Abstract
To develop a model for the biology and treatment of CD30+ anaplastic large cell lymphoma (ALCL), we transplanted leukemic tumor cells from a 22-month-old girl with multiple relapsed ALCL. Tumor cells were inoculated intraperitoneally into a 4-week-old SCID/bg mouse and produced a disseminated tumor within 8 weeks; this tumor was serially transplanted by subcutaneous injections to other mice. Morphology, immunohistochemistry, and molecular genetics which demonstrated the NPM-ALK fusion protein, resulting from the t(2;5)(p23;q35), confirmed the identity of the xenograft with the original tumor. The tumor produced transcripts for interleukin-1α, tumor necrosis factor-α, and interferon-γ which could explain the patient’s B-symptoms. Treatment of mice with monoclonal antibody (HeFi-1) which activates CD30 antigen administered on day 1 after tumor transplantation prevented tumor growth. Treatment with HeFi-1 after tumors had reached a 0.2 cm3 volume caused tumor growth arrest and prevention of tumor dissemination. We conclude that transplantation of CD30+ ALCL to SCID/bg mice may provide a valuable model for the study of the biology and design of treatment modalities for CD30+ ALCL.
Anaplastic large cell lymphoma (ALCL) was first described in 1985 as a CD30+ large cell lymphoma with distinctive anaplastic cell morphology, characteristically infiltrating the paracortical region and sinuses of lymph nodes. 1 It was subsequently shown that this tumor affects primarily children and is associated with frequent extranodal disease, especially skin involvement. 2 There is a recurrent translocation, t(2;5)(p23;q35), resulting in the fusion of the nucleophosmin gene (NPM) on chromosome 5q35 to a novel tyrosine kinase-encoding gene designated anaplastic lymphoma kinase (ALK) on chromosome 2p23 observed in 30 to 70% of all ALCLs. 3-7 Although patients whose tumors express the NPM-ALK protein have a statistically better prognosis, some children with NPM-ALK-positive tumors have disease resistant to multiple chemotherapy regimens. According to a recent study conducted by Children’s Cancer Group, pediatric patients with systemic ALCL have only a 41% five year disease-free survival and a 62% overall five year survival (MEK, personal communication). Thus novel therapies for treatment resistant ALCL are needed.
To address this issue, we developed a xenograft model of treatment resistant ALCL. SCID/bg mice were chosen for this purpose because they have impairment of natural killer cell activity in addition to the lack of B and T cell function. The model was found to closely resemble the primary tumor in histopathology and in clinical behavior with widespread organ involvement. The transcription of NPM-ALK and of various cytokines which could explain the patient’s symptoms was demonstrated. Finally, the model was used to test the efficacy of immunotherapy directed against the CD30 antigen expressed by the tumor cells.
Materials and Methods
Patient
The patient is a 22-month-old Caucasian female who presented with 2-month history of fever, cervical adenopathy, and weight loss. The diagnosis was at first difficult to establish because of the admixture of small and large atypical cells. Over the next 2 months the patient developed progressive lymphadenopathy. CAT scans revealed supraclavicular nodes extending into the mediastinum and enlarged paratracheal, axillary, subcarinal, and periaortic lymph nodes. A pleural effusion contained small, CD30+, cytologically malignant cells, with frequent mitoses. Cytogenetics revealed a t(2;5)(p23;q35) translocation. Bone marrow aspirate showed 7% malignant cells. Cerebrospinal fluid was negative for tumor cells. The patient was treated with adriamycin, prednisone, and vincristine. She tolerated the first course of chemotherapy well, and a CAT scan on day 30 showed persistent matted nodes in the supraclavicular region and a persistent mass in the anterior mediastinum. Biopsy of the mediastinum was interpreted as scar tissue. Five days later the patient developed fever and gum swelling. Biopsy of the gum showed recurrent lymphoma. She developed rapidly increasing cervical adenopathy. High-dose etoposide and cyclophosphamide were administered but complicated by Salmonella enteritis. CAT scan showed partial response, and a second round of chemotherapy was administered. Subsequent CAT scan showed tumor reduction, but before the next cycle of chemotherapy she developed fever and rapidly progressive bilateral cervical adenopathy. Two cycles of steroids, high-dose Ara-C, and cisplatin (DHAP) achieved a good clinical response. The patient is currently completing bone marrow transplantation from a matched unrelated donor.
Tumor Cell Preparation
A peripheral blood sample containing circulating tumor cells was obtained with informed consent from the patient at the time of the diagnosis. Peripheral blood mononuclear cells were isolated by gradient centrifugation using Ficoll Paque Plus (Pharmacia Biotech, Uppsala, Sweden), washed twice in PBS and resuspended in RPMI 1640 (BioWhittaker, Walkersville, MD).
