Abstract
Thrombomodulin is a cell surface anticoagulant that is expressed by endothelial cells and epidermal keratinocytes. Using immunohistochemistry, we examined thrombomodulin expression during healing of partial-thickness wounds in human skin and full-thickness wounds in mouse skin. We also examined thrombomodulin expression and wound healing in heterozygous thrombomodulin-deficient mice, compound heterozygous mice that have <1% of normal thrombomodulin anticoagulant activity, and chimeric mice derived from homozygous thrombomodulin-deficient embryonic stem cells. In both human and murine wounds, thrombomodulin was absent in keratinocytes at the leading edge of the neoepidermis, but it was expressed strongly by stratifying keratinocytes within the neoepidermis. No differences in rate or extent of reepithelialization were observed between wild-type and thrombomodulin-deficient mice. In chimeric mice, both thrombomodulin-positive and thrombomodulin-negative keratinocytes were detected within the neoepidermis. Compared with wild-type mice, heterozygous and compound heterozygous thrombomodulin-deficient mice exhibited foci of increased collagen deposition in the wound matrix. These findings demonstrate that expression of thrombomodulin in keratinocytes is regulated during cutaneous wound healing. Severe deficiency of thrombomodulin anticoagulant activity does not appear to alter reepithelialization but may influence collagen production by fibroblasts in the wound matrix.
Thrombomodulin is an anticoagulant glycoprotein that is expressed on the surface of vascular endothelial cells and epidermal keratinocytes. 1-3 As its name implies, thrombomodulin functions to modulate the activity of the hemostatic protease thrombin. In endothelium, thrombomodulin inhibits thrombin’s procoagulant effects while markedly enhancing thrombin-dependent activation of anticoagulant protein C. 1 Thrombomodulin also promotes thrombin-dependent activation of thrombin-activatable fibrinolysis inhibitor (TAFI), a carboxypeptidase that inhibits activation of plasminogen in fibrin clots. 4-6 Although thrombomodulin was originally identified as an endothelial protein, 7-9 it is also expressed by epidermal keratinocytes and some other types of cells. 10-12 The function of thrombomodulin in epidermis is unknown, but its expression is tightly regulated during keratinocyte differentiation. 3,11,13 Keratinocyte thrombomodulin is able to bind to thrombin and promote activation of protein C. 11-15
Because thrombin may affect wound healing by stimulating the proliferation of keratinocytes and fibroblasts, 16 we hypothesized that thrombomodulin may regulate reepithelialization and/or collagen deposition during cutaneous wound healing. In a previous study, we examined effects of increased keratinocyte thrombomodulin activity on wound healing in transgenic mice that express human thrombomodulin under the direction of a keratinocyte promoter. 14 These mice, which had up to 300% of normal thrombomodulin activity in epidermis, exhibited normal wound reepithelialization but had evidence of impaired collagen deposition during cutaneous wound healing. 14
The goals of the present study were to determine whether expression of thrombomodulin is regulated during cutaneous wound healing in humans and mice, and to determine whether wound healing is altered in thrombomodulin-deficient mice. Because mice with complete absence of thrombomodulin do not survive the embryonic period, 17 we chose to compare wound healing in wild-type mice and three groups of mice, generated by homologous recombination, that have moderate or severe thrombomodulin deficiency. Our results demonstrate that expression of thrombomodulin in keratinocytes during cutaneous wound healing is regulated similarly in humans and mice, but that deficiency of thrombomodulin anticoagulant activity does not alter reepithelialization of cutaneous wounds.
Materials and Methods
Cutaneous Wounds in Humans
After obtaining informed consent, punch biopsies (3–5 mm) of skin graft donor sites were performed in eight adult patients who required partial-thickness skin grafting for management of burn wounds. Donor sites were biopsied at the time of harvest (day 0), and on days 5, 10, and 30 after harvest. Local anesthesia was achieved by subdermal injection of 1% Xylocaine before biopsy, and biopsy sites were treated with topical antimicrobials. The protocol was approved by the University of Iowa Human Subjects Review Committee.
