Abstract
Uroplakins are the characteristic integral membrane proteins in terminally differentiated, superficial urothelial asymmetric unit membrane. Brenner tumors of the ovary and Walthard cell nests of Fallopian tubes have been considered to represent urothelial differentiation in the female genital tract, but no definitive differentiation marker has been demonstrated supporting such a conclusion. An immunohistochemical analysis was performed to assess the expression of uroplakins in these lesions as well as in various benign and neoplastic lesions and normal tissues of the female genital tract. Focal expression of uroplakins was observed on the luminal surface of ovarian Brenner tumor cells forming microcysts in all 5 cases examined. In contrast, uroplakins were slightly expressed in only 1 of 12 cases of Walthard cell nests, even in the presence of microcyst formation. Uroplakins were not expressed in other benign or malignant lesions or normal tissues of the female genital tract. These results support the hypothesis that the Brenner tumor and possibly Walthard cell nests represent urothelial (transitional cell) differentiation.
Brenner tumors comprise 1 to 2% of ovarian neoplasms; the vast majority are benign and measure less than 2 cm. Microscopically, they are composed of sharply demarcated, solid to partly cystic epithelial cell nests surrounded by a stroma composed of tightly packed, spindle-shaped cells. The epithelial cells are polygonal with pale, eosinophilic cytoplasm and oval nuclei that display distinct nucleoli and longitudinal grooving, the so-called coffee-bean appearance. Walthard cell nests are most commonly found on the peritoneal surface of the Fallopian tubes and also in the mesovarium and occasionally in the ovarian hilus. Because of the histological similarity of the epithelium of Brenner tumors and Walthard cell nests to the urothelium of the lower urinary tract, it has been suggested that Brenner tumors and Walthard cell nests represent urothelial (transitional cell) differentiation. This has further been supported by electron microscopic analysis showing similarities between them, including deeply indented nuclei, sparse granular endoplasmic reticulum, abundant free ribosomes, presence of glycogen particles, secondary lysosomes, moderate numbers of desmosomes, markedly tortuous, interdigitating cell membranes, and large complex intercellular spaces. 1-4 Both Brenner tumors and normal urothelium immunohistochemically stain for carcinoembryonic antigen 5-7 and involucrin. 8 However, none of these features are restricted only to urothelium.
The defining characteristic feature of terminal urothelial differentiation is the presence of the asymmetric unit membrane on the luminal surface and in intracytoplasmic fusiform vesicles. 9 These structures have not been observed in Brenner tumors or in Walthard nests by ultrastructural examination. 1-4
The asymmetric unit membrane 10,11 is characteristic of urothelium and is so named because of its highly unusual ultrastructure. The luminal leaflet of the plasmalemma is almost twice as thick as the cytoplasmic leaflet (9 versus 4 nm). In the superficial cells of the urothelium, discoid cytoplasmic vesicles lined with asymmetric unit membrane are formed in the Golgi complex. 11,12 The luminal plasma membrane of the superficial cells consists of hexagonal subunits which form scallop-shaped plaques typically 0.3 to 0.5 nm in diameter when viewed by transmission and scanning electron microscopy. These plaques contain four major integral membrane proteins called uroplakins Ia, Ib, II, and III, which form 16 nm “twisted-ribbon”-shaped particles arranged in a well-ordered hexagonal lattice with p6 symmetry. 13 The existence of asymmetric unit membrane plaques and their protein constituents, uroplakins, is urothelium-specific and differentiation-dependent. Possible functions of the asymmetric unit membrane include serving as a permeability barrier, as a means of stabilizing the apical bladder surface during expansion and contraction, and as a molecular device to change the surface area of the plasma membrane.
The expression patterns of uroplakins changed in carcinogen-treated rat bladder epithelium, even before tumor appearance, during the hyperplastic phase of the process. 14 In addition to superficial cells, intermediate cells of N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide (FANFT)-induced simple hyperplastic urothelium occasionally stained positive for uroplakins as well as occasional cells in papillomas and carcinomas. Furthermore, staining for uroplakins was occasionally decreased to absent in the luminal-lining cells of FANFT-induced papillary and nodular hyperplasia and of uracil-induced papillomas. Cells lining the bladder lumen in these lesions generally are not terminally differentiated urothelial superficial cells, but rather resemble intermediate and basal cells. The changes in uroplakin expression in experimental bladder carcinogenesis demonstrate the altered differentiation associated with the process.
