Abstract
The effects of intrinsic aging on the cutaneous wound healing process are profound, and the resulting acute and chronic wound morbidity imposes a substantial burden on health services. We have investigated the effects of topical estrogen on cutaneous wound healing in healthy elderly men and women, and related these effects to the inflammatory response and local elastase levels, an enzyme known to be up-regulated in impaired wound healing states. Eighteen health status-defined females (mean age, 74.4 years) and eighteen males (mean age, 70.7 years) were randomized in a double-blind study to either active estrogen patch or identical placebo patch attached for 24 hours to the upper inner arm, through which two 4-mm punch biopsies were made. The wounds were excised at either day 7 or day 80 post-wounding. Compared to placebo, estrogen treatment increased the extent of wound healing in both males and females with a decrease in wound size at day 7, increased collagen levels at both days 7 and 80, and increased day 7 fibronectin levels. In addition, estrogen enhanced the strength of day 80 wounds. Estrogen treatment was associated with a decrease in wound elastase levels secondary to reduced neutrophil numbers, and decreased fibronectin degradation. In vitro studies using isolated human neutrophils indicate that one mechanism underlying the altered inflammatory response involves both a direct inhibition of neutrophil chemotaxis by estrogen and an altered expression of neutrophil adhesion molecules. These data demonstrate that delays in wound healing in the elderly can be significantly diminished by topical estrogen in both male and female subjects.
Acute and chronic wound healing morbidity in the elderly imposes a substantial burden on health services. 1 Healing of an acute wound involves a reduction in wound size secondary to contraction and re-epithelialization and is accompanied by an increase in collagen deposition. 1 Delays in acute cutaneous wound healing lead to local infection, wound dehiscence, and a transition toward a chronic, nonhealing wound. An increase in local protease levels, in particular the neutrophil-derived serine protease elastase, has been associated with certain pathological conditions characterized by excessive tissue breakdown, including age-related acute and chronic wound healing states. 2,3 Elastase is capable of degrading a wide variety of structural and functional proteins deposited in wounds such as proteoglycans, collagen, and fibronectin. 4,5 Fibronectin is an essential component of wound repair, influencing re-epithelialization, collagen deposition, and wound contraction. 6 Moreover, we have recently reported that impaired age-related wound healing states are characterized by reduced local levels of fibronectin, secondary to degradation by elastase, which itself increases as a function of higher neutrophil counts in the wounds of the aged. 3,7 Therapeutic reduction of these raised elastase levels (for example, by reducing neutrophil numbers) could alter the balance towards matrix deposition, as opposed to degradation, and thus increase the rate of wound healing.
The cellular and molecular mechanisms underlying age-related changes in wound healing and elastase up-regulation are poorly understood. One contributing factor that may play a role is the level of local or systemic estrogen. For example, normal female skin undergoes profound changes postmenopausally, including a decrease in dermal collagen and reduced skin thickness, both of which can be reversed by topical estrogen application. 8 Furthermore, systemic hormone levels profoundly influence wound healing: post-menopausal females taking systemic hormone replacement therapy heal standardized acute wounds more rapidly than age-matched controls, 9 which is in agreement with previous animal studies. 10 The presence of the estrogen receptor in normal skin fibroblasts, as well as in wound fibroblasts and inflammatory cells of both young and aged males and females (Ashcroft G, unpublished observation) suggests that local estrogen levels may influence cutaneous physiology, including the wound healing process. Moreover, it is well-documented that sex hormones modulate immune responses, and an increase in the inflammatory response in specific diseases such as gingivitis can be related to reduced estrogen levels. 11
To investigate this further, we determined the effects of topical estrogen, applied immediately before skin excision and for 24 hours post-wounding to the wound edge, on the rate of wound healing in both elderly females and males. Health status-defined subjects have been used in this study to establish that any differences observed in wound healing rates are secondary to age per se, and not to coincidental pathology. In this model, topical estrogen significantly influenced the extent of wound healing in elderly men and women, associated with a reduction in neutrophil elastase and concomitant raised levels of fibronectin and collagen. The results suggest a novel approach to the prophylactic treatment of acute wound healing and potential extension to the treatment of chronic wound healing states. Moreover, the mechanistic basis for these observations involves estrogen-mediated inhibition of neutrophil chemotaxis in addition to down-regulation of cell surface adhesion molecules.
Methods
Subjects and Skin Biopsies
Approval for this study was given by the South Manchester Ethics Committee. Following informed consent, 36 health status-defined subjects 12 (18 females randomized to estrogen or placebo and 18 males randomized to estrogen or placebo, see Table 1 ▶ ) underwent two 4-mm punch biopsies from the left upper inner arm (a non-sun-exposed site) following local infiltration with 1 ml 1% lignocaine. Immediately before the biopsies were made, the skin to be biopsied was covered by a 5 × 4 cm standardized adhesive hormone replacement patch (Evorel active patch, 25 μg estradiol/24 hours, or identical placebo patch; Janssen Pharmaceuticals, Buckinghamshire, UK) through which the two punch biopsies were made. The patch was covered by a Multisorb dry gauze dressing (Smith & Nephew, York, UK) and both were removed after 24 hours. Thus, the edges of the punch biopsy wound and the immediately adjacent skin were exposed to estradiol in subjects who received the active patch.
Table 1.
Subject Characterization
| Male | Female | |||
|---|---|---|---|---|
| Placebo | Estrogen | Placebo | Estrogen | |
| Age | 71.8 ± 8.9 | 69.6 ± 3.6 | 72.5 ± 7.1 | 76.3 ± 5.6 |
| Estrogen (pmol/L) | 100 ± 14.7 (30 pg/ml) | 92 ± 16.6 | <50 (<15 pg/ml) | <50 |
| Progesterone (nmol/L) | <2 | <2 | <2 | <2 |
| Testosterone (nmol/L) | 13 ± 3.5 | 15.9 ± 3.9 | NA | NA |
| SHBG (nmol/L) | 46.9 ± 27.4 | 47.3 ± 14.2 | NA | NA |
Hormonal profiles of subjects (prebiopsy) participating in the study. No differences were observed between pre- and post-biopsy values. Values represent means ± SD. NA, not applicable. SHBG, sex hormone-binding globulin.
