Abstract
The Iip35 isoform of the major histocompatibility complex (MHC) class II-associated invariant chain (Ii) contains an endoplasmic reticulum (ER) targeting motif, but in B cell lines the ER retention is ineffective and a fraction of Iip35 is transported through the Golgi complex associated with class II molecules. We found Iip35 (but not Iip33, the major form of Ii) to be phosphorylated in B cell lines, as well as in transfected HeLa cells. The phosphorylation of Iip35 was found to be necessary for the exit of Iip35–class II complexes out of the ER. This requirement suggests that phosphorylation may change the interaction with factors responsible for ER retention/retrieval, and we did find that phosphorylated Iip35 associates with 14-3-3 proteins, a family of adaptor proteins that are involved in coordinating signal transduction pathways. This finding raises the intriguing possibility that the exit of Ii–class II complexes from the ER is regulated by intracellular signaling events.
Major histocompatibility complex (MHC) class II molecules are cell surface glycoproteins that present peptides to CD4+ T cells. In the endoplasmic reticulum (ER) class II molecules become associated with a type II transmembrane protein termed the invariant chain (Ii) (1). The association with Ii prevents binding of peptides and nascent proteins in the ER, and targeting information in the cytoplasmic tail of Ii directs Ii-class II complexes to endosomal or lysosomal compartments where association between class II molecules and antigenic peptides occur (2). Conflicting data have been presented as to whether a majority of complexes reach the endosomal system directly from the trans-Golgi network (TGN) (3, 4), or via the cell surface (5–7).
Alternative splicing and the use of two different initiation codons result in four distinct translation products being produced from the human Ii gene (8). A 16-amino acid N-terminal extension in Iip35 (and p43) contains a double-arginine motif that has been shown to maintain Iip35 in the ER. (To avoid confusion we have here used the presently prevailing nomenclature to name the different Ii isoforms. Thus Iip33 denotes the chain we have previously called Iip31, while Iip35 denotes the chain we have previously called Iip33.) This is most likely done by a retrieval mechanism (9), similarly to how type I transmembrane proteins are retrieved to the ER by a double lysine motif (10). Despite this retrieval motif, Iip35 is transported out of the ER both in B cells and in HeLa cells cotransfected with Iip33 and class II molecules (5, 11, 12). A recent report on Iip35 trafficking in B cells has suggested that the transported Iip35-class II complexes may reach the endosomal system directly from the TGN, while Iip33-class II complexes traverse the cell surface to a large extent (5).
Iip33, but not Iip35, has been reported to be phosphorylated (13) and the fact that phosphorylation can influence the internalization and sorting of molecules both at the plasma membrane and in the TGN (14–17), lead us to investigate a potential role of phosphorylation for the differential transport of the two Ii isoforms. In this report we show that it is Iip35, not Iip33, which is phosphorylated, both in B cell lines and in transfected HeLa cells. We could not find any evidence that phosphorylation influenced how Iip33 and Iip35 reach the endosomal system from the TGN, but phosphorylation appeared to be required for the exit of Iip35-DR complexes from the ER. In addition, Iip35 was found to be associated with 14-3-3 proteins in a phosphorylation-dependent manner both in B cells and in transfected HeLa cells. 14-3-3 proteins have been shown to control intracellular signaling pathways (reviewed in ref. 18) and the association between Iip35 and 14-3-3 suggests that exit from the ER of Ii-class II complexes may be modulated by signal transduction events.
MATERIALS AND METHODS
DNAs and Antibodies.
cDNAs encoding DRA*0101, DRB1*0301, Iip33, and Iip35 in expression vector pCMU have been described (9, 19). Serine (S) to alanine (A) or aspartic acid (D) tail mutants of Iip35 were constructed by oligonucleotide cassette cloning (9).
mAbs DA6.147 (20) and PIN.1.1 (21) recognize the cytoplasmic tails of DRα and Iip33/35, respectively. OKT8 recognizes CD8α. Antiserum K455 recognizes MHC class I (22) Antiserum K2 was raised against the peptide KESLELEDPSSGLGVTKQDL, derived from the lumenal domain of Ii. mAb Bü45 (The Binding Site, San Diego) recognizes the lumenal domain of Iip33/35. An antiserum against 14-3-3 (K-19) was obtained from Santa Cruz Biotechnology.
Cell Culture and Transfection.
HeLa cells, K44A HeLa cells, the Epstein–Barr virus-transformed B cell line JY, and the Burkitt lymphoma B cell line Raji were maintained as described (7). Twenty-five micrograms of DNA (DRA and DRB, 8 μg each; Iip33, 6 μg; Iip35, 3 μg. Omitted DNAs were substituted with an irrelevant cDNA in the same vector) was transfected into HeLa cells by calcium phosphate precipitation (7). For cells expressing the dynamin mutant, tetracycline was removed 2–3 hr before transfection and culture was continued in the absence of the drug. Cells were harvested 60–72 hr after transfection.
