Abstract
The early growth response 1 (Egr-1 or NGFI-A) gene product is a zinc finger protein transcription factor which has been implicated in the regulation of genes differentially expressed during the development of vascular disease. Egr-1 activity is regulated by alterations in the amount of protein, as well as protein-protein interactions with positive and negative transcriptional cofactors. NGFI-A-binding protein 2 (NAB2) is an example of a negative transcriptional cofactor capable of binding directly to Egr-1 and repressing Egr-1-mediated transcription. In this study, we show that NAB2 is rapidly and transiently expressed in vascular smooth muscle cells (VSMC) in response to the model agonist phorbol 12-myristate 13-acetate (PMA). This induction occurs at the protein as well as mRNA level, and the time course of induction trails closely behind that of Egr-1. NAB2 expression in VSMC is capable of inhibiting Egr-1 dependent gene expression in response to either PMA or fibroblastic growth factor-2 (FGF-2). In an in vivo model of mechanical arterial injury NAB2 levels also increase transiently in VSMC at a time when Egr-1 is elevated. It is possible that NAB2 is part of a negative-feedback mechanism which serves to down-regulate Egr-1-mediated gene transcription in injured VSMC.
Egr-1, also known as nerve growth factor induced-A (NGFI-A), Krox24, or zif268, is the prototype of a family of zinc finger transcription factors. 1-3 Egr-1 is rapidly and transiently induced by a variety of extracellular stimuli, including growth factors, cytokines, and injurious stimuli. 1-3 Members of the family have been implicated in commitments to proliferate, differentiate, or engage cell death pathways in a variety of cell types. Egr-1 may induce multiple genes relevant to vascular biology, including platelet-derived growth factor (PDGF) A-chain, 4,5 PDGF B-chain, 6 tissue factor, 7 basic fibroblastic growth factor (bFGF), 8 transforming growth factor-β1 (TGF-β1), 6,9 apoprotein AI, 10 tumor necrosis factor-α (TNF-α), 11 intercellular adhesion molecule 1 (ICAM-1), 12 5-lipoxygenase, 13,14 and urokinase-type plasminogen activator (u-PA). 6,15 Analysis of mice with targeted mutations in Egr-1 suggests that some of these genes are authentic targets for this transcription factor. 16
Following vascular injury, a series of cellular changes occur in the vessel wall that can result in pathology. These changes result, at least in part, from the proteins that are inducibly expressed by injured endothelium and smooth muscle cells. Previous studies demonstrated that Egr-1 can be found at sites of vascular injury, and it was suggested that inducible Egr-1 expression in response to vessel injury may coordinate the expression of multiple genes involved in the pathogenesis of vascular disease. 6,17,18 In more recent studies, it was demonstrated that Egr-1 induction in endothelial cells following injury was triggered by release and paracrine activation by FGF-2. 19 Using antisense approaches, Egr-1 was directly implicated in the migration and proliferation of smooth muscle cells following vascular injury. 20 These findings suggest that Egr-1-mediated gene transcription plays a key role in changing the functional characteristics of the vessel wall following injury.
The ability of Egr family members to induce changes in gene expression is closely regulated under normal conditions. Similar to some other transcription factors, Egr-1 can associate with corepressor proteins that can repress transcription of Egr-1 target genes. NGRI-A binding corepressors (NAB1) 21,22 and NAB2 23 are members of a novel family of relatively selective transcriptional repressors capable of down-regulating Egr-1 and Egr-2 (Krox 20)-mediated transcription. These proteins bind directly to Egr-1 via a region that is highly conserved among the NAB family and across species. In the mouse, NAB1 is constitutively expressed in low levels in most tissues and does not appear to be highly regulated by Egr-1 inducible stimuli. 21 NAB1 repression is not activator-specific and it is a general transcriptional regulator. 22 In contrast, NAB2 constitutively expressed at highest levels within the brain and thymus and is inducible by growth factors such as serum and nerve growth factor (NGF), which are known to augment Egr-1 expression. 23 These observations suggest that NAB2 may function as an important inducible regulator of gene expression. 23
This study is the first demonstration of NAB expression within cells of the vasculature. Basal expression of these two transcriptional repressors may help to maintain vascular smooth muscle cells (VSMC) in a quiescent state. Our finding that NAB2 is markedly induced following VSMC injury, in vivo, suggests that it may play a regulatory role in the vessel wall. Inducible Egr-1-dependent gene expression may be tightly controlled by up-regulated expression of NAB2. The coordinated regulation of this transcription factor and its repressor suggests that this system may play a role in maintaining vascular homeostasis.