All subjects were taking no medication, had normal medical histories and examinations, chest X-ray, electrocardiogram, and hematological, lipid, and biochemical profiles. Subjects were all nonsmokers with normal dietary histories and body mass indices. For the female groups estrogen and progesterone levels were measured; for male groups estrogen and progesterone levels, mean testosterone, and mean sex hormone-binding globulin (SHBG) were determined (Table 1) ▶ . No significant differences were observed between mean prebiopsy values, 24-hour post-wounding values, and re-excision values. Prolactin levels and prostatic specific antigen levels (males only) were within normal limits. All hormone levels were determined by routine radioimmunoassays.
Each initial biopsy of normal skin was bisected and one-half embedded in Optimal Cutting Temperature (OCT) compound (Miles Inc., Elkhart, IN), frozen over liquid nitrogen, and stored at −70°C. The other half was snap-frozen in liquid nitrogen and stored at −70°C. From each of the four groups of subjects, five subjects underwent re-excision of the wounds at day 7 post-wounding, and four subjects at day 80 post-wounding. The left upper inner arm was cleaned with isopropyl alcohol, elliptical excisions of the wounds were made following infiltration with 1% lignocaine, and two sutures were used to close the gap. One wound was bisected; half was processed in OCT and liquid nitrogen and the other half fixed in 10% formalin prior to paraffin embedding and sectioning for histology. The second wound, microdissected from underlying fat and surrounding normal skin, was bisected and snap-frozen for biochemical analysis.
Image Analysis of Wounds
In vivo wound areas as determined by planimetry (tracing around the wound edge) were quantified by image analysis using an Olympus Vanox camera and PC image capture system. For all wound areas measured the wound edge was easily visible, despite the presence of a central crust in some cases at day 7. Paraffin-embedded 7-μm sections were stained with H&E and Masson’s Trichrome. Cross-sectional wound areas were determined by image analysis. Immunostaining was performed on 7-μm cryosections for fibronectin (a rabbit anti-human fibronectin antibody, 1:100; Pasteur, Lyon, France), collagen I (rabbit anti-human antibody, 1:20; Pasteur), neutrophils (mouse anti-human CD15; 1:10 dilution; Serotec, Oxford, UK), monocyte/macrophages (anti-human CD14; 1:20 dilution, Serotec) and estrogen receptor (mouse anti-human, 1:50; Oncogene Science, Cambridge, UK). For the determination of cell numbers, two measurements of the upper wound taken immediately below the epidermis or dense clot, and two from the deep aspect of the wound, at a final magnification of ×25. All images were captured with a Leitz Aristoplan camera under standardized conditions.
The intensity of immunostaining for collagen I and fibronectin, relative to normal skin, in each case was scored on the following 10-point scale by two observers blinded to specimen identity: −5, no staining; −4, 0–25%; −3, 26–50%; −2, 51–75%; −1, 76–99%; 0, normal skin from each individual, 1, 101–125%; 2, 126–150%; 3, 151–175%; 4, 176–200%; 5, 200%+. This scoring system has been found to yield reproducible results in our hands. 9
Hydroxyproline Assay
As an indication of total collagen content, hydroxyproline concentration was determined in individual bisected day 7 and day 80 wounds. 13 Tissues were hydrolyzed in 2 ml of 6 mol/L HCl for 3 hours at 130°C and the solution neutralized to pH 7 with 2.5 mol/L NaOH. After diluting 20-fold with distilled water, 2 ml was mixed with 1 ml of 0.05 mol/L chloramine T solution and incubated for 20 minutes at room temperature. After adding 1 ml of 3.15 mol/L perchloric acid, the mixture was incubated at room temperature for 5 minutes. One milliliter of 20% p-dimethylamino-benzaldehyde was added and the solution incubated for 20 minutes at 60°C. Absorbance of each sample at 557 nm was determined and the amount of hydroxyproline assessed by comparison to a standard curve (0–5 μg hydroxyproline).
Mechanical Properties of Day 80 Wounds
Wound stiffness at day 80 was determined using the nondisruptive dimensional analysis system (DAS). Previous studies have correlated wound breaking strength values (ultimate pressure at failure) to wound stiffness using this system. The system applies a multiaxial load (negative pressure) to the wound and measures the deformation due to the load of two reflective targets placed at the wound edges, using a high resolution camera and video processor. Pressure was applied to a maximum of 100 mm Hg and then released. Stiffness was measured between 20 and 80 mm Hg.
Fibronectin Zymography
Tissue proteases responsible for fibronectin degradation were identified by zymography 14 using fibronectin-containing acrylamide gels (12% acrylamide and 0.33 mg/ml fibronectin, Central Blood Products Ltd., Edinburgh, UK). Homogenized tissue samples (20 μg dry weight) were subjected to electrophoresis under nonreducing conditions. 15 After electrophoresis, the gels were washed twice with 2.5% Triton-X100 for 1 hour to remove SDS. The gels were incubated for 18 hours at 37°C in developing buffer containing 50 mmol/L Tris/HCl, 150 mmol/L NaCl, and 5 mmol/L CaCl2, pH 7.4. At the end of incubation the gels were stained with 0.5% Coomassie brilliant blue and destained. Areas of protease activity appeared as clear zones against a dark blue background. Broad range prestained molecular weight standards (Bio-Rad, Hertfordshire, UK) were used as molecular weight markers. Separate lanes were loaded with 10 ng of human neutrophil elastase (ICN, York, UK). Image analysis was performed on scanned images in order to quantify band intensities using a Novatech image analysis package (Novatech, CA).
