Abstract
Different parts of the skin respond to ionizing radiation with different sensitivities. To examine the mechanisms underlying these different responses, we investigated various cellular parameters in the skin after exposure of mice to 5 Gy of ionizing radiation. Epidermal cells responded to radiation by undergoing growth arrest, whereas the cells in the matrix of hair follicles underwent apoptosis but not growth arrest. These distinct responses correlated with differential increases in p53 and p21 proteins in these two populations of cells; whereas an increase in p53 protein levels was observed in both epidermis and hair follicular matrix, especially in the latter, the induction of p21 was strong in the epidermis but absent in the follicular matrical cells. Studies using p53-null and p21-null mice demonstrated that the radiation-induced apoptosis in the hair follicles was fully dependent on p53, and growth arrest in the epidermis was only partially dependent on p53 but fully dependent on p21. These results indicate that two epithelial cell types respond to radiation by different pathways that are governed in part by the differential p53- and p21-dependent responses of these cells; high-level induction of p53 in the absence of p21 induction led to apoptosis, whereas intermediate induction of both p53 and p21 led to growth arrest.
Radiation therapy is one of the major approaches to cancer treatment. Although skin injury is reduced with the use of high energy external beams which have “skin sparing” effect, the radiation-induced damage to skin and its appendages is still among the deleterious reactions that limit the use of high dose radiation. The skin reactions early in the course of treatment with radiation include hair loss and epidermal erythema. 1 Different components of skin react to radiation with different sensitivities; loss of hair occurs after treatment with relatively low doses of radiation, indicating that the hair follicles are highly sensitive to ionizing radiation. 1-3 Despite intensive investigations of radiation damage responses of skin in the past, the molecular mechanisms for these responses are still not well elucidated.
Earlier studies have demonstrated that DNA is the target of ionizing radiation 4 and that death of cells, defined as loss of reproductive ability, is the ultimate consequence of radiation exposure if the cells cannot repair their damaged DNA. With few exceptions, radiosensitivity of cells is influenced by the stage of cellular differentiation and by cellular proliferating activity; less differentiated cells are more radiosensitive than highly differentiated cells and proliferating cells are more radiosensitive than nonproliferating cells. This phenomenon is termed the Law of Bergonie and Tribondeau. 3 The radiosensitivity of cells is also determined by the phase of cell cycle during which radiation is given; cells in G2/M phase are most sensitive to irradiation. 5,6 p53 plays a central role in radiation responses, including cell growth arrest and apoptosis. 7-10 The association of p53 with apoptosis is clearly demonstrated, not only in cell culture experiments, but also in animal studies. p53 is differentially induced in radiosensitive tissues such as spleen and thymus, and these tissues are highly susceptible to radiation-induced apoptosis. 11,12 p53 also regulates transcription of multiple genes, including p21, a cyclin/CDK inhibitor, which are believed to mediate p53-dependent cell cycle arrest induced by DNA damage. 13-15
In this study, we report that epidermal cells and hair follicular cells respond to ionizing radiation by distinct pathways. Following irradiation, epidermal cells underwent growth arrest, whereas the follicular matrical cells died through apoptosis. These different responses correlated with differential increases of p53 and p21 in these two cell populations. Whereas p53 was increased in both of the epidermis and hair follicular matrix, induction of p21 was only seen in the epidermis and was absent in the follicular matrix. In the absence of p53, apoptosis in the hair follicles was completely abrogated, but the growth arrest in the epidermis, where p21 was still induced, was only partially abrogated. The growth arrest in the epidermis in response to radiation was dependent upon p21, as in the p21-null mice the growth arrest was completely abrogated. However, radiation-induced apoptosis appears not to be affected by loss of function of p21; in the irradiated epidermis of p21-null mice, there was no increased induction in apoptosis following irradiation. These findings indicate that the differential responses of epidermal and hair matrix to radiation are governed at least in part by p53 and p21.