Metabolic Labeling, Phosphate Labeling, and Immunoprecipitation.
Cells were labeled in methionine-deficient DMEM with [35S]methionine (Trans-label; ICN) then chased in DMEM containing nonradioactive methionine, as indicated. Cells were lysed with 1% Nonidet P-40 in PBS containing protease inhibitors (Complete, Boehringer Mannheim). For phosphate labeling 1 mCi of [32P]orthophosphate (1 Ci = 37 GBq; ICN) was added to ≈1–2 × 105 cells in phosphate-free DMEM (GIBCO/BRL) for 2.5 hr, then washed and lysed as above. Immunoprecipitating antibodies were added and immunoprecipitates were harvested with protein A- or G-Sepharose. The beads were washed and samples were resuspended in the appropriate sample buffer before loading onto first-dimension nonequilibrium pH gradient electrophoresis (NEPHGE) gels containing ampholines (pH 3.5–10; Pharmacia) (23). Second-dimension slab gels were SDS/7.5–12.5% PAGE. In some cases, samples were analyzed only on the slab gels. Autoradiographs were scanned by using an Agfa ArcusII scanner and composites were printed on a Kodak XLS 8600 PS printer.
Immunoblotting.
Proteins were transferred to Immobilon-P membranes (Millipore) Membranes were blocked with PBS with 3% defatted milk and 0.05% Tween-20 for 2 hr, then probed with primary antibodies in the blocking buffer, washed in PBS with 0.3% Tween-20, and probed with secondary antibodies conjugated to horse-radish peroxidase or alkaline phosphatase (Bio-Rad). After washing, membranes were developed with enhanced chemoluminescence (Amersham) or with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (Promega).
Flow Cytometry.
Transfected K44A HeLa cells were detached from the petri dishes with 2 mM EDTA in PBS. Cell suspensions were washed with PBS containing 1% BSA at 4°C and incubated with mAbs Bü45 or OKT8 for detection of Ii or CD8α. After washing, the cells were stained with fluorescein-conjugated goat anti-mouse IgG before analysis on a Becton Dickinson LYSIS II instrument.
RESULTS
Intracellular Transport of Iip35 Is Influenced by Complex Formation with DR or Iip33.
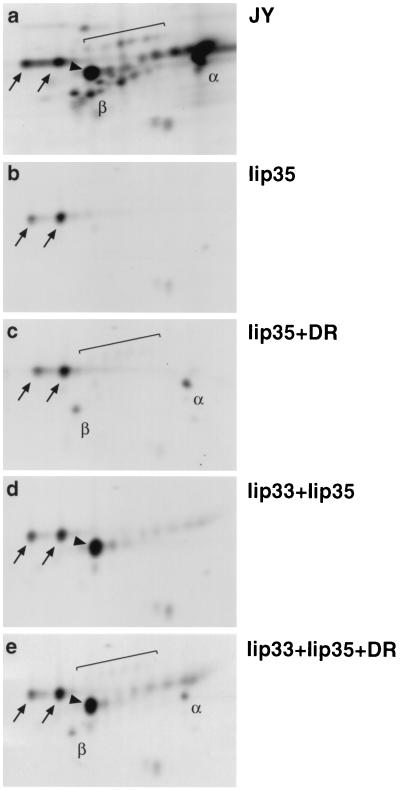
To study the requirements for transport of Iip35 we analyzed immunoprecipitates from JY B cells and transfected HeLa cells by two-dimensional gel electrophoresis. Ii was immunoprecipitated from lysates of metabolically labeled and chased cells by using mAb PIN.1.1, reactive with the cytoplasmic N-terminal part of both Iip33 and Iip35. In the precipitate from JY cells (Fig. 1a) the majority of Iip35 was present in its core-glycosylated form. However, a small but distinct set of Iip35-derived spots had acquired modifications indicative of transport out of the ER. These modifications largely represent addition of sialic acid to N- and O-linked carbohydrates (24).
Figure 1.
Iip35 can exit the endoplasmic reticulum if associated with DR molecules or Iip33. Cells were labeled with [35S]-methionine for 2 hr, followed by a 2-hr incubation in nonradioactive medium before lysis and immunoprecipitation with mAb PIN.1.1. Samples from JY cells (a); or HeLa cells transfected with Iip35 (b); Iip35 and DR3 (c); Iip33 and Iip35 (d); or Iip33, Iip35, and DR3 (e) were analyzed by two-dimensional gel electrophoresis. Arrows indicate the core-glycosylated Iip35-spots, brackets the sialylated Iip35 spots. Core-glycosylated Iip33 is indicated by an arrowhead. α, DRα; β, DRβ. Acidic proteins are located to the right, basic proteins to the left.
