Abstract
The cyclin-dependent kinase inhibitor p27Kip1 is a powerful molecular determinant of cell cycle progression. Loss of expression of p27Kip1 has been shown to be predictive of disease progression in several human malignancies. In this study we investigated the expression of two key cell cycle regulators, p27Kip1 and cyclin E, in the progression of transitional cell carcinoma of the bladder. An immunohistochemical analysis was conducted in a series of 50 bladder tumor specimens, including 3 metastatic lymph nodes, and 7 normal bladder specimens, using specific antibodies against the two regulators of the cell cycle, p27Kip1 and cyclin E. The degree of immunoreactivity was correlated with the pathological tumor grade, stage, and patient survival. A uniformly intense immunoreactivity for p27Kip1 and cyclin E was observed in epithelial cells of normal bladder tissue. Malignant bladder tissue demonstrated a heterogeneous pattern of significantly reduced p27Kip1 and cyclin E immunoreactivity, compared with normal urothelium (P < 0.01). In addition, there was progressive loss of expression of both cell cycle proteins with increasing tumor grade and pathological stage. Expression of p27Kip1 was significantly lower in the poorly differentiated tumors (grades III) compared to well and moderately differentiated (grades I and II) tumors (P = 0.004). Moreover, the expression of cyclin E was lower in grade III tumors compared to grade I and II lesions, although this difference failed to reach statistical significance. Most significantly, Kaplan-Meier plots of patient survival show increased mortality risk associated with low levels of p27Kip1 (P = 0.001) and cyclin E (P = 0.002) expression. This is the first evidence that loss of expression of p27Kip1 and cyclin E in human bladder transitional cell carcinoma cells correlates with advancing histological aggressiveness and poor patient survival. These results have clinical importance, because they support a role for p27Kip1 and cyclin E as novel predictive markers of the biological potential of bladder tumors that will enable identification of those tumors most likely to progress to muscle invasive disease and of patient survival.
Transitional cell carcinoma of the bladder is a common malignancy of the genitourinary tract and is the second most prevalent cancer among middle-aged and elderly men. 1 The management of this tumor depends on an accurate assessment of the tumor’s biological potential, and the ability to identify those tumors most likely to progress to muscle invasive disease would greatly facilitate effective treatment of the disease. Although the pathological grade of the tumor is an important variable in bladder cancer management, a true prognostic marker to identify the likelihood of tumor progression and ultimate patient prognosis has yet to be identified.
During the past several years, advances made in our understanding of the cell cycle regulatory machinery have indicated that disruption of the normal cell cycle is a critical step in cancer development. 2-9 Abnormalities of various components of the cell cycle have been identified in several types of human cancer. 10-24 As the major regulatory events leading to cell proliferation and differentiation occur within the G1 phase of the cell cycle, attention has been focused on altered expression of the G1 cyclins and cyclin-dependent kinases (Cdk) as key events in tumorigenesis. 8-10,25-27
The G1 cyclins, including three D-type cyclins and cyclin E, regulate the progression of cells through the G1 phase of the cell cycle through interactions with specific Cdks. Each of these cyclin/Cdk complexes is activated at a specific point during G1 and has a specific set of substrates. Cyclin E is a late G1 cyclin, which, along with its catalytic subunit Cdk2, is involved in phosphorylation of the Rb protein. The activation of the cyclin E/Cdk2 complex is the rate-limiting event for cell transition into the S phase of the cell cycle. Overexpression of cyclin E accelerates the G1-to-S phase transition, and increased expression of multiple cyclin E-related proteins has been reported in several human malignancies. 11,13,28-31 The activity of the cyclinE/Cdk2 complex is primarily regulated by the Cip/Kip family of Cdk inhibitors (CKI), which include the p21Waf1, p27Kip1, and p57Kip2 proteins. The p27Kip1 protein appears to be the major regulator of cyclin E, and several studies have demonstrated the importance of this protein in cell growth and differentiation. 4,6-8,32-34 Modulation of p27Kip1 activity appears to be mediated mainly by the antimitogenic effects of transforming growth factor-β (TGF-β), in addition to cell-to-cell contact and agents that elevate adenosine 3′,5′-cyclic phosphate. 29,32
Overexpression of p27Kip1 in mammalian cells induces a G1 block in the cell cycle. Conversely, defective regulation at the p27 checkpoint in the cycle can result in uncontrolled cellular proliferation. Several investigators have demonstrated that lost or decreased p27Kip1 expression is a prognostic marker in several human malignancies, including breast, 11-14 colorectal, 15-17 prostate, 18-20 esophageal, 21-22 gastric, 23 and pulmonary carcinomas. 24 No tumor-specific mutation of the p27Kip1 gene has been found in humans. 10 In addition, low levels of p27Kip1 in certain tumors have been attributed to tumor-specific enhanced proteosome-mediated degradation of the protein. 15,35 This has been hypothesized to be a hallmark of more aggressive tumors, indicating that loss of p27Kip1 expression and cyclin E overexpression may be markers of more invasive tumors with a poorer prognosis.