In rat and human transitional cell carcinomas, the number of cells that express uroplakins is markedly reduced or absent. 14,15 However, expression is still present in human metastatic transitional carcinoma. 15
The aim of the present investigation was to demonstrate evidence of urothelial differentiation in Brenner tumors and Walthard nests by immunohistochemical analysis for uroplakins. Other benign or malignant lesions as well as normal tissues of the female genital tract were also studied by the same methods.
Materials and Methods
The material included five typical Brenner tumors, 16 twelve Walthard cell nests, and two of each of the following lesions: serous cyst adenoma, serous cyst adenocarcinoma, mucinous cyst adenoma, mucinous cyst adenocarcinoma, and granulosa cell tumors of the ovary, hyperplastic endometrium, endometrial adenocarcinoma, squamous cell carcinoma and adenocarcinoma of the cervix, and mesothelial inclusion cysts of the Fallopian tube. Normal urinary bladder, cervical, endometrial, myometrial, placental, Fallopian tube, and ovarian tissues were also examined. Specimens came from patients treated between 1993 and 1995 at the University of Nebraska Medical Center or between 1997 and 1998 at the Nagoya Midori Municipal Hospital.
These surgically excised tissues were fixed in 4% phosphate-buffered formaldehyde and processed routinely for paraffin embedding. The avidin-biotin-peroxidase complex method 17 was used for immunohistochemical analysis of uroplakin. After deparaffinization, unstained slides were treated with microwave heating in antigen retrieval solution (BioGenex; San Ramon, CA) for 10 minutes. Anti-asymmetric unit membrane (AUM) antibody that reacts with all four uroplakins (generously provided by Dr. Tung-Tien Sun) was made against highly purified bovine AUM 18 and applied at a 1:104 dilution. Anti-rabbit IgG secondary antibody and Vectastain ABC kit was purchased from Vector Laboratories, Inc. (Burlingame, CA). Diaminobenzidine was used as a substrate and then counter stained with hematoxylin.
Results
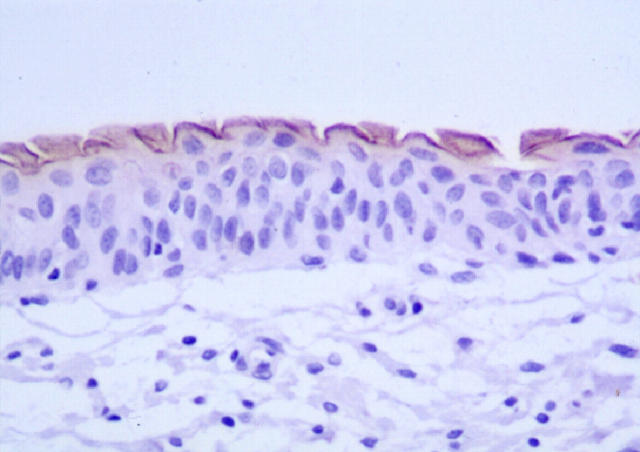
Figure 1 ▶ shows typical uroplakin staining of normal human urinary bladder. The luminal surface of the superficial cells has distinct and consistent expression of uroplakins. In general, the cytoplasm did not show uroplakin expression.
Figure 1.
Immunohistochemical stain for uroplakins in normal human urinary bladder. Note the positive continuous expression on the luminal surface of the superficial cells.
All five Brenner tumors also showed positive staining for uroplakins. However, it was mostly present focally on the luminal surface of the small cysts (Figure 2a) ▶ present in four of the tumors. Tumor cells lining the lumens had positive staining. There was no apparent histological difference between uroplakin-positive microcystic areas compared to those that were negative. Neither was there a relationship with positivity and thickness of the tumor cell layers around the cystic areas. One of the Brenner tumors had diffusely positive staining on the cell membrane as well as lumens (Figure 2 ▶ b). In addition, the cytoplasm of some tumor cells stained weakly positive.
Figure 2.