SDS-PAGE and Immunoblotting
Protein samples (20 μg dry weight) extracted as described above were subjected to electrophoresis on a 12% acrylamide gel. Polypeptides were transferred to nitrocellulose paper (0.45 μm pore size, Bio-Rad) by electrophoresis at 20V for 30 min (Bio-Rad semidry transfer blot apparatus). The transferred proteins were incubated with polyclonal anti-human neutrophil elastase antibody (Calbiochem Co., Nottinghamshire, UK) diluted 1:500 for 2 hours at room temperature, followed by incubation with horseradish peroxidase conjugated goat anti-rabbit IgG (Sigma, Poole, UK) at 1:3000 dilution for 1 hour at room temperature. Antibody binding was visualized using the enhanced chemiluminescence kit according to manufacturer’s instructions (Amersham Int., Buckinghamshire, UK). Separate lanes were loaded with 500 ng and 50 ng of human neutrophil elastase (ICN).
Elastase Determination
Frozen tissue samples (20 μg dry weight) and human neutrophil elastase (0.01–0.3 μg/ml) were incubated for up to 1 hour at 37°C in 200 μl of 0.1 mol/L Hepes buffer, pH 7.5, containing 0.5 mol/L NaCl, 10% dimethylsulfoxide, and 0.1 mmol/L elastase substrate (methoxy succinyl-ala-ala-pro-val-p-nitroanilide; Calbiochem). 16 Substrate degradation was determined by measuring OD410 (Dynatech MR5000, Nashville, TN). A standard curve for degradation was prepared from the elastase data. Results were expressed as ng/ml elastase activity/20 μg dry weight.
Neutrophil Extraction and Flow Cytometry
Neutrophils were isolated from freshly drawn blood from healthy volunteers using Polymorphprep (Gibco, Paisley, UK). Neutrophils were resuspended in RPMI 1640 (Biofluids, Rockville, MD) at a concentration of 1.2 × 106/ml and were incubated for 12 hours at 37°C in a humidified atmosphere (5% CO2) in the presence or absence of estrogen at concentrations of 3, 30, and 300 pg/ml (ie, 10 pmol/L, 100 pmol/L, and 1 nmol/L, respectively). For flow cytometry, cells were stained with antibodies according to standard protocols and analyzed on a FACScan (Becton Dickinson, Mansfield, MA). Antibodies used were as follows: anti-CD18-FITC, anti-CD15, anti-PECAM, anti-L-selectin (PharMingen Corp., San Diego, CA). Those unconjugated antibodies were detected using appropriate FITC- and TRITC-labeled secondary antibodies (Sigma).
Chemotaxis Assays
Chemotaxis of neutrophils was assayed in a 12-well chemotaxis chamber (Corning Costar Transwell Plate, Rockville, MD), each bottom well containing 400 μl of fMet-Leu-Phe (0.1 μM) or control medium. Neutrophils were resuspended in chemotaxis buffer (Hanks’ medium; 0.5% bovine serum albumin) at a final concentration of 3 × 106/ml, 100 μl added to the upper chamber, and incubated for 90 minutes at 37°C in a humidified atmosphere (5% CO2). Cells that migrated across the membrane (pore size 3 μm) were fixed in 10% paraformaldehyde with 100 mmol/L EDTA in PBS and counted using a Coulter Counter (Beckman Coulter, Fullerton, CA).
Statistical Analysis
Data were normalized where appropriate and differences between means determined using an independent Student’s t-test or analysis of variance. These data are represented as means ± SD. A nonparametric Mann-Whitney U test was used where data did not follow a normal distribution. P < 0.05 is considered significant.
Results
The Effect of Estrogen on Wound Healing
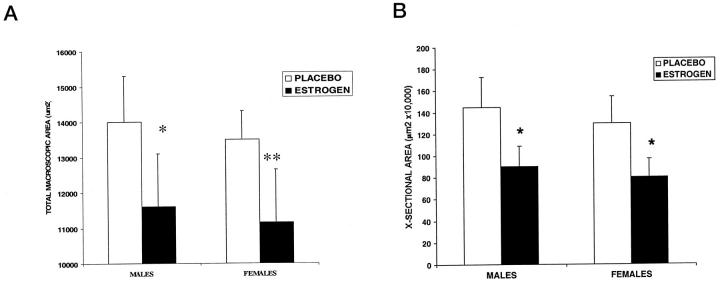
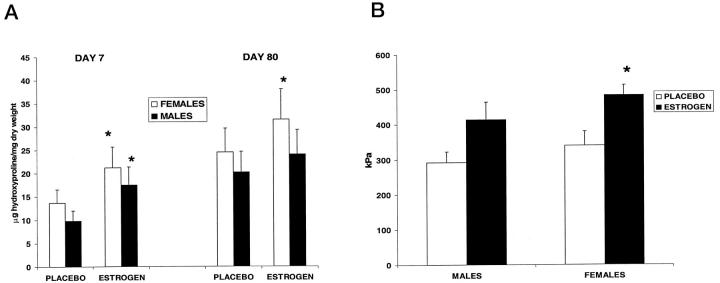
Topical estrogen applied to normal elderly skin immediately before wounding and for 24 hours post-wounding to the wound margins increased the extent of wound healing in both elderly males and females (Figure 1) ▶ . Specifically, wound size was significantly reduced for estrogen-treated wounds (Figure 2a) ▶ both in vivo and when histological cross-sectional areas of H&E-stained sections were determined (Figure 2b) ▶ . Collagen levels, determined by both semiquantitative assessment and hydroxyproline levels, were consistently increased at both day 7 and day 80 post-wounding in both sexes treated with estrogen compared to placebo (Table 2 ▶ , Figure 3a ▶ ). When comparing males and females, for all conditions of treatment there was a trend among females to deposit more collagen. In order to determine the effects of estrogen on the mechanical properties of day 80 wounds, we assessed the stiffness of wounds, which correlates to ultimate breaking strength. There was a trend for estrogen-treated wounds to show increased stiffness compared to placebo, and this was statistically significant in females (Figure 3b) ▶ .