In HeLa cells, Iip35 expressed by itself (Fig. 1b) did not acquire any carbohydrate modifications. In contrast, when Iip35 was coexpressed with HLA-DR (DR) molecules (Fig. 1c) the sialylated forms appeared, indicating transport of Iip35-containing complexes out of the ER. Coexpression of Iip35 with Iip33 (Fig. 1d) resulted in barely detectable transport of Iip35 (see also Fig. 3B), while coexpression with both DR and Iip33 did not appear to increase the exit of Iip35 from the ER compared with cotransfection with DR alone (compare Fig. 1 c and e). These results confirm that the complex formation with DR enables Iip35 to be transported out of the ER despite its retrieval/retention motif.
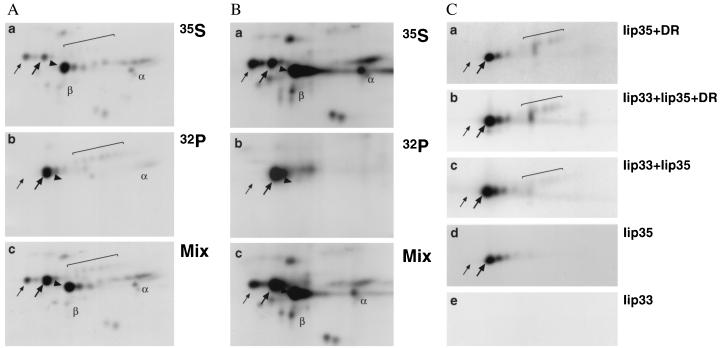
Figure 3.
Iip35 is phosphorylated. (A) JY cells were either labeled with [35S]methionine for 2 hr, followed by a 2-hr chase in nonradioactive medium, or labeled with [32P]phosphate for 2.5 hr before lysis and immunoprecipitation with mAb PIN.1.1. Methionine-labeled (a) or phosphate-labeled (b) immunoprecipitates were split in two parts that were analyzed either separately or after mixing (c). The three panels were exposed for equal lengths of time. (B) Raji cells were either labeled with [35S]methionine for 2.5 hr, or labeled with [32P]phosphate for 2.5 hr before lysis and immunoprecipitation with antiserum K2. Samples were analyzed as in A. (C) Transfected HeLa cells were labeled with [32P]phosphate for 2.5 hr before lysis and immunoprecipitation with PIN.1.1. Precipitates from HeLa cells transfected with Iip35 and DR3 (a); Iip33, Iip35, and DR3 (b); Iip33 and Iip35 (c); Iip35 (d); or Iip33 (e) are shown. Spots are marked as in Fig. 1, except that the phosphorylated Iip35 spots are indicated with thick arrows while the left-most core-glycosylated Iip35 spots are labeled with thin arrows. The core-glycosylated Iip33 spots are not labeled by phosphate, but their location in precipitates from 35S-labeled cells are indicated in A and B.
Iip35-DR Complexes Do Reach Proteolytic Compartments in the Late Endocytic Pathway.
To show that the sialylated Iip35 did reach the endosomal system we took advantage of the fact that Ii degradation in the endosomal/lysosomal system is partially inhibited by the protease inhibitor leupeptin, leading to the formation of leupeptin-induced protein (LIP) fragments (11, 25). Immunoprecipitates from metabolically labeled leupeptin-treated JY cells and transfected HeLa cells were analyzed by two-dimensional gel electrophoresis. In the precipitates from HeLa cells transfected with Iip33 and DR, a series of mainly basic spots migrating at 21–23 kDa was prominent (Fig. 2a). In contrast, the precipitates from Iip35-DR transfected cells gave rise to a small, but distinct group of more basic, slightly slower migrating LIP fragments (Fig. 2b). In precipitates from HeLa cells expressing both Iip33 and Iip35 together with DR (Fig. 2c), as well as in precipitates from JY cells (Fig. 2d), both types of LIP fragments were visible. Analysis by immunofluorescence staining showed that Iip35 could be detected in endosomal compartments of HeLa cells transfected with Iip35 and DR in the presence, but not in the absence of leupeptin (not shown). These results show that a population of Iip35/DR complexes do reach proteolytic compartments in the endocytic pathway.
Figure 2.
Iip35-DR3 complexes do reach the endosomal system. Cells were labeled as in Fig. 1, except that leupeptin (250 μg/ml) was included in the medium during labeling and chase. After lysis DR molecules (and associated Ii) was precipitated by using mAb DA6.147. Samples form HeLa cells transfected with Iip33 and DR3 (a); Iip35 and DR3 (b); Iip35, Iip33, and DR3 (c); or JY cells (d) were analyzed. Spots are marked as in Fig. 1. The thin vertical arrow indicates LIP fragments derived from Iip35, the thick vertical arrow indicates LIP fragments derived from Iip33.