The potential significance of p27Kip1 and cyclin E proteins in bladder cancer progression has yet to be investigated. In this study we performed an immunohistochemical analysis of bladder tumor specimens to characterize the expression of these key cell cycle regulators in transitional cell carcinoma of the bladder. Our findings demonstrate that down-regulation of p27Kip1, as well as cyclin E protein expression within the same tumor cell populations, correlate with tumor grade, pathological stage, and patient survival.
Materials and Methods
Tissue Selection
Fifty bladder tumor specimens were obtained from 47 patients with histologically confirmed transitional cell carcinoma of the bladder. These specimens included 26 cases obtained from patients undergoing radical cystoprostatectomy for localized disease, 1 partial cystectomy, 16 transurethral bladder tumor resections, and 7 transurethral bladder biopsies. Three lymph nodes with metastatic lesions from a single patient were also analyzed. Seven normal bladder tissue specimens were obtained from six patients and served as controls. These included four biopsy specimens from cancer-free patients and three specimens from histologically normal areas of bladder in patients who underwent radical cystoprostatectomy for an isolated invasive grade III lesion. All control specimens were determined to be tumor-free on histopathological analysis. All specimens were paraffin-embedded sections obtained from the archives of the Department of Pathology at the University of Maryland School of Medicine. The tumor histological grade was determined by our pathologist (A. B.) before immunohistochemical analysis. This study included 8 grade I lesions, 18 grade II lesions, and 21 grade III lesions of transitional cell carcinoma of the bladder. Classified by pathological tumor stage, there were 5 Tis, 9 Ta, 15 T1, 6 T2, 9 T3, and 3 T4 tumors. Patient age ranged from 33 to 87 years, with a mean of 64 years. Patient follow-up ranged from 0.5 to 7.2 years, with a median of 3.2 years.
Immunohistochemical Analysis
Tissue specimens were deparaffinized and dehydrated with xylene and graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol at room temperature for 25 minutes. Specimens were washed in phosphate buffered saline solution three times (5 minutes each). Nonspecific binding sites were blocked with 10% goat serum and slides were subsequently incubated with a rabbit polyclonal antibody raised against the human p27Kip1 protein (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The antibody against the p27Kip1 protein was raised against the peptide sequence (amino acids 181–198, mapping at the COOH terminus of human p27Kip1). Sections were rinsed with phosphate buffered saline, incubated with the biotinylated anti-rabbit secondary antibody at room temperature for 45 minutes, and then treated with the biotin-avidin peroxidase system (Santa Cruz Biotechnology). Positive immunoreactivity was visualized with the 3,3-diaminobenzidine reaction. Hematoxylin was used for counterstaining. Immunohistochemical analysis for cyclin E expression was performed using the same system used for p27Kip1 expression. The antibody against the cyclin E protein was also a rabbit polyclonal antibody raised against the cyclin E protein (1 μg/ml; Santa Cruz Biotechnology). The cyclin E antibody was raised against the epitope corresponding to a partial amino acid sequence of human cyclin E.
Quantitation of Immunostaining
Cancerous urothelium was identified and examined for p27Kip1 and cyclin E immunostaining. Cell counts were performed by two independent observers (J. J. D. and N. K.) at 400× magnification using a 10×10 counting grid via an eyepiece. Observers were blinded to the pathological grade of the tumor. Three different fields were counted by each observer, and an index of protein expression was calculated as a percentage (number of positively immunostained cells/total number of tumor cells counted). The relative intensity of immunostaining was graded subjectively by each observer as 0, +1, +2, and +3, corresponding to negative, weak, moderate, and strong immunoreactivity, respectively.