Animals
Four to six-week-old SCID/bg mice were obtained from Taconic Farms (Germantown, NY) and housed in autoclaved microisolator cages in an air-filtered laminar flow cabinet within the Animal Research Facility of the Beth Israel Deaconess Medical Center. Food was irradiated, and water and bedding were autoclaved before use. All procedures were performed under aseptic conditions.
Tumor Implantation and Growth
A tumor cell suspension prepared from peripheral blood cells (2 × 10 7 cells) was injected intraperitoneally (i.p.) to produce a tumor in mouse 1. Subsequent passages of tumor from mouse 1 were performed by serial transplantation of small pieces of tumor subcutaneously into the left flank of SCID/bg mice using a 13-gauge trocar. Initial transplantation groups were composed of 3 or 4 mice. Subsequent experimental therapeutic groups were composed of 16 treated mice and 11 control mice.
Histology and Immunohistology
For histological examination, tumor tissue and organs were fixed in 10% buffered formalin and embedded in paraffin; 4-μm sections were stained with hematoxylin and eosin. Immunohistochemistry for CD30, CD15, EMA, ALK1, CD3, CD4, CD8, CD20, CD57, and EBV-LMP was performed using a three-step immunoperoxidase technique using biotinylated secondary antibodies, streptavidin conjugated with horseradish peroxidase and diaminobenzidine (chromogen).
Flow Cytometry
Single cell suspensions obtained from transplanted tumor cells were analyzed for expression of cell surface antigens using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). Cells were labeled directly with murine monoclonal antibodies against CD30, CD15, CD3, CD8, CD19, and CD5, conjugated with fluorescein isothiocyanate or phycoerythrin (Caltag, Burlingame, CA).
RNA Extraction and cDNA Synthesis
Total RNA from cells in suspension was isolated using the TRIzol extraction kit (Gibco BRL, Life Technologies, Gaithersburg, MD). For RNA extraction from tissues, the extraction was preceded by a homogenization step. cDNA was generated using Superscript II (Gibco BRL, Life Technologies) and 10 pmol of dT18-primer in 29 μl, according to the manufacturer’s instructions. Successful cDNA synthesis was confirmed by amplification of an 839-bp fragment of β-actin (Clontech, Palo Alto, CA).
Polymerase Chain Reaction for Expression of Cytokine mRNA
Detection of mRNA for human cytokines, cytokine receptors, and transcription factors was performed using commercially prepared primers and controls for interleukin (IL)-1α, IL-2, IL-2 receptor, IL-4, IL-5, IL-6, IL-6 receptor, IL-7, IL-8, IL-10, transforming growth factor (TGF)-β, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, c-myc, G-CSF, and G-CSF receptor (Clontech) according to the manufacturer’s instructions. For IL-9 mRNA detection, previously described primers and polymerase chain reaction (PCR) conditions were used. 8 10 μl of PCR products were electrophoresed through 2% agarose gels in Tris-borate EDTA buffer, pH 7.4. Gels were stained with ethidium bromide and visualized with ultraviolet light using a Gel Doc 1000 Analyzer (Bio-Rad, Hercules, CA).
Molecular Analysis for NPM-ALK Transcripts
cDNAs were amplified by PCR using the primers NPM S1 and ALK A1 as previously described. 3 The specific primer sequences were NPM-S1: TCCCTTGGGGGCTTTGAAATAACACC; ALK-A1: CGAGGTGCGGAGCTTGCTCAGC. PCR was preceded by a 5-minute incubation at 94°C. Cycle conditions were as follows: Denaturation at 94°C for 45 seconds, annealing at 60°C for 45 seconds, and elongation at 72°C for 2 minutes for 35 cycles and final extension at 72°C for 5 minutes. The resulting PCR products were fractionated through a standard 2% agarose gel and visualized with ethidium bromide staining by exposure to ultraviolet light using a Gel Doc 1000 Analyzer (Bio-Rad, Hercules, CA).
In Vivo Experiments
Mice were transplanted with same passage tumor cells per individual experiment to study the anti-tumor effects of the anti-CD30 antibody HeFi-1 (National Cancer Institute, Frederick Cancer Research and Development Center, Biological Resources Branch, Frederick, MD). 9 Mice were injected i.p. with 10 μg of anti-CD30 antibody (HeFi-1) or mouse IgG1 (Sigma, St. Louis, MO) in 0.2 ml of PBS as a control, every other day for 10 days, for a total of 5 injections starting on day 1 or day 6 after tumor implantation. The size of the subcutaneous tumors was measured with a caliper and tumor volume was calculated according to the formula V = d × D × π/2, where d is the smaller diameter and D the larger diameter. Mice were then monitored for tumor development and progression. Treated and control mice were euthanized and necropsied for evidence of tumors, including histology and immunohistochemistry. Experimental groups included 8 mice for each treatment protocol and 11 mice in the control group. Tumor volumes in both groups were compared by Wilcoxon rank-sum test for statistical significance.