Iip35 Is the Phosphorylated Form of Invariant Chain.
To investigate the phosphorylation of Ii, JY cells were separately labeled with [35S]methionine and [32P]orthophosphate. Labeled proteins were immunoprecipitated with PIN.1.1. One half of the immunoprecipitates were analyzed separately (Fig. 3 Aa and Ab), while the other halves were mixed together before analysis by two-dimensional gel electrophoresis (Fig. 3Ac). Surprisingly, we could find no evidence for phosphorylation of Iip33 and instead it became clear that Iip35 was phosphorylated. Thus, the more acidic spots of the nonsialylated forms of Iip35, as well as the sialylated forms of Iip35 were labeled with [32P]phosphate. In addition, DRα appeared to be labeled to some extent, in agreement with what has been described (26). Because phosphorylation of Iip33 was originally described in the Burkitt lymphoma B cell line Raji (12), we reanalyzed the Ii phosphorylation in this cell line as outlined above. An antiserum directed against a lumenal epitope of Ii was used for the immunoprecipitations. Comparison of the gel representing 35S-labeled material (Fig. 3Ba) with the gel containing both 35S- and 32P-labeled material (Fig. 3Bc) shows that also in Raji cells Iip35 appears to be the main phosphorylated form of Ii. The poor immunoprecipitation of sialylated invariant chain is due to the reactivity of the antiserum, not to differences in Ii transport between Raji and JY cells.
We also phospholabeled HeLa cells transfected with Iip35 and DR (Fig. 3Ca); Iip33, Iip35, and DR (Fig. 3Cb); Iip33 and 35 (Fig. 3Cc); Iip35 (Fig. 3Cd) and Iip33 (Fig. 3Ce) and analyzed immunoprecipitated material as above. The results show that Iip35 (but not Iip33) was phosphorylated also in transfected cells and that the phosphorylation was independent of complex formation with DR and/or Iip33.
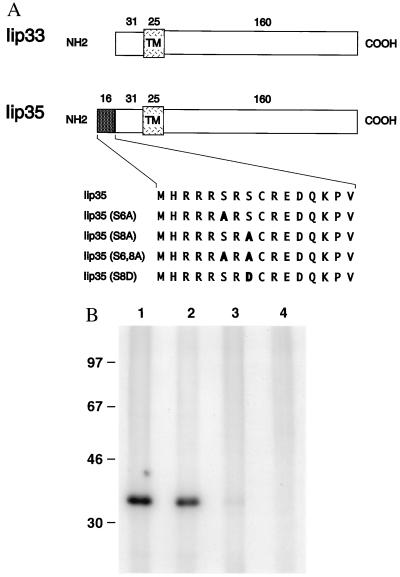
Serine 8 (S8) in the Cytoplasmic Tail of Iip35 Is the Predominantly Phosphorylated Amino Acid.
To investigate if any of the two serine residues unique to Iip35 was phosphorylated, we introduced serine to alanine mutations (S6 and S8; Fig. 4A). HeLa cells transfected with different mutants were labeled with [32P]phosphate and Ii was immunoprecipitated with PIN.1.1. This antibody apparently recognized and precipitated all the mutant chains equally well (see Fig. 7). Fig. 4B shows that the mutation at position S8 greatly reduced phospholabeling of Iip35, indicating that this is the predominant site for phosphorylation (compare lane 3 with lane 1). However, the reproducible presence of weak phospholabeling of this mutant suggests that S6 is also phosphorylated, though to a lesser extent. When both serine residues were mutated (lane 4) no phosphorylation was detected, indicating that there are no other residues than serines S6 and S8 that are phosphorylated in Iip35.
Figure 4.
Mutational analysis of phosphorylated amino acid residues. (A) Constructs used to map the phosphorylated residues. (B) Transfected HeLa cells were labeled with [32P]phosphate for 2.5 hr before lysis and immunoprecipitation with PIN.1.1. Cells transfected with Iip35 (lane 1); Iip35(S6A) (lane 2); Iip35(S8A) (lane 3) or Iip35(S6, 8A) (lane 4) were analyzed by SDS/PAGE. Molecular size markers are located to the left.
Figure 7.
