Abstract
Granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) and/or their receptors are increasingly detected in solid human tumors, although little is known about their function in tumor growth and invasion. We analyzed RNA and protein expression of both factors and their receptors in 22 human gliomas (WHO grade II, III, and IV) and derived cell cultures. G-CSF, GM-CSF, and/or their receptors were expressed in all tumors and derived cell cultures, but coexpression of both factors and receptors was almost exclusively found in grade IV glioblastomas and thus correlated with advanced tumor stage. The functional significance of G-CSF and GM-CSF as regulators for glioma cells was demonstrated by 1) stimulation of proliferation and migration in tumor cells expressing one or both receptors by the corresponding factor; 2) inhibition of growth and migration of glioma cells expressing G-CSF, GM-CSF, and their receptors by neutralizing antibodies to both factors. These results indicate a significant role for both factors in the autocrine regulation of growth and migration in late-stage malignant gliomas and suggest a shift from paracrine to autocrine regulation with tumor progression. The implication of G-CSF and GM-CSF in glioblastoma growth regulation could make these factors further prognostic indicators and raises questions concerning their use in cancer therapy.
Gliomas are the most common primary malignant brain tumors in adults. Their most malignant form, the glioblastoma multiforme, forms highly vascularized, uniformly fatal neoplasms. Glioma cells, like all tumor cells, are characterized by their uncontrolled growth and increasing escape from regulatory mechanisms of the environment. Current research on brain tumor biology focuses on the mechanisms underlying the stimulation of tumor proliferation and/or angiogenesis. In the past several years a number of growth factors have been identified that stimulate glioma cell growth and glioma-induced angiogenesis through autocrine or paracrine mechanisms ( ref 1 and references therein, and refs 2-4 ). These new insights into the biology of gliomas offer promising therapeutic concepts that are currently under investigation. 5-8 To date the prognosis of patients with malignant glioma remains poor, offering a median survival time of only 1 year, despite aggressive treatments, including surgical resection and radio- and chemotherapy. 9,10 The latter two treatments are limited by two major complications, neutropenia and sepsis. The hematopoietic growth factors granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) were shown to be of therapeutic value in different conditions of primary and secondary bone marrow dysfunction. 11 Consequently they now find widespread clinical application in reducing the duration and severity of neutropenic periods in routine cancer therapy. 12-15
G-CSF and GM-CSF were originally identified as factors controlling proliferation, maturation, and functional activity of granulocytes, macrophages, and their precursors. 16,17 Whereas GM-CSF, a 22-kd glycoprotein, was first defined by its effect in vitro on granulocyte and macrophage colony formation, it is now clear that this factor also acts on multipotent stem cells. 18 GM-CSF binds to a dimeric receptor consisting of a ligand-specific α subunit and a β subunit that is shared between GM-CSF, interleukin 3 (IL-3), and interleukin 5 (IL-5) receptor. 19,20 G-CSF, a 30-kd glycoprotein, was first defined as a granulocyte stimulator and leukemic differentiation factor. The factor also acts on undifferentiated stem cells, has some macrophage-stimulating activity, and, synergistically with other factors, stimulates megakaryocyte colony formation. G-CSF binds to a group of three receptors that are products of the same gene and differ from each other by different mRNA processing at their 3′ end. 21,22 Besides their roles as growth and differentiation factors in the hematopoietic system, G-CSF and GM-CSF seem to have a much broader spectrum of activities. Both factors are produced by fibroblasts, 23,24 keratinocytes, 25,26 and endothelial cells 27 and stimulate the growth of fibroblast precursor cells 27 and keratinocytes. 28,29 In addition, both G-CSF and GM-CSF can stimulate endothelial cell proliferation and migration and may therefore play a role in angiogenesis. 30,31
The use of G-CSF and GM-CSF in patients with malignant tumors relies on data showing no influence of either growth factor on tumor growth. 32-34 Recently there has been an increasing number of reports describing the expression of G-CSF and/or GM-CSF by nonhematopoietic human tumor cells, 35-38 suggesting a potential role of these factors in tumor growth and invasion. 36,39,40
Previous studies on the expression of either G-CSF or GM-CSF alone in gliomas provided rather contradictory results, claiming that the factors were either expressed exclusively in vitro but not in vivo 41,42 or were expressed in vivo without showing an autocrine stimulatory function in vitro. 38,43-45 These studies analyzed the expression of both factors in a limited number of established human and rodent glioma cell lines and compared it with the expression in patient tissue samples. To date there is no conclusive study on the expression of G-CSF and GM-CSF and their receptors in tumor tissue and derived cells in culture or on the functional role of both factors in glioma progression. Here we demonstrate the expression of G-CSF and GM-CSF and their receptors in glioma tissues of different WHO grading and derived cell cultures and show the functional significance of both factors in the stimulation of tumor cell proliferation and migration in an autocrine mechanism.
Materials and Methods
Cell Lines and Tumor Tissues
Tumor tissue was obtained intraoperatively and either immediately frozen in liquid nitrogen for cryosectioning and RNA extraction or transferred to RPMI 1640 medium with 10% fetal calf serum (FCS) for further cultivation in vitro. The tumor tissue was minced mechanically, and 2 ml cell/tissue pellet was cultivated in one 75-mm tissue culture flask in RPMI medium with 10% FCS. Fresh medium was added twice a week. After they reached confluence cells were passaged at a split ratio of 1:2. For RNA extraction and further experiments, most cultures were used in low passages (passage 1–11); selected cultures were also used in higher passages. Mycoplasma contamination of the cell lines was excluded with the Boehringer Mannheim Mycoplasma test kit.
Cell Identification and Characterization
The glial origin of the cultured cells was confirmed by staining with an anti-glial fibrillary acidic protein (anti-GFAP) antibody (GF12.24; Progen, Heidelberg, Germany). Only cell cultures that showed a homogeneous GFAP staining in all cells were used for these studies. The glioma cell origin of all cultures used in functional studies was additionally confirmed by a homogeneous staining against endothelial growth factor (EGF) receptor (Dako, Hamburg, Germany). Furthermore, endothelial and neuronal cell contamination of these cultures could not be detected after staining with an anti-platelet/endothelial cell adhesion molecule antibody (clone WM59; Pharmingen, San Diego, CA), anti-factor VII antibody (clone F8/86; Dako), and an antibody against neurofilament protein 160 kd (clone NF403; Progen, Heidelberg, Germany) and 70 kd as well as 200 kd (clone 2S11; Progen). Interestingly, cultures originating from different tumor tissues differ in their morphologies and cell densities in vitro.
Conditioned Media and Enzyme-Linked Immunosorbent Assay
For preparation of conditioned media, 6 × 10 4 cells/cm 2 were seeded into 6-cm dishes in RPMI, 10% FCS. After 24 hours, the medium was shifted to RPMI, 0% FCS, and 4 days later the conditioned medium was harvested, centrifuged for 10 minutes at 10,000 × g, and stored in aliquots at −70°C. G-CSF and GM-CSF enzyme-linked immunosorbent assays (ELISAs) were performed in conditioned media, using commercially available kits, according to the manufacturers’ instructions (GM-CSF EIASA from Medigenix, Brussels, Belgium; G-CSF ELISA from Amersham, Braunschweig, Germany).
Proliferation Assay
Cells were seeded in eight replicas on 96-well plates at a cell density of 1 × 10 4 cells in RPMI, 10% FCS. After 24 hours, the medium was shifted to RPMI, 0% FCS, and 0, 100, and 150 ng/ml G-CSF and GM-CSF (factors with an activity of 10,000 U/μg were a generous gift of Boehringer Mannheim GmbH, Mannheim, Germany), or, alternatively, 2 μg/ml anti-G-CSF blocking antibody (clone G 61.8.1; from Oncogene Research, Cambridge, MA) or 2 μg/ml anti-GM-CSF antibody (clone GM 4.1.9; from Oncogene Research) or both antibodies together were added. As a control antibody 2 μg/ml of an mouse monoclonal IgG antibody directed against an unrelated protein was added. Forty-eight hours after growth factor or antibody addition, bromodesoxyuridine (BrdU) (BrdU labeling and detection kit III; Boehringer Mannheim GmbH) was added to the wells for 24 hours at a final concentration of 10 mmol/L, and proliferation was analyzed colorimetrically based on BrdU incorporation. The assay was processed according to the manufacturers’ instructions. Optical density was determined, and the mean value of the control samples containing no growth factor or antibody was arbitrarily set to 1. Values are means of three independent experiments (eight replicas each) with SD.