Cutaneous Wounds in Mice
Wound healing was studied in the following groups of mice: 1) heterozygous thrombomodulin-deficient (TM+/−) mice (n = 5) that were generated from crosses of TM+/− mice 17 with C57BL/6 mice; 2) wild-type (TM+/+) mice (n = 13) that were either C57BL/6 mice obtained from the Jackson Laboratory (Bar Harbor, ME) or wild-type littermates from crosses of TM+/− mice with C57BL/6 mice; 3) compound heterozygous (TMpro/−) mice (n = 12) that have one null thrombomodulin allele and one mutant allele (replacement of glutamic acid 404 with proline); 18 4) TM−/− chimeric mice (n = 15) that were generated by injecting homozygous thrombomodulin-null embryonic stem (ES) cells into the blastocele of either C57BL/6 or ROSA 26 embryos. 19 ROSA 26 mice constitutively express β-galactosidase, which facilitates detection of non-ES-derived cells in chimeric mice. 20
Cutaneous wounds were created as described previously. 14 Sixty different wounds were created in a total of 45 mice. Mice (3–4 months of age) were anesthetized with sodium pentobarbital (50 mg/kg ip). Hair was shaved from the dorsal surface, and one or two full-thickness wounds (5–10 mm in diameter) were created through the panniculus carnosis. Wounds were allowed to heal for 3, 7, or 30 days, and then the mice were sacrificed and the wounds were removed for histological analysis. The protocol was approved by the University of Iowa, Veterans Affairs, and Massachusetts Institute of Technology Animal Care and Use Committees.
Histological Analysis
Human tissue was fixed in 10% formalin and embedded in paraffin. Immunoperoxidase staining for human thrombomodulin was performed using monoclonal antibody TM1009 (Dako Corp., Carpinteria, CA) as described previously. 3,11 Mouse wounds were bisected; one half of each wound was fixed in 10% formalin, and the other half was frozen in O.C.T. compound (Allegiance Health Care Corp., Omaha, NE). Formalin-fixed, paraffin-embedded sections of all wounds were stained with Masson’s trichrome or analyzed for murine thrombomodulin by immunoperoxidase staining, using the monoclonal antibody 273-34A 21 (generously provided by Dr. Stephen J. Kennel, Oak Ridge National Laboratory, Oak Ridge, TN) as described previously. 14
To detect β-galactosidase activity, frozen sections were fixed for 5 minutes in acetone and washed two times with phosphate-buffered saline. Sections were then incubated in phosphate-buffered saline containing 35 mmol/L K3Fe(CN)6, 35 mmol/L K4Fe(CN)6, 2 mmol/L MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) (Gold Biotechnology, St. Louis, MO) and counterstained with nuclear fast red.
