by RT-PCR.
The activation of several genes has been reported extensively in liver cancers, such as those encoding for α-fetoprotein, glutamine synthetase, 32 and insulin-like growth factor II. In contrast with those markers, HIP/PAP expression was not detectable during fetal liver development. 31,33 In this context, it is important to emphasize that, in agreement with previous study, 34 there was no correlation between α-fetoprotein and HIP/PAP serum levels, which suggests that both inductions occur by independent mechanisms.
Cholangiocarcinoma tissues were only available for immunohistochemical analysis, but HIP/PAP protein was also frequently expressed in the tumors (8/20, 40%). Our observations thus demonstrate that HIP/PAP gene activation is a frequent genetic feature of tumor liver cells engaged in either the hepatocyte or cholangiolar pathways. The role of HIP/PAP in liver carcinogenesis is as yet unknown, and only a subset of HCC and cholangiocarcinoma tumors express HIP/PAP. However, HIP/PAP serum levels are also increased in a subset of cirrhotic patients without malignancies (25/51, 49%), and HIP/PAP is expressed in ductular cells characterized by cytokeratin 7 and OV6 immunostainings. Taken together, these results suggest that HIP/PAP is expressed not only in hepatocellular carcinoma, but also in cholangiocarcinoma as well as in ductular cells. As these ductular cells have been proposed to represent potential progenitor cells with a bipotential capacity to differentiate into bile duct cells and hepatocytes, they might constitute potential precursors for a subset of liver cancers with either hepatocyte or cholangiolar phenotypes. This observation, seen alongside the expression of HIP/PAP in tumor cells with either hepatocyte or cholangiolar phenotypes, leads us to suggest that HIP/PAP may be implicated in hepatocyte or cholangiolar differentiation and proliferation.
Footnotes
Address reprint requests to Laurence Christa, Institut National de la Santé et de la Recherche Médicale U-370 and Liver Unit, Centre Hospitalier Universitaire Necker, 156 rue de Vaugirard, 75742 Paris cedex 15, France. E-mail: christa@necker.fr.
Supported by Institut National de la Santé et de la Recherche Médicale, Association pour la Recherche contre le Cancer, Ligue Nationale contre le Cancer, Fondation de France.
References
- 1.Lasserre C, Christa L, Simon MT, Vernier P, Bréchot C: A novel gene (HIP) activated in human liver cancer. Cancer Res 1992, 52:5089-5095 [PubMed] [Google Scholar]
- 2.Drickamer K: Calcium-dependent carbohydrate-recognition domains in animal proteins. Curr Opin Struct Biol 1993, 3:393-400 [Google Scholar]
- 3.Frigerio JM, Dusetti NJ, Garrido P, Dagorn JC, Iovanna JL: The pancreatitis associated protein III (PAPIII), a new member of the PAP gene family. Biophys Biophys Acta 1993, 1216:329-331 [DOI] [PubMed] [Google Scholar]
- 4.Narushima Y, Unno M, Nakagawa KI, Mori M, Miyashita H, Suzuki Y, Nogushi N, Takasawa S, Kumagai T, Yonekura H, Okamoto H: Structure, chromosomal localization, and expression of mouse genes encoding type III Reg, Reg IIIa, Reg IIIb, Reg IIIc. Gene 1997, 185:159-168 [DOI] [PubMed] [Google Scholar]
- 5.Itoh T, Teraoka H: Cloning and tissue-specific expression of cDNAs for the human and mouse homologues of rat pancreatitis-associated protein (PAP). Biophys Biophys Acta 1993, 1172:184-186 [DOI] [PubMed] [Google Scholar]
- 6.Orelle B, Keim V, Masciotra L, Dagorn JC, Iovanna JL: Human pancreatitis-associated protein: messenger RNA cloning, and expression in pancreatic diseases. J Clin Invest 1992, 90:2284-2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe T, Yonemura Y, Yonekura H, Suzuki Y, Miyashita H, Sugiyama K, Morizumi M, Unno S, Tanaka O, Kondo H, Bone AJ, Takasawa S, Okamoto H: Predominant β-cell replication, and amelioration of surgical diabetes by Reg protein. Proc Natl Acad Sci USA 1994, 91:3589-3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christa L, Carnot F, Simon MT, Levavasseur F, Stinnakre MG, Lasserre C, Clement B, Devinoy E, Bréchot C: HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am J Physiol 1996, 271:G993-G1002 [DOI] [PubMed] [Google Scholar]
- 9.Iovanna JL, Keim V, Nordback I, Montalto G, Camarena J, Letoublon C, Levy P, Berthezene P, Dagorn JC: Serum levels of pancreatitis-associated protein as indicators of the course of acute pancreatitis. Gastroenterology 1994, 106:728-734 [DOI] [PubMed] [Google Scholar]
- 10.Katsumata N, Chakraborty C, Myal Y, Schroedter IC, Murphy LJ, Shiu RPC, Friesen HG: Molecular cloning and expression of peptide 23, a growth hormone-releasing hormone-inducible pituitary protein. Endocrinology 1995, 136:1332-1339 [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty C, Vronkatis M, Molnar P, Schroedter IC, Katsumata N, Murphy LJ, Shui RPC, Friesen HG: Expression of pituitary peptide 23 in the rat uterus: regulation by estradiol. Mol Cell Endocrinol 1995, 108:149-154 [DOI] [PubMed] [Google Scholar]
