Abstract
Pterin-4a-carbinolamine dehydratase (PCD) is a bifunctional protein also known as DCoH (dimerization co-factor of hepatocyte nuclear factor 1 (HNF1)). PCD/DCoH modulates the DNA binding specificity of HNF1, thus acting on its transcriptional activity. In addition, it participates in the recycling of tetrahydrobiopterin (BH4), an essential cofactor of several metabolic reactions. We investigated colorectal tumors and colorectal tumor cell lines as compared to normal colon samples in search of a potential differential expression of PCD/DCoH. Immunohistochemistry was conducted on 20 human colorectal tumors and 20 normal samples using a specific polyclonal antibody. Immunoblotting and RT-PCR analysis for PCD/DCoH and HNF1 were also performed on both human tissues and CACO-2 and HT-29 cell lines. All of the 20 tumors and both colon cancer cell lines presented a strong and widespread immunoreactivity for PCD/DCoH, contrasting with the absence of expression in the normal epithelia. We thus report the massive overexpression of PCD/DCoH in colon tumors, which is in striking contrast with the absence of staining in normal counterparts. The sharp contrast in the expression of a modulator of transcriptional activity between tumoral and normal cells may have a physiopathological role. PCD/DCoH could potentially be a new marker of malignant colon cells in vivo.
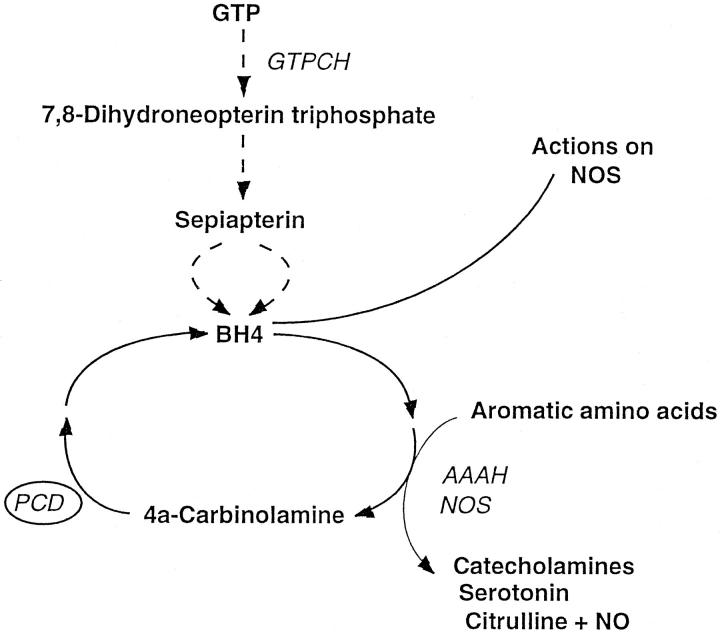
Pterin-4a-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 (PCD/DCoH) is a bifunctional protein of 11 kd 1,2 involved both in the recycling of tetrahydrobiopterin (BH4) (Figure 1) ▶ and the dimerization of hepatocyte nuclear factor 1 (HNF1). 3,4
Figure 1.
Synthetic and recycling pathways of tetrahydrobiopterin (BH4). Presented here is a simplified version; the intermediate reactions are not shown. GTPCH, Guanosine triphosphate-cyclohydrolase I; PCD, Pterin-4a-carbinolamine dehydratase; AAAH, Aromatic amino acid hydroxylases; NOS, Nitric oxide synthase.
BH4 is an essential cofactor of several monooxygenases such as the aromatic amino acid hydroxylases, 5 glycol ether mono-oxygenase, 6 and nitric oxide synthase. 7 Because the three aromatic acid hydroxylases metabolize phenylalanine, tyrosine, and tryptophan and synthesize the precursors of the neurotransmitters dopamine, norepinephrine, epinephrine, and serotonin, a lack of BH4 causes neurological disorders and mental retardation associated with hyperphenylalaninemia or phenylketonuria. 8,9 In human pathology, a deficit of PCD is associated with a rare, mild form of hyperphenylalaninemia characterized by the excretion of 7-substituted pterins in the urine of affected patients. 10-15 In addition, local PCD deficiency is also associated with the depigmentation disorder vitiligo in affected epidermis through inhibition of melanogenesis by the accumulating 7-substituted pterins. 16,17
Hepatocyte nuclear factors (HNF) 1α and 1β constitute a family of homeodomain-containing transcription factors. 18 HNF1α and HNF1β (also known as v-HNF1) are homologous proteins that bind their target DNA as homo- or heterodimers. The dimerization cofactor of HNF1 (DCoH/PCD) maximizes the transcriptional activity of HNF1 by stabilizing its dimers 19 and modulates the binding of HNF1 to nucleic acids: DCoH/PCD allows binding of HNF1 to sequences that deviate considerably from the consensus palindrome, thus profoundly modifying the DNA binding specificity of HNF1. 20
HNF1α can activate the expression of its target genes when cotransfected into some cell lines that do not normally express HNF1α, but the amount of activation depends on the type of recipient cell used. 19,21-23 A tissue-specific protein may thus be required for its function and DCoH/PCD is a likely candidate. It should be noted in this context that DCoH/PCD is expressed in a highly tissue-specific manner. 24
Given, on one hand, the important role of PCD/DCoH as an enhancer and modifier of transcriptional activity, and on the other hand its involvement in the recycling of an essential cofactor of nitric oxide synthase and several other metabolic enzymes, we studied its potential role in carcinogenesis by determining whether its expression differed in colorectal tumors and transformed cell lines as compared to normal colon samples.
Materials and Methods
Tissue Sampling
Human colorectal cancer tissue samples were obtained at surgery (n = 20) (Table 1) ▶ . The normal tissues (n = 20) were obtained either from the same surgical specimens at distance from the tumor (n = 17) or from patients undergoing surgery for diverticular colon disease (n = 3).
Table 1.