Results
Expression of Thrombomodulin during Cutaneous Wound Healing in Humans
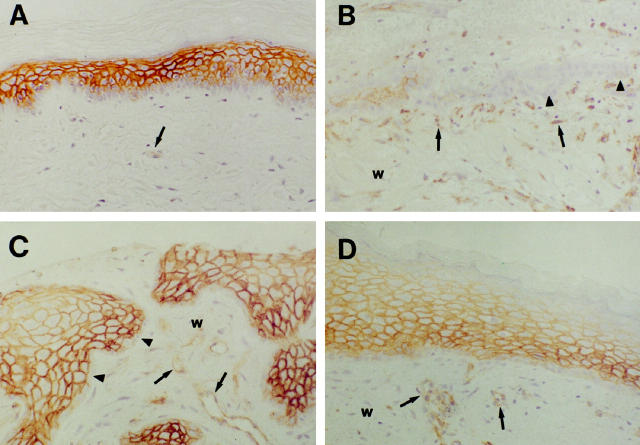
To determine the pattern of expression of thrombomodulin in keratinocytes during cutaneous wound healing, partial-thickness wounds from skin graft donor sites were biopsied at the time of harvest and at various times of healing, up to 30 days after harvest. In unwounded human epidermis, thrombomodulin staining was absent in basal keratinocytes and most intense in suprabasal spinous keratinocytes, and diminished superficially within granular and cornified keratinocytes (Figure 1A) ▶ . Thrombomodulin was also detected in endothelium within the dermis. On day 5 of healing, tongues of neoepidermis were seen invading the provisional wound matrix (Figure 1B) ▶ . Keratinocytes at the margins of the neoepidermal tongues stained weakly or did not stain for thrombomodulin, but keratinocytes within areas of neoepidermal stratification stained strongly for thrombomodulin. On day 10 of healing, the neoepidermal tongues were more highly stratified, and neoepidermal staining for thrombomodulin was more prominent (Figure 1C) ▶ . The marginal layer of neoepidermal keratinocytes remained thrombomodulin-negative. By day 30 of healing, wounds were completely reepithelialized, and thrombomodulin staining was prominent in suprabasal keratinocytes (Figure 1D) ▶ .
Figure 1.
Immunohistochemical localization of thrombomodulin during wound healing in human skin. In unwounded human epidermis (A), thrombomodulin staining was absent in basal keratinocytes, most intense in suprabasal spinous and granular keratinocytes, and diminished in cornified keratinocytes. On day 5 of healing (B), keratinocytes at the margins of neoepidermal tongues did not stain for thrombomodulin (arrowheads), but keratinocytes within areas of neoepidermal stratification stained strongly for thrombomodulin. On day 10 of healing (C), staining for thrombomodulin within the neoepidermis was even more prominent, but keratinocytes at the margins of the neoepidermis continued to stain only weakly for thrombomodulin (arrowheads). By day 30 (D), wounds were completely reepithelialized, and thrombomodulin staining was prominent in suprabasal keratinocytes. w, wound matrix; small arrows, endothelium. Magnification, ×200.
Expression of Thrombomodulin during Cutaneous Wound Healing in Mice
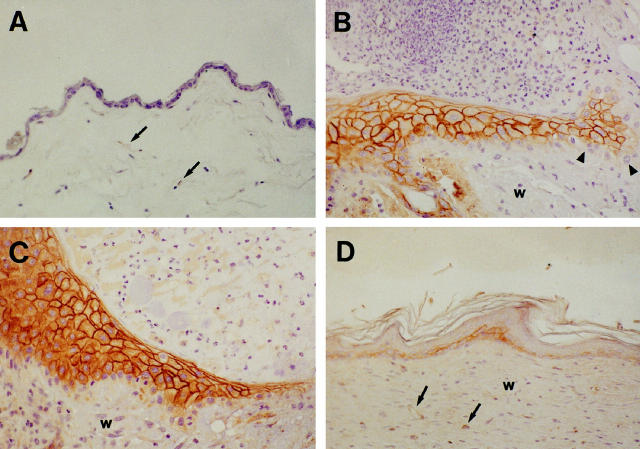
To determine whether expression of thrombomodulin is regulated during cutaneous wound healing in mice, we examined full-thickness wounds in wild-type (TM+/+) mice. Compared to human epidermis, the epidermis of unwounded mouse skin was less stratified, and epider- mal staining for thrombomodulin was much less promi-nent (Figure 2A) ▶ . On day 3 of healing, strong staining for thrombomodulin was observed in keratinocytes within the stratifying neoepidermis (Figure 2B) ▶ . No staining for thrombomodulin was detected in keratinocytes in the marginal layer of the neoepidermis, which interacts directly with the provisional wound matrix (Figure 2B) ▶ . This pattern of thrombomodulin expression was similar to that observed in human partial-thickness wounds after 5 days of healing, except that expression of thrombomodulin by keratinocytes within the interior of the neoepidermis was even more exuberant in mouse wounds than in human wounds. On day 7 of healing of mouse wounds, a highly stratified, thrombomodulin-positive neoepidermis extended over most of the wound matrix (Figure 2C) ▶ . On day 30, mouse wounds were completely reepithelialized, with some residual hyperkeratinization and a more prominent thrombomodulin staining pattern than that seen in unwounded mouse skin (Figure 2D) ▶ . Thus, although thrombomodulin is expressed only weakly in unwounded mouse epidermis, its expression in keratinocytes is markedly up-regulated during cutaneous wound healing.