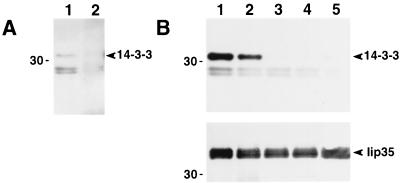
Phosphorylated forms of Iip35 are associated with 14-3-3 proteins. (A) Ii (lane 1) or MHC class I molecules (lane 2) were immunoprecipitated from JY cell lysates by using mAb PIN.1.1 or rabbit antiserum K455. Samples were separated by SDS/PAGE, then transferred to membranes. The membranes were probed with anti-14-3-3 antiserum. (B) Ii was immunoprecipitated from HeLa cells transfected with Iip35 (lane 1); Iip35(S6A) (lane 2); Iip35(S8A) (lane 3); Iip35(S6, 8A) (lane 4) or Iip35(S8D) (lane 5). Samples were separated and blotted as in A. The membranes were probed with anti-14-3-3 antiserum (Upper) and with PIN.1.1 (Lower). Molecular mass (kDa) is indicated to the left.
In the Absence of Functional Dynamin Iip35 Accumulates at the Cell Surface.
Dynamin is necessary for the formation of clathrin-coated vesicles from the plasma membrane, but not from the TGN. In cells expressing a dominant negative form of dynamin, K44A, clathrin-mediated endocytosis from the cell surface is inhibited, while clathrin-dependent transport from the TGN to the endosomal system appears to be normal (27). When expressed in K44A-expressing HeLa cells, Iip33 accumulates at the plasma membrane, and biosynthesis experiments suggest that ≈80% of Iip33 reaches the endosomal system via the plasma membrane in these cells (7). If phosphorylation was essential for the direct delivery of Iip35 from the TGN to the endosomal system, we would expect that the Iip35(S6, 8A) mutant, and possibly also the other mutants, would accumulate at the plasma membrane, whereas the cells expressing wild-type Iip35 would not.
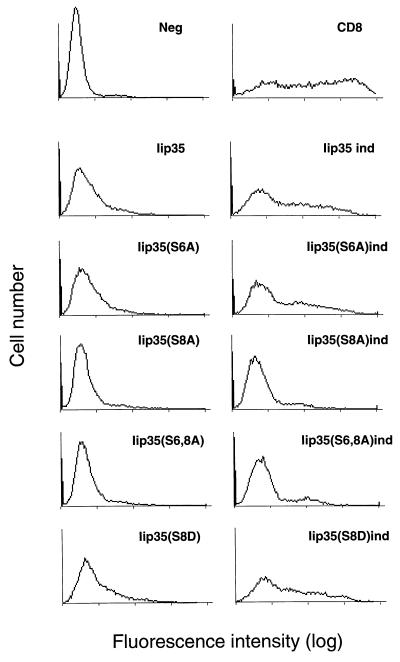
Iip35 or the different mutated constructs were transfected (together with DR) into K44A HeLa cells with or without induction of the mutant dynamin. Cells were analyzed for cell surface expression of Ii by flow cytometry after staining with Bü45, reactive with the extracellular domain of the molecule. Fig. 5 shows that noninduced cells expressing wild-type Iip35 or Iip35(S6A) were weakly stained with Bü45, while the cells expressing Iip35(S8A) and Iip35(S6, 8A) were stained at background level. When induced cells (Fig. 5, ind) were analyzed, the level of Ii staining was distinctly increased in the wild-type and Iip35(S6A) expressing cells, while the level of staining in Iip35(S8A) and Iip35(S6, 8A) was distinctly lower and only marginally increased above background. A fourth mutant, Iip35(S8D), where the serine in position 8 was mutated to aspartic acid (to mimic the negative charge of a phosphoserine) behaved similarly to the wild-type Iip35. None of the Iip35 forms could be detected in endosomal compartments by immunofluorescence in cells expressing mutant dynamin even after leupeptin treatment (not shown), and therefore the differences in surface staining between the different constructs did not appear to reflect differences in endosomal sorting.
Figure 5.
Cell surface staining of Iip35. K44A HeLa cells were supertransfected with different Iip35 constructs together with DR3 and CD8 without (Left) or with (ind, Right) induction of the mutant dynamin. Invariant chain at the cell surface was labeled with mAb Bü45 and analyzed by flow cytometry. Stainings of cells transfected with DR3 and the indicated Iip35 constructs are shown. The negative control represents cells stained with secondary antibody alone. The of level CD8 staining (by using mAb OKT8) was equivalent in all transfectants.
Phosphorylation Is Required for Iip35 Exit from the ER.