Cell Migration Assay
Cells were seeded in RPMI, 10% FCS, in two replicas at a density of 75,000 cells per well on six-well plates. Twenty-four hours after the cells had reached confluence the monolayer was wounded by scraping with a cell scraper of 1 cm width. The borders of the cell-free area were marked. Medium was shifted to RPMI, 0% FCS, and 100 ng/ml G-CSF or 100 ng/ml GM-CSF or, alternatively, 2 μg/ml anti-G-CSF antibody (clone G 61.8.1) or 2 μg/ml anti-GM-CSF antibody (clone GM 4.1.9) was added. As a control antibody, 2 μg/ml of a mouse monoclonal IgG antibody directed against an unrelated protein was added. Cell migration was documented by phase-contrast microscopy, with photos taken at time point 0 (ie, immediately after scraping), as well as 24 and 48 hours later. Migration was quantified by measuring the migration distance on photomicrographs at a magnification of ×200. The values with standard deviations are means of two experiments with two replica platings each.
Antibodies and Immunohistochemistry
Cells were grown on slides, and, alternatively, frozen 6-μm sections were mounted on 3-aminopropyl-triethoxy-silane-coated slides and air dried. Specimens were fixed either in 4% paraformaldehyde for 20 minutes at room temperature, followed by 5 minutes in 70% EtOH and 5 minutes in 100% EtOH, or in acetone for 15 minutes at −20°C and subsequently air dried. PFA-fixed slides were digested with pronase E (1 mg/ml) for 3 minutes at room temperature. Slides were incubated overnight at 4°C with the first antibody, then washed and incubated at room temperature for 30 minutes with the second, biotinylated anti-Ig, antibody. Antibody detection (Vectastain; from Vector Laboratories, Camon Wiesbaden, Germany) using a horseradish peroxidase-conjugated streptavidin complex resulted in an intense red precipitate. The cells were faintly counterstained with Mayer’s hematoxylin, air-dried, and mounted. The monoclonal antibodies LMM741 (from Pharmingen, Hamburg, Germany) and K12B7 (from Serotec, Camon Wiesbaden, Germany) were used to detect the G-CSF receptor and the α subunit of the GM-CSF receptor, respectively. For staining of G-CSF and GM-CSF, monoclonal anti-G-CSF antibody (clone G 61.8.1; from Oncogene Research) and anti-GM-CSF antibody (clone GM 4.1.9; from Oncogene Research) were used. The GM-CSF antibody required slides fixed with 4% paraformaldehyde. All other antibodies were used after acetone fixation of frozen sections. For control of general nonspecific staining the secondary antibody alone was used.
Oligonucleotide Primers
Sense and antisense primers were synthesized according to the sequences extracted from genbank. The primers used were as follows:
G-CSF (bp 93–118, sense) CACAGTGCACTCTGGACAGTGCAGG
(bp 479–508, antisense) TAGACCGTCGTCTACCTTCTTGACCCTTAC
G-CSF receptor (bp 1141–1160, sense) CCTGGAGCTGAGAACTACCG
(bp 1431–1450, antisense) GCCACCAGAAGAGTCTTTCG
GM-CSF (bp 42–62, sense) TGGCCTGCAGCATCTCTGCA
(bp 344–364, antisense) ACACGTTGGGTCTGATAGTG
GM-CSF receptor β (bp 981-1000, sense) AATACATCGTCTCTGTTCAG
(bp 1297–1317, antisense) TCACTCCACTCGCTCCAGAT
β-actin (bp 2608–2627, sense) GAAGTGTGACGTGGACATC
(bp 2945–2965, antisense) ACTCGTCATACTCCTGCTTG
All oligonucleotide primer pairs spanned intron-exon splice sites, ensuring that PCR products did not derive from any DNA present in the RNA preparations. The identity of the PCR amplification products was confirmed by size and restriction digest.
Factor and receptor expression levels were analyzed semiquantitatively by comparison with the expression of β-actin mRNA.
RNA Isolation and RT-PCR
Total RNA was isolated as described by Chomczynski and Sacchi 46 from glioma cell cultures and frozen brain tissue. RT-PCR was performed using the Perkin-Elmer (Branchburg, NJ) Geneamp RT-PCR kit.
Reverse transcription was done in a volume of 100 μl, using 10 μg of total RNA; 2.5 U/μl MuLV reverse transcriptase; 1 mmol/L each of dATP, dGTP, dCTP, and dTTP, as well as 2.5 μM Oligo(dT)16 in 1 × PCR buffer II; and 1 U/μl RNase inhibitor. The 100-μl PCR reaction contained 9 μl of the RT reaction and 2.5 U AmpliTaq DNA polymerase. MgCl2 concentration and temperature were optimized for each primer set:
G-CSF: 2.75 mmol/L MgCl2, 35 cycles of 94°C 1 minute, 72°C 1 minute 30 s
G-CSF receptor: 2 mmol/L MgCl2, 35 cycles of 94°C 1 minute, 60°C 1 minute 30 s, 72°C 1 minute 30 s
GM-CSF: 2 mmol/L MgCl2, 25 cycles of 94°C 1 minute 30 s, 60°C 2 minutes, 72°C 3 minutes
GM-CSF receptor β: 2 mmol/L MgCl2, 35 cycles of 94°C 1 minute, 60.5°C 1 minute 30 s, 72°C 1 minute 30 s
β-actin: 2 mmol/L MgCl2, 25 cycles of 94°C 1 minute, 54°C 1 minute, 72°C 1 minute
RT-PCR experiments were done in triplicate; each culture passage is indicated in Table 1 ▶ , and representative results are shown in Figure 1 ▶ . The results of the GM-CSF PCR were also identical for higher cycle numbers. The lower number was chosen to obtain optimal signal strength for all probes tested.
Table 1.