Patient and Tissue Characteristics of the Adenocarcinomas
| Case | Age (years) | Sex | Localization of tumor | Degree of differentiation | Dukes’ stage | PCD/DCoH expression |
|---|---|---|---|---|---|---|
| 1 | 62 | F | Left Colon | Moderate | A | ++ |
| 2 | 71 | F | Right Colon | Moderate | B | ++ |
| 3 | 48 | M | Left Colon | Moderate | B | ++ |
| 4 | 73 | M | Right Colon | Moderate | B | ++ |
| 5 | 74 | M | Left Colon | Moderate | B | ++ |
| 6 | 62 | M | Left Colon | Moderate | B | ++ |
| 7 | 80 | M | Left Colon | Moderate | B | ++ |
| 8 | 61 | F | Left Colon | Moderate | B | ++ |
| 9 | 76 | M | Left Colon | Well | B | ++ |
| 10 | 76 | M | Right Colon | Well | B | ++ |
| 11 | 76 | F | Left Colon | Well | B | ++ |
| 12 | 65 | F | Left Colon | Moderate | B | ++ |
| 13 | 71 | F | Right Colon | Moderate | C | ++ |
| 14 | 52 | M | Right Colon | Moderate | C | ++ |
| 15 | 63 | M | Left Colon | Moderate | C | ++ |
| 16 | 63 | M | Left Colon | Moderate | C | ++ |
| 17 | 64 | M | Left Colon | Well | C | ++ |
| 18 | 75 | F | Left Colon | Well | C | ++ |
| 19 | 71 | F | Left Colon | Moderate | D | ++ |
| 20 | 76 | F | Left Colon | Well | D | ++ |
F, female; M, male; ++, staining in >50% of the epithelial cells.
All tissues were fixed immediately after removal in 4% formaldehyde freshly prepared from paraformaldehyde for 8 to 12 hours. They were then progressively dehydrated, embedded in paraffin, cut into 8-μm sections, collected on gelatin-coated glass slides, and kept at room temperature until use.
Tissue samples for polymerase chain reaction analysis (the same 20 tumors and 20 normal colon samples) were placed in liquid nitrogen immediately after removal and stored at −70°C until extraction.
Antibody
Recombinant PCD/DCoH was produced in bacterial cells using the pGEMEX-2 expression vector (Promega, Madison, WI) containing the PCD/DCoH coding sequence under the control of an IPTG-inducible promoter. Generation of this vector, pHDH14, and subsequent purification of PCD/DCoH from overproducing cells was performed according to Köster et al. 25 The antiserum was raised in rabbits against recombinant PCD/DCoH (performed by DAKO A/S, Denmark). The polyclonal rabbit antibodies (F3862) were purified by affinity chromatography using a HiTrap protein A-Sepharose column purchased from Pharmacia Biotech (Uppsala, Sweden). The antibody (F3862) was tested for anti-PCD specificity and sensitivity against rat liver extracts. Further characterization of the antiserum was performed by Western blot analysis and immunohistochemistry. 50
Immunohistochemistry
After deparaffinization and rehydration, endogenous peroxidase activity was blocked in 2% H2O2 for 30 minutes, and nonspecific binding was blocked in 5% normal sheep serum (NSS) in phosphate buffered saline (PBS) for 15 minutes.
Primary antibodies were diluted at 1/4000 in 1% NSS/PBS-azide. The slides were incubated with the primary antibodies in a humid chamber for 48 hours at 4°C. After 48 hours, the unbound primary antibody was removed by rinsing with PBS. The slides were incubated with 1.5% normal goat serum in PBS-azide for 15 minutes followed by an incubation with biotinylated antirabbit IgG (Vector Laboratories, Burlingame, CA) at 1/200 dilution in PBS for 30 minutes. Following two PBS rinses, Vectastain ABC Complex (Vector Laboratories, Burlingame, CA) solution was applied to the tissue sections for 1 hour. The immunoreaction was visualized by using peroxidase substrate 3,3′ diaminobenzidine tetrahydrochloride (0.05% in PBS and 0.03% H2O2) for 2 to 3 minutes. The reaction was stopped by PBS rinsing. After counterstaining with hematoxylin and subsequent dehydration, the sections were observed and photographed with a light microscope. A quantification of the importance of the staining was evaluated by counting the number of labeled cells in each sample using the following criteria: 0, no positive staining; +, staining in less than 50% of the epithelial cells; ++, staining in more than 50% of the epithelial cells.
The staging of the tumors was established according to the Dukes’ classification system. The histological grading of the levels of differentiation of the tumors was established according to the glandular appearance, the presence or absence of nuclear polarity and nuclear pleomorphism. 26
Controls
Several controls were performed to validate the specificity of the antibody used in this study. First, adult rat tissues known to express PCD/DCoH (liver, kidney, and brain tissues) 27,28 were used as positive controls. Further controls consisted in the replacement of the primary antibody by normal rabbit serum and preincubation of the diluted immunoserum overnight at 4°C with 8 μg/ml of recombinant PCD/DCoH. Immunohistochemical staining of the samples by the preincubated and the nonpreincubated antibodies was performed at the same time and in parallel. A fourth control consisted in immunoblotting performed on colon tissue extracts: two normal colonic samples and two colorectal tumors were used for this purpose. Tissues were homogenized in 20 volumes of buffer (Tris HCl 20 mmol/L, pH 6.8; SDS 1%; mercaptoethanol 5%) in a glass-Teflon homogenizer. The homogenates were treated with ultrasound and then boiled. Protein concentrations were determined using bicinchoninic acid after trichloroacetic precipitation as described in Brown et al 29 and by amido black staining of samples dotted on nitrocellulose membranes as described in Nakamura et al. 30 For immunoblotting, equal amounts of protein of each normal and tumoral sample were separated by SDS-PAGE gel electrophoresis and transferred to a nitrocellulose membrane by semidry electrophoretic transfer (Bio-Rad, Hercules, CA). Nonspecific binding was blocked with 5% skimmed milk in TTBS (50 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 0.05% Tween 20, and 0.1% NaN3) by overnight incubation at 4°C. The membranes were then incubated with the antibodies for 48 hours at 4°C. After three successive washes in PBS, PBS-Tween and hypertonic PBS for 15 minutes each, the membranes were incubated with 1/200 anti-rabbit IgG alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, MO) for 1 hour at room temperature followed by three washes. Bound antibodies were detected in alkaline phosphatase buffer pH 9.55 (Tris-HCl 0.1 mol/L, NaCl 0.1 mol/L, MgCl2 2 mmol/L, ZnCl2 1 μmol/L, diethanolamine 25 mmol/L) containing 0.34 mg/ml Nitro blue tetrazolium and 0.136 mg/ml 5-bromo-4-chloro-3 indoyl phosphate. The same immunoblotting procedure was performed using the antibody preincubated overnight with the antigenic peptide.