Materials and Methods
Mice and Irradiation
All mice were bred and held in the AAALAC-approved McArdle Laboratory Animal Care Facility. Except where noted, experiments were carried out using the inbred FVB/N mouse strain. FVB/N mice carrying the p53-knockout allele were obtained from Dr. Anne Griep (University of Wisconsin). The mice with p53-knockout allele were originally generated by Dr. Tyler Jacks. 16 The p53-null mice (homozygous for the p53-knockout allele or p53−/−) were produced by interbreeding p53+/− mice and genotyped by polymerase chain reaction (PCR). The p21-null mice (129/Sv-B6) were also obtained from Dr. Tyler Jacks. 14
Irradiation was carried out on 8-day-old mice with γ-ray from a 137Cesium (137Cs) source at a dose rate of 3.1 Gray (Gy)/min. A single dose of 5 Gy was delivered to the whole body of mice individually. The mice were then sacrificed at 4, 24, or 48 hours after irradiation. Groups of unirradiated mice were used as controls. One hour before sacrifice, mice were injected with 5-bromo-2′-deoxyuridine (BrdU, Sigma, St. Louis, MO, Cat# B-5002) at a dose of 100 mg/g body weight, to label the cells undergoing DNA synthesis in vivo. Skin samples were obtained from the dorsal area and fixed in 10% buffered formalin. Skin sections of 5 μm in thickness were cut from paraffin-embedded samples. For each time point, 3 to 5 mice were studied. For long term observation of radiation effects on the skin, the mice were kept for 14 days after irradiation and hair loss was monitored.
Immunohistochemistry for BrdU
The procedure for BrdU staining was described previously. 17 Briefly, the skin sections were deparaffinized in xylenes and rehydrated in graded alcohol and phosphate buffered saline (PBS). Endogenous peroxidase was quenched by treatment of skin sections with 3% hydrogen peroxide for 15 minutes. BrdU was detected using the protocol provided with the BrdU staining kit (Cat# HCS24, Oncogene Research Products, Calbiochem, San Diego, CA). Tissue sections were digested with trypsin and treated with a denaturing solution. After incubation with biotinylated mouse anti-BrdU antibody (3 hours), and streptavidin-peroxidase, the slides were exposed to the peroxidase substrate (DAB) mixture for 5 minutes and counterstained with hematoxylin. In the epidermis, the total numbers of cells and the number of BrdU-positive cells per microscopic field were counted in 10 randomly selected fields (400× magnification) of each skin section, and sections from 3 mice for each time point were examined. In the hair follicles, 10 vertically cut follicular bulbs that showed both derma papilla and matrixes were examined and the number of BrdU-positive cells counted.
Immunohistochemistry for p53 and p21
The tissue slides were deparaffinized, rehydrated, and quenched the same way as for BrdU staining. The slides were heated in a boiling 0.01 mol/L citrate buffer, pH 6.0, by a microwave oven for 20 minutes to unmask antigens. Tissue sections were blocked with 5% nonfat dry milk/PBS and 5% normal goat serum for 30 minutes. After blocking, rabbit anti-mouse p53 antibody (CM5, Cat# NCL-p53-CM5p, Novocastra Laboratories), diluted 1:500, or anti-mouse p21 antibody (M-19, Cat# sc-471, Santa Cruz Biotechnology, Santa Cruz, CA), diluted to 1.5 μg/ml, was added and incubated for 3 hours at room temperature in a humid chamber. After incubation with secondary antibody (30 minutes), then with Vectastain ABC reagents (30 minutes), the slides were exposed to DAB substrate. p53- and p21-positive cells were examined, photographed, and counted.
TUNEL Assay
Apoptosis in the skin sections before and after irradiation was analyzed by TUNEL assay. The protocol for this assay was described previously. 18 Briefly, paraffin-embedded sections were also deparaffinized, and rehydrated, analyzed for the presence of DNA fragmentation using an in situ apoptosis detection kit (Apoptag, Intergen (Oncor), Purchase, NY, #S7110-KIT, fluorescein). The procedure was carried out following the manufacturer’s instructions except that the incubation of samples with the fluorescein-conjugated anti-digoxigenin antibody was increased to 1 hour. The slides were examined by UV microscopy and photographed with Kodak Ektachrome P1600 film.