Materials and Methods
Cell Culture
Bovine aortic smooth muscle cells (BASMC) were harvested and grown in Dulbecco’s modified Eagle’s medium (Life Technologies), pH 7.4, containing 50 μg/ml streptomycin, 50 IU/ml penicillin, and 10% calf serum at 37°C and 5% CO2. 24 At confluence, approximately every 4 days, the cells were passaged by being rinsed twice with phosphate-buffered saline (PBS; pH 7.4), followed by a 3-minute incubation at 37°C with 0.05% trypsin and 0.02% EDTA in Hanks’ balanced salt solution (BioWhittaker) before resuspension in growth medium. All experiments were completed with cells between passages 3 and 8. Cells were growth-arrested 12 hours before phorbol 12-myristate 13-acetate (PMA) induction with 1% calf serum. At the time of induction, 1 μg of PMA (Sigma) dissolved in ethanol was added to each 100-mm plate containing 10 ml of growth arrest medium.
Northern Blot Analysis
Total RNA was extracted from confluent cells at various time points after PMA exposure using the Trizol reagent in accordance with the manufacturer’s instructions (Life Technologies, Inc.). RNA samples (10 μg) were separated by a 1% formaldehyde/agarose gel and transferred to a Hybond nylon membrane (Amersham). The membrane was UV cross-linked (Stratalinker UV Crosslinker, Stratagene) and hybridized overnight with NAB1, NAB2, PDGF A-chain, or Egr-1 cDNA probes that had been random-primer labeled (Megaprime DNA labeling systems, Amersham) with [α32P]dCTP (DuPont). The blot was washed with 0.5× SSC several times at 65°C and exposed to film at −70°C for 1 week. Ethidium bromide staining confirmed even membrane loading.
Nuclear Extract
Compartmental extracts from cells grown in tissue culture were prepared by a modification of the method by Dignam et al. 25 At confluence, monolayers were washed twice with PBS at 4°C and collected in 50 ml conical tube on ice using a rubber policeman. Cells were pelleted and lysed by the addition of 20 μl/100-mm plate of Buffer A (10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.1 mmol/L dithiothreitol, 0.5% Nonidet P-40, 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1.5 μg/ml pepstatin A, 0.2 mmol/L levamisole, 10 mmol/L β-glycerophosphate, and 0.5 mmol/L benzamidine) and placed on ice for 10 minutes. The tubes were vortexed and the nuclei pelleted at 14,000 rpm in a microcentrifuge for 20 minutes at 4°C. The nuclei were resuspended in 20 μl/100-mm plate Buffer A and lysed by the addition of 20 μl/100-mm plate Buffer B (20 mmol/L HEPES, pH 7.9, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.1 mmol/L dithiothreitol, 0.5 mmol/L PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1.5 μg/ml pepstatin A, 0.2 mmol/L levamisole, 10 mmol/L β-glycerophosphate, and 0.5 mmol/L benzamidine). Samples were vortexed at 4°C for 20 minutes and centrifuged for 5 minutes at 14,000 rpm. Supernatant containing nuclear proteins was removed and an equal volume of Buffer C (20 mmol/L HEPES, pH 7.9, 100 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 20% glycerol, 0.1 mmol/L dithiothreitol, 0.5 mmol/L PMSF, 0.1 μg/ml leupeptin, 1 μg/ml aprotinin, 1.5 μg/ml pepstatin A, 0.2 mmol/L levamisole, 10 mmol/L β-glycerophosphate, and 0.5 mmol/L benzamidine) was added and mixed. Aliquots were immediately frozen on dry ice and were stored at −80°C.
Western Blot Analysis
Nuclear extracts (10 μg) were denatured in sample buffer containing 12 mmol/L Tris pH 6.8, 5% glycerol, 0.4% SDS, 2.9 mmol/L 2-mercaptoethanol, and 0.02% bromophenol blue at 90°C for 5 minutes. Protein samples were separated on an 8 to 10% SDS polyacrylamide gel and transferred to nitrocellulose membrane (Protran, Schleicher & Schuell) using Towbin buffer and an electrophoretic blotting apparatus (trans-Blot Cell, Bio-Rad). The membranes were probed with polyclonal antibodies to Egr-1, Sp1, Sp3 (Santa Cruz Biotechnology), and NAB2 (provided by J. Milbrandt) at dilutions of 1:1,000 to 1:10,000, followed by enhanced chemiluminescent detection (Amersham) using 1:10,000 horseradish peroxidase-linked donkey anti-rabbit secondary antiserum (Amersham). Blots were exposed to film for 1 to 30 minutes.