Figure 2.
Immunohistochemical localization of thrombomodulin during wound healing in mouse skin. In unwounded mouse skin, epidermal staining for thrombomodulin was very weak (A). On day 3 of healing (B), a neoepidermal tongue extended from the margin of the wound (left side of image) into the wound matrix (right side of image). Strong staining for thrombomodulin was observed in keratinocytes in areas of stratification within the neoepidermis, but keratinocytes at the margins of the neoepidermis (arrowheads) did not stain for thrombomodulin. On day 7 of healing (C), a highly stratified, thrombomodulin-positive neoepidermis extended over most of the wound matrix. On day 30 (D), mouse wounds were completely reepithelialized, with some residual hyperkeratinization and a more prominent thrombomodulin staining pattern than that seen in unwounded mouse skin. w, wound matrix; small arrows, endothelium. Magnification ×200.
Cutaneous Wound Healing in Thrombomodulin-Deficient Mice
The observation that expression of thrombomodulin is regulated similarly during cutaneous wound healing in humans and mice suggested that the mouse would be an appropriate animal model for examining effects of thrombomodulin gene dosage on cutaneous wound healing. Therefore, full-thickness wounds were created in three groups of mice with varying degrees of thrombomodulin deficiency. The first group consisted of wild-type (TM+/+) mice. The second group consisted of heterozygous thrombomodulin-deficient (TM+/−) mice that were generated from matings of TM+/− mice with C57BL/6 mice. TM+/− mice have approximately 50% thrombomodulin anticoagulant activity compared with TM+/+ mice. 17 The third group consisted of compound heterozygous (TMpro/−) mice that have one null allele and one mutant thrombomodulin allele. TMpro/− mice have <1% thrombomodulin anticoagulant activity compared with TM+/+ mice. 18 Wounds were allowed to heal for 3, 7, or 30 days and then were harvested and analyzed histologically. No differences in rate or extent of reepithelialization were observed between TM+/+, TM+/−, or TMpro/− mice. On day 3, all wounds exhibited marked neoepidermal proliferation at the margin of the provisional wound matrix (not shown). On day 7, all wounds exhibited a highly stratified neoepidermis extending from the margin of the wound and overlying much of the wound matrix (Figure 3, A, C, E) ▶ , and by day 30 all wounds were completely reepithelialized (Figure 3, B, D, F) ▶ . We did observe some differences between the three groups of mice in collagen staining by Mason’s trichrome within the wound matrix. The overall intensity of collagen staining was similar in TM+/+, TM+/−, and TMpro/− mice, but foci of increased collagen deposition were apparent in the wound matrices of TM± and TMpro/− mice compared to TM+/+ mice on day 7 (compare Figure 3, A, C, E ▶ ). On day 30, dense collagen staining was observed in all three groups of mice, but collagen bundles tended to be thicker and denser in TM+/− and TMpro/− mice (Figure 3, D, F) ▶ than in TM+/+ mice (Figure 3B) ▶ .
Figure 3.