Results
Ionizing Radiation Selectively Induces Growth Arrest in Epidermis and Apoptosis in Hair Follicular Matrixes
Hair loss or alopecia is one of the earliest responses following exposure to ionizing irradiation and occurs with low dose exposure. This observation suggests that hair follicles are sensitive to radiation-induced damage. We compared radiation responses, eg, cell growth arrest and apoptosis, in the hair follicle and epidermis. We found that cells in the epidermis and hair follicular matrix respond differently to ionizing radiation. In the epidermis, radiation induced significant growth arrest, as indicated by the decrease in the number of cells positive for BrdU (Figure 1, a and b ▶ , and Table 1 ▶ ). This growth arrest was most severe 24 hours after 5 Gy of radiation, with the BrdU-positive cells reduced 10-fold. However, growth arrest was not readily seen in the proliferating epithelial compartment of the follicular matrix. Continued BrdU incorporation in these cells was still observed at 24 hours after irradiation (Fig. 1, e and f ▶ , and Table 1 ▶ ). The follicular matrical cells, however, were highly sensitive to radiation-induced apoptosis. Four hours after irradiation with 5 Gy, a large number of the cells in the matrix were undergoing apoptosis, as evidenced by fluorescent staining in the TUNEL assay (Figure 2, e and f) ▶ . These apoptotic cells also were readily seen with H&E staining based upon their pyknotic properties and were in the same region where cells were rapidly dividing (Figure 1f) ▶ . By 24 and 48 hours, the follicular matrix appeared smaller and the total number of cells per bulb were about 20 to 30% less than untreated follicular bulbs (data not shown). In these follicular matrical areas, the percentage of BrdU-positive cells was equivalent to that of untreated control (Table 1) ▶ . The increase in apoptosis, however, was not detected in the epidermis following irradiation (Figure 2, a and b) ▶ . These responses were also demonstrated in the skin sections from mice treated with 3 or 10 Gy. From these data we conclude that epidermal cells respond to radiation by undergoing growth arrest while follicular matrical cells undergo apoptosis.
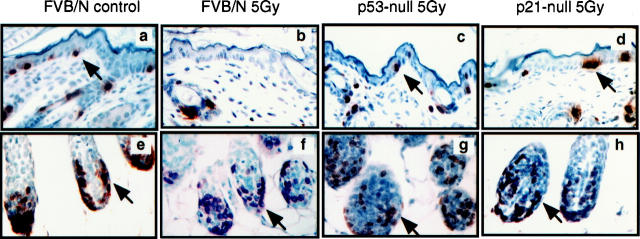
Figure 1.
Comparison of levels of DNA synthesis in the epidermis and hair follicular matrix from normal FVB/N control, p53-null, and p21-null mice after treatment with ionizing radiation (5 Gy). Shown are high power magnification (×400) images of epidermal sections (a–d) and hair follicle sections (e–h) from unirradiated normal mice (a and e), irradiated normal mice (b and f), irradiated p53-null mice (c and g) and irradiated p21-null mice (d and h). The skin sections from these mice were stained immunohistochemically for BrdU using peroxidase-based detection. Sections were counterstained with hematoxylin. Arrows indicate examples of BrdU-positive (brown-stained nuclei) cells. Sections from unirradiated control mice for p53-null and p21-null are not included because the BrdU labeling was similar to that of the unirradiated normal mouse quantified in Table 1 ▶ .
Table 1.
Inhibition of DNA Synthesis in the Epidermis and Hair Follicles after Irradiation
| Mouse strain | Tissue | % BrdU-positive cells* | |
|---|---|---|---|
| Unirradiated | Irradiated† | ||
| Normal | Epidermis | 4.5 ± 0.2 | 0.4 ± 0.1 |
| Normal | Hair follicular matrix | 43 ± 2.2 | 41 ± 3.9 |
| p53-null | Epidermis | 4.5 ± 0.4 | 2.9 ± 0.6 |
| p21-null | Epidermis | 4.4 ± 0.3 | 4.7 ± 0.5 |
*Average numbers ± SE from skin sections of 3 mice.
†24 hours after irradiation (5 Gy).
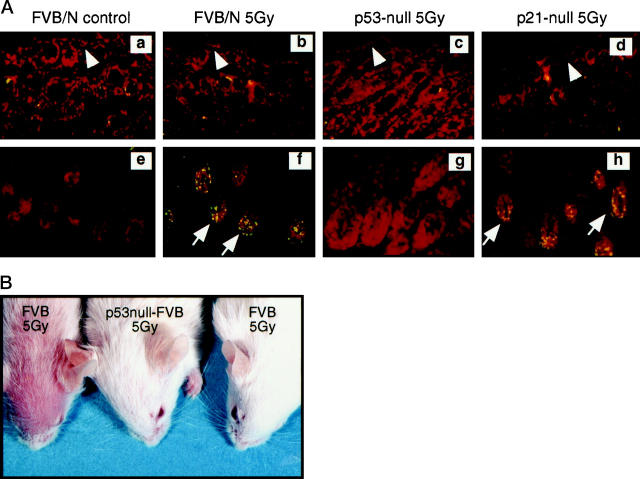
Figure 2.