RNA Extraction and Reverse Transcription
Total RNAs were obtained with RNA Now (Biogentex Inc., Seabrook, TX) and quantitated by absorbance at 260 and 280 nm from each tumor tissue and normal colon. After submitting the RNA to an additional step of DNA digestion by deoxyribonuclease (GIBCO BRL, Ghent, Belgium) for 10 minutes, cDNA was generated by reverse transcription using the Superscript system (GIBCO BRL) according to the manufacturer’s manual. The obtained cDNA was stabilized with a 2-mmol/L EDTA solution.
Polymerase Chain Reaction
The primers used for the amplification of PCD/DCoH were: forward primer 22-39, CTGAGCGCTGAGGAGAGG3′ and reverse primer 237-220, GGTGCTCAGCGTGATGTG3′, which amplify a 216-bp target sequence. The primers used for the amplification of HNF1 were: forward primer 791-811, TCTACAACTGGTTTGCCAACC3′ and reverse primer 1101-1082, GGCTTCTGTACTCAGCAGGC3′. They amplify a 311-bp target sequence. Care was taken to choose primer sequences across an intron sequence so that DNA fragments would not be amplified.
PCR was carried out in a Perkin Elmer Cetus Thermal Cycler (Perkin Elmer Corp., Norwalk, CT) using Goldstar DNA Polymerase (Eurogentec) according to the manufacturer’s manual. Conditions consisted of 35 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute for both sets of primers. The 35 cycles were followed by a final extension at 72°C for 10 minutes. Eight microliters of PCR products were electrophoresed on a 1.2% agarose gel (GIBCO BRL Life Technologies, Paisley, UK) containing ethidium bromide and visualized by UV transillumination.
Known positive and negative samples were included in each PCR as technical controls. These controls were satisfactory in all cases.
Colon Carcinoma Cell Lines: PCR Analysis and Immunoblotting
Two human colon adenocarcinoma cell lines, CACO-2 and HT-29, were obtained from American Type Culture Collection (Manassas, VA). CACO-2 cells were cultured in 20% fetal calf serum, and 80% MEM Eagle with 2 mmol/L L-glutamine and Earle’s balanced salt solution adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mmol/L nonessential amino acids, and 1.0 mmol/L sodium pyruvate. HT-29 cells were cultured in 10% fetal bovine serum and 90% McCoy’s 5a medium with 1.5 mmol/L L-glutamine. All cell cultures were protected from infection by 50 U/ml of penicillin and 50 μg/ml of streptomycin. All culture media were purchased from GIBCO-BRL Life Technologies. The cells were kept in 5% CO2 atmosphere at 37°C. Both confluent and nonconfluent CACO-2 and HT29 cells were harvested by rubber policeman, divided into two groups, and centrifuged. One group was used for RNA preparation; the pellet was homogenized in RNA Now (Biogentex Inc.) and total RNA was extracted according to the manufacturer’s manual. The second part was used for immunoblotting. The cell pellet was homogenized by sonication in 20 volumes of the electrophoresis sample buffer (see above). PCR analysis and immunoblotting experiments were carried out as for the surgical tissue samples.
Results
Controls
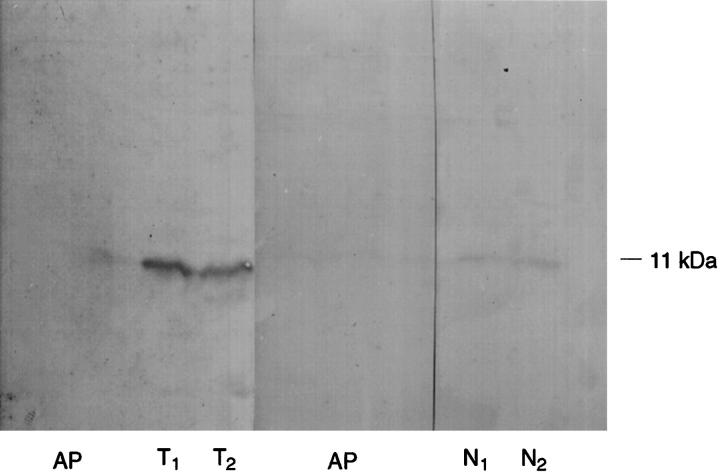
All controls proved to be satisfactory. The immunoreactivity observed both on the histological slides and in the immunoblotting experiments disappeared completely when the antibody was preabsorbed overnight with the antigenic peptide (Figures 2, 3a, 3b, and 8) ▶ ▶ ▶ .
Figure 2.
Immunoblotting for PCD/DCoH on normal colon and colon tumor extracts. T: tumor extracts, N: normal colon extracts, AP: immunoblotting using the antibody preincubated overnight with the control peptide (recombinant PCD/DCoH purified from bacterial cells). The 11-kd protein recognized by the antibody most probably corresponds to PCD/DCoH. The immunostaining is much stronger on tumoral tissues and disappears completely on both tumoral and normal extracts after preincubation.
Figure 3.
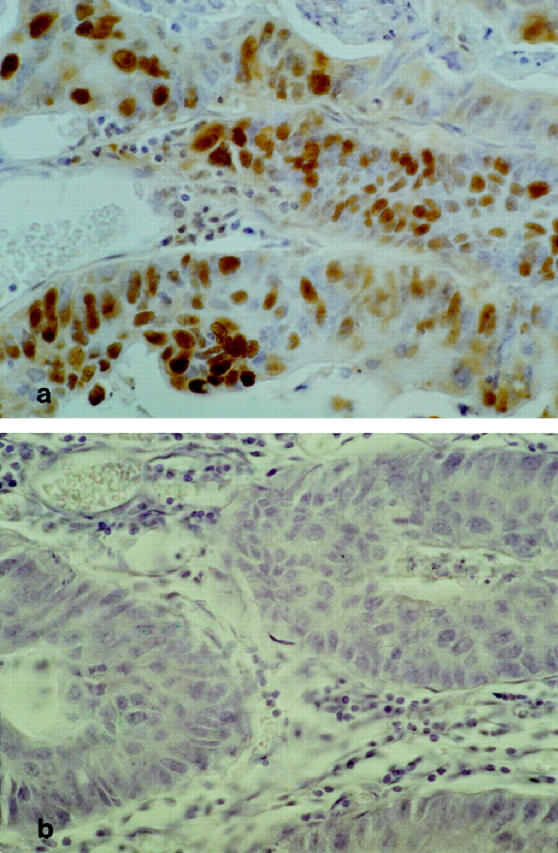
a: Positive staining for PCD/DCoH in a colon carcinoma. Original magnification, ×280. The majority of the neoplastic cells are stained. b: Staining with PCD/DCoH antibody, preincubated overnight with the control peptide. Same tumor as in a; the immunopositivity has disappeared. Original magnification, ×280.