Expression of G-CSF, GM-CSF, and Their Receptors in Cell Cultures (mRNA) and Their Original Tumor Tissue (Protein)
| Tumor stage | G-CSF | G-CSF receptor | GM-CSF | GM-CSF receptor | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA | Prot. | RNA | Prot. | RNA | Prot. | RNA | Prot. | |||
| 1 | NCH37§§ p11,28,38 | Glio. IV | ++ | * | ++ | ** | ++ | * | +(+) | * |
| NCH48§§p10 | Glio. IV | − | (+) | +(+) | − | |||||
| NCH57 p3 | Glio. IV | +(+) | ** | + | ** | (+) | * | ++ | * | |
| NCH60 p3 | Glio. IV | ++ | nd | + | nd | (+) | nd | + | nd | |
| NCH61§§ p15 | Glio. IV | +(+) | ** | +(+) | ** | − | − | ++ | ** | |
| NCH65 p4 | Glio. IV | + | nd | (+) | nd | − | nd | + | nd | |
| NCH69 p1 | Glio. IV | (+) | − | − | +(+) | |||||
| NCH70 p3 | Glio. IV | (+) | − | − | (+) | |||||
| NCH77§§ p6,24 | Glio. IV | +(+) | ** | ++ | ** | − | − | ++ | * | |
| NCH92 p3 | Glio. IV | +(+) | * | ++ | ** | +(+) | ** | ++ | ** | |
| 2 | NCH45 p6 | Astro. III/glio. IV | − | − | +(+) | + | ||||
| NCH46§§ p12 | Astro. III/glio. IV | − | − | ++ | (+) | |||||
| 3 | NCH76 p7 | Astro. III | (+) | * | +(+) | * | − | − | − | − |
| NCH84 p3 | Astro. III | − | +(+) | − | +(+) | |||||
| 4 | NCH91 p5,12 | Astro. II–III | (+) | ** | (+) | − | − | − | +(+) | * |
| NCH20 | Astro. II–III | ** | * | − | − | |||||
| NCH88 | Astro. II–III | ** | (*) | − | − | |||||
| NCH110 | Astro. II–III | ** | (*) | − | ** | |||||
| 5 | NCH83 p4 | Astro. II | − | +(+) | − | (+) | ||||
| NCH85 p1 | Astro. II | (+) | ** | ++ | − | − | ++ | |||
| NCH94 p3 | Astro. II | − | ++ | − | +(+) | |||||
| NCH58§§ p3,12 | Astro. II | − | − | − | + | |||||
| N.B. | Normal brain | − | − | − | − | − | − | (+) | − |
Samples with histopathological diagnosis are grouped according to decreasing malignancy. 1: Glioblastoma WHO grade IV (Glio.). 2: Glioblastoma WHO grade IV, developing within an astrocytoma WHO grade III. 3: Astrocytoma WHO grade III (Astro.). 4: Astrocytoma progressing from WHO grade II to grade III. 5: Astrocytoma WHO grade II. Glioma cell cultures were analyzed at early and later passages (p). Expression levels are estimated semiquantitatively in comparison to β-actin mRNA expression. nd, Not determined; −, no expression; +, weak expression; ++, stronger expression than +. Protein expression in the tumor tissue is indicated by * (weak expression) and ** (stronger expression than *). N.B., Normal brain. The cultures used for functional studies are marked by §§. For NCH20, 88, and 110, only protein data from tumor tissue are shown.
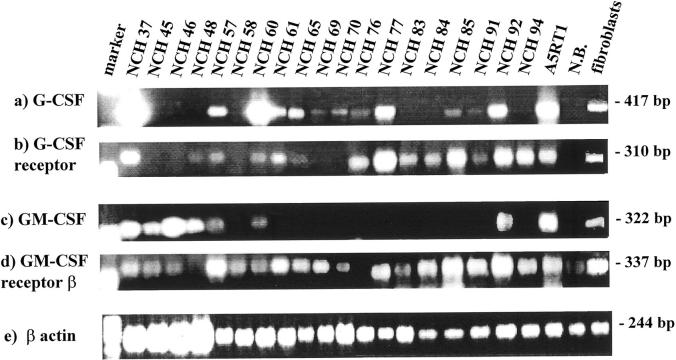
Figure 1.
Expression of G-CSF (a), G-CSF receptor (b), GM-CSF (c), GM-CSF receptor β (d) and β-actin mRNA (e) in cultures of human gliomas (passages 1–12) determined by semiquantitative RT-PCR (DNA marker, φX174/HaeIII digest). N.B., normal brain.
Results
mRNA Expression of G-CSF and GM-CSF and Their Receptors in Glioma Cell Cultures
Expression of GM-CSF and G-CSF as well as their receptors has been demonstrated in established human glioma cell lines. 38 However, a controversy remained in the literature concerning the relevance of these data for primary glioma cultures and tumors in vivo. 42,43 Thus we investigated the expression of both factors and receptors in early and selected late passage cultures of gliomas representing different stages of tumor progression. mRNA expression was analyzed in cultures of four astrocytomas of grade II, one of grade II-III, two of grade III, and 12 glioblastomas of grade IV, in comparison to normal brain tissue (Table 1 ▶ and Figure 1 ▶ ). A-5RT1, a keratinocyte (HaCaT-ras) tumor cell line, 47,48 with a high level of expression of G-CSF and GM-CSF, and primary human skin fibroblasts served as a positive control.
The RT-PCR data show that all glioma-derived cell cultures constitutively express G-CSF and/or GM-CSF and/or their receptors in vitro, though with a greatly diverging spectrum (Figure 1) ▶ . The G-CSF receptor is expressed in 14/19 (74%) of tumor cultures, whereas 12/19 (63%) express G-CSF (Table 1) ▶ . The GM-CSF receptor is expressed in almost all (17/19, 89%) tumor cell cultures tested, with the exception of NCH 48, a glioblastoma-derived, and NCH 76, a grade II astrocytoma-derived culture. Expression of the GM-CSF receptors is also found, though weakly, in normal brain tissue. This is most likely due to contamination with blood-derived cells present in the surgical specimen, because normal brain tissue was negative in immunohistochemistry (Figure 2e) ▶ . The growth factor GM-CSF is only expressed in 7/19 (37%) tumor cell cultures (in NCH 37, 45, 46, 48, 57, 60, 92), which were all derived from glioblastomas in the most advanced tumor stage. Coexpression of G-CSF and its receptor was found in 10/19, and coexpression of GM-CSF and its receptor in 6/19 tumor cell cultures. This factor and receptor expression were detected in early culture passages but were also maintained in later passages, as tested for some tumor cell cultures (eg, NCH 37 up to passage 38).
Figure 2.
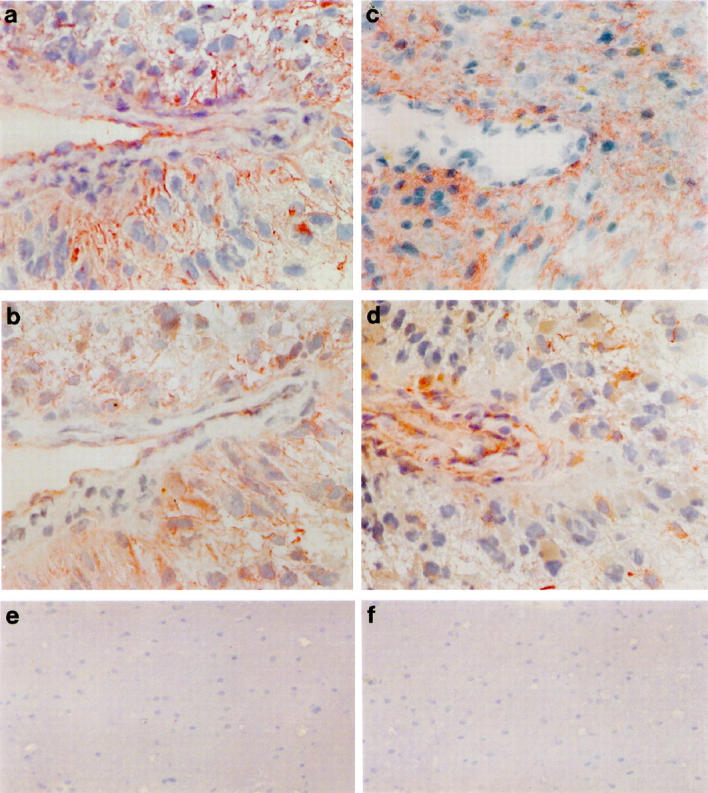
Immunocytochemical staining of NCH 92 glioblastoma tissue sections with antibodies directed against G-CSF (a), the G-CSF receptor (b), GM-CSF (c), and the α subunit of the GM-CSF receptor (d). Both factors and their receptors show a cytoplasmic staining with accentuation of cell borders and an accumulation around blood vessles. Magnification ×360. Immunocytochemical staining of normal brain tissue sections with antibodies directed against the G-CSF receptor (e) and the GM-CSF receptor (f). Normal brain tissue does not express the G-CSF (e) or GM-CSF (f) receptor or the corresponding factors (data not shown). Magnification of ×210 was chosen to show the negative staining in a larger area of the tissue.
Importantly, a clear correlation existed between the coexpression of GM-CSF and its receptor and tumor stages (Table 1) ▶ . Expression of the G-CSF and GM-CSF receptors alone, ie, without the respective factors, occurred predominantly in low-grade gliomas. Coexpression of G-CSF and its receptor was found in some low-grade gliomas as well as in high-grade glioblastomas. However, coexpression of GM-CSF and its receptor is found exclusively in cultures derived from grade IV glioblastoma. Most of the latter cultures also coexpress G-CSF and its receptor. Thus our data show that coexpression of the growth factors with their receptors is predominantly found in the more progressed tumor stages, and this is particularly true of GM-CSF and its receptor.