A:
Comparison of apoptosis induced by 5 Gy of ionizing radiation in the epidermis (a–d) and hair follicles (e–h) from unirradiated (a and e) and irradiated (b and f) normal mice, irradiated p53-null mice (c and g) and irradiated p21-null mice (d and h). These skin sections were tested with the TUNEL assay using a fluorescein-conjugated antibody for detection. Sections were counterstained with propidium iodide. Sections from unirradiated p53-null and p21-null control mice are not included because they were not different from sections of unirradiated normal mice. The epidermis is indicated by arrowheads; apoptotic nuclei (stained green) in hair follicular matrix are indicated by arrows. B: Alopecia at 14 days after irradiation (5 Gy) in p53-sufficient mice but not in p53-null mice.
Levels of p53 Are Increased in Both Epidermis and Matrixes of Hair Follicles After Irradiation
To understand the role of p53 in response to radiation in different compartment of skin, we stained skin sections immunohistochemically for p53 protein. Following irradiation, p53 protein levels were increased in both the hair follicle and epidermis (Figure 3) ▶ . The most significant increase in p53 levels was seen in the matrical cells of hair follicles (Figure 3, c and d) ▶ . Increases in p53 protein were not detected in the more differentiated cells of the hair sheath above the follicular matrix. In the irradiated epidermis, cells with increased levels of p53 were found throughout the whole tissue, though more frequently in the poorly differentiated stratum basale (Figure 3, a, b) ▶ . These results indicate that the p53 induction occurs in both the epidermis and hair follicles in response to radiation and is influenced by the differentiation stage of cells. The increases in p53 proteins seen in both epithelial cell types in response to radiation, although they suggest that p53 may mediate radiation responses in these cells, do not fully explain the difference in responses between them.
Figure 3.
Detection and comparison of the increases in p53 protein in the epidermis (a and b) and follicular matrix (c and d) from normal mice after irradiation. Shown are micro-images of the skin sections from un-irradiated mice (a and c) and mice sacrificed 4 hours after irradiation (c and d). These sections were stained for p53 immunohistochemically using peroxidase-based detection and counterstained with hematoxylin. p53-positive (brown) nuclei are indicated by arrows. Images for epidermis and hair follicles for each mouse (a and c, b and d) were taken from the same skin sections.
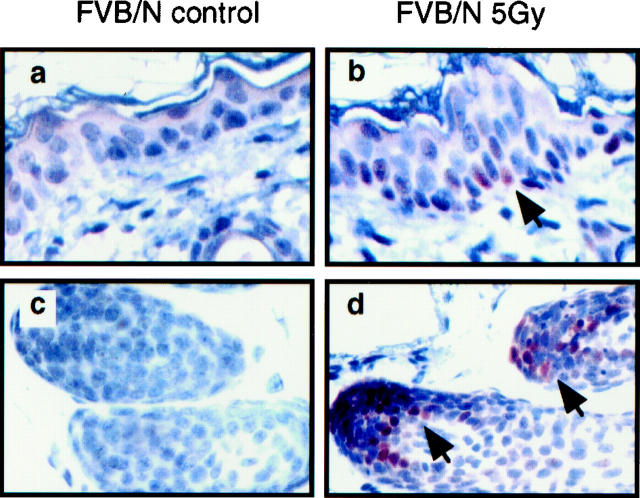
p21 Induction Is Dependent on Cell Type and Differentiation State
To investigate whether there are differences in the induction of factors by p53 that might be related to the different responses in the epidermis and follicular matrix, we measured the levels of p21 protein, a cell cycle regulator that is often seen to be induced in response to radiation and is known to be induced in its expression by p53. We found a significant disparity in the induction of p21 in the epidermis and follicular matrix. The number of p21 positive cells increased in the epidermis following irradiation (Figure 4, a and b) ▶ . These p21-positive cells were present both in the basal layer where growth arrest is observed and also in the suprabasal layers. In contrast, p21-positive cells were not detected in the matrix of hair follicles (Figure 4, d and e) ▶ where apoptosis but not growth arrest was induced in response to radiation. Given the strong induction of p53, it was surprising that the matrical cells were resistant to induction of p21 in response to radiation. A few p21-positive cells were also observed in the upper portion of the hair follicular bulb, a region that lies outside of the proliferating compartment (Figure 4) ▶ . Likewise, more p21-positive cells were also observed in the differentiated or partially differentiated cells in the hair sheath in the upper layer of the dermas (data not shown). Interestingly, in these differentiating hair follicular cells where p21 was induced by irradiation, p53 was not significantly induced, indicating the induction of p53 and p21 can be uncoupled in these cells.