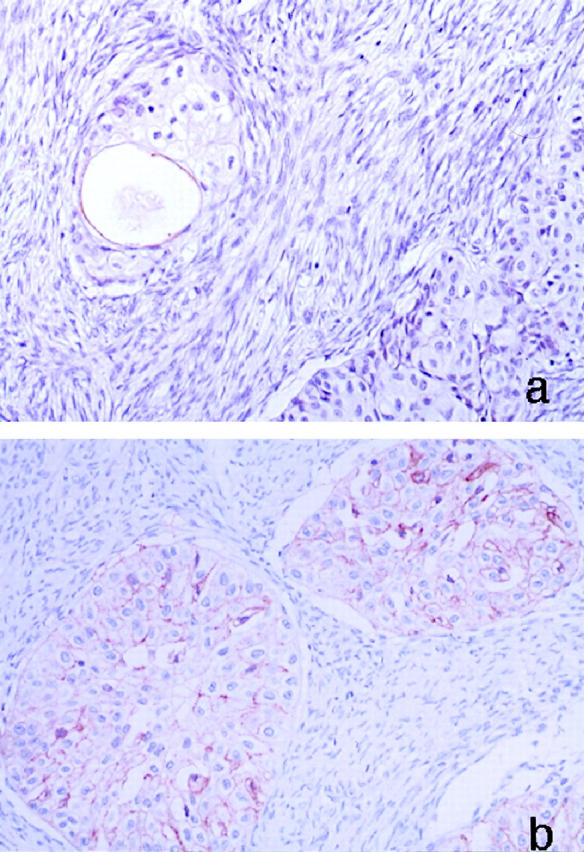
Immunohistochemical stain for uroplakins in a Brenner tumor. a: Expression is observed at the luminal surface of the microcyst. The staining is focally discontinuous. b: Diffuse expression of uroplakins on the cell membrane is seen in one case.
Expression of uroplakins was not observed in 11 cases of Walthard nests despite the histological appearance of multiple cell layers around microcysts similar to transitional cells and similar to the microcysts seen in the Brenner tumors. Only one case of Walthard nest showed slight uroplakin expression on the luminal surface of the cysts (Figure 3,a and b) ▶ .
Figure 3.
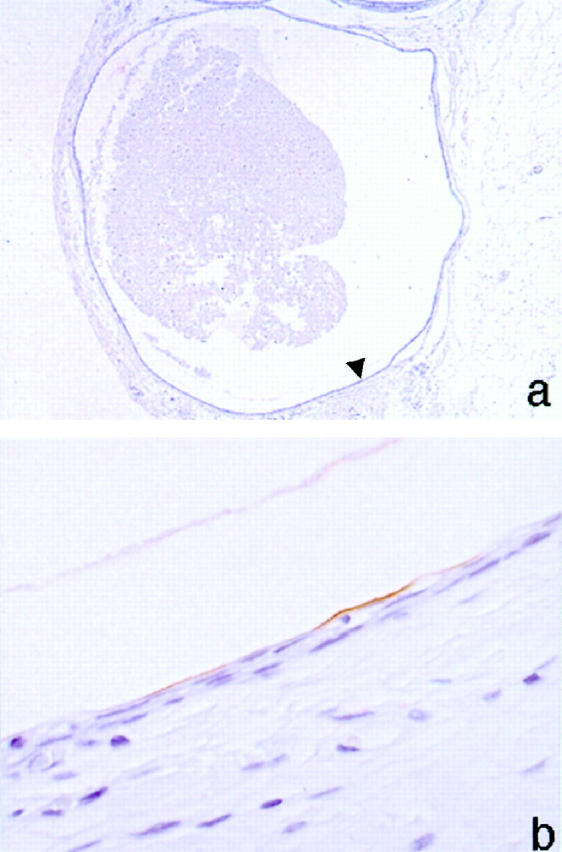
Immunohistochemical stain for uroplakins in a Walthard nest showing only faint luminal uroplakin expression. a: Low power field of Walthard cell nests. Arrowhead shows positive lumen. b: High power field of uroplakin-positive lumen.
Uroplakins were not expressed in any of the normal tissues or any of the other benign or malignant lesions examined including the mesothelial inclusion cyst of the Fallopian tube. The squamous epithelium of the cervix was likewise negative for uroplakin staining.