Statistical Analysis
Student’s t-test was used to analyze the differences in p27Kip1 and cyclin E expression between normal bladder and transitional cell carcinoma of grades I, II, and III. The values are presented as means ± SE. Differences in mean values were considered significant at a P value <0.05. Kaplan-Meier estimates of survival functions were generated among groups of patients with tumors of low versus high p27Kip1 and cyclin E immunostaining indices. 36 Differences in Kaplan-Meier curves for patient survival (after cystectomy or transurethral resection) between groups were compared by the log-rank test.
Results
The level of p27Kip1 immunoreactivity was high in the transitional epithelial cells of normal bladder specimens (Figure 1A) ▶ . Diffuse staining was seen within the nucleus and in the cytoplasm of the normal urothelium. In marked contrast, malignant bladder tissue demonstrated a heterogeneous pattern of significantly reduced p27Kip1 immunoreactivity. More impressively, there was progressive loss of p27Kip1 expression with increasing tumor grade. The data summarized in Table 1 ▶ reveal the quantitative analysis of p27Kip1 immunoreactivity related to the histological grade and pathological stage of the bladder tumor specimens. These results clearly indicate a significant decrease in the levels of p27Kip1 immunostaining with increasing histological grade, as well as pathological stage of transitional cell carcinoma of the bladder (P < 0.01).
Figure 1.

Photomicrographs of immunoreactivity patterns of p27Kip1 expression in normal bladder and malignant bladder tissue. Immunohistochemical analysis was performed as described in Materials and Methods. Immunostaining against p27Kip1 protein revealed intense immunoreactivity among the epithelial cells of normal bladder urothelium (A). B-D indicate sections of malignant bladder tissue with increasing tumor grade, exhibiting progressive loss of p27Kip1 expression. B shows a grade I transitional cell carcinoma of the bladder indicating moderate immunoreactivity (+2) of the p27Kip1 protein within the cancer foci. C reveals a grade II bladder tumor, and D a section of a grade III bladder tumor, indicating a characteristically weak immunoreactivity (+1) and loss of p27Kip1 protein expression, respectively. D shows a high grade transitional cell carcinoma of the bladder in which malignant foci exhibit complete loss of p27Kip1 expression (arrow). Original magnifications, ×100.
Table 1.
Correlation of p27Kip1 Expression and Cyclin E Expression with Histological Grade and Pathological Stage in Transitional Cell Bladder Tumors
| Tumor grade | Normal (7) | Grade I (8) | Grade II (18) | Grade III (21) | + LN (3) | |
|---|---|---|---|---|---|---|
| % p27Kip1 positivity | 63.6 ± 2.1 | 40.3 ± 6.6* | 37.9 ± 3.2* | 23.1 ± 3.7* | 25.3 ± 5.6* | |
| % cyclin E positivity | 57.9 ± 7.8 | 27.0 ± 8.1* | 19.3 ± 3.9* | 16.5 ± 3.5* | 33.5 ± 4.9* |
| Tumor stage | Tis (5) | Ta (9) | T1 (15) | T2 (6) | T3 (9) | T4 (3) |
|---|---|---|---|---|---|---|
| % p27Kip1 positivity | 49.5 ± 8.2 | 38.3 ± 2.3* | 37.9 ± 4.7* | 20.7 ± 4.2* | 17.8 ± 5.3* | 2.5 ± 1.2* |
| % cyclin E positivity | 43 ± 6.4 | 16.2 ± 1.7* | 21.6 ± 5.6* | 10.1 ± 5.6* | 14.6 ± 4.6* | 10.5 ± 6.9* |
Values represent the mean percentage of p27Kip1 and cyclin E immunoreactive cells calculated as the number of stained cells divided by the total number of cells counted, ± SE. Numbers in parentheses indicate the number of specimens analyzed in each group.
+ LN, lymph nodes positive for metastatic bladder tumor cells.
*Values significantly different from normal urothelium (P < 0.01).