Figure 1.
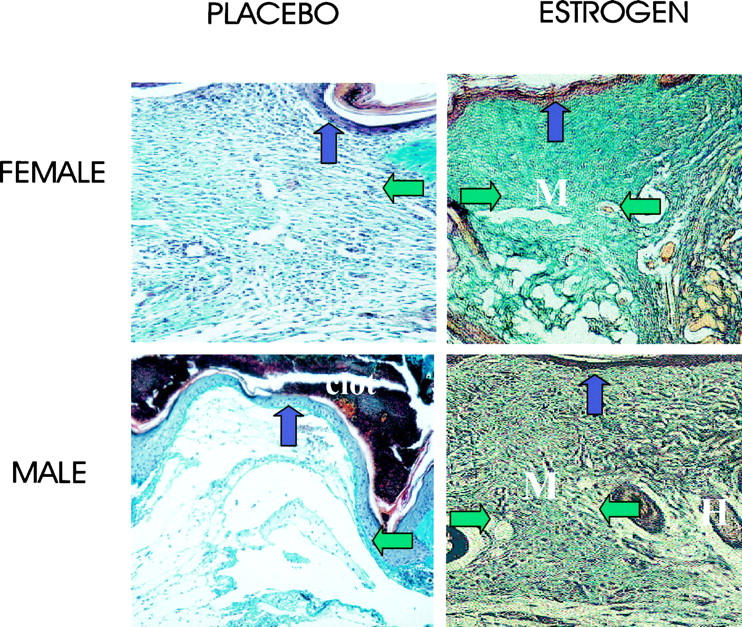
The deposition of matrix at day 7 was enhanced in elderly males and females by topical estrogen. Estrogen treatment stimulates matrix deposition (green fibers; M, matrix; H, hair follicle) in day 7 Masson’s Trichrome-stained sections. In placebo-treated wounds the granulation tissue is immature with fibrin deposits and little collagen. Horizontal arrows demarcate the wound edges (which, because of the increase in wound size, extend beyond the left field of view in the placebo-treated group). Vertical arrows indicate the epidermis. Original magnification, ×16.
Figure 2.
Estrogen treatment was associated with reduced wound areas at day 7. A: Macroscopic areas as determined by planimetry (as described in Methods) were significantly reduced with estrogen treatment at day 7 post-wounding. *P = 0.04; **P = 0.02; n = 5 per group. B: Cross-sectional day 7 wound areas as assessed histologically using image analysis. Estrogen significantly reduced the wound cross-sectional area as determined using image analysis. *P < 0.05; n = 5 per group.
Table 2.
Immunostaining Scores for Collagen I and Fibronectin, Compared to Normal Skin from the Same Subject
| Collagen I (d7) | Collagen I (d80) | Fibronectin (d7) | |
|---|---|---|---|
| M-placebo | −4* (SD 1) | −1 (SD 1.4) | +1.2* (SD 0.4) |
| M-estrogen | −1.4 (SD 0.5) | +0.8 (SD 1.2) | +3 (SD 0.7) |
| F-placebo | −3* (SD 0.7) | −0.8* (SD 1.3) | +1.8* (SD 0.8) |
| F-estrogen | −1.2 (SD 0.4) | +1.3 (SD 0.5) | +3.2 (SD 0.8) |
All values represent means; n = 5 (d7) and n = 4 (d80); SD, standard deviation; * P < 0.05. M, male; F, female. There was no significant staining for fibronectin in any section at day 80.
Figure 3.
Estrogen increased wound collagen deposition and increased wound strength at day 80. A: Hydroxyproline levels were increased significantly in day 7 wound tissue by estrogen treatment for both males and females. At day 80 the difference between placebo and estrogen was only significant in females. *P < 0.05; n = 5 per group (d7) and n = 4 per group (d80). B: Mechanical properties of day 80 wounds assessed by the DAS showed an increase in wound stiffness at day 80 in the estrogen-treated wounds, which was significant only in females. *P < 0.05, n = 4 per group.
Estrogen Receptor Localization
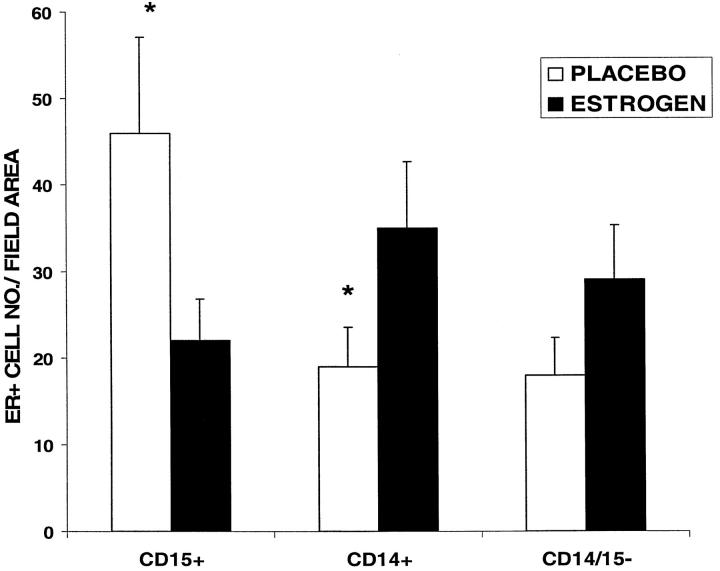
There was a significant increase in cells staining positively for both CD15 (neutrophils) and the estrogen receptor (ER) in the placebo versus the estrogen-treated wounds (Figure 4) ▶ . In contrast, the number of ER+ cells that were monocytes (CD14) was decreased in the placebo-treated wounds compared to those treated with estrogen. Those cells that were CD15− and CD14−, but were positive for the ER, resembled fibroblasts morphologically, with no significant difference in numbers between treatment groups. No gender differences were observed in ER staining in any of the wounds.