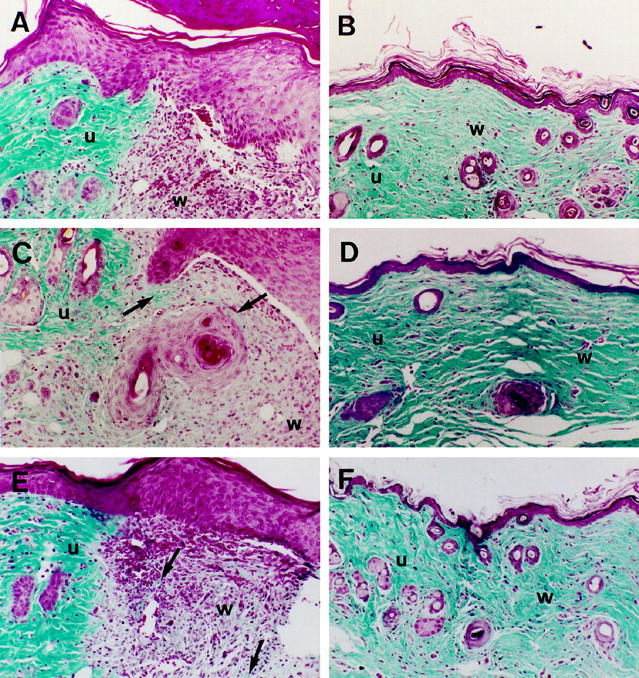
Cutaneous wound healing in thrombomodulin-deficient mice. No differences in rate or extent of reepithelialization were observed between TM+/+ (A, B), TM+/− (C, D), or TMpro/− (E, F) mice. On day 7, foci of increased staining for collagen (arrows) were apparent in the wound matrices of TM+/− (C) and TMpro/− (E) mice compared to TM+/+ (A) mice. On day 30, dense collagen staining was observed in all three groups of mice, but collagen bundles tended to be thicker in TM+/− (D) and TMpro/− (F) mice than in TM+/+ mice (B). u, unwounded skin; w, wound matrix. Masson’s trichrome stain; magnification, ×150.
Expression of Thrombomodulin and Cutaneous Wound Healing in Chimeric Mice
The experiments described above in thrombomodulin-deficient mice demonstrated that reepithelialization of cutaneous wounds proceeded essentially normally in mice that have as little as 1% of normal thrombomodulin anticoagulant activity. In an earlier study, we found that reepithelialization of cutaneous wounds also proceeded normally in transgenic mice with up to 300% of normal epidermal thrombomodulin activity. 14 Therefore, large differences in thrombomodulin anticoagulant activity do not appear to have major consequences for wound reepithelialization. Because homozygous deficiency of thrombomodulin results in embryonic lethality in mice, 17 however, it is still not known whether keratinocytes that lack thrombomodulin completely can function normally during cutaneous wound healing. To address this question, we examined cutaneous wound healing in chimeric mice that were generated by injection of TM−/− ES cells into blastoceles from C57BL/6 or ROSA 26 mice. The degree of chimerism, based on coat coloration, of TM−/− chimeric mice varies between 40% and 80%. 19 In unwounded skin, patchy epidermal staining for β-galactosidase was observed in TM−/− ROSA 26 chimeric mice, which indicated that both TM+/+ and TM−/− keratinocytes were present in the epidermis (not shown). Like wounds in TM+/− and TMpro/− mice, wounds in TM−/− chimeric mice exhibited a prominent neoepidermis after 3–7 days of healing (Figure 4) ▶ . In some TM−/− chimeric mice, a majority of neoepidermal keratinocytes stained strongly for thrombomodulin (Figure 4A) ▶ , whereas in other TM−/− chimeric mice there was a relative paucity of thrombomodulin-positive keratinocytes in the neoepidermis (Figure 4B) ▶ . In all TM−/− chimeric mice, both thrombomodulin-positive and thrombomodulin-negative keratinocytes were detected within the stratified portion of the neoepidermis.
Figure 4.

Immunohistochemical localization of thrombomodulin during cutaneous wound healing in TM−/− chimeric mice after 3–7 days of healing. In some TM−/− chimeric mice, a majority of neoepidermal keratinocytes stained strongly for thrombomodulin (A), whereas in other TM−/− chimeric mice there was a relative paucity of thrombomodulin-positive keratinocytes in the neoepidermis (B). Arrowheads, magnification, thrombomodulin-positive keratinocytes; magnification, ×250.