Although the intensity of p27Kip1 immunoreactivity was uniformly high in the transitional urothelium of normal bladder specimens (+3 intensity), a moderate degree of variability in the staining intensity was observed among cancer specimens of the same histological grade. Well differentiated transitional cell carcinoma specimens (grade I, Figure 1B ▶ ) showed a moderate level of immunoreactivity for p27Kip1 (+2 intensity), with immunostaining limited to scattered areas of the cancerous epithelium. In contrast, poorly differentiated bladder tumors (grades II and III) exhibited immunostaining ranging from weak (+1) to total loss of protein expression. (Figure 1, C and D) ▶ . The results, summarized in Table 2 ▶ , reveal the distribution of immunostaining intensity among the various bladder tissue specimens analyzed. Approximately 89% of grade II tumor specimens included in this study demonstrated either very weak (72%) or no (16.7%) expression, whereas total loss of p27Kip1 staining was observed in 67% of grade III bladder tumors.
Table 2.
Expression of p27Kip1 Protein in Normal and Malignant Bladder Tissue
| Type of specimen | Number of specimens | Distribution of p27Kip1 staining intensity (% of specimens in group) | |||
|---|---|---|---|---|---|
| Negative (0) | Weak (+1) | Moderate (+2) | Strong (+3) | ||
| Normal | 7 | 0 | 0 | 0 | 100 |
| Grade I | 8 | 0 | 25 | 62.5 | 12.5 |
| Grade II | 18 | 17 | 72 | 11 | 0 |
| Grade III | 21 | 67 | 33 | 0 | 0 |
| +LN | 3* | 33 | 67 | 0 | 0 |
*These 3 specimens were from the same patient.
+LN, lymph nodes identified as positive for bladder tumor cell metastasis.
As the p27Kip1 protein appears to be the major regulator of cyclin E during the late G1 phase of the cell cycle, it was interesting to investigate the expression of cyclin E in the same bladder tumor specimens that had shown a progressive loss of p27Kip1 with increasing histological grade. Although the overall number of cyclin E immunoreactive cells was not as high as that for the p27Kip1 protein, a similar pattern of significantly reduced cyclin E immunoreactivity was observed in cancerous foci compared to normal bladder specimens. Significantly enough, as with p27Kip1 immunoreactivity, there was progressive loss of cyclin E expression with advancing histological grade of the bladder tumor (Figure 2, A–D) ▶ . Quantitative analysis of the data, summarized in Table 1 ▶ , shows a significant correlation between loss of cyclin E expression and increasing tumor grade. Table 1 ▶ also indicates a close relationship between cyclin E levels and pathological tumor stage. In addition, the intensity of cyclin E protein expression correlated with the intensity of p27Kip1 protein immunoreactivity in the same specimens of transitional cell carcinoma of the bladder. As shown in Table 3 ▶ , strong immunoreactivity (+3) was observed in all normal bladder specimens, whereas most well differentiated grade I tumors exhibited moderate immunoreactivity (+2; 50%). Poorly differentiated tumors were characterized by significant (29%) or total loss (71%) of cyclin E expression.
Figure 2.
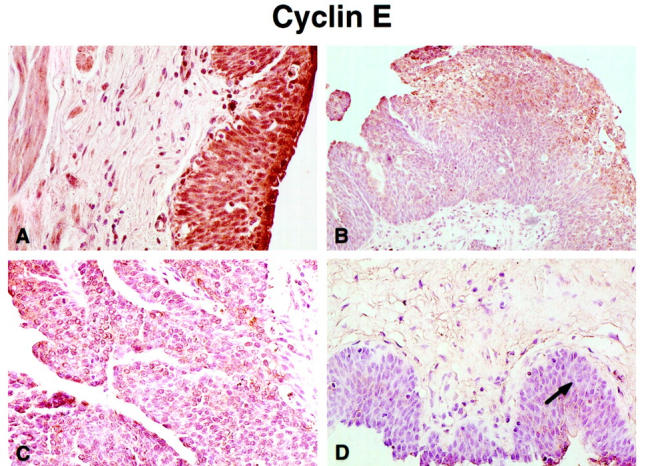
Immunoreactivity patterns of cyclin E expression in normal bladder and malignant bladder tissue. Immunostaining using the rabbit polyclonal antibody against the cyclin E protein revealed uniformly abundant expression among the epithelial cells of the normal bladder urothelium. Original magnification, ×100 (A). B shows a grade I transitional cell carcinoma of the bladder indicating moderate immunoreactivity (+2) for cyclin E protein within the cancer foci. Original magnification, ×50. C and D reveal sections of a grade II bladder tumor, and a grade III bladder tumor, indicating weak immunoreactivity (+1) and loss of cyclin E expression, respectively. Arrow indicates high grade, malignant bladder epithelial cells exhibiting complete loss of cyclin E immunoreactivity (D). Original magnifications, ×100 (C and D).