Expression of G-CSF, GM-CSF, and Their Receptors at the in Vivo Protein Level
The expression of growth factors by tumor cells in tissue culture is often considered an in vitro artifact with doubtful biological significance. Thus we analyzed the expression of these factors and their receptors on frozen sections of the original tumors and three additional grade II-III astrocytomas, as well as normal brain tissue, by immunocytochemical staining with antibodies against G-CSF, G-CSF receptor, GM-CSF, and the α subunit of the GM-CSF receptor (Figure 2 ▶ and Table 1 ▶ ). Specificity of the antibodies against G-CSF and GM-CSF was confirmed by competition with the factor in immunohistochemistry and by Western blot analysis, respectively. In normal brain, expression of both growth factors and receptors was negative (Figure 2, e and f ▶ , and data not shown). In tumors, the expression of growth factors and receptors was weak but clearly discernible in the cytoplasm, with more intense staining at the cell membrane for G-CSF and both growth factor receptors. The staining pattern for both factors and receptors was heterogeneous, with noticeable differences in staining intensity, especially for the receptors. Interestingly, the expression of G-CSF and GM-CSF in grade IV glioblastoma was also frequently localized around blood vessels (Figure 2, a and c) ▶ , often with colocalization of their receptors (Figure 2, b and d) ▶ . Collectively, we could demonstrate a good correlation between the mRNA expression of G-CSF, GM-CSF, and their respective receptors in cultured glioma cells and the protein staining in tumor sections (Table 1) ▶ . Expression of GM-CSF receptor at both the mRNA (β subunit tested) and protein (α subunit tested) levels demonstrated the presence of the high-affinity receptor consisting of both subunits.
Stimulation of Proliferation by G-CSF and GM-CSF
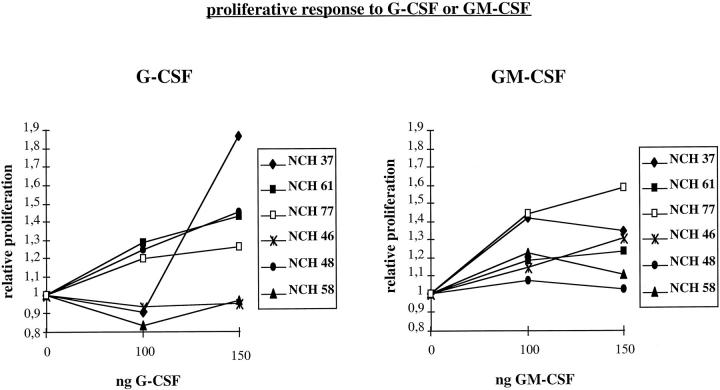
Even though glioma cells express G-CSF, GM-CSF, and the corresponding receptors, this has a biological significance only when the receptors are functionally active. Therefore six tumor cell lines with a different expression profile for both growth factors and receptors (labeled §§ in Table 1 ▶ ) were functionally analyzed: NCH 37 expresses both factors and both receptors, NCH 61 and NCH 77 G-CSF and both receptors, NCH 46 GM-CSF and weakly its receptor, NCH 48 GM-CSF and weakly the G-CSF receptor, and NCH 58 only the GM-CSF receptor. These cell lines were exposed to increasing amounts (0, 100, and 150 ng/ml) of G-CSF and GM-CSF in serum-free media, and the proliferative response was determined using a BrdU incorporation assay. All cell lines expressing the respective receptors showed a dose-dependent stimulation of cell proliferation in response to G-CSF and/or GM-CSF (Figure 3) ▶ . NCH 37, 61 and NCH 77 expressing both receptors showed an increase in proliferation in the presence of both G-CSF and GM-CSF, whereas NCH 46 and 48, expressing only one receptor, responded solely to the respective growth factors GM-CSF and G-CSF, respectively. NCH 58, expressing only the GM-CSF receptor, responded exclusively to GM-CSF, though weakly.
Figure 3.
Proliferative responses of glioma cells NCH 37 (expressing both factors and both receptors), NCH 61 and NCH 77 (both expressing G-CSF, G-CSF receptor, and GM-CSF receptor), NCH 46 (expressing GM-CSF and the GM-CSF receptor), NCH 48 (expressing GM-CSF and the G-CSF receptor), and NCH 58 (expressing the GM-CSF receptor) to increasing amounts of G-CSF and GM-CSF as determined after 24 hours by BrdU incorporation. For each assay eight wells were measured in three independent experiments. Standard deviations are ≤0.1.
Functional Significance of Autocrine Growth Stimulation
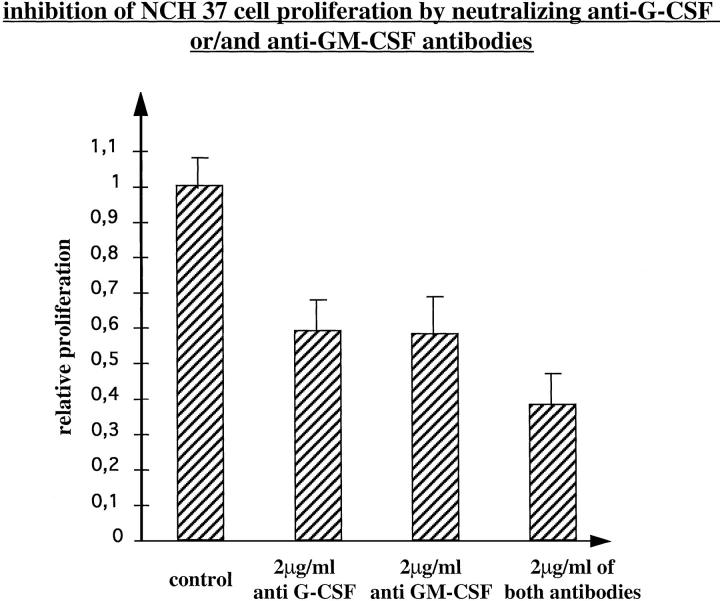
To further demonstrate the functional role of G-CSF and GM-CSF for autocrine growth regulation in glioma cells, specific blocking antibodies were used. NCH 37 cells that express G-CSF, GM-CSF, and both receptors were selected for these studies. The release of growth factors by NCH 37 cells into the culture media was confirmed by ELISA, demonstrating high amounts of G-CSF (4052.5 ± 65 pg/ml) and GM-CSF (1319 ± 109 pg/ml). NCH 58 cells were used as a control because they did not express or secrete any factor. In serum-free NCH 37 cultures blocking antibodies (1 and 2 μg/ml) against G-CSF and GM-CSF clearly inhibited cell proliferation (Figure 4) ▶ . Inhibition was strongest at 2 μg/ml, a concentration that, according to the manufacturer’s description, is capable of neutralizing the amount of growth factor secreted by NCH 37. The two antibodies resulted in a similar inhibition of cell proliferation and, when applied together, in an even greater growth inhibition. On the other hand, the antibodies had no effect on the proliferation of NCH 58 cells that do not express G-CSF or GM-CSF, excluding unspecific inhibitory effects (data not shown). The addition of 2 μg/ml of an unrelated control antibody also had no effect on cell proliferation (data not shown).
Figure 4.
Proliferative responses of NCH 37 (expressing both factors and both receptors) to 2 μg/ml neutralizing antibodies against G-CSF or GM-CSF or both, measured by BrdU incorporation. Values are means of eight wells measured in three independent experiments ± SD.