Figure 4.
Estrogen receptor localization in acute wounds. Quantification of estrogen receptor-positive (ER+) cells in specific subgroups at day 7 post-wounding showed a significant increase in ER+CD15+ (neutrophil) cells and decrease in ER+ CD14+ (monocyte) cells in the placebo versus estrogen-treated wounds. *P < 0.05, n = 5 per group. ER+ CD14− CD15− cells were considered to be fibroblasts on morphological grounds and were found to be increased (not significantly) in the estrogen-treated wounds. No gender differences were observed. The data represented graphically are that of the male groups.
Reduced Fibronectin Is Associated with Increased Neutrophil Elastase Activity
We determined whether wound tissue possessed fibronectin-degrading activity. Using zymography, all tissue extracts from day 7 acute wounds degraded fibronectin, showing a major band at approximately 30 kd, comigrating with commercial neutrophil elastase, but there was consistently less degradation in the estrogen-treated groups (Figure 5a) ▶ . As an in vivo correlate, wound fibronectin levels determined by scoring immunostaining were markedly increased with estrogen treatment compared to placebo in all the day 7 wounds of both male and female subjects (Figure 5b ▶ and Table 2 ▶ ), with minimal staining in any section at day 80. Western blotting confirmed the 30-kd protease activity seen on fibronectin zymograms as elastase, which was present only in placebo-treated groups (Figure 5c) ▶ . Parallel to these findings, neutrophil elastase activity was quantified using a synthetic elastase substrate degradation assay. This showed that estrogen treatment significantly reduced elastase activity in day 7 wounds compared to placebo (<50 ng elastase per 20 μg dry weight of tissue for the placebo groups concurring with the Western blot data; Figure 5d ▶ ). At day 80 post-wounding no elastase activity could be detected for any specimen.
Figure 5.
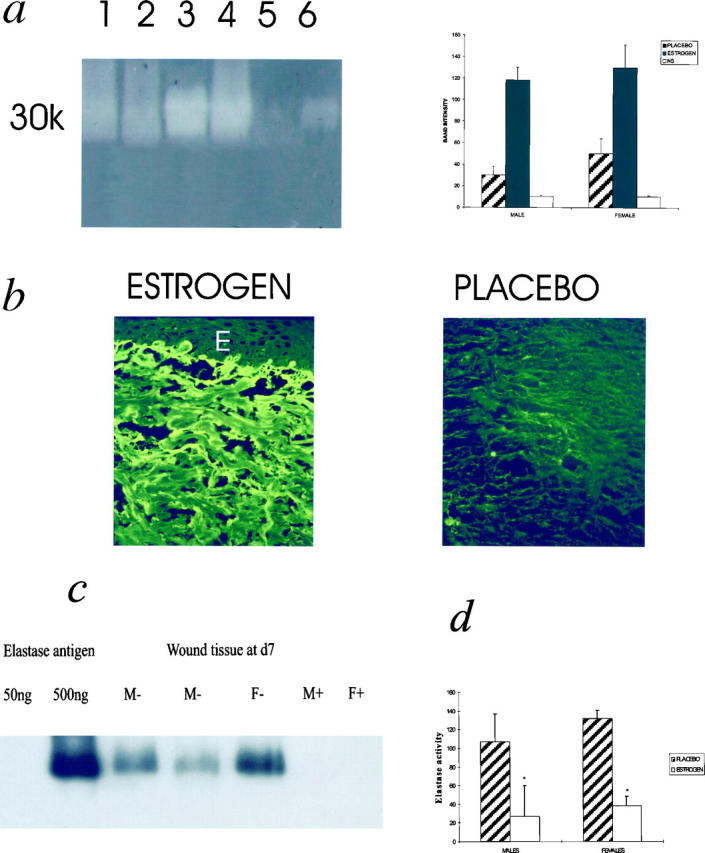
a: Factors capable of fibronectin breakdown in acute wounds are reduced with estrogen treatment. Fibronectin degradation is decreased in acute wounds of aged humans treated with estrogen compared to placebo. Degradation bands appear as pale regions in the gel. Lane 1, female-estrogen; 2, male-estrogen; 3, female-placebo; 4, male-placebo; 5, female skin; 6, 10 ng human neutrophil elastase. NS, normal skin. Band intensities were quantified using image analysis. Results are depicted as means ± SD. Each bar represents the average of band intensities from n = 5 subjects and thus the gel is a general representation. b: Fibronectin immunostaining is markedly increased at day 7 in estrogen-treated wounds. A marked increase in immunostaining for fibronectin was observed in the wounds of the estrogen-treated subjects for both males and females, compared to placebo. E, epidermis. In b no epithelium was present over the wound but photograph of section was taken below clot. Original magnification, ×25. c: Protease responsible for fibronectin degradation confirmed as elastase in acute wound tissue. Immunoblotting with an anti-human neutrophil elastase antibody confirmed that day 7 healing wounds in placebo-treated groups contained elastase, which localized to the degradation band seen in a parallel zymogram. Experiment shown is representative of n = 8 males, n = 8 females. Tissue from placebo groups contained >50 ng elastase/20 μg dry weight, whereas tissue from estrogen-treated groups contained <50 ng/20 μg dry weight. The sensitivity of this assay was >80 ng elastase, whereas the zymography is known to be a more sensitive assay for protease detection (in Figure 4A ▶ 10 ng elastase can be detected). M, males; F, females; +, estrogen; −, placebo. d: Elastase activity in acute wound tissue is reduced with estrogen treatment. Elastase activity was quantified using a synthetic elastase substrate degradation assay. *P < 0.05; n = 5 for each group. Elastase activity is expressed as ng/ml activity per 20 μg dry weight.