Figure 4.
Detection of p21 protein induction in epidermis (a–c) and hair follicles (d–f) from normal (FVB/N) and p53-null mice. Shown are skin sections from un-irradiated normal mice (a and d), irradiated normal (b and e) and irradiated p53-null (c and f) mice sacrificed at 24 hours that were stained immunohistochemically for p21 using peroxidase-based detection and counterstained with hematoxylin. p21-positive (brown) nuclei are indicated by arrows. Nonspecific reaction of the secondary antibody with the stratum cornea is occasionally seen. Sections from unirradiated p53-null mice were not included because they had a similar staining pattern to the unirradiated p53-sufficient mice (a and d), as in the unirradiated normal mice, very few p21-positive cells were present.
Loss of p53 Leads to a Full Abrogation of Radiation-Induced Apoptosis in Hair Follicles but only Partial Abrogation of Growth Arrest in the Epidermis
p53 is a crucial factor in both cell growth arrest and apoptosis induced by DNA damage agents. To determine whether loss of p53 affects responses of skin to radiation, experiments were performed on p53-null mice. In the untreated p53-null mice, differentiation-related apoptotic-like events in the epidermis and hair follicles was limited to cells within the terminally differentiated compartments (data not shown). The numbers of these apoptotic cells were similar to those seen in the p53-sufficient mice, indicating that loss of p53 does not affect differentiation related apoptotic-like events. However, loss of p53 completely abrogated radiation-induced (5 Gy) apoptosis in the follicular matrix (Figure 2, a, g) ▶ . Correlative to this absence in radiation-induced apoptosis, alopecia that occurs in p53-sufficient mice after irradiation did not occur in irradiated p53-null mice (Figure 2i) ▶ .
In the p53-null epidermis, however, the radiation-induced growth arrest was only partially abrogated (Figure 1c ▶ and Table 1 ▶ ). Although the reduction in the number of BrdU positive cells was less than that seen in the p53-sufficient epidermis, the reduction was still statistically significant. 17 Thus, both p53-dependent and p53-independent responses govern growth arrest in the epidermis after irradiation.
p53-Independent p21 Induction in the Epidermis
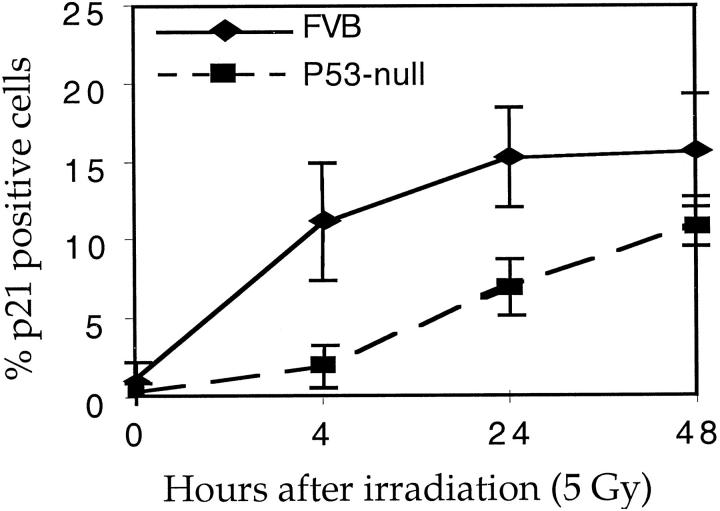
As indicated above, growth arrest in response to radiation in the epidermis was still evident in p53-null mice. We examined, therefore, the levels of p21 protein in the p53-null mice after irradiation. We found that, in the epidermal cells, p21 levels were still increased in p53-null mice after irradiation, although the levels of induction were lower than that seen in p53-sufficient mice and the increase in p21 appeared to be delayed (Figures 4C and 5) ▶ ▶ . Thus p21 induction in the skin by ionizing radiation can be p53-independent as well as p53-dependent. As seen in the p53-sufficient mice, there was no increase of p21 in hair matrix cells in p53-null mice after irradiation, though again there was sporadic induction in the upper portion of the hair follicle.
Figure 5.
Time course of p21 induction in the epidermis of p53-sufficient and p53-null mice. Data were generated from skin sections of three mice at each time point, with 10 microscopic fields counted per section. Error bars indicate SE.