- 12.Livesey FJ, O’Brien JA, Li M, Smith AG, Murphy LJ, Hunt SP: A Schwann cell mitogen accompanying regeneration of motor neurons. Nature 1997, 390:614-618 [DOI] [PubMed] [Google Scholar]
- 13.Dusetti NJ, Montalto G, Ortiz EM, Masciotra L, Dagorn JC, Iovanna JL: Mechanism of PAP I gene induction during hepatocarcinogenesis: clinical implications. Br J Cancer 1996, 74:1767-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen BE, Zajac VF, Michalopoulos GK: Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology 1998, 27:1030-1038 [DOI] [PubMed] [Google Scholar]
- 15.Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F, Bannasch P: Oval cell lines OC/CDE6 and OC/CDE22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest 1994, 71:700-709 [PubMed] [Google Scholar]
- 16.Fausto NF: Liver stem cells. Arias IM Boyer JL Fausto NF Jakoby WB Schachter DA Shafritz DA eds. The Liver: Biology and Pathobiology, third edition. 1994, :pp 1501-1518 Raven Press, New York [Google Scholar]
- 17.Sigal SH, Brill S, Fiorino AS, Reid LM: The liver as a stem cell and lineage system. Am J Physiol 1992, 26:G139-G142 [DOI] [PubMed] [Google Scholar]
- 18.Alison M, Sarraf C: Hepatic stem cells. J Hepatol 1998, 29:676-682 [DOI] [PubMed] [Google Scholar]
- 19.Thorgeirsson SS: Hepatic stem cells. Am J Pathol 1993, 142:1331-1333 [PMC free article] [PubMed] [Google Scholar]
- 20.Fu XX, Su CY, Lee Y, Hintz R, Biempica L, Snyder R, Ragler CE: Insulin like growth factor II expression and oval cell proliferation associated with hepatocarcinogenesis in woodchuck virus carriers. J Virol 1988, 62:3422-3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER, Thorgeirsson SS: Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology 1992, 16:1327-1333 [DOI] [PubMed] [Google Scholar]
- 22.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V: Hepatic OV-6 expression in human liver disease, and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol 1998, 29:455-463 [DOI] [PubMed] [Google Scholar]
- 23.Desmet V, Roskams T, Van Eyken P: Ductular reaction in the liver. Pathol Res Pract 1995, 191:513-524 [DOI] [PubMed] [Google Scholar]
- 24.Sell S: Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology 1998, 27:317-331 [DOI] [PubMed] [Google Scholar]
- 25.Sirica AE, Gainey TW, Harrel MB, Caran N: Cholangiocarcinogenesis and biliary adaptation responses in hepatic injury. Sirica AE Longnecker DS eds. Biliary and Pancreatic Ductal Epithelia: Pathobiology and Pathophysiology. 1997, :pp 229-290 Marcel Dekker New York [Google Scholar]
- 26.Tarsetti F, Lenzi R, Salvi R, Schuler E, Rijhsinghani K, Lenzen R, Tavolon N: Liver carcinogenesis associated with feeding of ethionine in a choline-free diet: evidence against a role of oval cells in the emergence of hepatocellular carcinoma. Hepatology 1993, 18:596-603 [PubMed] [Google Scholar]
- 27.Roskams T, De Vos R, Desmet V: “Undifferentiated progenitor cells” in focal nodular hyperplasia of the liver. Histopathology 1996, 28:291-299 [DOI] [PubMed] [Google Scholar]
- 28.Factor VM, Radaeva SA, Thorgeirsson SS: Origin and fate of oval cells in Dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol 1994, 145:409-422 [PMC free article] [PubMed] [Google Scholar]
- 29.Sirica AE, Gainey TW, Mumaw VR: Ductular hepatocytes: evidence for a bile ductular cell origin in furan-treated rats. Am J Pathol 1994, 145:375-383 [PMC free article] [PubMed] [Google Scholar]
- 30.Roskams T, van den Oord JJ, De Vos R, Desmet VJ: Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol 1990, 137:1019-1025 [PMC free article] [PubMed] [Google Scholar]
- 31.Lasserre C, Colnot C, Bréchot C, Poirier F: HIP/PAP gene, encoding a C-type lectin overexpressed in primary liver cancer, is expressed in nervous system as well as in intestine, and pancreas of the post-implantation mouse embryo. Am J Pathol 1999, 154:7601-7610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christa L, Simon MT, Flinois JP, Gebhardt R, Bréchot C, Lasserre C: Overexpression of glutamine synthetase in human primary liver cancer. Gastroenterology 1994, 106:1312-1320 [DOI] [PubMed] [Google Scholar]
- 33.Lasserre C, Simon MT, Ishikawa H, Diriong S, Nguyen VC, Christa L, Vernier P, Bréchot C: Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem 1994, 224:29-38 [DOI] [PubMed] [Google Scholar]
- 34.Montalto G, Iovanna JL, Soresi M, Dusetti N, Carroccio A, Barthelemy-Bialas S, Cartabellota A, Dagorn JC: Clinical evaluation of pancreatitis associated protein as a serum marker of hepatocellular carcinoma: comparison with α-fetoprotein. Oncology 1998, 55:421-425 [DOI] [PubMed] [Google Scholar]