Anti-Tumor Activity of HeFi-1 in Vitro
The anti-tumor activity of HeFi-1 on human ALCL tumor cell growth in vitro was determined by 3H-thymidine incorporation. Single cell suspensions from short-term tumor tissue cultures were split 24 hours before assays were performed. Cells were resuspended in 10% fetal calf serum (FCS) in RPMI, supplemented with L-glutamine and penicillin/streptomycin at a final concentration of 5 × 10 4 cells/ml. The cell suspension was plated at 0.2 ml/well in 96-well round bottom plates (Corning Glass Works, Corning, NY). HeFi-1 or IgG1 isotype (Sigma) as a control, was added in soluble form at a concentration of 10 μg/ml. After 72 hours, wells were pulsed with 1 μCi 3H-thymidine/well (New England Nuclear Research Products, Boston, MA). Cells were harvested onto glass fiber filters with a cell harvesting system (PhD Cell Harvester, Cambridge Technology, Inc., Cambridge, MA) and 3H-thymidine incorporation was measured in a Wallac 1409 liquid scintillation counter (Wallac Inc., Gaithersburg, MD). Each experiment was performed in triplicate. Results were statistically analyzed using paired t-test and Statview software package.
Results
Xenografted Tumor Cells Grow in SCID/bg Mice
Lymphoma cells obtained from the ALCL patient were inoculated i.p. into one 4-week-old female SCID/bg mouse and produced disseminated tumor in 8 weeks. The established tumor was transplanted by subcutaneous injection into four SCID/bg mice which all developed rapidly growing lymphomas manifested by palpable tumor masses within 4 to 6 days. At necropsy, diffuse lymphadenopathy involving mediastinal, retroperitoneal, cervical, axillary, and submandibular nodes, as well as tumor involving the spleen, liver, lung, and pancreas were detected.
Histopathology
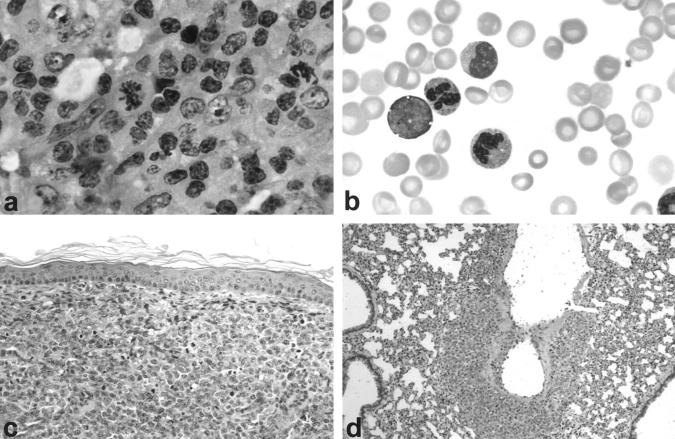
The cytological features of the established tumor were similar to those of the original ALCL tumor with anaplastic, predominantly large tumor cells with round or bean-shaped nuclei, a high nuclear to cytoplasmic ratio, and numerous mitoses (Figure 1, a–c ▶ and Figure 4a ▶ ). Microscopic evaluation of a wide range of organs revealed systemic massive lymphomatous involvement of the lungs (Figure 1d) ▶ , pancreas, kidneys, and gastrointestinal tract as well as peripheral blood involvement. Invasion of the central nervous system and bone marrow was not observed.
Figure 1.
a: Lymph node histology of ALCL patient. H&E, ×600. b: Circulating ALCL tumor cells in the peripheral blood. Giemsa stain, ×1000. c: Human ALCL tumor with anaplastic morphology infiltrating dermis of SCID/bg mouse. H&E stain, ×200. d: Tumor cells infiltrating walls of a pulmonary blood vessel. H&E stain, ×200.
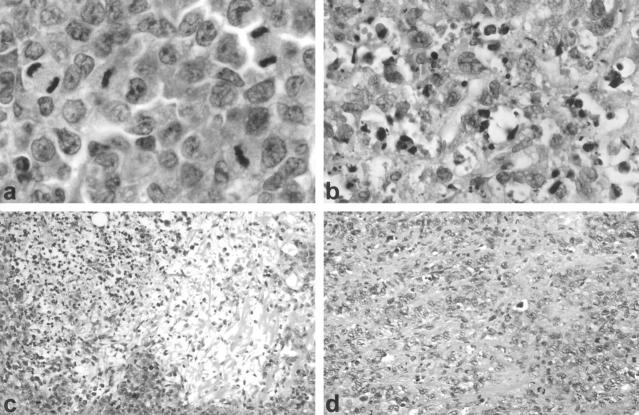
Figure 4.