Figure 8.
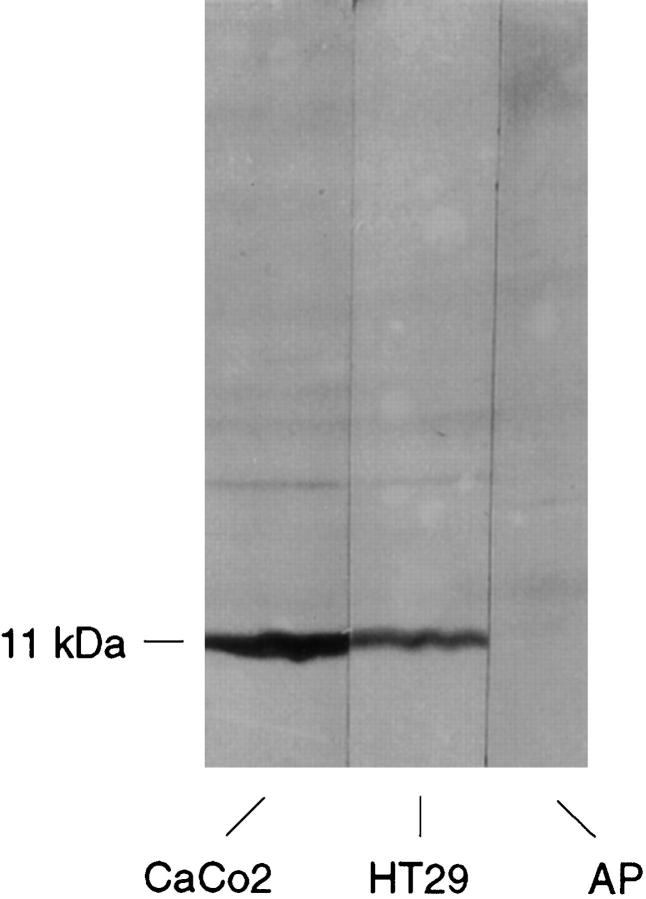
Immunoblotting for PCD/DCoH on CACO2 and HT29 cell line extracts. The specific band of 11 kd probably corresponds to PCD/DCoH. The signal disappears completely when the antibody used has been preincubated overnight with the antigenic peptide (AP).
PCD/DCoH Expression in Normal Colon
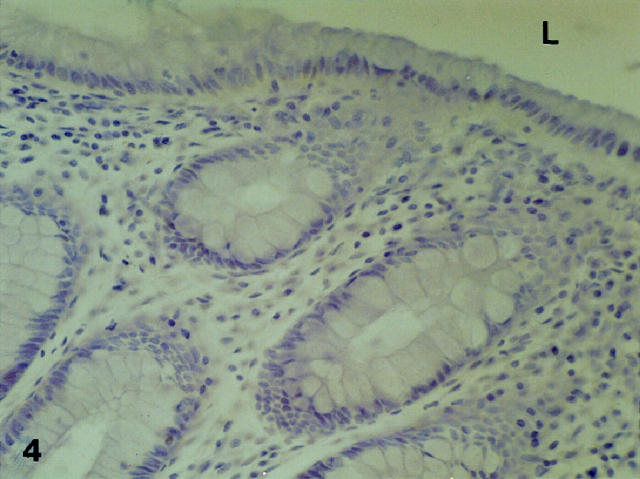
At low and high magnification, the mucosa was free of all immunostaining (Figure 4) ▶ whereas in the deeper layers, immunopositivity was observed in the submucosal and myenteric nervous plexuses (Figure 5) ▶ . The vascular structures, lymphocyte clusters and basal membranes were all negative. However, some inflammatory cells, in particular mastocytes, were stained. At immersion, very few at most 1 or 2 in a 15 × 10 mm tissue sample) immunopositive cells, always restricted in their localization to the bottom layers of the crypts, were detected (results not shown). These cells were typically triangular in shape, evoking by their morphology the neuroendocrine cells of the colon.
Figure 4.
Immunostaining for PCD/DCoH in normal mucosa. The epithelium is free of staining. Original magnification, ×210. L, lumen.
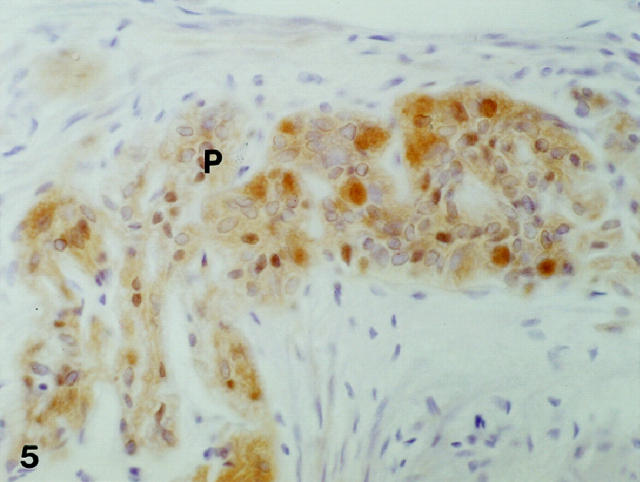
Figure 5.
Immunopositivity for PCD/DCoH is observed in the myenteric nervous plexuses (P). Original magnification: ×280.
The presence of PCD/DCoH messenger RNA was detected in these samples by reverse transcription-polymerase chain reaction amplification (Figure 6) ▶ . Immunoblotting experiments also detected the presence of an 11-kd protein in these tissue samples (Figure 2) ▶ . It is important to note that all samples contained the full thickness of the bowel wall including the nervous plexuses.