Transient Transfection Analysis and Assessment of Reporter Activity
Rat aortic smooth muscle cells, passages 6 to 8, were seeded into 100-mm plates in Waymouth’s medium (Life Technologies), pH 7.4, containing 10% fetal bovine serum (FBS). At 40 to 50% confluence, the cells were washed twice with phosphate-buffered saline, pH 7.4, and incubated in Waymouth’s medium containing 0.25% FBS for 24 hours to facilitate growth arrest. The cells were transfected with 10 μg of chloramphenicol acetyltransferase reporter vector EBS3fos-CAT 26 and 1 μg of pcDNA3 or NAB2-pcDNA3 27 using FuGENE6 in accordance with the manufacturer’s instructions. The cells were exposed to PMA (100 ng/ml) or FGF-2 (Sigma) (25 ng/ml) for 24 hours before cell harvest. CAT activity was normalized to the concentration of protein in the lysates as previously described. 5,19 Data are reported as mean values with standard error bars. Student’s t-test (unpaired, two-tailed) was used to calculate significance levels between NAB2 and pcDNA3 groups.
Arterial Injury Model
Carotid arteries from anesthetized male Sprague-Dawley rats (400 g) were denuded with a 2F Fogarty balloon catheter as previously described. 28 After injury, smooth muscle cells migrate onto the denuded surface, forming an intimal lesion over several weeks at the site of injury. De-endothelialized segments of arteries were identified by injection of Evans blue (0.3 ml of 5% solution in saline) 10 minutes before the animal was sacrificed at the indicated times. Arterial segments were perfusion fixed with phosphate-buffered (0.1 mol/L, pH 7.4) paraformaldehyde.
In Situ Hybridization
In situ hybridization was performed on en face preparations of vessel segments as previously described. 29 A mouse NAB1 cDNA in pBluescript (Stratagene) was linearized with HindIII and transcribed with T3 polymerase to make antisense probe or linearized with BamHI and transcribed with T7 polymerase to make sense probe. A mouse NAB2 cDNA in pCite3 was linearized with NcoI and transcribed with Sp6 polymerase to make antisense probe or linearized with XhoI and transcribed with T7 polymerase to make sense probe. Vessel segments were treated with proteinase K (1 μg/ml) at 37°C, prehybridized for 2 hours at 55°C in 0.3 mol/L NaCl, 20 mmol/L Tris (pH 7.5), 5 mmol/L EDTA, 1X Denhardt’s solution, 10% dithiothreitol, and 50% formamide, and incubated with the appropriate [35S]uridine 5′-triphosphate (UTP)-labeled riboprobe for 16 hours. After washing the slides were coated with autoradiographic emulsion (Kodak, NTB2), exposed for 3 weeks, and developed. All specimens were examined under dark-field illumination after nuclear counterstaining with hematoxylin. Images were photographed and digitalized. The radiolabeled probe appears as white grains.
Results
PMA-Activated BASMC Transiently Increase NAB2 mRNA
PMA was used as a model agonist to study the inducible expression of transcriptional regulators. Growth-arrested BASMC were incubated with 100 ng/ml PMA for various time periods and then harvested for total RNA. Northern Blot analysis revealed that Egr-1 steady-state mRNA levels peaked at 1/2 hours after PMA addition and then decreased back to undetectable levels by 6 hours (Figure 1) ▶ . NAB2 levels lagged behind Egr-1 levels by approximately 1/2 hour, reaching a peak at 1 hour and then tapering back to baseline values by approximately 6 hours. In contrast, NAB1 is constitutively expressed by cultured VSMC and its level did not change significantly over time after the addition of PMA. Steady-state levels of PDGF A-chain, a growth factor whose expression can be induced by Egr-1, 4-6 began to increase approximately 2 hours after PMA exposure, peaked at 4 hours, and decreased back to baseline levels by 24 hours. This experiment suggests that NAB2 mRNA is increased in VSMC following activation. Moreover, the relative time course compared to Egr-1 and PDGF A-chain suggests that NAB2 may be involved in the regulation of Egr-1-mediated vascular gene expression.
Figure 1.