Human ALCL tumor in HeFi-1 treated mice. a: Viable tumor with numerous mitoses in mouse treated with control IgG isotype antibody. H&E, ×1000. b: Tumor removed from HeFi-treated mouse at day 20 showed many dead tumor cells (×600). c: Tumor cell depletion (×200). d: Fibrosis (×200).
Immunophenotypic Characterization of the Tumor
Immunophenotyping on paraffin sections of the transplanted ALCL displayed tumor cell expression of CD30, CD3, and ALK-1, confirming expression of the NPM-ALK fusion protein and identity with the original human ALCL tumor. As in the original tumor, CD4, CD8, EMA, CD15, CD20, CD57, and LMP were not expressed. Analysis of cell surface antigens of the mouse tumor by flow cytometry confirmed expression of CD30 and CD3. CD19, CD4, CD8, and CD5 antigen expression was absent.
Molecular Gene Expression
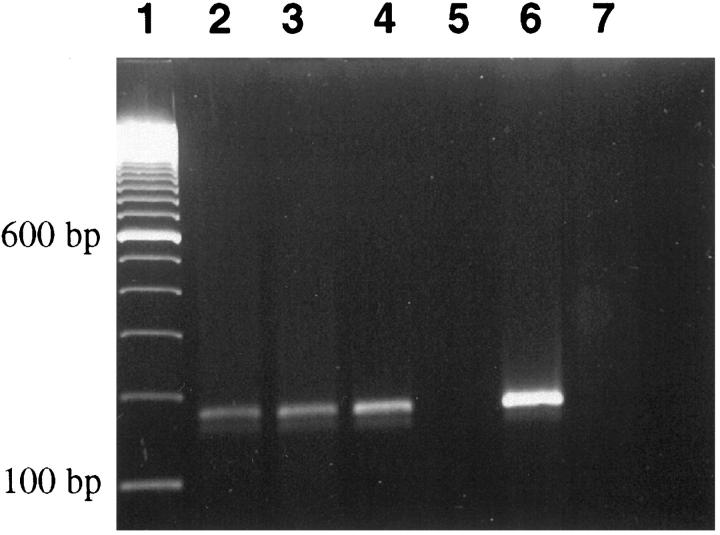
Reverse transcriptase (RT)-PCR of the original patient tumor and mouse xenografts showed the predicted 177-bp product for NPM/ALK (Figure 2) ▶ . Evaluation of mRNA expression by RT-PCR revealed transcripts for IL-1α, IL-2 receptor, IL-6 receptor, G-CSF receptor, IL-8, IL-10, TGF-β, IFN-γ, TNF-α, and c-myc. No expression of message was found for IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, or G-CSF (data not shown).
Figure 2.
RT-PCR for NPM-ALK transcripts: lane 1, molecular size marker; lane 2, original blood sample of patient; lane 3, human ALCL xenograft from SCID/bg mouse; lane 4, short term tissue culture of explanted tumor cells; lane 5, negative control (no template); lane 6, t(2;5)-positive control ALCL cell line (Karpas 299); lane 7, t(2;5) negative Hodgkin cell line (KM-H2).