Loss of p21 Completely Abrogates Growth Arrest in the Epidermis but Does Not Affect Apoptosis
Because induction of p21 in the epidermis correlates with growth arrest and absence of p21 induction in the hair follicular matrix correlates with apoptosis, we speculated that p21 induction may lead cells away from the apoptosis pathway and towards the growth arrest pathway; hence, induction of p21 may indirectly help cells to survive. To test this hypothesis, we performed irradiation experiments on p21-null mice. Loss of p21 fully abrogated growth arrest in the epidermis (Figure 1D ▶ and Table 1 ▶ ). However, apoptosis in the hair follicles and epidermis appeared to be similar to that seen in wild-type mice (Figure 2h) ▶ indicating that p21 does not prevent cells from undergoing apoptosis, even though it mediates growth arrest.
Discussion
In this study, we have shown that different cell populations of skin respond to radiation differently. Epidermal cells react to radiation-induced DNA damage by undergoing growth arrest, whereas hair follicular matrical cells respond to radiation by undergoing apoptosis. The apoptosis correlated with increases in p53 protein levels in the matrical cells, whereas cell growth arrest in the epidermal cells correlated with the induction of both p53 and p21. Interestingly, p21 could be induced at least partially in the absence of p53 function in the epidermis. The radiation-induced apoptosis in the hair follicles was completely dependent upon p53, whereas the radiation-induced growth arrest in the epidermis was only partially dependent upon p53 but wholly dependent upon p21.
Increase of p53 Levels and Skin Responses
Growth arrest and apoptosis both can be mediated by p53. In this study, absence of p53 completely abrogated radiation-induced apoptosis in the follicular matrical cells, indicating that apoptosis induced by DNA damage in this cell type is fully dependent on p53. Apoptosis can occur through p53-independent pathways. 19,20 In this regard we noted that the apoptotic-like process associated with terminal differentiation of both the epithelial and hair follicular cells was p53-independent (data not shown). p53 also plays a crucial role in mediating growth arrest in the epidermis, because loss of p53 function severely compromised this response. This growth arrest, however, was not completely p53-dependent, as some growth arrest still occurred in p53-null epidermis after irradiation. Thus, p53-independent mechanisms must play a role in this process, at least in the epidermis. Because p21 is still induced in the p53-null mice and knockout of p21 led to the complete abrogation of growth arrest, p53-independent induction of p21 must contribute to the p53-independent growth arrest.
It has been argued that p53 levels determine which pathway the cells will choose; low levels of p53 induce growth arrest and high levels of p53 induce apoptosis. 21 At a very low level, p53 induces neither apoptosis nor growth arrest; instead, it induces cellular differentiation. 22 The correlation between high p53 levels and induction of apoptosis in the matrix is consistent with the aforementioned hypothesis that high levels of p53 induce apoptosis. Interestingly, however, in the same follicular matrical cell population where there were increased p53 levels, cells were still undergoing DNA synthesis, ie, there was a failure to undergo growth arrest. This indicates that the pathway downstream of p53 for growth arrest was not intact or active in these cells.
Different Skin Responses to Radiation in Relation with p21 Induction
p21 is a cyclin/CDK inhibitor that mediates growth arrest downstream of p53. Levels of p21 in epidermis were increased after treatment with 5 Gy of radiation, but no induction of p21 was seen in the hair follicular matrix. This result correlated with growth arrest in the epidermis and the absence of growth arrest in the hair follicles. Coincidentally, there was a reverse correlation between p21 induction and apoptosis. We initially speculated that p21 might be acting as a survival factor by directing cells towards growth arrest, thereby circumventing induction of an apoptotic response. This speculation was supported by previous reports that p21 deficiency made cells more susceptible to cell death induced by DNA damage. 23,24 Recently, p21 was found to protect cells from p53-mediated apoptosis in melanoma cells. 25 Polyak et al 26 proposed that p21 can inhibit apoptosis by causing growth arrest. We did not see an increase in apoptosis in the epidermis of p21-null mice irradiated with 5 Gy ionizing radiation. One possible explanation for the absence of apoptosis in the p21-null irradiated epidermis is that other survival factors present in that tissue effectively block the apoptosis pathway even in the absence of p21. In this regard, the basement membrane has been found to suppress apoptosis. 27,28 Another possibility is that the pro-apoptotic signals induced in some cells in response to radiation, eg, bax, fail to be induced in the epidermis.