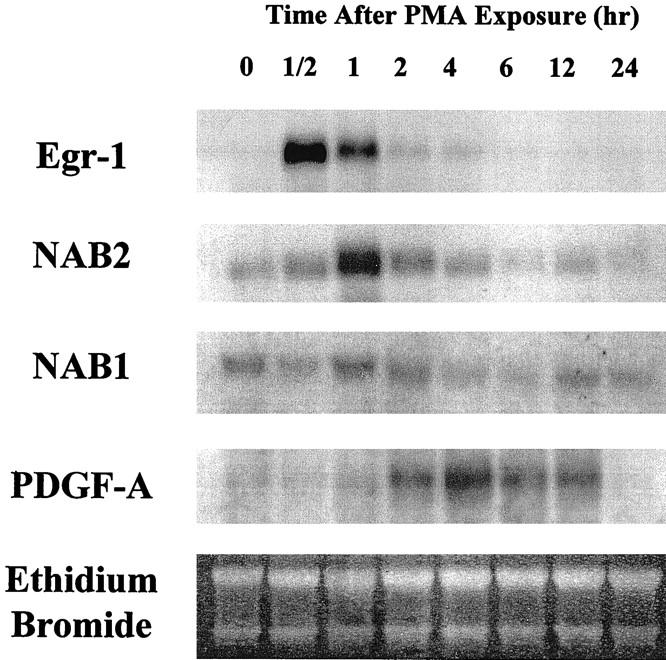
Northern blot analysis comparing the time course of steady-state mRNA levels after PMA activation. Growth-arrested BASMC were exposed to PMA (100 ng/ml) for increasing time periods and harvested for total RNA. RNA was resolved on a 1% agarose gel and transferred to nylon membrane before hybridization with a radiolabeled NAB2 cDNA probe. The same blot was stripped and probed sequentially for Egr-1, PDGF A-chain, and NAB1. Ethidium bromide stained membrane confirmed equivalent RNA loading.
PMA-Activated BASMC Transiently Increase NAB2 Nuclear Protein
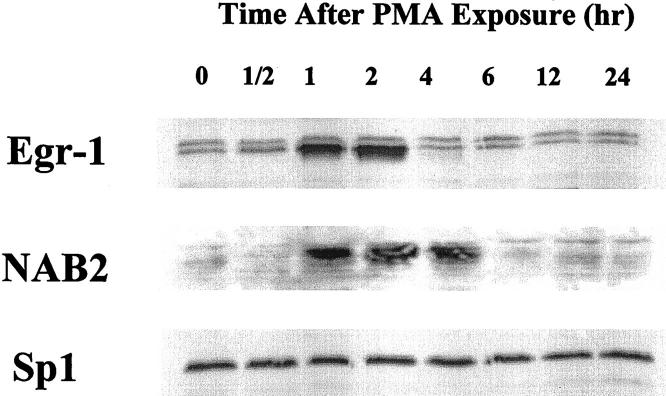
To confirm that increased NAB2 mRNA corresponded with increased nuclear protein levels, Western blot analysis was preformed on nuclear extracts from BASMC after PMA injury. Egr-1 nuclear protein increased from baseline levels to maximum levels approximately 1 to 2 hours after PMA exposure (Figure 2) ▶ . By 4 hours protein levels have returned back to baseline. NAB2 nuclear protein peaked at a slightly later time point, approximately 2 hours after PMA exposure, and returned back to baseline levels by 6 hours. Nuclear Sp1 (and Sp3, data not shown) did not change with PMA exposure confirming equivalent protein loading. This experiment is consistent with our Northern Blot analysis and suggests that NAB2 nuclear protein levels are dramatically induced in BASMC following injury with a time course that follows Egr-1 induction.
Figure 2.
Western blot analysis comparing the time course of inducible nuclear proteins. Nuclear protein was prepared from BASMC exposed to PMA (100 ng/ml) over time. Approximately 10 μg of protein/lane was resolved by 8% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane before immunoblotting with polyclonal antibodies to Egr-1, NAB2, and Sp1. Bound antibodies were detected using a horseradish peroxidase-based chemiluminescence method.
Effect of NAB2 on Egr-1-Dependent Gene Expression
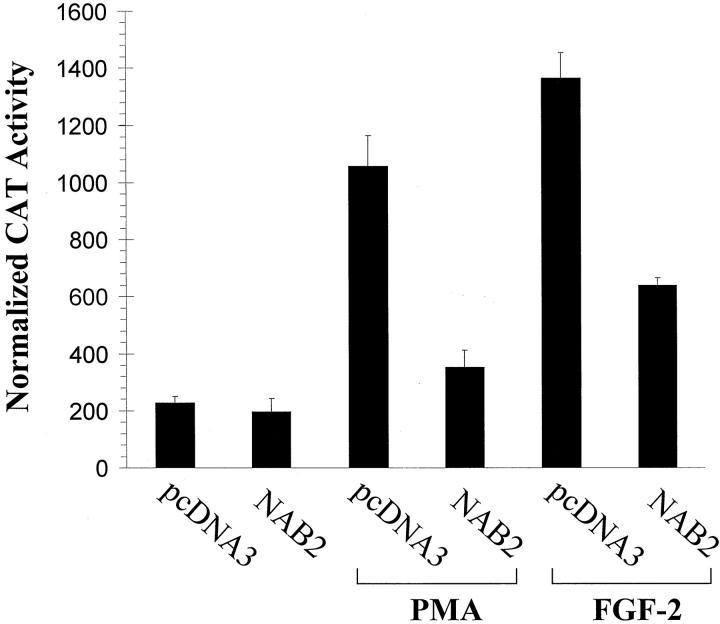
To determine whether NAB2 can modulate the capacity of Egr-1 to activate gene expression induced by PMA, we performed transient transfection analysis in SMC using the CAT-reporter construct, EBS3fos-CAT. This construct is driven by a fragment of the c-fos promoter and contains three tandem Egr-1 consensus binding sites upstream of the transcriptional start site. PMA induced Egr-1-dependent CAT expression over fourfold (Figure 3) ▶ . This effect was inhibited by prior cotransfection with a CMV-promoter-driven NAB2 expression construct, P < 0.05 (Figure 3) ▶ , but not by empty expression vector pcDNA3. NAB2 had little effect on the activity of the reporter in the absence of agonist (Figure 3) ▶ .