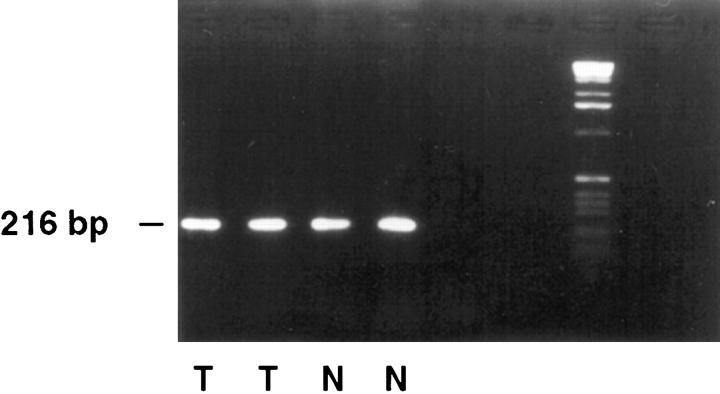
Figure 6.
RT-PCR analysis of PCD/DCoH in tumoral and normal colon. PCD/DCoH messenger RNA is present in colon tumors (T) and total normal colon (N) including the muscular layers.
PCD/DCoH Expression in the Colon Carcinomas
Patient characteristics are given in Table 1 ▶ .
All of the 20 tumoral samples were immunoreactive in the majority of their epithelial neoplastic cells (Figures 3a and 7, a–c ▶ ▶ , and Table 1 ▶ ). The immunoreactivity was massive in all samples, independent of the degree of differentiation, localization, or Dukes’ stage. In all samples, clusters of homogeneously stained malignant cells alternated with heterogeneous and variable staining in other groups of cells. There were also very rare neoplastic regions of tissue without staining. In zones of homogeneous staining, both the nucleus and the cytoplasm were often marked even if some cells stained uniquely in their cytoplasm or their nucleus. In the heterogeneous zones, any combination seemed equally possible. The immunostaining of the nuclei was independent of their size. The frequent images of cell mitosis were very rarely marked (Figure 7a) ▶ . Normal-looking mucosa adjacent to tumoral structures (Figure 7b) ▶ resembled in its lack of staining normal mucosa at distance from the tumors. As in the normal tissues, the vascular structures and lymphocyte clusters were negative.
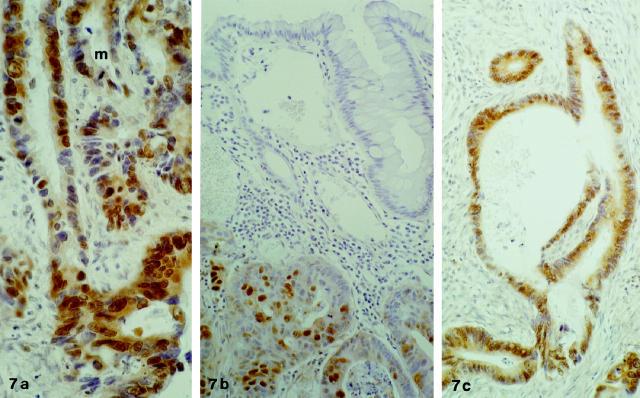
Figure 7.
a: Immunostaining for PCD/DCoH in a tumoral glandular structure. Presence of cytoplasmic and nuclear staining. Note an image of mitosis in the upper portion (m) which shows no immunostaining. Original magnification, ×280. b: PCD/DCoH immunopositivity in an adenocarcinoma showing mostly but not exclusively nuclear staining. The upper part of the field shows adjacent, normal-looking mucosa which contrasts with its lack of staining. Original magnification, ×180. c: Massive PCD/DCoH immunopositivity in a tumoral glandular structure contrasting with the lack of positivity in the surrounding stroma. Original magnification, ×180.
All of the tumoral samples were positive in RT-PCR analysis of messenger RNA for PCD/DCoH (Figure 6) ▶ .
Immunoblotting experiments on both Caco-2 and HT29 colon carcinoma cell lines (confluent and nonconfluent samples) showed the presence of a specific 11-kd band which disappeared when the antibody was preincubated with the antigenic peptide (Figure 8) ▶ .
Polymerase Chain Reaction Amplification of HNF1
HNF1 messenger RNA was present in both tumoral and normal tissue specimens (Figure 9) ▶ and in both colon carcinoma cell lines in vitro (results not shown).
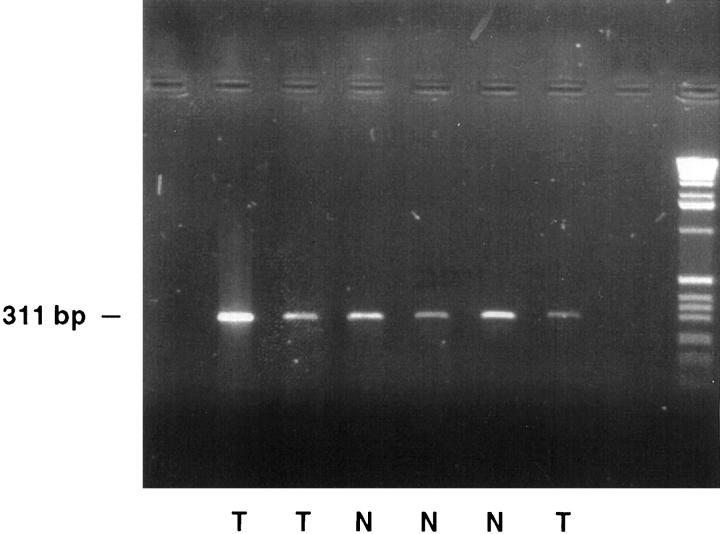
Figure 9.
RT-PCR analysis of HNF1 in tumoral and normal colon. HNF1 messenger RNA is present in colon tumors (T) and normal colon (N).
Discussion
We report in this article the existence of an abundant expression of PCD/DCoH both in two colon cancer cell lines and in the malignant epithelial cells of human colon cancer specimens. The immunostaining in these specimens is intense, widespread, and limited to the malignant cells, contrasting with the near absence of immunohistochemically detectable PCD/DCoH in the normal epithelial colon cell counterparts.