Cell Migration in Response to G-CSF and GM-CSF
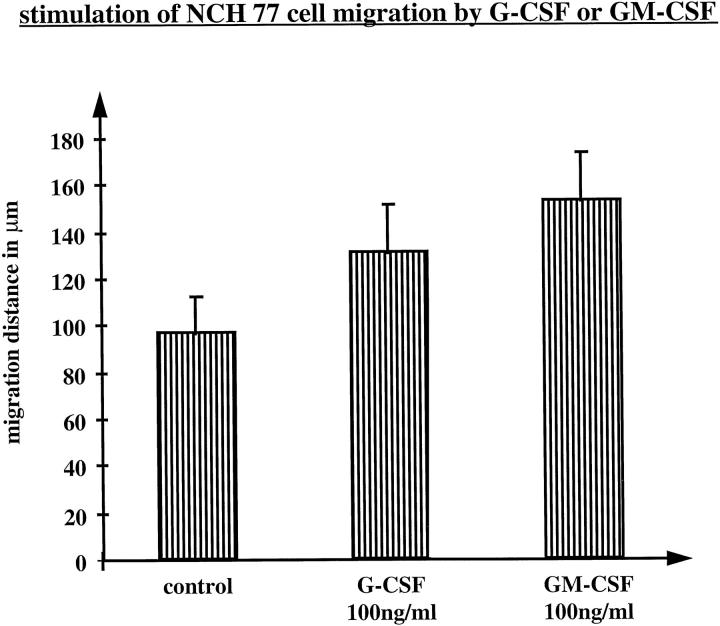
Because G-CSF and GM-CSF are also known as stimulators of cell migration for endothelial cells, 30 keratinocytes, 28,29 as well as certain tumor cells 37,40,41 their effect on migration was tested on NCH 77 cell cultures expressing both G-CSF and GM-CSF receptors. Postconfluent cultures were wounded and subsequently incubated in serum-free media with 100 ng/ml G-CSF or GM-CSF for 24 hours. Tumor cell migration was documented by phase-contrast microscopy (Figure 5) ▶ and quantified by measuring the migration distance (Figure 6) ▶ . Migration of NCH 77 cells was clearly stimulated by G-CSF and in an even more pronounced way by GM-CSF (Figures 5, c and d, and 6) ▶ ▶ .
Figure 5.
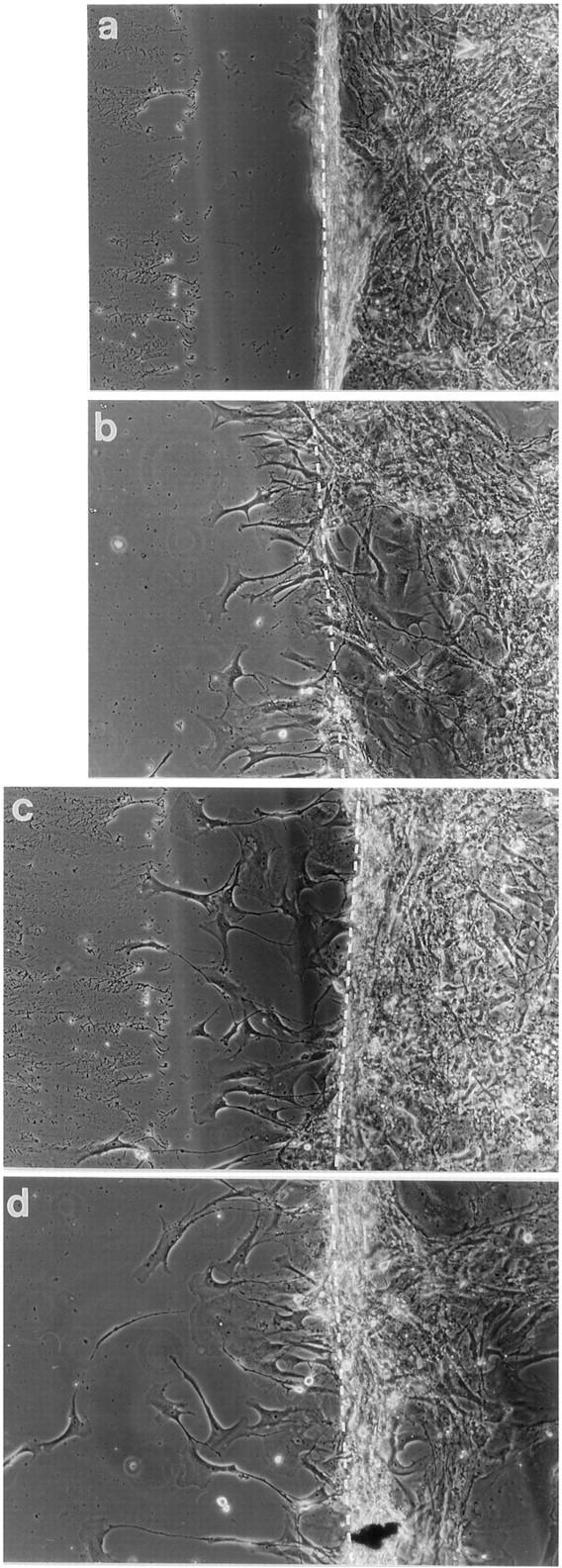
Stimulation of migration of NCH 77 glioma cells (expressing G-CSF, G-CSF receptor, and GM-CSF receptor) by G-CSF and GM-CSF (100 ng/ml). A confluent monolayer of cells was disrupted by scraping (a) (t = 0 hours), and cells were incubated for 24 hours in RPMI (b), RPMI + 100 ng/ml G-CSF (c), and RPMI + 100 ng/ml GM-CSF (d). Magnification is ×200.
Figure 6.
Migratory response of NCH 77 glioma cells (expressing G-CSF, G-CSF receptor, and GM-CSF receptor) to G-CSF and GM-CSF (100 ng/ml). Cell migration was quantified by measuring the migration distance on photomicrographs at a magnification of ×200. The values with standard deviations are means of two experiments with two replica platings each.
Autocrine Regulation of Cell Migration
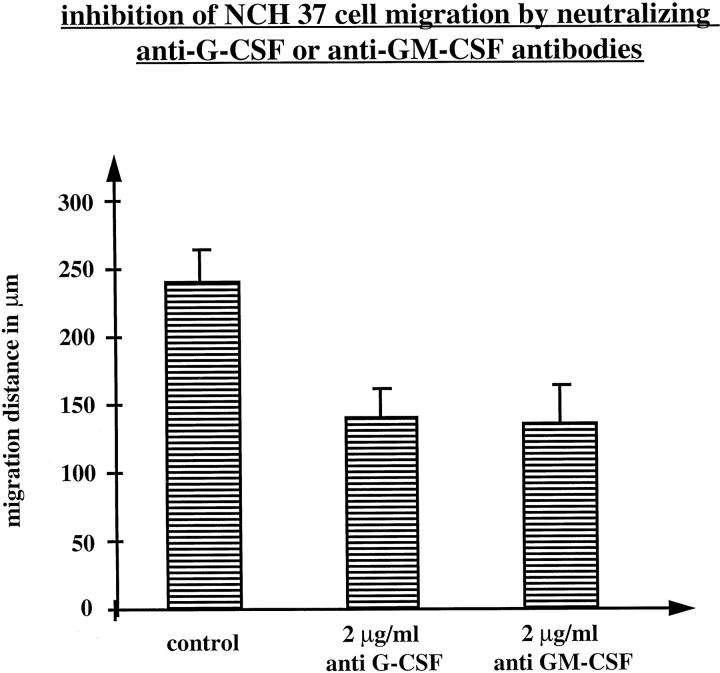
To investigate the role of both factors as autocrine stimulators of tumor cell migration, we analyzed the migration of NCH 37 glioblastoma cells (expressing G-CSF, GM-CSF, and both receptors) in the presence of neutralizing antibodies to both growth factors. Again, postconfluent cultures of NCH 37 cells were wounded and then incubated for 24 h in serum-free media in the presence of 2 μg/ml anti-G-CSF or anti-GM-CSF blocking antibodies. Cell migration in phase-contrast microscopy demonstrated clear differences in cell morphology and a more pronounced spontaneous migration of NCH 37 than that seen with NCH 77 cells, concerning both the migration distances as well as the number of cells migrating into the wounded area (Figure 7) ▶ . This pronounced migratory activity was markedly inhibited by both blocking antibodies (Figure 8) ▶ , whereas the addition of 2 μg/ml of an unrelated control antibody had no effect (data not shown). Thus our data clearly indicate an autocrine mechanism for both G-CSF and GM-CSF in regulating the migration of these glioblastoma cells.