Impact of Estrogen on the Inflammatory Response and in Vitro Mechanisms of Action
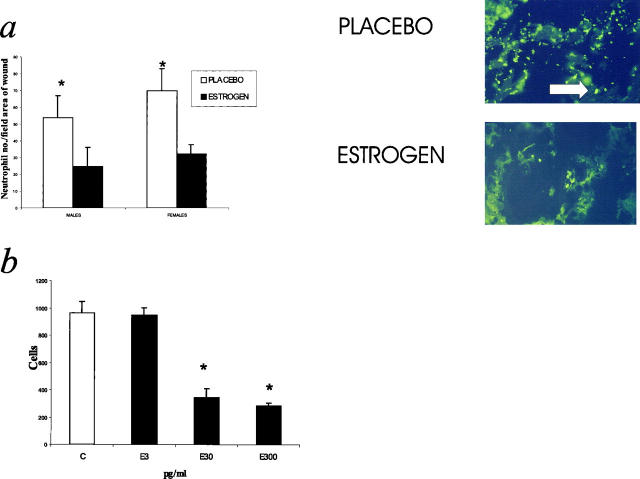
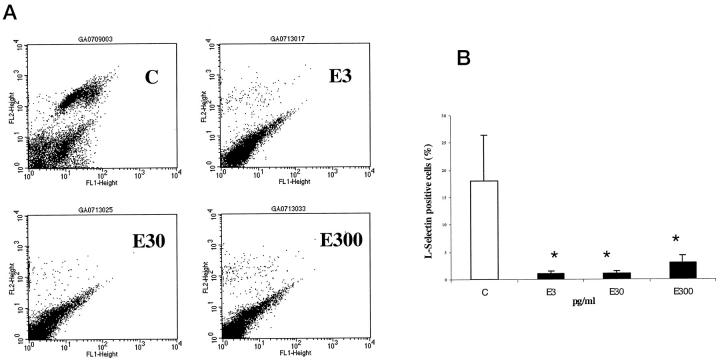
Neutrophil numbers, quantified by image analysis of immunostained sections, were significantly decreased in the estrogen-treated wounds at day 7 post-wounding (Figure 6a) ▶ . At day 80, no immunolocalization of neutrophils was observed in any section. No differences in circulating neutrophil numbers were detected between any of the four groups (data not shown). Estrogen at concentrations of 30 and 300 pg/ml significantly impaired the chemotaxis of neutrophils to the classical stimulant fMet-Leu-Phe compared to untreated neutrophils (Figure 6b) ▶ . The inhibition was a consistent response independent of donor gender. FACS analysis of cell surface adhesion molecule expression showed that estrogen at all concentrations down-regulated the expression of L-selectin (Figure 7, a and b) ▶ , but not PECAM, CD18, or CD15 (data not shown). Again, these estrogen-induced responses were observed irrespective of donor gender.
Figure 6.
Neutrophil numbers were reduced in day 7 estrogen-treated wounds. a: Quantification of neutrophils in day 7 wounds. Estrogen treatment significantly reduced the numbers of local neutrophils in day 7 wounds *P < 0.05; n = 5 for each group. The immunostaining panels represent the center of day 7 wounds stained with an antibody to CD15. Original magnification, ×25. Arrows indicate CD15+ cells. b: Estrogen directly inhibited neutrophil chemotaxis. Estrogen treatment of neutrophils inhibited chemotaxis to fMLP at 30 and 300 pg/ml. n = 8. C, control.
Figure 7.
Estrogen reduces the subpopulation of neutrophils expressing L-selectin. A: Representative FACS analysis of single-labeled neutrophils with L-selectin antibody. C, control; E3, estrogen 3 pg/ml, E30, estrogen 30 pg/ml; E300, estrogen 300 pg/ml. L-selectin cell population observed in the upper right quadrant at 10 2 (FL2) in control panel, and is largely absent after estrogen treatment for 12 hours. B: Reduction of L-selectin cell surface expression detected by FACS after estrogen treatment. n = 8. C, control. Gender had no effect on either parameter when male and female responses were assessed independently. *P < 0.05.