The significance of this striking difference between neoplastic and normal colon epithelial cells could be two-way. PCD/DCoH, acting as either a reductive enzyme and/or a transregulator of transcription factors, may confer on these cells a growth advantage, allowing them to outgrow other neoplastic cells within the tumor to become the predominant cell type through clonal expansion. Alternatively, excess PCD/DCoH in the malignant cells might be a bystander, produced coincidentally as a result of another alteration which is the cause of the selective growth advantage. 31 In either event, the fact that the expression of the protein is restricted to the neoplastic cells may permit its possible utilization as a tumoral marker.
As an enzyme, PCD is involved in the recycling of BH4 (Figure 1) ▶ . Several teams have reported effects of BH4 on the proliferation of cell lines: it causes an increase in cell proliferation in PC12 rat pheochromocytoma cell line and enhances the proliferation of transformed fibroblasts and rat C6 glioma cells. 32 It was also shown that immature, proliferating murine erythroleukemia cells contain much more BH4 than do the terminally differentiated counterparts. 33 In rat thymocytes, BH4 biosynthesis and accumulation were increased and peaked just before the early stages of the S phase of the cell cycle. 34,35 In culture, BH4 was required for the proliferation of some but not all mouse erythroleukemia cell lines. 36 However, reduction of endogenous BH4 levels did not alter the growth of either MOLT-4 leukemia or MCF-7 breast adenocarcinoma cells in culture. 37
Another pathway to explore is the involvement of BH4 as an essential cofactor of nitric oxide synthase (NOS). 38,39 BH4 helps stabilize the active dimeric state of this enzyme and prevents and reverses the inhibition of NOS by nitric oxide. 40,41 Even though a relative loss of NOS has been reported in colorectal neoplasia as compared to normal colon by some authors, 42,43 others have reduced the growth of a human colon adenocarcinoma xenograft by selectively inhibiting inducible NOS (iNOS). 44 Nitric oxide may thus have a functional role in promoting solid tumor growth and progression. Moreover, a cohort of supporting metabolic enzymes can be coinduced along with iNOS in a series of human tumor cell lines including colorectal ones. 45 One such enzyme is GTP-cyclohydrolase-I involved in the biopterin biosynthetic pathway (Figure 1) ▶ . Coinduction of these auxiliary metabolic pathways may play an important role in determining the capacity of tumors for NO activation. 45
Thus, it appears that BH4 and any enzyme involved in its recycling may have an influence on cell proliferation and the role of PCD/DCoH in this context may be important.
Alternatively, PCD/DCoH is a transregulator of the transcription factors HNF1α and β. Selective transcriptional activation of specific genes is regulated by the concerted action of tissue-restricted transcription factors such as HNF1, expressed in the liver but also in the kidney, stomach, and intestine of several species. 46-48 The formation of homo- or hetero-dimers of transcription factors may lead to altered DNA-binding specificity or altered transcriptional activity. This phenomenon may include cofactor proteins regulating the dimerization process such as PCD/DCoH.
PCD/DCoH stabilizes HNF/DNA complexes and promotes interactions with suboptimal DNA target sequences such as the TATA box region. The hypothesis is that the association between PCD/DCoH and HNF results in the appearance of an activation surface of HNF1 that is not expressed when DCoH is absent. 4 There exist also interactions of HNF1 with RNA, but these interactions are completely abolished when HNF1 is complexed with PCD/DCoH. 20
The PCD/DCoH dependent increase in the stability of HNF1/DNA complexes may have biological importance. It gives greater opportunity to DNA-bound HNF1 to interact with the transcriptional machinery before it is released from DNA. A yet further action of PCD/DCoH could be the liberation of HNF1 unspecifically bound to RNA, rendering it available for DNA binding. 20
It has been proposed that the differential expression of the cofactor in specific cell lines might explain the varying transactivation potential of HNF1 depending on the recipient cell used. 49 Along the same lines, one can speculate that the presence of PCD/DCoH observed in the malignant colon cells of our human tissue specimens may modulate the transactivation potential of HNF1 in these tumor cells.
The presence of HNF1 messenger RNA in the tumoral samples studied renders plausible the cooperative hypothesis between PCD/DCoH and the transcriptional role of HNF1 in these cells. Furthermore, the intranuclear localization of PCD/DCoH supports this idea of the involvement of the protein in the regulation of transcription. In contrast, the cytoplasmic localization, also observed, may rather be in favor of an enzymatic action in the cytoplasmic metabolism.
Moreover, PCD/DCoH may also interact with factors distinct from HNF1. In the Xenopus, for instance, in the early stages of the embryo, DCoH is present whereas HNF1 is not detectable. 24 Also, in the developing larvae of Xenopus, PCD/DCoH is present in the gut. However, in the adult, this expression disappears from the gut whereas HNF1, already present in the larvae, continues to be expressed. 24 It may be that, in the development of the Xenopus, the presence of PCD/DCoH is related to the actively growing and developing gut. 24 Thus, PCD/DCoH may act as an oncofetal protein, no longer present in adult normal colon but increasing to fetal levels in colon tumors.
The excess of PCD/DCoH observed in colon cancer may thus be important via its enzymatic activity in the recuperation of BH4 and/or as a cofactor protein regulating the dimerization process of transcription factors. It is also tempting to speculate that the two biological roles of dehydratase/dimerization cofactor may be related, because, after binding to HNF1, the protein retains its enzymatic activity. 20 Therefore, not only one cannot eliminate either of the two activities of the molecule, but the dehydratase activity may even be involved in HNF1 transcriptional activation.
Another interesting question to ask in future studies is whether the increase in PCD/DCoH staining is due to increased mRNA expression or a posttranscriptional mechanism leading to an increase in the half-life of the protein.
In conclusion, both functions of PCD/DCoH seem, at this stage, to have potential importance in carcinogenesis. Therefore, this first report of a massive expression of this protein in a tumoral tissue while its expression is absent from the corresponding normal tissue may have physiopathological, diagnostic, and even therapeutic implications and deserves further investigations.
Footnotes
Address reprint requests to Dr. Rally Eskinazi, Département de Gastroentérologie, Hôpital Erasme, Université Libre de Bruxelles, 808 Route de Lennik, B-1070, Brussels, Belgium. E-mail: rally.eskinazi@skynet.be.