Figure 7.
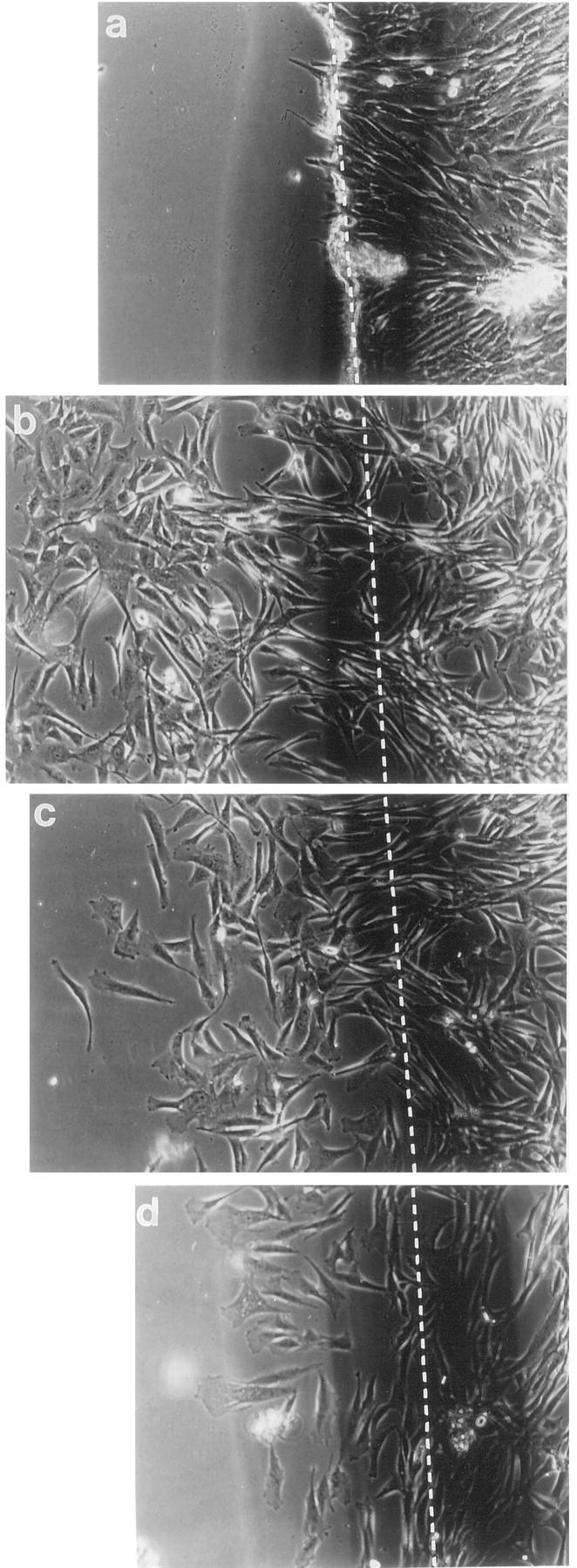
Inhibition of migration of NCH 37 glioma cells (expressing G-CSF, GM-CSF, and both receptors) by neutralizing antibodies to G-CSF or GM-CSF (2 μg/ml). A confluent monolayer of cells was disrupted by scraping (a) (t = 0 hours), and cells were incubated for 24 hours in RPMI (b), RPMI + 2 μg/ml anti-G-CSF antibody (c), and RPMI + 2 μg/ml anti-GM-CSF antibody (d). Magnification is ×200.
Figure 8.
Migratory response of NCH 37 glioma cells (expressing G-CSF, GM-CSF, and both receptors) to neutralizing anti-G-CSF and anti-GM-CSF antibodies (2 μg/ml). Cell migration was quantified by measuring the migration distance on photomicrographs at a magnification of ×200. The values with standard deviations are means of two experiments with two replica platings each.
Discussion
Gliomas are among the fastest growing and most malignant human tumors. The development of a highly malignant tumor phenotype is understood as an increasing independence of the tumor cells from their environment, involving alterations in many cellular and humoral factors. 49 Two factors that are expressed in solid tumors 35-37,39 and potentially associated with tumor cell proliferation and migration 36,40,41 as well as tumor induced angiogenesis, 31,48 are G-CSF and GM-CSF. So far there have been only a few studies on their expression in human gliomas, with controversial data obtained in established cell lines or in tumor tissues. 38,42-44 The expression of GM-CSF was found in established glioma cell lines but not in glioma tumor specimens, 42,43 whereas G-CSF expression was shown in low-grade astrocytomas in vitro and in vivo but not in grade IV glioblastomas. 45
Whereas both factors and receptors are absent in normal brain, our data show the expression of G-CSF and GM-CSF in glioma tissue samples as well as in derived cell cultures at both the mRNA and protein levels. Moreover, in contrast to earlier negative reports, 42,43,45 we find the expression of both G-CSF and GM-CSF receptor in vivo in the majority of tumors analyzed as well as in derived cultures. These tumor cultures were thoroughly tested for their homogeneous astrocytic composition, and only those showing homogeneous staining for GFAP were used in these studies. Expression of the G-CSF and GM-CSF receptors alone, ie, without the respective factors, is found predominantly in low-grade gliomas. This indicates that these tumor cells can respond to the respective factors but still require their production by stromal cells; ie, they are dependent on paracrine growth stimulation. 50 Our data suggest that with tumor progression cells become increasingly independent of the growth-regulatory mechanisms of the surrounding tissue by producing the growth factors themselves. G-CSF is expressed in some low-grade as well as in most high-grade gliomas, suggesting that the expression of G-CSF is activated realtively early during tumor progression. GM-CSF, on the other hand, is turned on exclusively in grade IV glioblastomas and is, in most cases, coexpressed with the GM-CSF and often with the G-CSF receptor. Whereas a G-CSF autocrine regulatory mechanism is already found in some low-grade gliomas, coexpression of GM-CSF and its receptor seems to be associated exclusively with the most aggressive and angiogenic glioblastoma phenotype.
G-CSF and GM-CSF were originally described as growth and differentiation factors of myeloid cells. 18 Thus their expression in human gliomas may well contribute to the inflammatory infiltrate that characterizes this tumor type, 43 although a correlation between increased macrophage numbers and expression of GM-CSF was not confirmed (data not shown). But there are other, equally important functions G-CSF and GM-CSF may have in human gliomas.
Both G-CSF and GM-CSF stimulate the proliferation of glioma cells in vitro that express the respective receptors, demonstrating the functionality of the receptors and the efficacy of the factors in growth regulation. Moreover, the inhibition of proliferation by neutralizing antibodies substantiates the hypothesis that both factors act as part of an autocrine growth regulatory mechanisms that may also function in vivo.
Previous reports on different tumor models indicated that G-CSF and GM-CSF may promote tumor progression by enhancing cell migration and thus favoring invasion and metastasis. Young et al 51 described a stimulation of the migratory and metastatic potential in Lewis lung carcinoma cells by GM-CSF. Others showed the association of GM-CSF expression with a metastatic phenotype in solid tumors of mouse and human origin. 40,52,53 Here we demonstrate that G-CSF and GM-CSF stimulate glioma migration of cells expressing the respective receptors. Moreover, the significant inhibition of cell migration caused by blocking antibodies in culture strongly suggests that both factors are active in an autocrine regulatory manner. From these data we conclude that the constitutive expression of G-CSF and GM-CSF and their receptors in glioma cells increases their migratory capacity and thus may contribute to their highly invasive phenotype. The exclusive coexpression of G-CSF and GM-CSF and their receptors in grade IV glioblastoma, the most malignant and invasive astrocytoma type, supports this hypothesis.