Discussion
In this study we found that expression of thrombomodulin in keratinocytes during cutaneous wound healing is regulated similarly in humans and mice. In both species, very little thrombomodulin was detected in the marginal layers of keratinocytes that interact directly with components of the wound matrix. In contrast, expression of thrombomodulin in wound keratinocytes was strongly up-regulated in areas of stratification within the interior of the neoepidermis, particularly during the first 10 days of healing. This tightly regulated pattern of thrombomodulin expression in wound keratinocytes is reminiscent of that seen in unwounded human epidermis and other types of stratifying squamous epithelium, in which thrombomodulin is absent in the basal epithelial layer but expressed strongly in suprabasal keratinocytes. 3;11 These findings suggest that keratinocyte thrombomodulin may not interact directly with components of the provisional wound matrix, but instead may regulate thrombin or interact with other proteins within the neoepidermis itself.
We had hypothesized that thrombomodulin might regulate wound reepithelialization through one of several potential mechanisms. First, thrombomodulin might inhibit the effects of thrombin on keratinocyte thrombin receptors such as PAR-1 or PAR-3, 22 resulting in decreased thrombin-stimulated keratinocyte proliferation. 23 Second, thrombomodulin might inhibit local thrombin generation, indirectly causing decreased thrombin-stimulated keratinocyte proliferation. Third, thrombomodulin might increase activation of TAFI, 4 resulting in decreased generation of plasmin and a decreased rate of reepithelialization. Plasminogen is activated to plasmin during wound healing, 24 and reepithelialization of cutaneous wounds is delayed in plasminogen-deficient mice. 25 Fourth, the extracellular lectin-like domain of thrombomodulin, which has been proposed to function in cell-cell adhesion, 26 might support keratinocyte adhesion during reepithelialization.
Because complete deficiency of thrombomodulin produces embryonic lethality in mice, 17 we chose to examine effects of thrombomodulin on wound reepithelialization in mice with either moderate (TM+/−) or severe (TMpro/−) deficiency of thrombomodulin anticoagulant activity. TM+/− mice have an apparently normal phenotype under ambient conditions, but they are susceptible to increased deposition of cross-linked fibrin in response to hypoxia. 27 TMpro/− mice exhibit a more severe thrombotic phenotype than TM+/− mice, with spontaneous deposition of fibrin in the heart, lung, and spleen. 27 In contrast to these major effects of thrombomodulin deficiency on fibrin deposition, however, we found that reepithelialization of full-thickness cutaneous wounds proceeded normally in both TM+/− and TMpro/− mice. These results suggest that inhibition of thrombin by thrombomodulin is not a major mechanism of regulation of wound reepithelialization in mice. Our findings are consistent with a previous report of normal wound reepithelialization in mice lacking the major thrombin receptor PAR-1. 28
One limitation of wound healing studies in TM+/− and TMpro/− mice is that, although these mice have decreased thrombomodulin anticoagulant activity, they still express approximately 50% of wild-type levels of thrombomodulin protein. Therefore, effects of thrombomodulin that may be independent of thrombin binding, such as potential effects of the amino-terminal lectin-like domain, cannot be assessed in these mice. To try to circumvent this problem, we also examined wound healing in TM−/− chimeric mice. We found that reepithelialization proceeded normally in TM−/− chimeric mice, and that both thrombomodulin-positive and thrombomodulin-negative keratinocytes were present within the neoepidermis. These observations indicate that keratinocytes that lack thrombomodulin completely can participate in normal reepithelialization. Because some thrombomodulin-positive keratinocytes were present in the chimeric mice, however, we cannot completely exclude the possibility that the lectin-like domain, or another thrombin-independent domain of thrombomodulin, is necessary for normal wound reepithelialization. This question could be addressed more definitively by generating animals with skin-specific deletion of the thrombomodulin gene.
