Abstract
A simple, economical radioassay system employing disposable polypropylene microcentrifuge tubes was developed. Plastic adapters permitted automatic operation in liquid scintillation spectrometers. Counting efficiencies of 3H, 14C, 32P, and 125I in liquid scintillation cocktails and of 32P by Cerenkov radiation (at lower efficiency in absence of added scintillator) were comparable to those in standard vials. Multipurpose use of the microtubes made the system versatile and expedient, e.g., collection of precipitates and radioassay in the same container. Collection of radioimmune precipitates was aided by a carrier inorganic precipitate, Mg2P2O7.
Full text
PDF


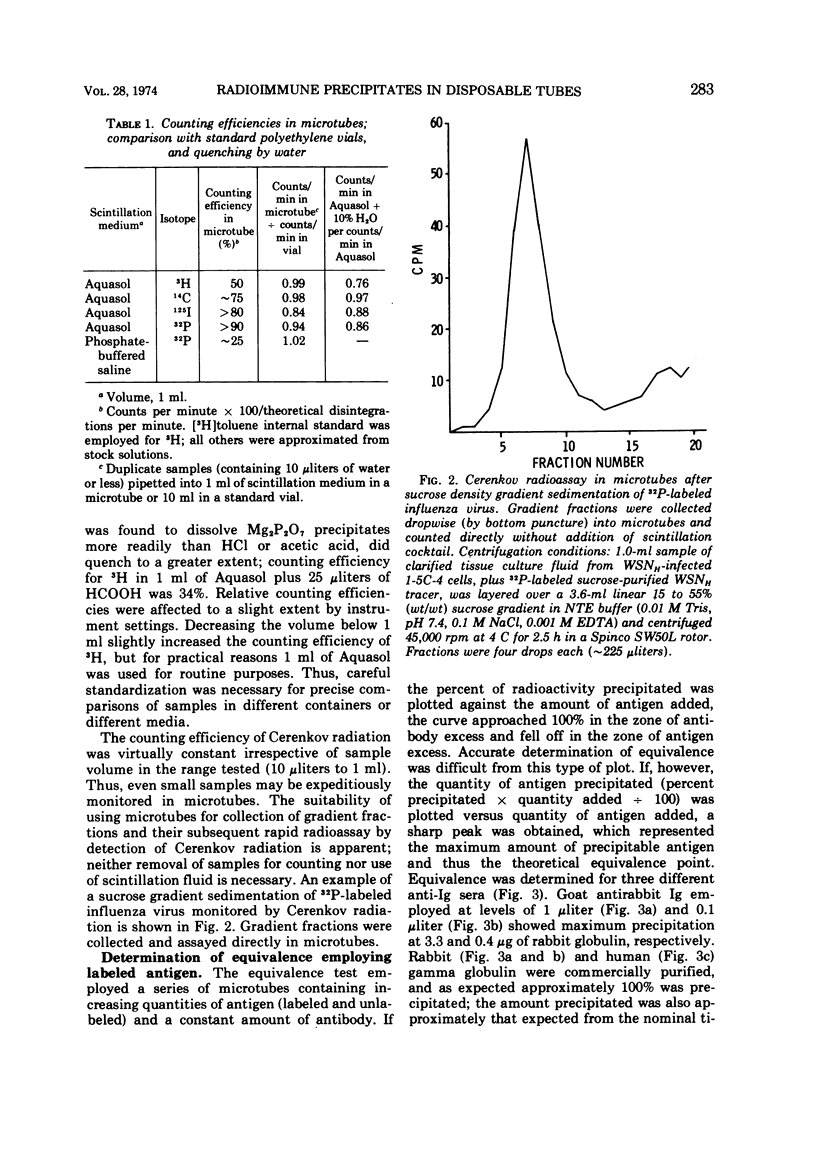
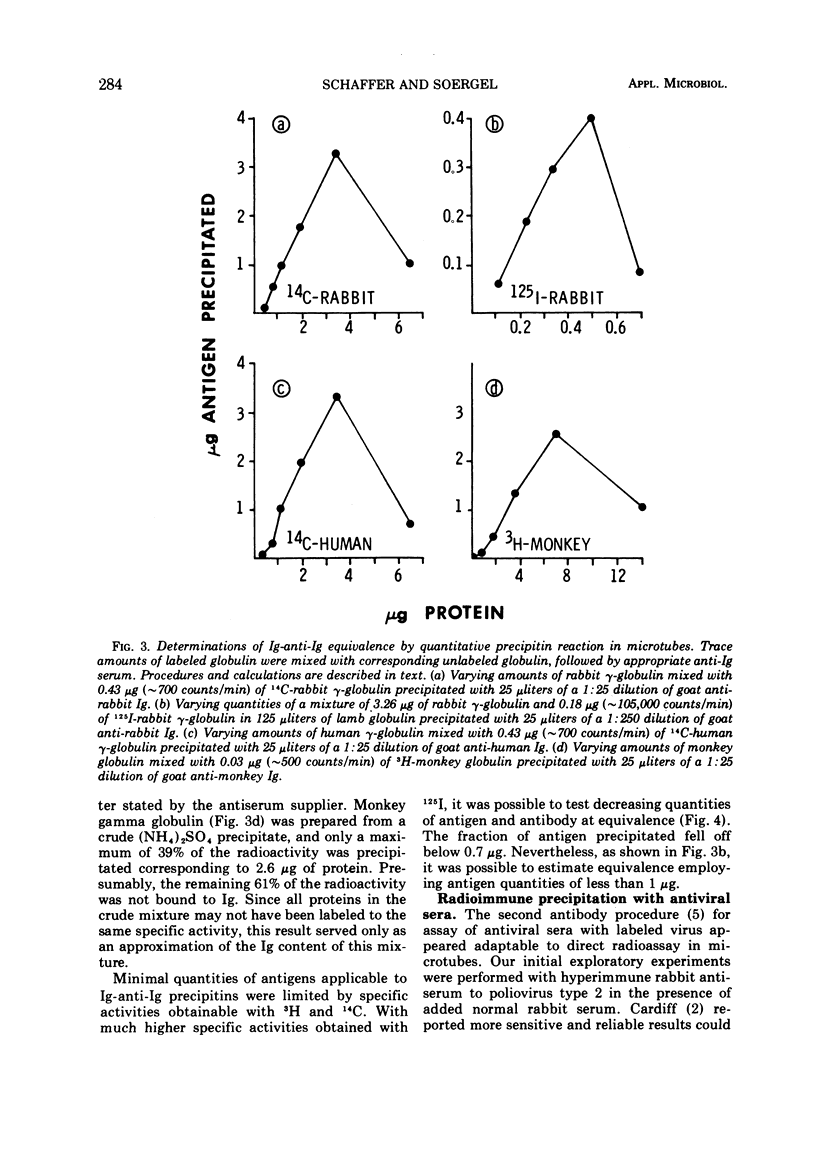