In addition to their excessive invasive tumor growth, glioblastomas are highly vascularized. They are characterized by a pronounced angiogenesis, 1 supposedly induced by the strong expression of vascular endothelial growth factor, a potent angiogenic factor. 54 In addition to their stimulation of growth and migration, G-CSF and GM-CSF may also contribute to this angiogenic reaction by stimulating endothelial cell proliferation and migration, as shown in cell culture and the rabbit cornea assay. 30,31 The immunohistochemical identification of both factors in glioma tissue and their frequent accumulation around blood vessels may be interpreted as a contribution to the induction of angiogenesis and tumor vascularization. To further confirm this hypothesis we are currently investigating in detail the observed induction of an angiogenic response by G-CSF in a nude mouse transplantation model (Mueller and Fusenig, unpublished results).
Collectively, our data lead us to conclude that the coexpression of G-CSF and GM-CSF and their receptors in glioma cells may have multiple effects: 1) The growth factors are part of an autocrine stimulatory mechanism for enhancing tumor cell proliferation. 2) G-CSF and GM-CSF stimulate tumor cell migration and may thereby contribute to tumor invasion. 3) Both factors might also act in a paracrine fashion on stromal cells, thus contributing to tumor-induced angiogenesis. In vivo experiments counteracting the function of G-CSF and GM-CSF in malignant gliomas, either by neutralizing antibodies, as used in our in vitro experiments, or by antisense technology, might open a new therapeutic possibility.
Such new therapeutic strategies are of great interest because the treatment of patients with malignant glioma still remains a challenging problem. In addition to radical surgery, radiation and, rarely, chemotherapy are administered, but the latter two treatment modalities are often limited by a severe neutropenia. G-CSF and GM-CSF, the factors that we have shown to play a role in glioma cell proliferation and migration, are increasingly used to combat this myelosuppression. 15 As both factors are usually injected subcutaneously or intravenously, the concentration of factor in the brain should be relatively low, not necessarily resulting in an enhancement of tumor growth in those gliomas that express the respective receptors. Nevertheless, in light of our data more studies evaluating the effect of G-CSF and GM-CSF on glioma growth and invasion in vivo are warranted.
Acknowledgments
The authors thank Mrs. Annette Buttler for expert technical assistance and Mrs. Brigitte Nagel-Plagens for critically reading the manuscript.
Footnotes
Address reprint requests to Dr. N. E. Fusenig, Division of Carcinogenesis and Differentiation, German Cancer Research Center, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. E-mail: fusenig@dkfz-heidelberg.de.
Supported by EU grant BIO 4 CT and by a grant from the Verein zur Foerderung der Krebsforschung in Deutschland.
References
- 1.Jensen RL: Growth factor-mediated angiogenesis in the malignant progression of glial tumors: a review. Surg Neurol 1998, 49:189-196 [DOI] [PubMed] [Google Scholar]
- 2.Stachowiak MK, Moffet J, Maher P, Tucholski J, Stachowiak EK: Growth factor regulation of cell growth and proliferation in the nervous system. A new intracrine nuclear mechanism. Mol Neurobiol 1997, 15:257-283 [DOI] [PubMed] [Google Scholar]
- 3.Tang P, Steck PA, Yung WK: The autocrine loop of TGF-α/EGFR and brain tumors. J Neurooncol 1997, 35:303-314 [DOI] [PubMed] [Google Scholar]
- 4.Lamszus K, Schmidt NO, Jin L, Laterra J, Zagzag D, Way D, Witte M, Weinand M, Goldberg ID, Westphal M, Rosen EM: Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int J Cancer 1998, 75:19-28 [DOI] [PubMed] [Google Scholar]
- 5.Halfter H, Lofti R, Westermann R, Young P, Ringelstein EB, Stogbauer FT: Inhibition of growth and induction of differentiation of glioma cell lines by oncostatin M. Growth Factors 1998, 15:135-147 [DOI] [PubMed] [Google Scholar]
- 6.Campbell JW, Pollack IF: Growth factors in gliomas: antisense and dominant negative mutant strategies. J Neurooncol 1997, 35:275-285 [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Barth RF, Adams DM, Soloway AH: Intratumoral delivery of boronated epidermal growth factor for neutron capture therapy of brain tumors. Cancer Res 1997, 57:4333-4339 [PubMed] [Google Scholar]
- 8.Stratmann A, Machein MR, Plate KH: Anti-angiogenic gene therapy of malignant glioma. Acta Neurochir Suppl (Wien) 1997, 68:105-110 [DOI] [PubMed] [Google Scholar]
- 9.Black PM: Brain tumors. Part 1. N Engl J Med 1991, 324:1471-1476 [DOI] [PubMed] [Google Scholar]
- 10.Black PM: Brain tumors. Part 2. N Engl J Med 1991, 324:1555-1564 [DOI] [PubMed] [Google Scholar]
- 11.Groopman JE, Molina J-M, Scadden DT: Hematopoietic growth factors. Biology and clinical applications. N Engl J Med 1989, 321:1449-1459 [DOI] [PubMed] [Google Scholar]
- 12.Rosier J-F, Scalliet P: Use of hematopoietic growth factors in radiation therapy. Prog Growth Factors 1997, 3:3-6 [Google Scholar]
- 13.Mayordomo JI, Rivera F, Diaz-Puente MT, Lianes P, Colomer R, Lopez-Brea M, Lopez E, Paz-Ares L, Hitt R, Garcia-Ribas I: Improving treatment of chemotherapy-induced neutropenic fever by administration of colony stimulating factors. J Natl Cancer Inst 1995, 87:803-808 [DOI] [PubMed] [Google Scholar]
- 14.Rampling R, Steward W, Paul J, Macham MA, Harvey E, Eckley D: rhGM-CSF ameliorates neutropenia in patients with malignant glioma treated with BCNU. Br J Cancer 1994, 69:541-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Society of Clinical Oncology: American Society of Clinical Oncology recommendations for the use of hematopoietic colony stimulating factors: evidence based clinical practice guidelines. J Clin Oncol 1994, 12:2471–2508 [DOI] [PubMed]
- 16.Metcalf D: The granulocyte-macrophage colony-stimulating factors. Science 1985, 229:16-22 [DOI] [PubMed] [Google Scholar]
- 17.Aglietta M, Bussolino F, Piacibello W, Aprá F, Sanavio F, Stacchini A, Monzeglio C, Carnino F, Gavosto F: Human GM-CSF in vivo: identification of the target cells and of their kinetics of response. Int J Cell Cloning 1990, 8:283-292 [DOI] [PubMed] [Google Scholar]
- 18.Williams ME, Quesenberry PJ: Hematopoietic growth factors. Hematol Pathol 1992, 6:105-124 [PubMed] [Google Scholar]
- 19.Nicola NA, Wycherley K, Boyd AW, Layton JE, Cary D, Metcalf D: Neutralizing and nonneutralizing monoclonal antobodies to the human granulocyte-macrophage colony-stimulating factor receptor α-chain. Blood 1993, 82:1724-1731 [PubMed] [Google Scholar]
- 20.Kastelstein RA, Shanafelt AB: GM-CSF receptor: interactions and activation. Oncogene 1993, 8:231-236 [PubMed] [Google Scholar]
- 21.Tkatch LS, Tweardy DJ: Human granulocyte colony-stimulating factor (G-CSF), the premier granulopoietin: biology clinical utility, and receptor structure and function. Lymphokine Cytokine Res 1993, 6:477-488 [PubMed] [Google Scholar]