Discussion
These data implicate topical estrogen in increasing the extent of wound healing in the elderly by reducing wound size and stimulating matrix deposition. Our observation of a marked increase in wound collagen deposition with estrogen treatment is consistent with previous reports of topical estrogen application increasing dermal collagen content in normal aged skin. 8 Because the wound areas are reduced with estrogen treatment, it is possible that an increase in collagen density reflects compression of the existing granulation tissue, rather than increased production per se. However, preliminary studies in vitro suggest that estrogen stimulates fibroblast matrix production (Ashcroft G., unpublished observation), and the histological data suggest that compression of matrix is not the predominant factor. In females, the increase in collagen correlates to the mechanical properties of the day 80 wounds. Interestingly, not only do females appear to deposit increased quantities of collagen at days 7 and 80 in response to estrogen, but also in the placebo group compared to males. These gender-related trends warrant further investigation and may involve differences among individuals and between genders in tissue-specific expression of ER isoforms. 17
Fibronectin is an essential component of the early wound environment, directing keratinocyte migration, increasing fibroblast influx and collagen deposition, and stimulating wound contraction. 6 Previous studies have suggested that the process of aging in healthy human subjects is associated with an up-regulation of wound elastase secondary to increased local neutrophil numbers, and that elastase is the major protease responsible for fibronectin degradation in both chronic venous ulcer and acute wound tissue extracts. 3 In addition to its effects on fibronectin levels, persistent and chronically high levels of elastase expression may have other detrimental effects on wound healing, such as degradation of the dermal/epidermal junction 18 and the inhibition of fibroblast adhesion to fibronectin. 19 Moreover, an increase in fibronectin deposition and a decline in elastase activity are associated with the healing of venous ulcers. 20 We now show that estrogen treatment results in reduced wound tissue elastase activity, reduced in vitro degradation of fibronectin by elastase, and a parallel increase in fibronectin levels in vivo at day 7 post-wounding. Our study does not address the issue of different temporal kinetics of deposition since we have only assessed one early time point; however, our previous studies in an animal model of aging and in human elderly subjects suggest that aging is associated with a decrease in fibronectin at all time points with increased degradation in vitro. 3,7 These data do not exclude a direct effect of estrogen on fibronectin production, as opposed to degradation; however, previous studies have indicated that fibronectin production occurs normally both in aged dermal fibroblasts and in those from chronic venous ulcers. 21
Based on these data, we propose that one mechanism whereby estrogen accelerates wound healing may involve an estrogen-dependent down-regulation of wound elastase activity and therefore fibronectin degradation. This could be attributed to an indirect action via estrogen-mediated effects on the immune response and/or leukocyte transit. To investigate an effect on the inflammatory response, we quantified numbers of CD15+ neutrophils infiltrating the tissue at intervals after wounding and the results indicate that estrogen treatment reduces the numbers of local neutrophils. To understand the mechanisms underlying the observed reduction in wound neutrophil numbers in response to estrogen treatment, we determined the effects of estrogen exposure on neutrophil chemotaxis and on the expression of cell adhesion molecules potentially important in the transendothelial migration of neutrophils. Our in vitro studies suggest that estrogen directly inhibits the chemotaxis of neutrophils to a classical chemoattractant in a dose-dependent manner. These studies define a mechanism for estrogen-dependent interruption of neutrophil accumulation at the wound site and confirm a previous in vitro report of estrogen-mediated inhibition of neutrophil chemotaxis via a receptor-mediated system. 11 In addition, estrogen virtually abolishes the subpopulation of neutrophils expressing L-selectin. It has been reported that loss (or shedding) of cell surface L-selectin reduces the ability of neutrophils to localize at sites of inflammation, 22 and thus may represent a key mechanism whereby estrogen modulates the inflammatory response.
This estrogen-related modulation of neutrophil responses and subsequent local decrease in elastase levels may be important not only in wound healing but also in other pathophysiological conditions. In this context, it is interesting that estradiol inhibits myocardial infiltration by neutrophils after reperfusion injury, possibly by attenuating neutrophil adherence to coronary vascular endothelium. 23 It is noteworthy that the number of neutrophils is greater in the female placebo group compared to the male placebo group, similar to our previous report of studies conducted in control subjects biopsied without any patch. 9 This may reflect the increased levels of endogenous systemic estrogen in males and/or gender differences in neutrophil function. 24 Interestingly, this observation may be relevant to the increased incidence of coronary heart disease in females observed postmenopausally, in that neutrophils have been implicated as mediators of atherosclerosis. 25
In our proposed model, estrogen causes an early decrease in wound neutrophil numbers, mediated by a direct inhibition of chemotaxis and a decreased expression of L-selectin. The suppression in neutrophil accumulation results in reduced neutrophil elastase activity and fibronectin degradation. In a cascading fashion, the increase in local fibronectin enhances fibroblast influx and collagen deposition. Moreover, because elastase activates matrix metalloproteinases, 26 reduced elastase would minimize collagen degradation, favoring healing. Increased wound contraction would then occur by a combination of myofibroblast-mediated contraction and re-epithelialization. Previous studies in animal models have concentrated on the effects on wound repair of the systemic rather than topical administration of estrogen. 27 We have previously reported that, in a similar model to the one described here, systemic hormone replacement therapy in postmenopausal females is associated with significantly accelerated wound healing compared to age-matched controls (resembling the profile observed in young females 9 ). The application of topical estrogen to wounds is a practical extension of these findings. Estrogen applied topically to elderly skin immediately before wounding and for 24 hours post-wounding may reduce the incidence of acute wound complications associated with aging, such as dehiscence and infection. Moreover, because chronic wounds are acute wounds which fail to heal because of cyclical episodes of insult and inflammation, the use of topical estrogen as a therapeutic measure to influence the inflammatory response and accelerate healing can be envisioned. However, because neutrophils are one of the main lines of antibacterial host defense and estrogen modulates the neutrophil oxidative burst, 28 it will be important in future studies to demonstrate that estrogen does not increase the susceptibility of these or other types of wounds to infectious agents. Despite these reservations, we did not observe any clinical evidence of infection in estrogen-treated wounds in this study. This study does not address the questions of whether estrogen applied only to normal skin before wounding (and not to the wound itself), or estrogen applied only after wounding, would influence healing, nor whether different doses or application regimens would have enhanced effects. However, this study demonstrates that a single dose of topical estrogen has a major therapeutic effect. Topical application of estrogen to the skin of elderly patients as a prophylactic measure (for example, prior to and during elective surgery) or to acute and chronic wounds may prove to be a novel and cost-effective treatment.
Acknowledgments
We thank Sue Scobie of the South Manchester University Hospital Pharmacy Department and Dr. Julie Morris, University Statistician, for their help. Janssen Pharmaceuticals supplied the placebo and active Evorel patches.
Footnotes
Address reprint requests to Dr. Gillian Ashcroft, Oral Infection and Immunity Branch, National Institute of Dental and Craniofacial Research, Room 327, Building 30, National Institutes of Health, Bethesda, MD 20892. E-mail: gashcroft@ydir.nidcr.nih.gov.