Table 3.
Expression of Cyclin E Protein in Normal and Malignant Bladder Tissue
| Type of specimen | Number of specimens | Distribution of cyclin E staining intensity (% of specimens in group) | |||
|---|---|---|---|---|---|
| Negative (0) | Weak (+1) | Moderate (+2) | Strong (+3) | ||
| Normal | 7 | 0 | 0 | 0 | 100 |
| Grade I | 8 | 0 | 37.5 | 50 | 12.5 |
| Grade II | 18 | 17 | 67 | 17 | 0 |
| Grade III | 21 | 71 | 29 | 0 | 0 |
| +LN | 3* | 0 | 67 | 33 | 0 |
*These 3 specimens were from the same patient.
+LN, lymph nodes identified as positive for bladder tumor cells metastasis.
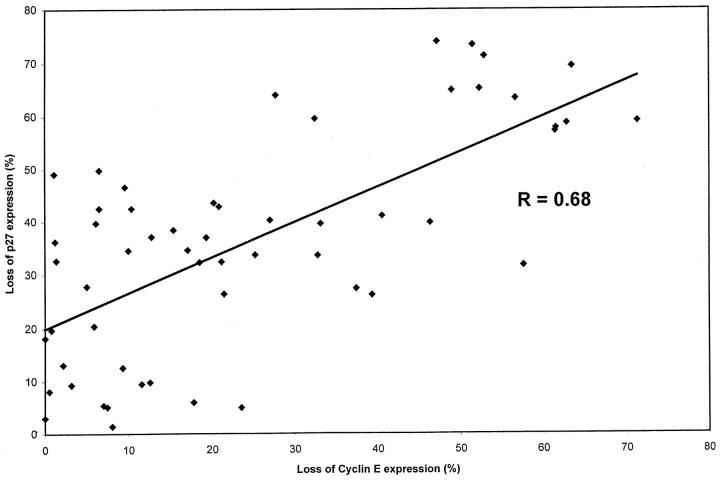
Analysis of the relationship between the expression of p27Kip1 and its cell cycle partner, cyclin E, in the same bladder tumors revealed that a direct correlation exists between loss of expression of both proteins in the same neoplastic foci; this correlation, however, is not very strong (r = 0.68; Figure 3 ▶ ).
Figure 3.
Relationship between loss of p27Kip1 protein expression and loss of cyclin E protein expression in our bladder tumor specimens. A direct correlation was detected between loss of expression of both proteins in the same neoplastic foci (correlation coefficient r = 0.68).
Figure 4 ▶ shows Kaplan-Meier plots of patient survival indicating increased mortality risk associated with low levels of p27Kip1 expression (P = 0.001) and low levels of cyclin E expression (P = 0.002) in primary bladder tumors. Stratification of survival was based on two levels of p27Kip1 and cyclin E immunoreactivity, defined as high (>30%) or low (<30%) expression. Fifty percent survival in patients with high p27Kip1 expression (n = 27) was reached at 40.4 months, compared to 13.7 months in those patients (n = 21) with low levels of p27Kip1 expression. Bladder cancer patients (n = 34) with tumors exhibiting low cyclin E expression reached 50% survival at 26.7 months. In contrast, 50% survival was not reached in those patients (n = 14) with a high level of cyclin E expression.
Figure 4.