Differentiation Stages, Differential Induction of p53 and p21, and Radiosensitivity
It has long been observed that poorly differentiated/undifferentiated cells are more sensitive than differentiated cells to radiation. Our results may provide a model to explain the different cellular sensitivities to radiation. Radiation induced higher increases in p53 levels in the matrical cells that underwent apoptosis than in the epidermal cells that underwent growth arrest. Conversely, p21 was not induced in the matrical cells but was strongly induced in the epidermal cells. It has been argued that the matrical cells in the hair follicles are less differentiated than cells of the epidermis, including epidermal basal cells, because the basal cells express the intermediate filaments keratins 5 and 10 but follicular matrical cells do not. The differential induction of p53 and p21 may therefore be related to the degree of cell differentiation. This hypothesis is supported by similar observations made by Potten and colleagues, who found in the murine intestinal epithelia a similar relationship between the degree of cell differentiation, levels of p53 and p21 induction, and cell fate. 29
In the context of this hypothesis, the differential induction of p21 is an intriguing factor. p21 induction was not observed in the matrical cells despite very high levels of p53. p21 expression is associated with cellular differentiation; its level can be found to increase during cellular differentiation. 30 One explanation for the absence of p21 induction in the less differentiated cells is that factors required for expression of p21 are absent in these cells, thereby preventing its induction via either the p53-dependent or the p53-independent radiation response pathways. Another explanation is that the p53 protein in the less differentiated cells is not transcriptionally active. Weinberg et al reported that, in terminally differentiating keratinocytes, low p53 protein levels were associated with significant transcriptional activities, whereas in the poorly differentiated keratinocytes, higher levels of p53 were present but the p53 was less active transcriptionally. 31
In the less differentiated cells of hair follicular matrix, apoptosis occurred through a p53-dependent pathway. Bax is a pro-apoptotic regulator that can be induced by p53 32 and may be involved in the induction of apoptosis in the hair follicles. 33 If p53 is not transcriptionally active in this matrical cells, however, it is unlikely that Bax is induced by p53. Using cells of testicular germ cell tumors, Burger reported that bcl-2 family proteins including bax were not induced by ionizing radiation, although p53 was induced in these cells, indicating that these proteins may not be involved in radiation-induced apoptosis in these poorly differentiated cells. 34 Further investigation of the p53-dependent induction of apoptosis in the matrical cells is needed. The role of p21 in the apoptotic response of hair follicles to radiation should also be further explored. We have shown that loss of p21 did not increase the sensitivity of cells to apoptosis, consistent with other studies with p21-deficient cells in which p21 was found not to affect apoptosis. 15 To test the role of p21 further, overexpression of p21, instead of p21 deletion, should be tested for its dominant effect on apoptotic responses in the hair follicles, ie, whether it is a survival factor in a tissue where apoptosis normally results from DNA damage.
Acknowledgments
We thank Amy Liem for assistance in breeding and maintenance of animals, Jane Weeks, Angie Buehl, and Harlene Edwards for processing tissue sections, and Dr. Normal Drinkwater for statistical analysis. We thank Dr. Anne Griep for providing p53-null FVB/N mice and Dr. Tyler Jacks for providing p21-null mice and for critically reading the manuscript.
Footnotes
Address reprint requests to Paul F. Lambert, McArdle Laboratory for Cancer Research, University of Wisconsin Medical School, 1400 University Avenue, Madison, WI 53706. E-mail: Lambert@oncology.wisc.edu.
Supported by a grant from the American Cancer Society (VM164) and by grants from the National Institutes of Health (CA22443 and CA07175).