Anti-Tumor Effect of HeFi-1, a Functional Anti-CD30 Antibody, in SCID/bg Mice Xenografted with ALCL Cells
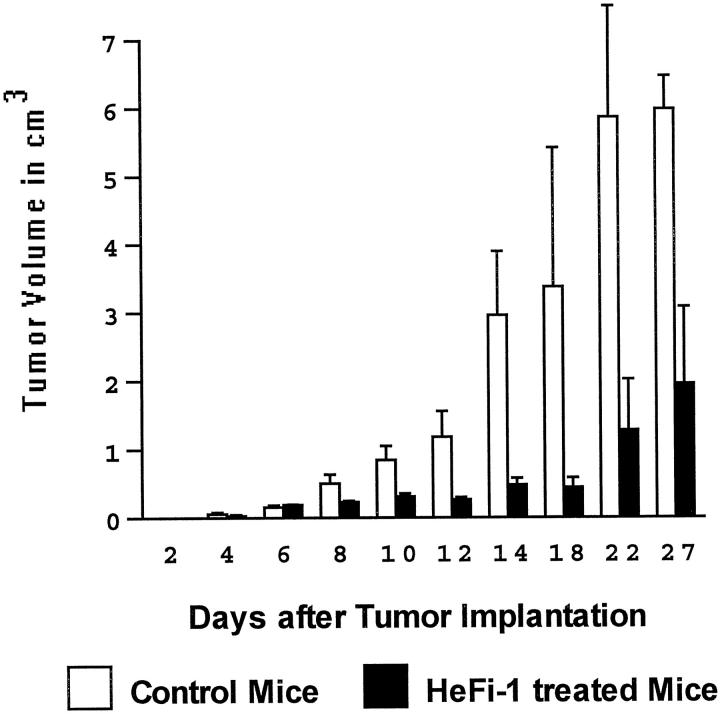
HeFi-1 is a monoclonal antibody raised against a functional epitope of the human CD30 antigen. 9 None of eight mice treated with 10 μg of HeFi-1 antibody within 24 hours of tumor transplantation and every other day for 10 days thereafter developed tumors by 30 days. All of eleven control animals injected with nonspecific IgG isotype matched control antibody developed tumors that reached a mean volume of 2.9 cm 3 by day 14, and produced disseminated tumor by 3 to 4 weeks. At necropsy, control mice were found to have widespread disease, while HeFi-1 treated mice were free of disease (P < 0.01). In the second experimental group, we tested the efficacy of HeFi-1 treatment in mice with established tumors. Mice bearing tumors of at least 0.2 cm 3 in volume, which on average corresponded to day 6 after tumor xenografting, were treated with HeFi-1 and experienced tumor growth arrest or regression, without tumor dissemination. Tumor volumes in control and treated animals compared at day 27 were significantly different (P < 0.025). All treated mice remained alive at 30 days (Figure 3) ▶ . Histological sections of tumors in treated mice revealed frequent dying or dead cells, cellular depletion, fibrosis, and sometimes calcification, consistent with a therapeutic effect achieved by HeFi-1 treatment (Figure 4, a–d) ▶ . In contrast, control mice with established tumors treated with control IgG antibody developed large tumors, which disseminated widely causing massive enlargement of lymph nodes, spleen, and other organs, and died or were moribund within 30 days.
Figure 3.
Anti-tumor effect of HeFi-1, a functional anti-CD30 antibody, in SCID/bg mice xenografted with ALCL cells. Mice were injected intraperitoneally with 10 μg of anti-CD30 antibody (HeFi-1) or mouse IgG1 in 0.2 ml of PBS, as a control, every other day for 10 days, for a total of 5 injections beginning on day 6 after tumor implantation. Tumor volumes in cm 3 are plotted at 0 to 27 days (mean ± SD).
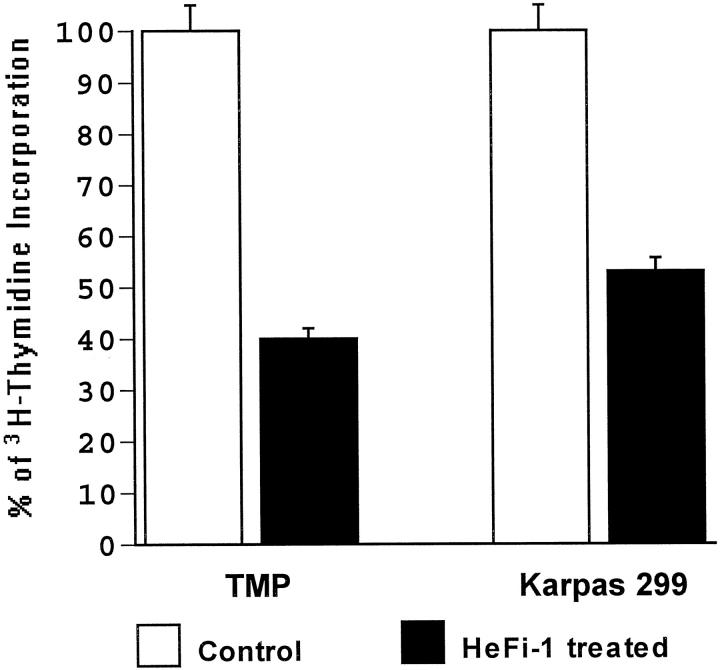
Growth Inhibition of ALCL Tumor Cells in Vitro
Incubation of tumor cells obtained from mouse xenografts with HeFi-1 significantly inhibited the proliferation of tumor cells (60%) as measured by 3H-thymidine incorporation (Figure 5) ▶ . An established t(2;5)+, CD30+ ALCL cell line (Karpas 299) was used for comparison and demonstrated a 47% inhibition under the same experimental conditions.
Figure 5.