Supported by the Fondation Erasme (to R. E. and J. L. V. L.), by an Interuniversity Poles of Attraction Programme from the Belgian State, Prime Minister’s Office, Federal Office for Scientific, Technical and Cultural Affairs, by the Fonds de la Recherche Scientifique Médicale Belge (3.4411.88, to A. R.), and by the Swiss National Science Foundation, grant 31-43380-95.
The first two authors contributed equally to this article.
References
- 1.Citron BA, Davis MD, Milstien S, Gutierrez J, Mendel DB, Crabtree GR, Kaufman S: Identity of 4a-carbinolamine dehydratase, a component of the phenylalanine hydroxylation system, and DCoH, a transregulator of homeodomain proteins. Proc Natl Acad Sci USA 1992, 89:11891-11894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thöny B, Neuheiser F, Hauer CR, Heizmann CW: Molecular cloning and recombinant expression of the human liver phenylalanine hydrox-ylase stimulating factor revealed structural and functional identity to the dimerization cofactor for nuclear transcription factor HNF1 alpha. Adv Exp Med Biol 1993, 338:103-106 [DOI] [PubMed] [Google Scholar]
- 3.Lazarus RA, Benkovic SJ, Kaufman S: Phenylalanine hydroxylase stimulator protein is a 4a-carbinolamine dehydratase. J Biol Chem 1983, 258:10960-10962 [Google Scholar]
- 4.Mendel DB, Khavari PA, Conley PB, Graves MK, Hansen LP, Admon A, Crabtree GR: Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science 1991, 254:1762-1767 [DOI] [PubMed] [Google Scholar]
- 5.Nichol CA, Smith GK, Duch DS: Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Ann Rev Biochem 1985, 54:729-764 [DOI] [PubMed] [Google Scholar]
- 6.Kaufman S, Pollock RJ, Summer GK, Das AK, Hajra AK: Dependence of an alkyl glycol-ether monooxygenase activity upon tetrahydropterins. Biochim Biophys Acta 1990, 1040:19–27 [DOI] [PubMed]
- 7.Bredt DS, Snyder SH: Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 1994, 63:175-195 [DOI] [PubMed] [Google Scholar]
- 8.Scriver CR, Kaufman S, Woo SLC: The hyperphenylalaninemias. Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic Basis of Inherited Disease, 1989, Vol. 1.:pp 495-546 McGraw-Hill, New York [Google Scholar]
- 9.Blau N, Thöny B, Heizmann CW, Dhondt JL: Tetrahydrobiopterin deficiency: from phenotype to genotype. Pteridines 1993, 4:1-10 [Google Scholar]
- 10.Curtius HC, Adler C, Rebrin I, Heizmann C, Ghisla S: 7-Substituted pterins: formation during phenylalanine hydroxylation in the absence of dehydratase. Biochem Biophys Res Commun 1990, 172:1060-1066 [DOI] [PubMed] [Google Scholar]
- 11.Adler C, Ghisla S, Rebrin I, Haavik J, Heizmann CW, Blau N, Kuster T, Curtius HC: 7-Substituted pterins in humans with suspected pterin-4a-carbinolamine dehydratase deficiency. Mechanism of formation via non-enzymatic transformation from 6-substituted pterins. Eur J Biochem 1992, 208:139-144 [DOI] [PubMed] [Google Scholar]
- 12.Davis MD, Kaufman S, Milstien S: Conversion of 6-substituted tetrahydropterins to 7-isomers via phenylalanine hydroxylase-generated intermediates. Proc Natl Acad Sci USA 1991, 88:385-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Citron BA, Kaufman S, Milstien S, Naylor EW, Greene CL, Davis MD: Mutation in the 4a-carbinolamine dehydratase gene leads to mild hyperphenylalaninemia with defective cofactor metabolism. Am J Hum Genet 1993, 53:768-774 [PMC free article] [PubMed] [Google Scholar]
- 14.Thöny B, Neuheiser F, Kierat L, Blaskovics M, Arn PH, Ferreira P, Rebrin I, Ayling J, Blau N: Hyperphenylalaninemia with high levels of 7-biopterin is associated with mutations in the PCBD gene encoding the bifunctional protein pterin-4a-carbinolamine dehydratase (PCD) and transcriptional coactivator (DCoH). Am J Hum Genet 1998, 62:1302-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thöny B, Neuheiser F, Kierat L, Rolland MO, Guibaud P, Schlüter T, Germann R, Heidenreich RA, Duran M, de Klerk JBC, Ayling JE, Blau N: Mutations in the pterin-4a-carbinolamine dehydratase gene (PCBD) are causative for a benign form of hyperphenylalaninemia. Hum Genet 1998, 103:162-167 [DOI] [PubMed] [Google Scholar]
- 16.Schallreuter KU, Wood JM, Ziegler I, Lemke KR, Pittelkow MR, Lindsey NJ, Gutlich M: Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. Biochim Biophys Acta 1994, 1226:181-192 [DOI] [PubMed] [Google Scholar]
- 17.Schallreuter KU, Wood JM, Pittelkow MR, Gutlich M, Lemke KR, Rodl W, Swanson NN, Hitzemann K, Ziegler I: Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 1994, 263:1444-1446 [DOI] [PubMed] [Google Scholar]
- 18.Tronche F, Yaniv M: HNF1, a homeoprotein member of the hepatic transcription regulatory network. Bioessays 1992, 14:579-587 [DOI] [PubMed] [Google Scholar]
- 19.Mendel DB, Hansen LP, Graves MK, Conley PB, Crabtree GR: HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev 1991, 5:1042-1056 [DOI] [PubMed] [Google Scholar]
- 20.Rhee KH, Stier G, Becker PB, Suck D, Sandaltzopoulos R: The bifunctional protein DCoH modulates interactions of the homeodomain transcription factor HNF1 with nucleic acids. J Mol Biol 1997, 265:20-29 [DOI] [PubMed] [Google Scholar]
- 21.Hansen LP, Crabtree GR: Regulation of the HNF-1 homeoproteins by DCoH. Curr Opin Genet Dev 1993, 3:246-253 [DOI] [PubMed] [Google Scholar]