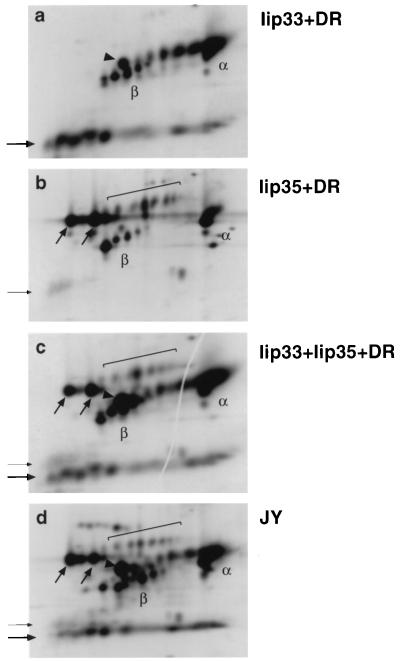
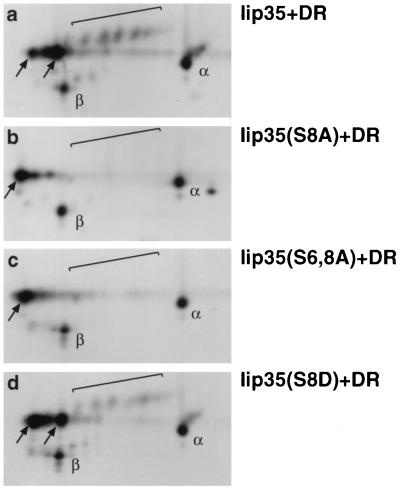
To determine whether the difference in cell surface expression was due to differences in intracellular transport rates, we metabolically labeled HeLa cells that had been transfected with DR together with different Iip35 constructs. Ii-DR complexes were immunoprecipitated with DA6.147, reactive with DRα, and immunoprecipitates were analyzed by two-dimensional gel electrophoresis. Fig. 6a shows that sialylated, transported forms of Ii (and DR) were readily visible in the cells expressing wild-type Iip35 and (to a somewhat smaller extent) in the cells expressing Iip35(S8D) (Fig. 6d). In contrast, no sialylation of Ii could be detected in the cells expressing Iip35(S8A) (Fig. 6b) or Iip35(S6, 8A) (Fig. 6c) even after longer exposures. The sialylation rate of Iip35(S6A) was similar to the rate in cells expressing wild-type Iip35 (not shown). We cannot totally rule out that alanine mutations in the Iip35 tail changes the conformation of the tail independently of the phosphorylation, but the fact that the mutation to aspartic acid results in a protein that can be transported suggests that it is the acidic charge of the phosphate, rather than any other property of the serine residue, that is essential for exit from the ER.
Figure 6.
Decreased exit from the ER of phosphorylation mutants. Transfected HeLa were labeled with [35S]methionine for 2 hr, followed by a 2-hr incubation in nonradioactive medium before lysis and immunoprecipitation with mAb DA6.147. Samples from cells transfected with Iip35 and DR3 (a); Iip35(S8A) and DR3 (b); Iip35(S6, 8A) and DR3 (c) or Iip35(S8D) and DR3 (d) were analyzed by two-dimensional gel electrophoresis. Spots are labeled as in Fig. 1.
Iip35 Associates with a 14-3-3 Protein in a Phosphorylation-Dependent Manner.
14-3-3 proteins bind a phosphoserine sequence motif (see below) present in many different proteins (18), and because the phosphoserines of Iip35 are surrounded by a similar sequence, we examined whether 14-3-3 might be associated with Iip35.
JY cells, or HeLa cells transfected with different Iip35 constructs alone, (Fig. 7B) or together with DR (not shown), were lysed and invariant chain was immunoprecipitated with PIN.1.1. After SDS/PAGE the samples were transferred to membranes and probed with a 14-3-3 antiserum (Fig. 7 A and B Upper) and with PIN.1.1 (Fig. 7B Lower). The 14-3-3 antiserum specifically detected a 31-kDa protein in the precipitate from JY cells and in the precipitates from HeLa cells transfected with phosphorylated forms of Iip35 (wild-type and S6A), but not in the precipitates from cells transfected with Iip35 chains where the S8 had been mutated. Complex formation with DR did not appear to affect the association with 14-3-3 (not shown). PIN.1.1 reacted equally well with all chains, ruling out that the differences in 14-3-3 reactivity was due to differences in the amounts of precipitated Iip35 chains. These experiments show that association between Iip35 and 14-3-3 requires phosphorylation, but not complex formation with DR.
DISCUSSION
The general function of the Ii chain is now understood, but the functional relevance of the different translation products derived from the Ii chain gene is still unclear.
A recent report (5) has suggested that Iip33-containing Ii-class II complexes reach the endosomal system via the plasma membrane (or early endosomes), while Iip35-containing Ii-class II complexes reach the later compartments in the endosomal system directly, without appearing at the cell surface. A possible explanation for this difference in transport routes could be the fact that Iip33 has been reported to be phosphorylated, while Iip35 has not (13). Phosphorylation has been shown to influence the internalization rate from the plasma membrane of several different cell surface molecules, including CD4 (14), the T cell receptor (15), and cytotoxic T lymphocyte-associated molecule 4 (CTLA-4) (28). Because phosphorylation has also been shown to be important for the sorting of furin (16) and the mannose-6-phosphate receptors (17) in the TGN we asked whether the differences between Iip33 and Iip35 in intracellular transport and delivery to the endosomal system might be due to differences in phosphorylation between the two molecules.