Discussion
The luminal plasma membrane of cells surrounding microcysts in Brenner tumors focally expressed uroplakins, and similar distribution of expression was observed in one Walthard nest. In contrast, staining for uroplakins was negative in normal tissue from the cervix, endometrium, myometrium, tubes, ovaries, and placenta sites. Likewise, no staining for uroplakins was detected in ovarian serous and mucinous cystadenomas, ovarian serous and mucinous adenocarcinomas, ovarian granulosa cell tumor, hyperplastic endometrium, endometrial adenocarcinoma, cervical squamous cell and adenocarcinoma or mesothelial inclusion cysts of the Fallopian tube.
Based on light microscopic and ultrastructural studies, Brenner tumors and Walthard cell nests have been considered to show urothelial differentiation, but asymmetric unit membranes have not been observed. An electron microscopic study by Roth 3 showed that the Walthard nests had a rather simple fine structure resembling the deeper layers of the urinary bladder epithelium, as evidenced by prominent intercellular spaces containing a profusion of cytoplasmic processes. However, oval or fusiform vesicles were not observed nor were luminal plaques. The Brenner tumors and bladder urothelium share similar immunohistochemical characteristics. Thus, both express chromogranin, neuron specific enolase, serotonin, carcinoembryonic antigen, epithelial membrane antigen and keratin, 7 but Brenner tumors and Walthard nests tended to be cytokeratin-7 positive whereas bladder urothelium was cytokeratin-20 positive. 19 Furthermore, involucrin, a protein precursor present in human stratum corneum, which has been found only in squamous epithelium and urothelium, was also observed in Brenner tumor and Walthard nests. 8 However, these ultrastructural and immunohistochemical changes are not specific to urothelium.
The presence of uroplakins, which are urothelial-specific differentiation proteins, as demonstrated in the present study, provides the first definitive evidence that the cells of the Brenner tumor show urothelial differentiation, not just morphologically resembling urothelial cells. The staining appeared specific for the Brenner tumors, as we did not observe it in any of the other normal tissues or benign and malignant lesions of the female genital tract, including several types of cystic lesions. The focal and variable expression of uroplakins in Brenner tumors suggests that terminal differentiation does not occur in all cells. The urothelial differentiation may be a result of urothelial metaplasia of ovarian celomic epithelium or mesothelium. 7 Because of the resemblance of the Walthard nests to urothelial cells, we expected positive staining for uroplakins in these cells, also. However, we observed it in only one case, possibly because these cells resemble more the deeper layers of the urothelium. The uroplakins are terminally differentiated proteins and are mainly expressed in the superficial cells. It has been suggested that Walthard nests are precursor lesions for Brenner tumors. 7 This seems unlikely, since the majority of Walthard nests are identified in extra-ovarian tissue and the cells seldom express uroplakins.
The histogenesis of Brenner tumors has been suggested to be most likely from differentiation of pluripotential celomic epithelium, 5,20,21 occasionally occurring with features of mucinous differentiation. However, similar tumors, with or without mucinous differentiation, have been reported in ovarian teratomas, 20,21 suggesting another possible histogenesis. It is likely that either of these pathways can occur as they are not mutually exclusive. The present study, although demonstrating the urothelial differentiation of Brenner tumors, does not resolve the question of histogenesis.
Acknowledgments
We gratefully acknowledge the advice and constructive comments of Dr. Eva Uzvolgyi (University of Nebraska Medical Center) and Dr. Tomoyuki Shirai (Nagoya City University), and the assistance of Michelle Moore with the preparation of this manuscript. We are especially grateful to Dr. Tung-Tien Sun (New York University) for providing the antibody used for the studies.
Footnotes
Address reprint requests to Samuel M. Cohen, Havlik-Wall Professor of Oncology, Department of Pathology and Microbiology, University of Nebraska Medical Center, 983135 Nebraska Medical Center, Omaha, NE 68198-3135. E-mail:scohen@unmc.edu.