Figure 3.
Effect of NAB2 expression on Egr-1-mediated gene transcription in VSMC. The Egr-1 responsive reporter construct, EBS3fos-CAT, was cotransfected with a CMV-driven NAB2 expression construct, NAB2, or an equivalent amount of control backbone, pcDNA3. Cells were exposed to PMA or FGF-2 to increase Egr-1 levels. Normalized CAT activity of at least three experiments is expressed as a mean ± SEM.
Since mechanical injury can release FGF-2 from preformed cellular stores 19 and endogenous FGF-2 can stimulate the vascular response to injury, in vitro and in vivo, in a paracrine manner, 19,30,31 we also investigated the effect of NAB2 on FGF-2-induced Egr-1 trans-activation potential. We have previously demonstrated that FGF-2 is a potent inducer of Egr-1 in vascular cells. 19 As with the model agonist, NAB2 repressed the capacity of this pathophysiologically important growth factor to induce Egr-1-dependent gene expression, P < 0.05(Figure 3) ▶ . These studies demonstrate that NAB2 can inhibit Egr-1 dependent reporter gene expression in VSMC.
Mechanical Injury Increases NAB2 in VSMC in Situ
VSMC gene expression in rat aorta after partial denudation with a balloon catheter was studied by in situ hybridization to establish if vascular NAB2 induction occurs following injury in vivo. Using this model we have previously shown that Egr-1 is induced early in VSMC following injury and increased expression lasts for at least 8 days. 5 Because of these findings, en face preparations of luminal smooth muscle cells migrating into the neointima were studied at 8 days, 2 weeks, and 6 weeks after injury. After 8 days low numbers of rapidly proliferating smooth muscle cells were visible in the neointima. At this time point an [35S]UTP-labeled NAB2 sense riboprobe used as a negative control showed low levels of background signal (Figure 4A) ▶ ; however, the NAB2 antisense probe showed significant levels of transcript (Figure 4B) ▶ . After 2 weeks of smooth muscle cell migration many more cells are visible within the neointima and NAB2 levels remained high (Figure 4C) ▶ . By 6 weeks, a time of minimal VSMC proliferation, NAB2 transcript had decreased (Figure 4D) ▶ . In a similar set of experiments NAB1 levels remained relatively constant after vessel injury (Figure 5, A–D) ▶ . These findings demonstrate that NAB2 expression can be induced by injury in the vessel wall. Although the time course was more prolonged, the transient increase in NAB2 mRNA changes detected in this in vivo rat model of vascular injury are consistent with our in vitro observations. In contrast to NAB2, NAB1 levels did not significantly change after vessel injury.
Figure 4.
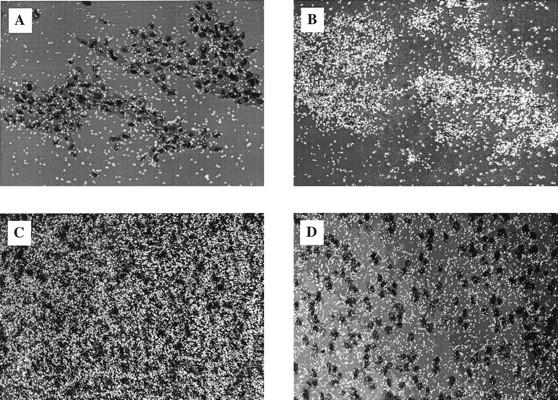
SMC gene expression over time in rat aorta after partial denudation of endothelium with a balloon catheter; en face preparations of luminal SMC are shown. A: After 8 days low numbers of smooth muscle cells were visible in the neointima. [35S]UTP-labeled NAB2 sense riboprobe showed low levels of background signal. B: Hybridization with a NAB2 antisense probe showed significant levels of transcript. C: Two weeks after injury many more SMC were visible within the neointima and NAB2 levels remained high. D: Six weeks after injury SMC number remained unchanged; however, NAB2 transcript was decreased. All photomicrographs were taken at 400× magnification.
Figure 5.