In contrast to what has been reported we found that Iip35, but not Iip33, was phosphorylated. This discrepancy with the published data may be explained by the fact that the migration of Iip33 and Iip35 is similar enough that comigration experiments (i.e., where 35S- and 32P-labeled material are mixed and analyzed in the same gel) are necessary to identify two chains with certainty, and this was not done in the original report (13). Mutational analysis showed that both the serine residues unique to the Iip35 tail (S6 and S8) could be phosphorylated, but that the majority of phosphorylation occurred in the S8 position. Though Iip35 contains a sequence motif that is sufficient to localize this molecule to the ER in transfected cells (9), Iip35 is not confined to the ER in the presence of Iip33 and class II molecules, but appears to be transported to the endosomal system. Interestingly, the majority of Iip35 that was transported out of the ER in the presence of class II molecules appeared to be phosphorylated, while nonphosphorylated Iip35 mutants did not show any signs of exit from the ER whether class II αβ chains were coexpressed or not. Mutation of phosphorylated amino acids to aspartic or glutamic acid can sometimes (29) (but not always; see ref. 30) mimic the phosphorylation event. An Iip35 mutant where the S8 residue was changed to aspartic acid [Iip35(S8D)] was transported out of the ER to some extent, suggesting that the negative charge common to the phosphate and the aspartic acid may be important for the egress of Iip35-class II complexes. The two modifications are chemically quite different, however, and the rate of transport out of the ER is not necessarily the same for the phosphorylated Iip35 and the S8D mutant of Iip35.
To examine the delivery of different Iip35 constructs to the endosomal system, we expressed Iip35 constructs in HeLa cells stably transfected with a dominant negative form of dynamin, K44A, under an inducible promoter. We have previously found that a majority of transfected Iip33 reaches the endosomal system via the cell surface in this system (7). When K44A expressing HeLa cells were transfected with DR and Iip35, we found that Iip35, like Iip33, accumulated at the plasma membrane, suggesting that a considerable part of Iip35 also traversed the cell surface. In these cells we could not detect any accumulation of Iip35 in endosomal compartments even after leupeptin treatment, but because a relatively minor part of Iip35 is transported out of the ER, lack of detection in the endosomal system does not allow the conclusion that no Iip35 reaches that location, only that the endosomal delivery is substantially decreased. Because only phosphorylated forms of Iip35 appear to be able to leave the ER, it is not possible to answer the question whether phosphorylation may influence the sorting of Iip35 in the TGN. However, Iip33 and Iip35 appear to be similarly affected by the inhibition of normal dynamin function, and we could not find any evidence to suggest that the two isoforms of Ii would reach the endosomal system by different routes (although we cannot rule out that quantitative differences may exist).
The phosphorylation of Iip35 prompted us to investigate whether this molecule might be associated with 14-3-3 proteins. 14-3-3 is a protein family that has been shown to interact with a large number of different proteins, many of which are involved in signal transduction or cell cycle regulation (18). Recent evidence has shown that 14-3-3 specifically bind phosphoserines in the sequence context R(S)X1,2SpX(P) (where X represents any amino acid, Sp represents phosphoserine and parentheses represent common but not mandatory amino acid residues). The cytoplasmic tail sequence of Iip35 fits well with this motif (see Fig. 4A). The 14-3-3 proteins can form homo- and heterodimers that have been suggested to function as adaptor proteins that can stabilize protein-protein interactions. 14-3-3 has not been directly shown to interact with molecules involved in intracellular transport, but these proteins can be found associated with the Golgi complex in mammalian cells (31) and overexpression of the yeast 14-3-3 homologs (encoded by the BMH1 and BMH2 genes) can rescue cells with mutations in the clathrin heavy chain gene, suggesting that 14-3-3 has a role in intracellular transport (32). Our finding that phosphorylated forms of Iip35 are associated with 14-3-3 suggests that 14-3-3 may influence the exit of Iip35-class II complexes from the ER, but it is not clear whether the increased transport of phosphorylated Iip35 is a direct result of association with 14-3-3. An alternative explanation could be that phosphorylated Iip35 is able to leave the ER independently of 14-3-3, but that association with this protein protects the phosphoserines from de-phosphorylation, thus maintaining Iip35 in a transport-competent state.