Although we did not detect abnormalities of wound reepithelialization in thrombomodulin-deficient mice, we did observe foci of increased collagen deposition in TM+/− and TMpro/− mice. We speculate that increased deposition of collagen in these mice may have resulted from increased stimulation of wound fibroblasts by thrombin. Thrombin’s effects on wound fibroblasts may be regulated not only by thrombomodulin expressed in keratinocytes, but also by thrombomodulin expressed in endothelial cells within granulation tissue. Another potential mechanism for increased collagen deposition in thrombomodulin-deficient mice is decreased thrombomodulin-dependent activation of protein C. Like thrombomodulin expressed in endothelium, thrombomodulin expressed in keratinocytes is capable of promoting activation of protein C. 11-15 In addition to its anticoagulant effects, activated protein C also has anti-inflammatory effects on monocytes and macrophages. 29 Therefore, decreased activation of protein C could result in increased cytokine-mediated stimulation of collagen deposition by fibroblasts in the wound matrix.
Acknowledgments
We thank Rochelle Erger, Jesse Courtier, Robert Lewis, Mary Maher-Sturm, Christine Bromley, and Jan Rodgers for technical assistance.
Footnotes
Address reprint requests to Dr. Steven R. Lentz, Department of Internal Medicine, C303 GH, University of Iowa, Iowa City, IA 52242. E-mail: steven-lentz@uiowa.edu.
Supported by the Office of Research and Development, Department of Veterans Affairs, National Institutes of Health grants HL-07344 and DK-25295, and the Roy J. Carver Charitable Trust.
References
- 1.Esmon CT: Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J 1995, 9:946-955 [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE: Thrombomodulin structure and function. Thromb Haemost 1997, 78:392-395 [PubMed] [Google Scholar]
- 3.Lager DJ, Callaghan EJ, Worth SF, Raife TJ, Lentz SR: Cellular localization of thrombomodulin in human epithelium and squamous malignancies. Am J Pathol 1995, 146:933-943 [PMC free article] [PubMed] [Google Scholar]
- 4.Bajzar L, Morser J, Nesheim M: TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem 1996, 271:16603-16608 [DOI] [PubMed] [Google Scholar]
- 5.Kokame K, Zheng XL, Sadler JE: Activation of thrombin-activable fibrinolysis inhibitor requires epidermal growth factor-like domain 3 of thrombomodulin and is inhibited competitively by protein C. J Biol Chem 1998, 273:12135-12139 [DOI] [PubMed] [Google Scholar]
- 6.Bajzar L, Nesheim M, Morser J, Tracy PB: Both cellular and soluble forms of thrombomodulin inhibit fibrinolysis by potentiating the activation of thrombin-activable fibrinolysis inhibitor. J Biol Chem 1998, 273:2792-2798 [DOI] [PubMed] [Google Scholar]
- 7.Esmon CT, Owen WG: Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA 1981, 78:2249-2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackman RW, Beeler DL, VanDeWater L, Rosenberg RD: Characterization of a thrombomodulin cDNA reveals structural similarity to the low density lipoprotein receptor. Proc Natl Acad Sci USA 1986, 83:8834-8838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen D, Dittman WA, Ye RD, Deaven LL, Majerus PW, Sadler JE: Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry 1987, 26:4350-4357 [DOI] [PubMed] [Google Scholar]
- 10.Yonezawa S, Maruyama I, Sakae K, Igata A, Majerus PW, Sato E: Thrombomodulin as a marker for vascular tumors. Comparative study with factor VIII and Ulex europaeus I lectin. Am J Clin Pathol 1987, 88:405–411 [DOI] [PubMed]
- 11.Raife TJ, Lager DJ, Madison KC, Piette WW, Howard EJ, Sturm MT, Chen Y, Lentz SR: Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J Clin Invest 1994, 93:1846–1851 [DOI] [PMC free article] [PubMed]