References
- 1.Ashcroft GS, Horan MA, Ferguson MWJ: A review: The effects of ageing on cutaneous wound healing in mammals. J Anat 1995, 87:1-27 [PMC free article] [PubMed] [Google Scholar]
- 2.Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MWJ: Age-related differences in the temporal and spatial regulation of Matrix Metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res 1997, 290:581-591 [DOI] [PubMed] [Google Scholar]
- 3.Herrick SE, Ashcroft GS, Ireland G, Horan MA, McCollum C, Ferguson MWJ: Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers is associated with matrix degradation. Lab Invest 1996, 77:281-288 [PubMed] [Google Scholar]
- 4.McDonald JA, Kelley DG: Degradation of fibronectin by human leucocyte elastase. J Biol Chem 1980, 255:8848-8858 [PubMed] [Google Scholar]
- 5.Kafienah W, Buttle DJ, Burnett D, Hollander AP: Cleavage of type I collagen by human neutrophil elastase. Biochem J 1998, 330:897-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark RAF: Overview and general considerations of wound repair. The Molecular and Cellular Biology of Wound Repair. Edited by R Clark, P Henson. New York, Plenum, 1988, pp 3–10
- 7.Ashcroft GS, Horan MA, Ferguson MWJ: Ageing is associated with reduced deposition of specific extracellular matrix components, an up-regulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Derm 1997, 108:430-437 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt JB, Binder M, Macheiner W, Kainz C, Gitsch G, Bieglmayer C: Treatment of skin ageing symptoms in perimenopausal females with estrogen compounds: a pilot study. Maturitas 1994, 20:25-30 [DOI] [PubMed] [Google Scholar]
- 9.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MWJ: Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nat Med 1997, 3:1209-1215 [DOI] [PubMed] [Google Scholar]
- 10.Roth GS, Harman SM, Lamberg SI: Altered ovarian regulation of wound healing during aging. Proc Soc Exp Biol Med 1981, 166:17-23 [DOI] [PubMed] [Google Scholar]
- 11.Ito I, Hayashi T, Yamada K, Kuzuya M, Naito M, Iguchi A: Physiological concentration of estradiol inhibits polymorphonuclear leukocyte chemotaxis via a receptor mediated system. Life Sci 1995, 56:2247-2253 [DOI] [PubMed] [Google Scholar]
- 12.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Keennes B, Muller-Hermelink HK, Steinmann GG: Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev 1984, 28:47-55 [DOI] [PubMed] [Google Scholar]
- 13.Woessner JF: The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961, 93:440-447 [DOI] [PubMed] [Google Scholar]
- 14.Herron G, Banda M, Clark E, Gavnilovic J, Werb Z: Secretion of metalloproteinases by stimulated capillary endothelial cells. J Biol Chem 1986, 261:2814-2818 [PubMed] [Google Scholar]
- 15.Laemmli U: Cleavage of proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 16.Nakajima K, Powers J, Ashe B, Zimmerman M: Mapping the extended substrate site of Cathepsin G and human leucocyte elastase. J Biol Chem 1979, 254:4027-4032 [PubMed] [Google Scholar]
- 17.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Lubahn DB, O’Donnell TF, Korach KS, Mendelsohn ME: Estrogen inhibits the vascular injury response in estrogen receptor α-deficient mice. Nat Med 1997, 3:545-548 [DOI] [PubMed] [Google Scholar]
- 18.Briggaman R, Schechter N, Fraki J, Lazuras G: Degradation of the epidermal-dermal junction by proteolytic enzymes from human skin and human polymorphonuclear leukocytes. J Exp Med 1984, 160:1027-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinnell F, Zhu M: Fibronectin degradation in chronic wounds depends on the relative levels of elastase, α1-proteinase inhibitor and α2-macroglobulin. J Invest Dermatol 1996, 106:335-341 [DOI] [PubMed] [Google Scholar]
- 20.Herrick SE, Sloan P, McGurk M, Freak L, McCollum C, Ferguson MWJ: Sequential changes in the histological pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992, 141:1085-1095 [PMC free article] [PubMed] [Google Scholar]
- 21.Herrick SE, Ireland G, Simon D, McCollum C, Ferguson MWJ: Venous ulcer fibroblasts compared to normal fibroblasts, show differences in collagen but not fibronectin production under both normal and hypoxic conditions. J Invest Dermatol 1996, 106:187-193 [DOI] [PubMed] [Google Scholar]
- 22.Harlan JM, Liu DY: Adhesion: its role in inflammatory disease. 1992:pp 98-103 W.H. Freeman, New York
- 23.Delyani JA, Murohara T, Nossuli TO, Lefer AM: Protection from myocardial reperfusion injury by acute administration of 17 beta-estradiol. J Mol Cell Cardiol 1996, 28:1001-1008 [DOI] [PubMed] [Google Scholar]
- 24.Spitzer JA, Zhang P: Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation 1996, 20:485-498 [DOI] [PubMed] [Google Scholar]
- 25.Schratzberger P, Dunzendorfer S, Reinisch N, Kahler CM, Herold M, Wiedermann CJ: Release of chemoattractants for human monocytes from endothelial cells by interaction with neutrophils. Cardiovasc Res 1998, 38:516-521 [DOI] [PubMed] [Google Scholar]
- 26.Ferry G, Lonchampt M, Pennel L, de Nanteuil G, Canet E, Tucker GC: Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett 1997, 3:111-115 [DOI] [PubMed] [Google Scholar]
- 27.Pallin B, Ahonen J, Zederfeldt B: Granulation tissue formation in oophorectomized rats treated with female sex hormones. II. Studies on the amount of collagen and on tensile strength. Acta Chir Scand 1975, 141:710-714 [PubMed] [Google Scholar]
- 28.Lim JS, Frenkel K, Troll W: Tamoxifen suppresses tumor promoter-induced hydrogen peroxide formation by human neutrophils. Cancer Res 1992, 52:4969-4972 [PubMed] [Google Scholar]