To our knowledge, this report is the first example where phosphorylation has been shown to influence the degree of ER retention or retrieval. Signal transduction events do control the phosphorylation of CD4, CD3γ, and CTLA-4, leading to changes in the cell surface expression of these molecules, and it is tempting to speculate that increased phosphorylation of Iip35 may result in more efficient exit of Ii-class II complexes from the ER in response to extracellular stimuli or during cell activation. Further experiments will be required to determine whether this is the case.
Acknowledgments
We thank D. Uranowski and K. Sedat for secretarial assistance, the DNA core lab at the R. W. Johnson Pharmaceutical Research Institute for their technical assistance, K. Wang for help with the use of dynamin mutant cells, and R. Teasdale for critical reading of the manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MHC, major histocompatibility complex; Ii, invariant chain; ER, endoplasmic reticulum; LIP, leupeptin induced protein; TGN, trans-Golgi network.
References
- 1.Cresswell P. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P R, Ploegh H L. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 3.Peters P J, Raposo G, Neefjes J J, Oorschot V, Leijendekker R L, Geuze H J, Ploegh H L. J Exp Med. 1995;182:325–334. doi: 10.1084/jem.182.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benaroch P, Yilla M, Raposo G, Ito K, Miwa K, Geuze H J, Ploegh H L. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warmerdam P A, Long E O, Roche P A. J Cell Biol. 1996;133:281–291. doi: 10.1083/jcb.133.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellino F, Germain R N. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Peterson P A, Karlsson L. J Biol Chem. 1997;272:17055–17060. doi: 10.1074/jbc.272.27.17055. [DOI] [PubMed] [Google Scholar]
- 8.Strubin M, Berte C, Mach B. EMBO J. 1986;5:3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutze M P, Peterson P A, Jackson M R. EMBO J. 1994;13:1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teasdale R D, Jackson M R. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Newcomb J R, Cresswell P. J Immunol. 1993;151:4153–4163. [PubMed] [Google Scholar]
- 12.Arunachalam B, Lamb C A, Cresswell P. Int Immunol. 1994;6:439–451. doi: 10.1093/intimm/6.3.439. [DOI] [PubMed] [Google Scholar]
- 13.Spiro R C, Quaranta V. J Immunol. 1989;143:2589–2594. [PubMed] [Google Scholar]
- 14.Shin J, Doyle C, Yang Z, Kappes D, Strominger J L. EMBO J. 1990;9:425–434. doi: 10.1002/j.1460-2075.1990.tb08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich J, Hou X, Wegener A M, Geisler C. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- 18.Aitken A. Trends Cell Biol. 1996;6:341–347. doi: 10.1016/0962-8924(96)10029-5. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson L, Peleraux A, Lindstedt R, Liljedahl M, Peterson P A. Science. 1994;266:1569–1573. doi: 10.1126/science.7985028. [DOI] [PubMed] [Google Scholar]
- 20.Guy K, Heyningen V V, Cohen B B, Deane D L, Steel C M. Eur J Immunol. 1982;12:942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- 21.Roche P A, Marks M S, Cresswell P. Nature (London) 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 22.Rask L, Lindblom J B, Peterson P A. Eur J Immunol. 1976;6:93–100. doi: 10.1002/eji.1830060205. [DOI] [PubMed] [Google Scholar]
- 23.Jones P P. In: Selected Methods in Cellular Immunology. Mishell B P, Shigii S P, editors. San Fransisco: Freeman; 1980. pp. 398–440. [Google Scholar]
- 24.Machamer C E, Cresswell P. Proc Natl Acad Sci USA. 1984;81:1287–1291. doi: 10.1073/pnas.81.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum J S, Cresswell P. Proc Natl Acad Sci USA. 1988;85:3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman J F, Strominger J L. Proc Natl Acad Sci USA. 1979;76:6304–6308. doi: 10.1073/pnas.76.12.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damke H, Baba T, Warnock D E, Schmid S L. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino J S, Saito T. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 29.Hurley J H, Dean A M, Sohl J L, Koshland D E, Jr, Stroud R M. Science. 1990;249:1012–1016. doi: 10.1126/science.2204109. [DOI] [PubMed] [Google Scholar]
- 30.Gould K L, Moreno S, Owen D J, Sazer S, Nurse P. EMBO J. 1991;10:3297–309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leffers H, Madsen P, Rasmussen H H, Honore B, Andersen A H, Walbum E, Vandekerckhove J, Celis J E. J Mol Biol. 1993;231:982–998. doi: 10.1006/jmbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- 32.Gelperin D, Weigle J, Nelson K, Roseboom P, Irie K, Matsumoto K, Lemmon S. Proc Natl Acad Sci USA. 1995;92:11539–11543. doi: 10.1073/pnas.92.25.11539. [DOI] [PMC free article] [PubMed] [Google Scholar]