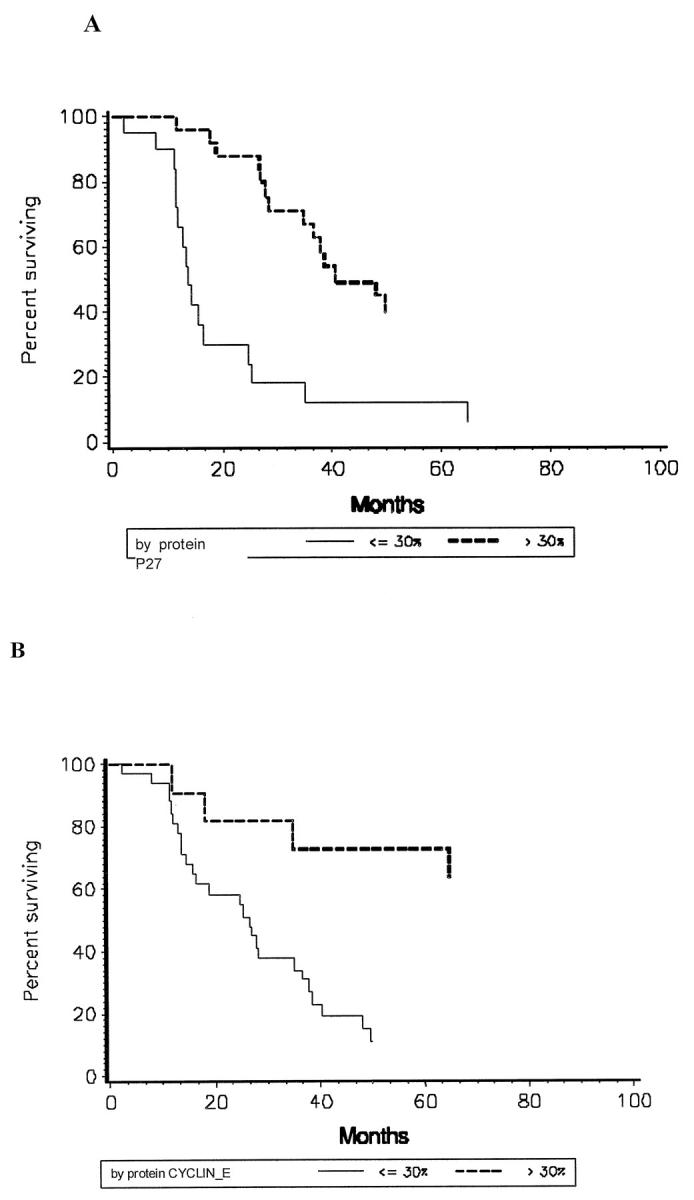
Kaplan-Meier curves for survival of bladder cancer patients (after cystectomy or transurethral resection) between tumor groups with high or low p27Kip1 protein expression (A) and high or low cyclin E protein expression (B).
Discussion
This study provides the first evidence of the predictive value of loss of two key cell cycle regulators, the CKI p27Kip1 and cyclin E, in bladder cancer progression. The expression of these two proteins in normal and malignant bladder tissue was correlated with tumor grade, pathological tumor stage, and patient survival. A significant loss of expression of both p27Kip1 and cyclin E proteins was detected in bladder cancer compared to normal bladder specimens. Significantly enough, our findings demonstrate a progressive loss of expression of each protein with increasing histological aggressiveness and pathological stage of bladder tumors. Most dramatically, loss of expression of p27Kip1 and cyclin E, as independent variables, correlated with a significantly increased mortality risk compared to those patients with bladder tumors that exhibited high expression of either protein. This positive correlation implies that with advancing tumor grade, loss of the key cell cycle regulators p27Kip1 and cyclin E may have novel prognostic value in human bladder cancer and may be used to alter treatment options offered to patients.
Abnormalities in various components of the cell cycle regulatory machinery have been previously reported in several types of human cancer. 10-25 More specifically, loss of expression of the CKI p27Kip1 has been shown to be associated with several tumor types. 13-18,20,22 Guo et al were the first to demonstrate a correlation between loss of p27Kip1 expression and increasing tumor grade in human prostate cancer. 20 Other investigators have subsequently described a significant association between loss of p27Kip1 immunoreactivity in prostate carcinoma and increased probability of recurrence and decreased survival. 18 In addition, a positive correlation between low or lost p27Kip1 expression and high-grade histology and/or a less favorable prognosis has been reported in other human malignancies, including breast, 11-14 colorectal, 15-17 esophageal, 21-22 gastric, 23 and pulmonary carcinomas. 24 This down-regulation of p27Kip1 is now hypothesized to be an essential step in the development and maintenance of the malignant phenotype in these human cancers.