Anti-tumor activity of HeFi-1 on human ALCL tumor cells in vitro. Human ALCL tumor cells isolated from mouse xenograft (TMP) were incubated with HeFi-1 or IgG1 an isotype control antibody for 72 hours and affect on growth was determined by 3H-thymidine incorporation. A t(2;5)-positive ALCL cell line (Karpas 299) was used as a positive control. Results of triplicate experiments are shown as percentages of controls (mean ± SD).
Discussion
We report the establishment of an ALCL tumor model in SCID/bg mice derived from a patient with systemic CD30+ ALCL resistant to chemotherapy. An alternative model was developed by Kuefer et al in lethally irradiated BALB/cByJ mice by transfer of bone marrow infected with a retroviral construct containing human NPM-ALK cDNA, but the resulting lymphomas lacked anaplastic morphology, were of B cell phenotype, and failed to express CD30 antigen. 10 We have shown that human ALCL tumor cells xenografted into SCID/bg mice and serially passaged through mice maintain their original morphological, immunohistochemical, and molecular features, as well as biological behavior similar to that of the patient’s original tumor. Using this model of human ALCL, we demonstrated the anti-tumor effects of an agonistic anti-human CD30 antibody on the development of CD30+ ALCL tumors in mice.
SCID mice have been widely used as xenograft recipients for human neoplastic tissue including hematological malignancies. 11-14 The SCID mutation is responsible for a lack of functional T and B lymphocytes, due to a congenital deficiency in the VDJ recombinase enzyme system involved in immunoglobulin (Ig) and T cell receptor gene rearrangement that effectively blocks both T and B cell maturation. 15 However, engraftment and maintenance of human hematopoietic tissue in SCID mice has been described as difficult 16-18 and its efficiency tends to vary depending on other factors including immunologically mediated tumor rejection. 19-21 Numerous methods for improving xenografting efficiency including γ-irradiation, administration of anti-NK-cell antibodies and immunosuppressive drugs have been used. 20 These methods were largely aimed at dampening the NK-mediated arm of the immune response but suffer from the disadvantage that they are cumbersome and often nonspecific. To avoid these difficulties we used SCID/bg mice. The beige mutation leads to selective impairment of NK-cell mediated immune function. 21 In combination with the SCID mutation, the beige mutation appears to provide favorable conditions for growth of human hematological malignancies in mice, thereby allowing the establishment and propagation of a reliable source of tumor cells from limited patient material. This is of particular importance since despite the ability of the tumor cells to grow indefinitely in vivo by serial passage through SCID/bg mice, lymphoma cells recovered from mouse tissues could not be maintained as a cell line in vitro, even in the presence of recombinant cytokines, a phenomenon which has been reported by others, 12 although primary cultures could be maintained for up to 3 months by fastidious culturing procedures.
Expression of cytokine and cytokine receptor genes is believed to play an important role in the regulation of normal and malignant lymphocyte proliferation. Numerous cytokines, including IL-1α, IL-2, IL-6, IL-7, and TNF-α have been identified as important autocrine growth factors in a variety of lymphoid neoplasms. 22,23 It was of interest that the present ALCL tumor expressed receptors for IL-2, IL-6, and G-CSF and transcripts for IL-1α, IL-6, TNF-α, and IFN-γ, which could explain the patient’s B symptoms.
Failure of conventional chemotherapy in CD30+ ALCL patients is accompanied by a poor prognosis. New immunological regimens targeting the CD30 molecule could offer alternative therapeutic approaches. The functional relevance of CD30 and CD30 ligand (CD30L) expression in CD30+ ALCLs is unclear at present. Recently, recombinant human CD30L was expressed on the surface of cultured cells and tested for biological activities on a variety of different CD30+ human lymphoma cell lines. It was demonstrated that CD30L+ cells have an antiproliferative effect on certain CD30+ ALCL cell lines. 24,25 Therefore, CD30L is capable of transducing signals through the specific cognate molecule CD30, making this a promising molecule to target for immunotherapy of these aggressive lymphomas.
The mechanism of action of HeFi-1 antibody in vivo is most likely a direct effect on tumor growth properties caused by activation of the CD30 signaling pathway. 24 The possibility of antibody dependent cellular cytotoxicity (ADCC) is unlikely because SCID/bg mice lack natural killer cells which are the main effectors of ADCC. Our in vitro proliferation assay demonstrates a direct growth inhibitory action of HeFi-1 on tumor cells obtained from murine xenografts. Preliminary studies in our laboratory have shown that the initial in vitro anti-proliferative effect of HeFi-1 is due to cell cycle arrest in late G1 rather than directly causing apoptosis (Levi E, Pfeifer W, Petrogiannis-Haliotis T, Wang Z, Kadin ME, manuscript in preparation). Our model should provide a better understanding of how HeFi-1 therapy affects tumor cell cycle and apoptosis in vivo.