- 12.Mizutani H, Hayashi T, Nouchi N, Ohyanagi S, Hashimoto K, Shimuzu M, Suzuki K: Functional and immunoreactive thrombomodulin expressed by keratinocytes. J Invest Dermatol 1994, 103:825-828 [DOI] [PubMed] [Google Scholar]
- 13.Raife TJ, Demetroulis EM, Lentz SR: Regulation of thrombomodulin expression by all-trans retinoic acid and tumor necrosis factor-α: differential responses in keratinocytes and endothelial cells. Blood 1996, 88:2043-2049 [PubMed] [Google Scholar]
- 14.Raife TJ, Lager DJ, Peterson JJ, Erger RA, Lentz SR: Keratinocyte-specific expression of human thrombomodulin in transgenic mice: effects on epidermal differentiation and cutaneous wound healing. J Invest Med 1998, 46:127-133 [PubMed] [Google Scholar]
- 15.Senet P, Peyri N, Berard M, Dubertret L, Boffa MC: Thrombomodulin, a functional surface protein on human keratinocytes, is regulated by retinoic acid. Arch Dermatol Res 1997, 289:151-157 [DOI] [PubMed] [Google Scholar]
- 16.Carney DH, Mann R, Redin WR, Pernia SD, Berry D, Heggers JP, Hayward PG, Robson MC, Christie J, Annable C: Enhancement of incisional wound healing and neovascularization in normal rats by thrombin and synthetic thrombin receptor-activating peptides. J Clin Invest 1992, 89:1469-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy AM, Rayburn HB, Rosenberg RD, Weiler H: Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci USA 1995, 92:850-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD: A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest 1998, 101:1983-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy AM, Hancock WV, Christie PD, Rayburn HB, Rosenberg RD: Intravascular coagulation activation in a murine model of thrombomodulin deficiency: effects of lesion size, age, and hypoxia on fibrin deposition. Blood 1998, 92:4188-4197 [PubMed] [Google Scholar]
- 20.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991, 5:1513-1523 [DOI] [PubMed] [Google Scholar]
- 21.Ford VA, Wilkinson JE, Kennel SJ: Thrombomodulin distribution during murine development. Rouxs Arch Dev Biol 1993, 202:364-370 [DOI] [PubMed] [Google Scholar]
- 22.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Coughlin SR: Role of the thrombin receptor in development and evidence for a second receptor. Nature 1996, 381:516-519 [DOI] [PubMed] [Google Scholar]
- 23.Talwar HS, Fisher GJ, Harris VA, Voorhees JJ: Agonist-induced hydrolysis of phosphoinositides and formation of 1,2-diacylglycerol in adult human keratinocytes. J Invest Dermatol 1989, 93:241-245 [DOI] [PubMed] [Google Scholar]
- 24.Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 [DOI] [PubMed] [Google Scholar]
- 25.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, Dano K: Impaired wound healing in mice with a disrupted plasminogen gene. Nature Med 1996, 2:287-292 [DOI] [PubMed] [Google Scholar]
- 26.Jackson DE, Mitchell CA, Mason G, Salem HH, Hayman JA: Altered thrombomodulin staining in blistering dermatoses. Pathology 1996, 28:225-228 [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg RD: Thrombomodulin gene disruption and mutation in mice. Thromb Haemost 1997, 78:705-709 [PubMed] [Google Scholar]
- 28.Connolly AJ, Suh DY, Hunt TK, Coughlin SR: Mice lacking the thrombin receptor, PAR1, have normal skin wound healing. Am J Pathol 1997, 151:1199-1204 [PMC free article] [PubMed] [Google Scholar]
- 29.Grey ST, Hancock WW: A physiologic anti-inflammatory pathway based on thrombomodulin expression and generation of activated protein C by human mononuclear phagocytes. J Immunol 1996, 156:2256-2263 [PubMed] [Google Scholar]