- 22.Shimoda K, Okamura S, Harada N, Kondo S, Okamura T, Niho Y: Identification of a functional receptor for granulocyte colony-stimulating factor on platelets. J Clin Invest 1993, 91:1310-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fibbe WE, van Damme J, Billiau A, Duinkerekn N, Lurvink E, Ralph P, Altrock BW, Kaushansky K, Willemze R, Falkenburg JHF: Human fibroblasts produce granulocyte-CSF, macrophage-CSF, and granulocyte-macrophage-CSF following stimulation by interleukin-1 and poly(rI)·poly(rC). Blood 1988, 72:860-866 [PubMed] [Google Scholar]
- 24.Hamilton JA, Piccoli DS, Cebon J, Layton JE, Rathanaswani P, McColl SR, Leizer T: Cytokine regulation of colony-stimulating factor (CSF) production in cultured human synovial fibroblasts. II. Similarities and differences in the control of interleukin-1 induction of granulocyte-macrophage CSF and granulocyte-CSF production. Blood 1992, 79:1413-1419 [PubMed] [Google Scholar]
- 25.Chodakewitz JA, Kupper TS, Coleman DL: Keratinocyte-derived granulocyte/macrophage colony-stimulating factor induces DNA synthesis by peritoneal macrophages. J Immunol 1988, 140:832-836 [PubMed] [Google Scholar]
- 26.Owsianowski M, Busch FW, Bonnekoh B, Orfanos CE: Long-term cultured adult human keratinocytes secrete granulocyte-macrophage colony-stimulating factor, but not interleukin-3, after cytokine exposure in vitro. Skin Pharmacol 1991, 4:158-164 [DOI] [PubMed] [Google Scholar]
- 27.Dedhar S, Gaboury L, Galloway P, Eaves C: Human granulocyte-macrophage colony stimulating factor is a growth factor active on a variety of cell types of non-hematopoietic origin. Proc Natl Acad Sci USA 1988, 85:9253-9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braunstein S, Kaplan G, Gottlieb AB, Schwartz M, Walsh G, Abalos RM, Tranquilino FT, Guido LS, Krueger JG: GM-CSF activates regenerative epidermal growth and stimulates keratinocyte proliferation in human skin in vivo. J Invest Dermatol 1994, 103:601-604 [DOI] [PubMed] [Google Scholar]
- 29.Olaniran AK, Baker BS, Garioch JJ, Powles AV, Fry LA: Comparison of the stimulatory effects of cytokines on normal and psoriatic keratinocytes in vitro. Arch Dermatol Res 1995, 287:231-236 [DOI] [PubMed] [Google Scholar]
- 30.Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, Aglietta M, Arese P, Mantovani A: Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989, 337:471-473 [DOI] [PubMed] [Google Scholar]
- 31.Bussolino F, Ziche M, Wang J, Alessi D, Mobidelli L, Cremona O, Bosai A, Marchisio P, Mantovani A: In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest 1991, 87:986-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacManus MP, McCormick D, Trimble A, Abram WP: Value of granulocyte colony stimulating factor in radiotherapy induced neutropenia: clinical and laboratory studies. Eur J Cancer 1995, 31A:302-307 [DOI] [PubMed] [Google Scholar]
- 33.Guillaume T, Sekhavat M, Rubinstein DB, Hamdan O, Symann ML: Transcription of genes encoding granulocyte-macrophage colony-stimulating factor, interleukin 3, and interleukin 6 receptors and lack of proliferative response to exogenous cytokines in nonhematopoietic human malignant cell lines. Cancer Res 1993, 53:3139-3144 [PubMed] [Google Scholar]
- 34.Foulke RS, Marshall MH, Trotta PP, Von Hoff DD: In vitro assessment of the effects of granulocyte-macrophage colony stimulating factor on primary human tumors and derived cell lines. Cancer Res 1990, 50:6264-6267 [PubMed] [Google Scholar]
- 35.Horii A, Shimamura K, Honjo Y, Mitani K, Miki T, Takashima S, Yoshida J: Granulocyte colony stimulating factor-producing tongue carcinoma. Head and Neck 1997, 19:351-356 [DOI] [PubMed] [Google Scholar]
- 36.Baba M, Hasegawa H, Nakayabu M, Shimizu N, Suszuki S, Kamada N, Tani K: Establishment and characteristics of a gastric cancer cell line (HuGC-OOHIRA) producing high levels of G-CSF, GM-CSF, and IL-6: The presence of autocrine growth control by G-CSF. Am J Hematol 1995, 49:207-215 [DOI] [PubMed] [Google Scholar]
- 37.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M: Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer 1994, 56:853-857 [DOI] [PubMed] [Google Scholar]
- 38.Nitta T, Sato K, Allegretta M, Brocke S, Lim M, Mitchell DJ, Steinman L: Expression of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor genes in human astrocytoma cell lines and in glioma specimens. Brain Res 1992, 571:19-25 [DOI] [PubMed] [Google Scholar]
- 39.Tsuzuki H, Fujieda S, Sunaga H, Noda I, Saito H: Expression of granulocyte colony stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res 1998, 58:794-800 [PubMed] [Google Scholar]
- 40.Pei X-H, Nakanishi Y, Takayama K, Yatsunami J, Bai F, Kawasaki M, Wakamatsu K, Tsuruta N, Mizuno K, Hara N: Granulocyte-colony stimulating factor promotes invasion by human lung cancer cell lines in vitro. Clin Exp Metastasis 1996, 14:351-357 [DOI] [PubMed] [Google Scholar]
- 41.Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J: Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res 1995, 55:3438-3443 [PubMed] [Google Scholar]
- 42.Murata J, Sawamura Y, Tada M, Sakuma S, Sudo M, Aida T, Abe H: Human glioblastoma cells produce granulocyte-macrophage colony-stimulating factor in vitro, but not in vivo, without expressing its receptor. Neurol Med Chir 1993, 33:603-609 [DOI] [PubMed] [Google Scholar]
- 43.Frei K, Piani D, Malipiero UV, Van Meir E, de Tribolet N, Fontana A: Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol 1992, 148:3140-3146 [PubMed] [Google Scholar]
- 44.Kikuchi T, Nakahara S, Abe T: Granulocyte colony-stimulating factor (G-CSF) production by astrocytoma cells and its effect on tumor growth. J Neurooncol 1996, 27:31-38 [DOI] [PubMed] [Google Scholar]
- 45.Stan AC, Walter GF, Welte K, Pietsch T: Immunolocalization of granulocyte colony-stimulating factor in human glial and primitive neuroectodermal tumors. Int J Cancer 1994, 57:306-312 [DOI] [PubMed] [Google Scholar]
- 46.Chomczynski P, Sacchi N: Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1978, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 47.Fusenig NE, Boukamp P: Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog 1998, 23:144-158 [DOI] [PubMed] [Google Scholar]
- 48.Mueller MM, Fusenig NE: Constitutive expression of G-CSF and GM-CSF in human skin carcinoma cells with functional consequence for tumor progression. Int J Cancer 1999 (in press) [DOI] [PubMed]
- 49.Aaronson SA: Growth factors and cancer. Science 1991, 254:1146-1153 [DOI] [PubMed] [Google Scholar]
- 50.Rak JW, Filmus J, Kerbel RS: Reciprocal paracrine interactions between tumor cells and endothelial cells: the angiogenesis progression hypothesis. Eur J Cancer 1996, 32A:2438-2450 [DOI] [PubMed] [Google Scholar]
- 51.Young MRI, Lozano Y, Coogan M, Wright MA, Young ME, Bagashi JM: Stimulation of the metastatic properties of Lewis-lung-carcinoma cells by autologous granulocyte-macrophage colony-stimulating factor. Int J Cancer 1992, 50:628-634 [DOI] [PubMed] [Google Scholar]
- 52.Takeda K, Hatakeyama K, Tsuchiya Y, Rikiishi H, Kumagai K: A correlation between GM-CSF gene expression and metastases in murine tumors. Int J Cancer 1991, 47:413-420 [DOI] [PubMed] [Google Scholar]
- 53.Ciotti P, Rainero ML, Nicol G, Spina B, Garré C, Casabona F, Santi PL, Bianchi-Scarrà G: Cytokine expression in human primary and metastatic melanoma cells: analysis in fresh bioptic specimens. Melanoma Res 1995, 5:41-47 [DOI] [PubMed] [Google Scholar]
- 54.Plate KH, Breier G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 1992, 359:845-848 [DOI] [PubMed] [Google Scholar]