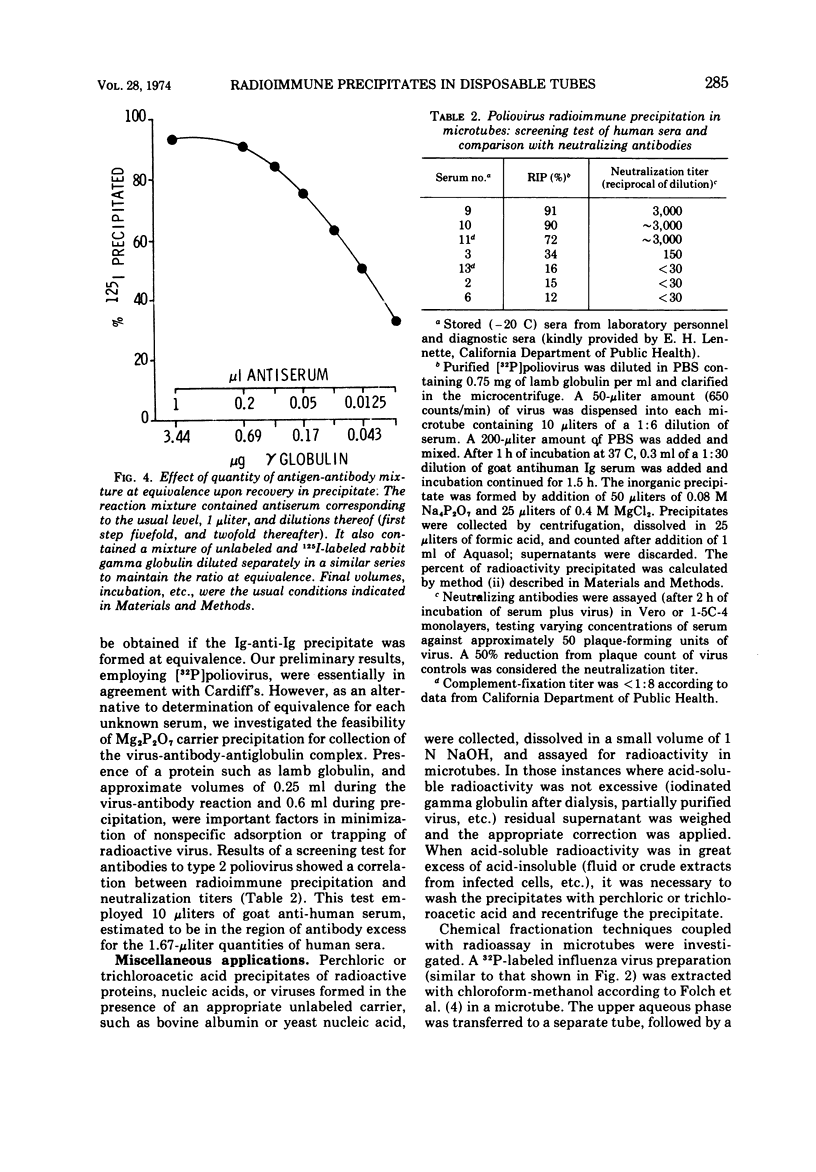


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Cardiff R. D. Quantitation of mouse mammary tumor virus (MTV) virions by radioimmunoassay. J Immunol. 1973 Dec;111(6):1722–1729. [PubMed] [Google Scholar]
- Clausen T. Measurement of 32P activity in a liquid scintillation counter without the use of scintillator. Anal Biochem. 1968 Jan;22(1):70–73. doi: 10.1016/0003-2697(68)90260-1. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GERLOFF R. K., HOYER B. H., McLAREN L. C. Precipitation of radiolabeled poliovirus with specific antibody and antiglobulin. J Immunol. 1962 Oct;89:559–570. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nayak D. P. Influenza virus: structure, replication and defectiveness. Fed Proc. 1969 Nov-Dec;28(6):1858–1866. [PubMed] [Google Scholar]
- Neame K. D., Homewood C. A. Inexpensive liquid scintillation counting of aqueous samples. Anal Biochem. 1974 Feb;57(2):623–627. doi: 10.1016/0003-2697(74)90119-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E., Straube D. C. Electrophoretic analysis of ribosomal and viral ribonucleic acids with a simple technique for slicing low-concentration polyacrylamide gels. Appl Microbiol. 1971 Oct;22(4):538–545. doi: 10.1128/am.22.4.538-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitcer E. M., Bruening G., Agrawal H. O. The multiplication of an influenza virus strain in a continuous line of mammalian cells. Arch Gesamte Virusforsch. 1967;20(1):137–141. doi: 10.1007/BF01245777. [DOI] [PubMed] [Google Scholar]