- 22.Rey-Campos J, Chouard T, Yaniv M, Cereghini S: vHNF1 is a homeo-protein that activates transcription and forms heterodimers with HNF1. EMBO J 1991, 10:1445-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo CJ, Conley PB, Hsieh CL, Francke U, Crabtree GR: Molecular cloning, functional expression, and chromosomal localization of mouse hepatocyte nuclear factor 1. Proc Natl Acad Sci USA 1990, 87:9838-9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogge von Strandmann E, Ryffel GU: Developmental expression of the maternal protein XDCoH, the dimerization cofactor of the homeoprotein LFB1 (HNF1). Development 1995, 121:1217-1226 [DOI] [PubMed] [Google Scholar]
- 25.Köster S, Thöny B, Macheroux P, Curtius HC, Heizmann CW, Pfleiderer W, Ghisla S: Human pterin-4a-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1: characterization and kinetic analysis of wild-type and mutant enzymes. Eur J Biochem 1995, 231:414-423 [DOI] [PubMed] [Google Scholar]
- 26.Lev R, Lee M: Colorectal carcinoma pathology. Rustgi AK eds. Gastrointestinal Cancers: Biology, Diagnosis and Therapy. 1995, :p 385 Lippincott-Raven Publishers, Philadelphia [Google Scholar]
- 27.Hauer CR, Rebrin I, Thöny B, Neuheiser F, Curtius HC, Hunziker P, Blau N, Ghisla S, Heizmann CW: Phenylalanine hydroxylase-stimulating protein/pterin-4a-carbinolamine dehydratase from rat and human liver. J Biol Chem 1993, 268:4828-4831 [PubMed] [Google Scholar]
- 28.Davis MD, Kaufman S, Milstien S: Distribution of 4a-hydroxytetrahydropterin dehydratase in rat tissues. FEBS Lett 1992, 302:73-76 [DOI] [PubMed] [Google Scholar]
- 29.Brown RE, Jarvis KL, Hyland KJ: Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem 1989, 180:136-139 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Tanaka T, Kuwahara A, Takeo K: Microassay for proteins on nitrocellulose filter using protein dye-staining procedure. Anal Biochem 1985, 148:311-319 [DOI] [PubMed] [Google Scholar]
- 31.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 32.Anastasiadis PZ, States JC, Imerman BA, Louie MC, Kuhn DM, Levine RA: Mitogenic effects of tetrahydrobiopterin in PC12 cells. Mol Pharmacol 1996, 49:149-155 [PubMed] [Google Scholar]
- 33.Tanaka K, Kaufman S, Milstien S: Tetrahydrobiopterin, the cofactor for aromatic amino acid hydroxylases, is synthesized by and regulates proliferation of erythroid cells. Proc Natl Acad Sci USA 1989, 86:5864-5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler I, Schwulera U, Ellwart J: Pteridines are produced during interleukin 2-induced T-cell proliferation and modulate transmission of this signal. Exp Cell Res 1986, 167:531-538 [DOI] [PubMed] [Google Scholar]
- 35.Schott K, Brand K, Hatakeyama K, Kagamiyama H, Maier J, Werner T, Ziegler I: Control of cell-cycle-associated tetrahydrobiopterin synthesis in rat thymocytes. Exp Cell Res 1992, 200:105-109 [DOI] [PubMed] [Google Scholar]
- 36.Kerler F, Ziegler I, Schmid C, Bacher A: Synthesis of tetrahydrobiopterin in Friend erythroleukemia cells and its modulator effect on cell proliferation. Exp Cell Res 1990, 189:151-156 [DOI] [PubMed] [Google Scholar]
- 37.Smith GK, Duch DS, Edelstein MP, Bigham EC: New inhibitors of sepiapterin reductase. J Biol Chem 1992, 267:5599-5607 [PubMed] [Google Scholar]
- 38.Marletta MA: Nitric oxide synthase: aspects concerning structure and catalysis. Cell 1994, 78:927-930 [DOI] [PubMed] [Google Scholar]
- 39.Marletta MA: Nitric oxide synthase structure and mechanism. J Biol Chem 1993, 268:12231-12234 [PubMed] [Google Scholar]
- 40.Baek KJ, Thiel BA, Lucas S, Stuehr DJ: Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem 1993, 268:21120-21129 [PubMed] [Google Scholar]
- 41.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B: Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J 1995, 14:3687-3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chhatwal VJ, Ngoi SS, Chan ST, Chia YW, Moochhala SM: Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Carcinogenesis 1994, 15:2081-2085 [DOI] [PubMed] [Google Scholar]
- 43.Moochhala S, Chhatwal VJ, Chan ST, Ngoi SS, Chia YW, Rauff A: Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis 1996, 17:1171-1174 [DOI] [PubMed] [Google Scholar]
- 44.Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AJ: Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res 1997, 57:3300-3304 [PubMed] [Google Scholar]
- 45.Nussler AK, Liu ZZ, Hatakeyama K, Geller DA, Billiar TR, Morris SM, Jr: A cohort of supporting metabolic enzymes is coinduced with nitric oxide synthase in human tumor cell lines. Cancer Lett 1996, 103:79-84 [DOI] [PubMed] [Google Scholar]
- 46.Bartkowski S, Zapp D, Weber H, Eberle G, Zoidl C, Senkel S, Klein-Hitpass L, Ryffel GU: Developmental regulation and tissue distribution of the liver transcription factor LFB1 (HNF1) in Xenopus laevis. Mol Cell Biol 1993, 13:421-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumenfeld M, Maury M, Chouard T, Yaniv M, Condamine H: Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development 1991, 113:589-599 [DOI] [PubMed] [Google Scholar]
- 48.Baumhueter S, Mendel DB, Conley PB, Kuo CJ, Turk C, Graves MK, Edwards CA, Courtois G, Crabtree GR: HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev 1990, 4:372-379 [DOI] [PubMed] [Google Scholar]
- 49.Nicosia A, Tafi R, Monaci P: Trans-dominant inhibition of transcription activator LFB1. Nucleic Acids Res 1992, 20:5321-5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Resibois A, Cuvelier L, Svoboda M, Heizmann CW, Thöny B: Immunohistochemical localization of pterin 4-alpha-carbinolamine dehydratase in rat peripheral organs. Histochem Cell Biol 1999, 111:381-390 [DOI] [PubMed] [Google Scholar]