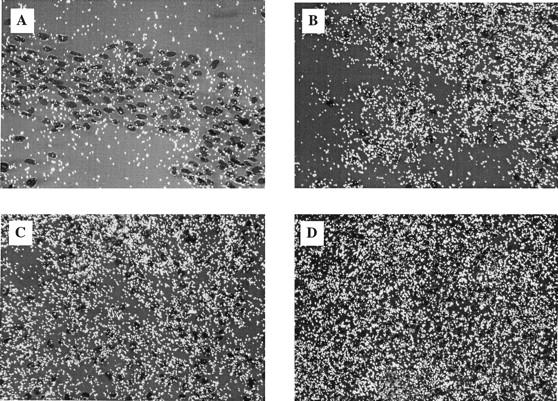
Postinjury en face preparations of luminal SMC labeled with NAB1 probes. A: [35S]UTP-labeled NAB1 sense riboprobe showed low levels of background signal. B: Hybridization with a NAB1 antisense probe showed significant levels of transcript at 8 days. C: Two weeks after injury many more SMC were visible within the neointima and NAB1 expression remained relatively constant. D: Unlike NAB2 transcript, NAB1 transcript did not decrease over time after injury and remained constant. All photomicrographs were taken at 400× magnification.
Discussion
Vascular diseases, such as atherosclerosis, are associated with changes in gene transcription that are mediated by alterations in the amount, specific activity and/or nuclear localization of various transcription factors. 32 An important transcriptional system involved in the regulation of vascular gene expression is the Egr family of zinc finger nuclear proteins. 17,18 Egr-1 and related family members, Egr-2 and Egr-3, are examples of “immediate-early” genes because they are rapidly and transiently induced by a large number of mitogenic, differentiative, and noxious stimuli. Once induced, Egr factors can bind to promoters containing the consensus sequence, 5′-GCG(G/T)GGGCG-3′, and increase or decrease transcription depending on the transcriptional milieu of the particular gene (reviewed in 3,33 ).
Of relevance to vascular biology is the observation that many stimuli associated with the development of vascular diseases, for example shear stress, 34 mechanical injury, 4,5 PDGF, 5 hypoxia, 35 reactive oxygen species, 36 and FGF, 37 are capable of inducing Egr-1 in vascular cells in vitro, and in some cases in vivo. Moreover, Egr-1 can activate the promoters of a variety of genes that are important in the pathogenesis of vascular diseases, 6,17,18 and vascular smooth muscle cell proliferation and regrowth after injury is dependent on activation of Egr-1. 20 Based on these data it has been hypothesized that Egr-1 may play a key regulatory role by linking injurious stimuli to the effector molecules that ultimately result in the expression of vascular pathology. 17,18
Transcription factors of the Egr family are regulated by mechanisms that involve changes in the amount of available Egr protein, and alterations in protein-protein interactions between Egr protein and transcriptional cofactors. Egr-1-mediated gene expression is dependent on both coactivators and corepressors. Cofactors, such as CREB-binding binding protein (CBP) and p300 increase the transcriptional efficiency of Egr-1 transactivation. 38 Corepressors such as NAB1 and NAB2 negatively regulate Egr-1 activity. NAB1 was identified using a yeast two-hybrid system by its ability to bind a 34-amino-acid inhibitory domain of Egr-1, called R1. 21 Deletion of R1 results in a marked increase in Egr-1 transcriptional activity and overexpression of NAB1 markedly decreases Egr-1 transcriptional activity. The related protein NAB2 was subsequently discovered because of its strong homology to NAB1. 23 NAB2 functions similarly to NAB1; however, there are important differences between these related proteins. For example, NAB1 is constitutively expressed in most cell types, whereas NAB2 is rapidly and transiently induced by many of the same stimuli that induce Egr-1. Furthermore, the pattern of tissue expression for NAB2 seems to be more tissue selective than NAB1. 23 Because of these differences NAB2 may play a negative feedback role by down-regulating the burst of Egr-1 activity that accompanies mitogenic, differentiative or noxious stimuli. 23
In this study we have established that NAB2 is rapidly and transiently expressed in VSMC in response to PMA in vitro and mechanical injury in vivo. NAB2 was expressed at the message and protein levels, and its time course closely followed Egr-1 expression. NAB2 functioned in VSMC by inhibiting Egr-1-mediated reporter expression in response to either a model agonist or FGF-2. We speculate that it has similar effects on authentic VSMC genes up-regulated by Egr-1. In contrast, NAB1 mRNA levels did not change significantly in response to PMA and it appears to have higher constitutive expression but less inducible expression than NAB2 in VSMC. It is possible that Egr-1 may increase NAB2 transcription directly, or perhaps both nuclear proteins are being induced by the same stimulus.