Previous work from this laboratory, using Ber-H2 antibody (which binds to a non-functional epitope on the human CD30 antigen) conjugated with saporin, a plant ribosome-inactivating toxin, demonstrated tumor growth delay and prolonged survival of ALCL xenografted mice. 26 The present study demonstrates that just a short course of HeFi-1 monoclonal antibody without a conjugated toxin can prevent growth and dissemination of established tumors and prolong survival of mice bearing a chemotherapy-resistant aggressive CD30+ ALCL. In a prior study by Tian et al it was shown that treatment of SCID mice bearing human ALCL tumor cells with repeated doses of HeFi-1 or M44 antibody significantly prolonged their survival. However, those investigators did not measure tumor burden before treatment and neither regression nor growth delay of established tumors was shown. 27 The current regimen has the advantage of avoiding the adverse effects of the toxin and potentially providing more efficient access to tumor cells using a smaller antibody molecule. The short course required to achieve an anti-tumor effect in this study also avoids the potential generation of human anti-mouse antibodies as would be desired in future clinical trials. In conclusion, our in vivo CD30+ ALCL tumor model demonstrates a method to study disseminated disease, beginning with limited human material. Furthermore, this model serves as a tool to elucidate the signaling mechanisms of CD30 activation in human tumor cells that could provide the basis for the selection of appropriate immunotherapeutic modalities in patients with chemotherapy resistant disease.
Acknowledgments
We thank Erwin Meluleni, Center for Animal Resources and Comparative Medicine, Harvard Medical School, Boston, MA, for excellent technical assistance.
Footnotes
Address reprint requests to Marshall E. Kadin, MD, Director of Hematopathology, Department of Pathology, YA 309, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215. E-mail: mkadin@caregroup.harvard.edu.
Supported by the Deutsche Krebshilfe (WP), Lymphoma Research Foundation of America (EL), Cure for Lymphoma Foundation, New York, NY (TP-H), and the American Cancer Society ROG-98-125-01 and the Leukemia Society of America, 6178-98 (MEK).
WP and EL contributed equally to this work.
References
- 1.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen J, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K: The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66:848-858 [PubMed] [Google Scholar]
- 2.Kadin ME, Sako D, Berliner N, Franklin W, Woda B, Borowitz M, Ireland K, Schweid A, Herzog P, Lange B, Dorfman R: Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood 1986, 68:1042-1049 [PubMed] [Google Scholar]
- 3.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 4.Kadin ME, Morris SW: The t(2;5) in human lymphomas. Leuk Lymphoma 1998, 29:249-256 [DOI] [PubMed] [Google Scholar]
- 5.Wellmann A, Otsuki T, Vogelbruch M, Clark HM, Jaffe ES, Raffeld M: Analysis of the t(2;5)(p23;q35) translocation by reverse transcription-polymerase chain reaction in CD30+ anaplastic large-cell lymphomas, in other non-Hodgkin’s lymphomas of T-cell phenotype, and in Hodgkin’s disease. Blood 1995, 86:2321-2328 [PubMed] [Google Scholar]
- 6.Kinney MC, Kadin ME: The pathologic and clinical spectrum of anaplastic large cell lymphoma and correlation with ALK gene dysregulation. Am J Clin Pathol 1999, 111(Suppl 1):S56-S58 [PubMed] [Google Scholar]
- 7.Falini B, Bigema B, Fizotti M, Pulfort K, Pileri SA, Delsol G, Carbone A, Paulli M, Magrini U, Menestrina F, Giardini R, Pilotti S, Mezzelani A, Ugolini B, Billi M, Pucciarini A, Pacini R, Pelicci PG, Flenghi L: ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am J Pathol 1998, 153:875-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruss HJ, Brach MA, Drexler HG, Bross KL, Herrmann F: Interleukin 9 is expressed by primary, and cultured Hodgkin, and Reed-Sternberg cells. Cancer Res 1992, 52:1026-1031 [PubMed] [Google Scholar]
- 9.Hecht TT, Longo DL, Cossman J, Bolen HB, Hsu SM, Israel M, Fisher RI: Production and characterization of a monoclonal antibody that binds Reed-Sternberg cells. J Immunol 1985, 134:4231-4236 [PubMed] [Google Scholar]