References
- 1.Hopewell JW: The skin: its structure and response to ionizing radiation. Int J Radiat Biol 1990, 57:751-773 [DOI] [PubMed] [Google Scholar]
- 2.Malkinson FD: Some principles of radiobiology: a selective review. J Invest Dermatol 1981, 77:32-38 [DOI] [PubMed] [Google Scholar]
- 3.Prasad KN: Handbook of Radiobiology, 2nd ed. New York, CRC Press, 1995
- 4.Hutchinson F: The molecular basis for radiation effects on cells. Cancer Res 1966, 26:2045-2052 [PubMed] [Google Scholar]
- 5.Dewey WC, Noel JS, Dettor CM: Changes in radiosensitivity and dispersion of chromatin during the cell cycle of synchronous Chinese hamster cells. Radiat Res 1972, 52:373-394 [PubMed] [Google Scholar]
- 6.Hall EJ: Radiobiology for the Radiologist, 3rd ed. Philadelphia, J.B. Lippincott Company, 1988
- 7.Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ, Jr: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992, 71:587-597 [DOI] [PubMed] [Google Scholar]
- 8.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB: Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 1992, 89:7491-7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canman CE, Chen CY, Lee MH, Kastan MB: DNA damage responses: p53 induction, cell cycle perturbations, and apoptosis. Cold Spring Harbor Symposia on Quantitative Biology 1994, 59:277-286 [DOI] [PubMed] [Google Scholar]
- 10.Levine AJ: p53, the cellular gatekeeper for growth and division (review). Cell 1997, 88:323-331 [DOI] [PubMed] [Google Scholar]
- 11.Midgley CA, Owens B, Briscoe CV, Thomas DB, Lane DP, Hall PA: Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci 1995, 108:1843-1848 [DOI] [PubMed] [Google Scholar]
- 12.MacCallum DE, Hupp TR, Midgley CA, Stuart D, Campbell SJ, Harper A, Walsh FS, Wright EG, Balmain A, Lane DP, Hall PA: The p53 response to ionising radiation in adult and developing murine tissues. Oncogene 1996, 13:2575-2587 [PubMed] [Google Scholar]
- 13.McDonald ER, 3rd,, Wu GS, Waldman T, El-Deiry WS: Repair defect in p21 WAF1/CIP1−/− human cancer cells. Cancer Res 1996, 56:2250-2255 [PubMed] [Google Scholar]
- 14.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ: Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995, 377:552-557 [DOI] [PubMed] [Google Scholar]
- 15.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82:675-684 [DOI] [PubMed] [Google Scholar]
- 16.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA: Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994, 4:1-7 [DOI] [PubMed] [Google Scholar]
- 17.Song S, Gulliver GA, Lambert PF: Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci USA 1998, 95:2290-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herber R, Liem A, Pitot H, Lambert PF: Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol 1996, 70:1873-1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace H, Clarke AR, Harrison DJ, Hooper ML, Bishop JO: Ganciclovir-induced ablation non-proliferating thyrocytes expressing herpesvirus thymidine kinase occurs by p53-independent apoptosis. Oncogene 1996, 13:55-61 [PubMed] [Google Scholar]
- 20.Arita D, Kambe M, Ishioka C, Kanamaru R: Induction of p53-independent apoptosis associated with G2M arrest following DNA damage in human colon cancer cell lines. Jpn J Cancer Res 1997, 88:39-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Ko LJ, Jayaraman L, Prives C: p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev 1996, 10:2438-2451 [DOI] [PubMed] [Google Scholar]
- 22.Lassus P, Ferlin M, Piette J, Hibner U: Anti-apoptotic activity of low levels of wild-type p53. EMBO J 1996, 15:4566-4573 [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YA, Elson A, Leder P: Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA 1997, 94:14590-14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan S, Chang JK, Smith ML, Duba D, Fornace AJ, Jr, O’Connor PM: Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene 1997, 14:2127-2136 [DOI] [PubMed] [Google Scholar]
- 25.Gorospe M, Cirielli C, Wang X, Seth P, Capogrossi MC, Holbrook NJ: p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene 1997, 14:929-935 [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B: Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev 1996, 10:1945-1952 [DOI] [PubMed] [Google Scholar]
- 27.Boudreau N, Sympson CJ, Werb Z, Bissell MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267:891-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau N, Werb Z, Bissell MJ: Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA 1996, 93:3509-3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson JW, Pritchard DM, Hickman JA, Potten CS: Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am J Path 1998, 153:899-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T: p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 1995, 9:935-944 [DOI] [PubMed] [Google Scholar]
- 31.Weinberg WC, Azzoli CG, Chapman K, Levine AJ, Yuspa SH: p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene 1995, 10:2271-2279 [PubMed] [Google Scholar]
- 32.Miyashita T, Reed JC: Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80(2):293-299 [DOI] [PubMed] [Google Scholar]
- 33.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R: Analysis of apoptosis during hair follicle regression (catagen) (see comments). Am J Pathol 1997, 151:1601-1617 [PMC free article] [PubMed] [Google Scholar]
- 34.Burger H, Nooter K, Boersma AW, Kortland CJ, van den Berg AP, Stoter G: Expression of p53, p21/WAF/CIP, Bcl-2, Bax, Bcl-x, and Bak in radiation-induced apoptosis in testicular germ cell tumor lines. Int J Radiat Oncol Biol Phys 1998, 41:415-424 [DOI] [PubMed] [Google Scholar]