In conclusion, we suggest that NAB1 and NAB2 are part of a negative transcriptional regulatory mechanism which serves to down-regulate Egr-1-mediated gene expression following vascular injury. Constitutive levels of the general repressor NAB1 may play a role in maintaining normal VSMC homeostasis. Vascular injury or growth factor stimulation cases a dramatic increase in the levels of Egr-1 and Egr-1-dependent genes. The inducible expression of NAB2 may play a role in returning the VSMC to a quiescent state. Thus dysfunction of this system may play a key role in the pathogenesis of vascular disease.
Acknowledgments
We thank J. Milbrandt for generously providing NAB cDNAs and antibodies. We also thank J. M. Drazen, K. Case, W. Atkinson, J. Svaren, J. Du, and D. H. Markowitz for their assistance and invaluable suggestions.
Footnotes
Address reprint requests to Tucker Collins, M.D., Ph.D., Vascular Research Division, Department of Pathology, Brigham and Women’s Hospital, 221 Longwood Avenue, Boston, MA 02115. E-mail: tcollins@bustoff.bwh.harvard.edu.
Supported in part by HL09613 (to ESS), grants from the National Health & Medical Research Council of Australia and NSW Department of Health (to LMK) and National Institutes of Health grants HL35716, HL45462, PO1 HL36028, and P50 HL56985 (to TC).
References
- 1.Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T, Le Beau MM, Adamson ED: A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 1988, 53:37-43 [DOI] [PubMed] [Google Scholar]
- 2.Milbrandt J: A nerve growth factor induced gene encodes a possible transcriptional regulatory factor. Science 1988, 238:797-799 [DOI] [PubMed] [Google Scholar]
- 3.Gashler A, Sukhatme VP: Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 1995, 50:191-224 [DOI] [PubMed] [Google Scholar]
- 4.Khachigian LM, Williams AJ, Collins T: Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem 1995, 270:27679-27686 [DOI] [PubMed] [Google Scholar]
- 5.Silverman ES, Khachigian LM, Lindner V, Williams AJ, Collins T: Inducible PDGF A-chain transcription in smooth muscle cells is mediated by Egr-1 displacement of Sp1 and Sp3. Am J Physiol 1997, 273:H1415-H1426 [DOI] [PubMed] [Google Scholar]
- 6.Khachigian LM, Linder V, Williams AJ, Collins T: Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science 1996, 271:1427-1431 [DOI] [PubMed] [Google Scholar]
- 7.Cui MZ, Parry GC, Oeth P, Larson H, Smith M, Huang RP, Adamson ED, Mackman N: Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR-1. J Biol Chem 1996, 271:2731-2739 [DOI] [PubMed] [Google Scholar]
- 8.Biesiada E, Razandi M, Levin ER: Egr-1 activates basic fibroblast growth factor transcription: mechanistic implications for astrocyte proliferation. J Biol Chem 1996, 271:18576-18581 [DOI] [PubMed] [Google Scholar]
- 9.Kim S-J, Glick A, Sporn MB, Roberts AB: Characterization of the promoter region of the transforming growth factor-β1 gene. J Biol Chem 1989, 264:402-408 [PubMed] [Google Scholar]
- 10.Kilbourne EJ, Widom R, Harnish DC, Malik S, Karathanasis SK: Involvement of early growth response factor Egr-1 in apolipoprotein AI gene transcription. J Biol Chem 1995, 270:7004-7010 [DOI] [PubMed] [Google Scholar]
- 11.Kramer B, Meichle A, Hensel G, Charnay P, Kronke M: Characterization of an Krox-24/Egr-1-responsive element in the human tumor necrosis factor promoter. Biochim Biophys Acta 1994, 1219:413-421 [DOI] [PubMed] [Google Scholar]
- 12.Maltzman JS, Carmen JA, Monroe JG: Transcriptional regulation of the Icam-1 gene in antigen receptor- and phorbol ester-stimulated B lymphocytes: role for transcription factor EGR1. J Exp Med 1996, 183:1747-1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.In KH, Asano K, Beier D, Grobholz J, Finn PW, Silverman EK, Silverman ES, Collins T, Fischer AR, Keith TP, Serino K, Kim SW, De Sanctis GT, Yandava C, Pillari A, Rubin P, Kemp J, Israel E, Busse W, Ledford D, Murray JJ, Segal A, Tinkleman D, Drazen JM: Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. J Clin Invest 1997, 99:1130-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman ES, Du J, DeSanctis GT, Radmark O, Samuelsson B, Drazen JM, Collins T: Egr-1 and Sp1 interact functionally with the 5-lipoxygenase promoter and its naturally occurring mutants. Am J Cell Mol Biol 1998, 19:316-323 [DOI] [PubMed] [Google Scholar]