Supported in part by grants CA32513 and CA36727 from the National Cancer Institute and DK39753 and DK47529 from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Roth LM: Fine structure of the Brenner tumor. Cancer 1971, 27:1482-1488 [DOI] [PubMed] [Google Scholar]
- 2.Cummins PA, Fox H, Langley FA: An ultrastructural study of the nature and origin of the Brenner tumor of the ovary. J Pathol 1973, 110:167-176 [DOI] [PubMed] [Google Scholar]
- 3.Roth LM: The Brenner tumor and the Walthard cell nest: an electron microscopic study. Lab Invest 1974, 31:15-23 [PubMed] [Google Scholar]
- 4.Bransilver BR, Ferenczy A, Richart RM: Brenner tumors and Walthard cell nests. Arch Pathol 1974, 98:76-86 [PubMed] [Google Scholar]
- 5.Shevchuk MM, Fenoglio CM, Richart RM: Histogenesis of Brenner tumors, II: histochemistry and CEA. Cancer 1980, 46:2617-2622 [DOI] [PubMed] [Google Scholar]
- 6.Shevchuk MM, Fenoglio CM, Richart RM: Carcinoembryonic antigen localization in benign and malignant transitional epithelium. Cancer 1981, 47:899-905 [DOI] [PubMed] [Google Scholar]
- 7.Santini D, Gelli MC, Mazzoleni G, Ricci M, Severi B, Pasquinelli G, Pelusi G, Martinelli G: Brenner tumor of the ovary: a correlative histologic, histochemical, immunohistochemical, and ultrastructural investigation. Hum Pathol 1989, 20:787-795 [DOI] [PubMed] [Google Scholar]
- 8.Walts AE, Said JW, Siegel MB, Banks-Schlegel S: Involucrin, a marker of squamous, and urothelial differentiation: an immunohistochemical study on its distribution in the normal and neoplastic tissues. J Pathol 1985, 145:329-340 [DOI] [PubMed] [Google Scholar]
- 9.Hicks RM, Ketterer B, Warren RC: The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Phil Trans R Soc Lond B 1974, 268:23-38 [DOI] [PubMed] [Google Scholar]
- 10.Hicks RM: The fine structure of the transitional epithelium of rat ureter. J Cell Biol 1965, 26:25-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koss LG: The asymmetric unit membranes of the epithelium of the urinary bladder of the rat: an electron microscopic study of a mechanism of epithelial maturation and function. Lab Invest 1969, 21:154-168 [PubMed] [Google Scholar]
- 12.Hicks RM: The function of the Golgi complex in transitional epithelium. J Cell Biol 1966, 30:623-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walz T, Häner M, Wu X-R, Henn C, Engel A, Sun T-T, Aebi U: Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed “twisted ribbon” structure. J Mol Biol 1995, 248:887-900 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa K, Sun T-T, Cohen SM: Analysis of differentiation-associated proteins in rat bladder carcinogenesis. Carcinogenesis 1996, 17:961-965 [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Wu X-R, Lin J-H, Sun T-T: Uroplakins, specific membrane proteins of urothelial umbrella cells, as histological markers of metastatic transitional cell carcinomas. Am J Pathol 1995, 147:1383-1397 [PMC free article] [PubMed] [Google Scholar]
- 16.Roth LM, Dallenbach-Hellweg G, Czernobilsky B: Ovarian Brenner tumors I. Metaplastic, proliferating, and of low malignant potential. Cancer 1985, 56:582-591 [DOI] [PubMed] [Google Scholar]
- 17.Hsu SM, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 18.Wu X-R, Manabe M, Yu J, Sun T-T: Large scale purification and immunolocalization of bovine uroplakins I, II and III. J Biol Chem 1990, 265:19170-19179 [PubMed] [Google Scholar]
- 19.Soslow RA, Rouse RV, Hendrickson MR, Silva EG, Longacre TA: Transitional cell neoplasms of the ovary and urinary bladder: a comparative immunohistochemical analysis. Int J Gynecol Pathol 1996, 15:257-265 [DOI] [PubMed] [Google Scholar]
- 20.Waxman M: Pure and mixed Brenner tumors of the ovary. Cancer 1979, 43:1830-1839 [DOI] [PubMed] [Google Scholar]
- 21.Nomura K, Aizawa S: A histogenetic consideration of ovarian mucinous tumors based on an analysis of lesions associated with teratomas or Brenner tumors. Pathol Int 1997, 47:862-865 [DOI] [PubMed] [Google Scholar]