- 10.Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, Morris SW: Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 1997, 90:2901-2910 [PubMed] [Google Scholar]
- 11.Itoh T, Shiota M, Takanashi M, Hojo I, Satoh H, Matsuzawa A, Moriyama T, Watanabe T, Hirai K, Mori S: Engraftment of human non-Hodgkin lymphomas in mice with severe combined immunodeficiency. Cancer 1993, 72:2686-2694 [DOI] [PubMed] [Google Scholar]
- 12.Cesano A, Hoxie JA, Lange B, Nowell PC, Bishop J, Santoli D: The severe combined immunodeficient (SCID) mouse model for human myeloid leukemias. Oncogene 1992, 7:827-836 [PubMed] [Google Scholar]
- 13.Visoneau S, Cesano A, Torosian MH, Miller EJ, Santoli D: Growth characteristics and metastatic properties of human breast cancer xenografts in immunodeficient mice. Am J Pathol 1998, 152:1299-1311 [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida M, Rybak RJ, Choi Y, Greenberg SJ, Barcos M, Kawata A, Matsuno F, Tsai H, Seon BK: Development of a severe combined immunodeficiency (SCID) mouse model consisting of highly disseminated human B-cell leukemia/lymphoma, cure of the tumors by systemic administration of immunotoxin, and development/application of a clonotype-specific polymerase chain reaction-based assay. Cancer Res 1997, 57:678-685 [PubMed] [Google Scholar]
- 15.Bosma GC, Custer RP, Bosma MJ: A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301:527-560 [DOI] [PubMed] [Google Scholar]
- 16.Charley MR, Tharp M, Locker J, Deng JS, Goslen Blake, Mauro T, McCoy P, Jegasothy B: Establishment of a human cutaneous T-cell lymphoma in C.B-17 SCID mice. J Invest Dermatol 1990, 94:381–384 [DOI] [PubMed]
- 17.Waller EK, Kamel OW, Cleary ML, Majumdar AS, Schick MR, Lieberman M, Weissmann IL: Growth of primary T-cell non-Hodgkin’s lymphomata in SCID-hu mice: requirement for a human lymphoid microenvironment. Blood 1991, 78:2650-2665 [PubMed] [Google Scholar]
- 18.Biondi A, Motta T, Garofalo A, Rossi V, Giudici G, Rizzo V, Pioltelli P, Corneo G, Barbui T, Parma A, Rambaldi A, Giavazzi R: Human T-cell lymphoblastic lymphoma expressing the T-cell receptor γ/δ established in immune-deficient (bg/nu/xid) mice. Leukemia 1993, 7:281-289 [PubMed] [Google Scholar]
- 19.Shibata S, Asano T, Ogura A, Hashimoto N, Hayakawa JI, Uetsuka K, Nakayama H, Doi K: SCID/bg mice as xenograft recipients. Lab Anim 1997, 31:163-168 [DOI] [PubMed] [Google Scholar]
- 20.Somasundaram R, Jacob L, Adachi K, Class R, Scheck S, Maruyama H, Herlyn D: Limitations of the severe combined immunodeficiency (SCID) mouse model for study of human B-cell responses. Scand J Immunol 1995, 41:384-390 [DOI] [PubMed] [Google Scholar]
- 21.Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, Schweitzer PA, Hesselton RM, Shultz LD: Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid/bg mice. Cell Immunol 1996, 171:186-199 [DOI] [PubMed] [Google Scholar]
- 22.Hsu SM, Waldron JW, Hsu PL, Hough AJ: Cytokines in malignant lymphomas: review and prospective evaluation. Hum Pathol 1993, 24:1040-1057 [DOI] [PubMed] [Google Scholar]
- 23.Ryffel B, Brockhaus M, Dürmüller U, Gudat F: Tumor necrosis factor receptors in lymphoid tissues and lymphomas. Am J Pathol 1991, 139:7-15 [PMC free article] [PubMed] [Google Scholar]
- 24.Grüss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG: Pleotrophic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood 1994, 83:2045-2056 [PubMed] [Google Scholar]
- 25.Younes A, Consoli U, Sell V, Clodi K, Kliche KO, Palmer JL, Grüss HJ, Armitage R, Thomas EK, Caniballas F, Andreeff M: CD30 ligand in lymphoma patients with CD30+ tumors. J Clin Oncol 1997, 15:3355-3362 [DOI] [PubMed] [Google Scholar]
- 26.Pasqualucci L, Wasik M, Teicher BA, Flenghi L, Bolognesi A, Stirpe F, Falini B, Kadin ME: Antitumor activity of anti-CD30 immunotoxin (Ber-H2/Saporin) in vitro and in SCID mice xenografted with human CD30+ anaplastic large cell lymphoma. Blood 1995, 85:2139-2146 [PubMed] [Google Scholar]
- 27.Tian ZG, Longo DL, Funakoshi S, Asai O, Ferris DK, Widmer M, Murphy WJ: In vivo antitumor effects of unconjugated CD30 monoclonal antibodies on human anaplastic large-cell lymphoma xenografts. Cancer Res 1995, 15:55(22):5335-5341 [PubMed] [Google Scholar]