- 15.Verde P, Boast S, Franze A, Robbiati F, Blasi F: An upstream enhancer and a negative element in the 5′ flanking region of the human urokinase plasminogen activator gene. Nucleic Acids Res 1988, 16:10699-10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan S, Zou Y, Gao Y, Zhai C, Mackman N, Lee S, Milbrandt J, Pinsky J, Kisiel W, Stern D: Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA 1998, 95:8298-8303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khachigian LM, Collins T: Inducible expression of Egr-1-dependent genes: a paradigm of transcriptional activation in vascular endothelium. Circ Res 1997, 81:457-461 [DOI] [PubMed] [Google Scholar]
- 18.Silverman ES, Collins T: Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol 1999, 154:665-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago FS, Lowe H, Day F, Chesterman CN, Khachigian LM: Egr-1 induction by injury is triggered by release and paracrine activation by fibroblastic growth factor-2. Am J Pathol 1999, 154:937-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago FS, Atkins DG, Khachigian LM: Vascular smooth muscle cell proliferation and regrowth after mechanical injury is dependent upon activation of Egr-1/NGFI-A. Am J Pathol 1999, in press [DOI] [PMC free article] [PubMed]
- 21.Russo MW, Sevetson BR, Milbrandt J: Identification of NAB1, a repressor of NGFI-A and Krox20 mediated transcription. Proc Natl Acad Sci USA 1995, 92:6873-6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, Milbrandt J: Nab1, a corepressor of NGFI-A (EGR-1), contains an active transcriptional repression domain. Mol Cell Biol 1998, 18:512-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J: NAB2, a corepressor of NGFI-A (Egr-1), and Krox20, is induced by proliferative, and differentiative stimuli. Mol Cell Biol 1996, 16:3545-3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunther S, Alexander RW, Atkinson WJ, Gimbrone MAJ: Functional angiotensin-II receptors in cultured vascular smooth muscle cells. J Cell Biol 1982, 92:289-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dignam JD, Lebovitz RM, Roeder RG: Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 1983, 11:1475-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gashler AL, Bonthron DT, Madden SL, Rauscher FJ, Collins T, Sukhatme VP: Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci USA 1992, 89:10984-10988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svaren J, Apel ED, Simburger KS, Jenkins NA, Gilbert DJ, Copeland NA, Milbrandt J: The Nab 2 and Stat6 genes share a common transcriptional termination region. Genomics 1997, 41:33-39 [DOI] [PubMed] [Google Scholar]
- 28.Clowes AW, Reidy MA, Clowes MM: Kinetics of cellular proliferation after arterial injury. I: smooth muscle growth in the absence of endothelium. Lab Invest 1983, 49:327-333 [PubMed] [Google Scholar]
- 29.Lindner V, Reidy MA: Expression of basic fibroblastic growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries: an en face study. Circ Res 1993, 73:589-595 [DOI] [PubMed] [Google Scholar]
- 30.Lindner V, Reidy MA: Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci USA 1991, 88:3739-3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA: Role of fibroblastic growth factor in vascular lesion formation. Circ Res 1992, 68:106-113 [DOI] [PubMed] [Google Scholar]
- 32.Collins T: Endothelial NF-κB, and the initiation of the atherosclerotic lesion. Lab Invest 1993, 68:499-508 [PubMed] [Google Scholar]
- 33.Liu C, Rangnekar VM, Adamson E, Mercola D: Suppression of growth and transformation of apoptosis by Egr-1. Cancer Gene Ther 1998, 5:3-28 [PubMed] [Google Scholar]
- 34.Khachigian LM, Anderson KR, Halon NJ, Gimbrone MA, Jr, Resnick N, Collins T: Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Thromb Vasc Biol 1997, 17:2280-2286 [DOI] [PubMed] [Google Scholar]
- 35.Gess B, Wolf K, Pfeifer M, Riegger GA, Kurtz A: In vivo carbon monoxide exposure and hypoxic hypoxia stimulate immediate early gene expression. Eur J Physiol 1997, 434:568-574 [DOI] [PubMed] [Google Scholar]
- 36.Nose K, Ohba M: Functional activation of the egr-1 (early growth response-1) gene by hydrogen peroxide. Biochem J 1996, 316:381-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delbridge GJ, Khachigian LM: FGF-1 induces platelet-derived growth factor A-chain gene expression in endothelial cells involves transcriptional activation by early growth response factor-1. Circ Res 1997, 81:282-288 [DOI] [PubMed] [Google Scholar]
- 38.Silverman E, Du J, Williams A, Wadgaonkar R, Drazen J, Collins T: cAMP-response-element-binding-protein-binding protein (CBP), and p300 are transcriptional co-activators of Egr-1. Biochem J 1998, 336:183-189 [DOI] [PMC free article] [PubMed] [Google Scholar]