In contrast, far less is known about the role of cyclin E in cell transformation and tumorigenesis. Cyclin E is a regulator cyclin of the G1-to-S phase transition of the cell cycle. p27Kip1 is able to inhibit the formation of active cyclin E/cdk2 complexes. 32 The mechanism by which p27Kip1 inhibits cdk activity is not fully understood, but it appears that binding of p27Kip1 to the cyclin D2/cdk4 complex sequesters the inhibitor away from the cyclin E/cdk2 complex, causing inactivation of the latter. This evidence suggests that the cyclin D2/cdk4 complex might act upstream of cyclin E/cdk2 by modulating the levels of free p27Kip1 that can interact with and inhibit the cyclin E/cdk2 complex. Many factors influence expression of cyclin E, including mutations in Rb or p16, overexpression of cyclin D1 or E2F, and alteration in the cyclin E proteolysis pathway. 13 Cyclin E overexpression has been associated with increasing tumor grade and patient mortality in breast cancer patients. 10,13,14
Although p27Kip1 is known to be an intimate biochemical partner of cyclin E in the cell cycle, the direct correlation between p27Kip1 levels and cyclin E expression in human cancer has yet to be defined. Our results clearly indicate that both cyclin E and p27Kip1 immunoreactivity is decreased in bladder cancer compared to normal bladder urothelium. Conceptually, cyclin E overexpression and p27Kip1 underexpression both result in increased cdk2 activity, and presumed loss of this checkpoint leads to aberrant cell proliferation. This concept is consistent with recent findings indicating that this pattern of p27Kip1 and cyclin E expression is associated with increasing tumor aggressiveness and increased patient mortality in breast cancer. 13 Furthermore, in vitro studies demonstrated that overexpression of cyclin E is associated with increased p27Kip1 expression. 28 Ectopic expression of cyclin D1 or cyclin E in several cell lines has been reported to lead to reduced levels of expression of the p27Kip1 protein. 17 On the basis of these findings, one could propose a feedback inhibitory loop between cyclin E and p27Kip1, the function of which is to maintain a homeostatic balance between the positive and negative regulators of the G1 phase of the cell cycle. Therefore, in response to loss of p27Kip1 expression during tumorigenic growth, there may be a cellular down-regulation of cyclin E in an attempt to offset the loss of the p27 checkpoint in the cell cycle. The present study strongly suggests the aggressiveness of a bladder tumor can be characterized in part by specific derangements in the cell-cycle progression machinery. Our results are also unique in that they describe a potential predictive role for these two cell cycle regulators in the progression of bladder cancer. However, knowledge of the overall expression profile of multiple proteins regulating cell cycle kinetics is needed before we fully understand the prognostic significance of the cell cycle in malignant development and tumor progression. Prospective studies that involve screening a larger number of patients with invasive disease (including those with metastatic lesions) will be required to establish the potential clinical usefulness of p27Kip1 and cyclin E as prognostic markers in bladder cancer.
The role of other cell cycle regulatory proteins in bladder cancer progression remains largely undefined. Loss of Rb immunoexpression has been considered a marker for invasive bladder cancer and has been associated with shorter patient survival. 37 Increased p53 immunoreactivity has been reported in higher grade and stage bladder cancers and has been associated with disease recurrence and decreased overall survival. 38 However, not all p53-positive bladder tumors recur or progress. The effects of p53 on the cell cycle are mediated through the regulation of p21Waf1 expression. Loss of p21Waf1 expression in p53-altered tumors has been shown to be a positive predictor of tumor recurrence and poorer patient survival than in those p53-altered tumors that have maintained p21Waf1 expression through p53-independent pathways. 39
Histologically determined tumor grade and stage are still the primary prognostic variables that dictate treatment strategies. Although these variables provide the physician with a characterization of tumor’s biological potential, a significant degree of tumor heterogeneity remains, even within various prognostic subgroups. 39 Therefore, accurate assessment of tumor aggressiveness is often a challenging task. To date, various potential bladder tumor markers have been investigated. Blood group antigens, tumor-associated antigens, proliferating antigens, oncogenes, epidermal growth factor receptor, peptide growth factors and their receptors, tumor angiogenesis and angiogenesis inhibitors, and cell adhesion molecules have been identified as potential prognostic markers for transitional cell carcinoma of the bladder. 39
In summary, the present study has identified potential new prognostic markers to provide predictive information of the natural history of bladder cancer and the likelihood of disease recurrence or progression. Our increasing understanding of the cell cycle machinery has made it apparent that altered expression of several of its components play an important role in loss of cell regulation. Therefore, the ultimate prognostic value of cell cycle regulatory proteins as tumor markers may involve the evaluation of multiple markers for each tumor specimen. Our findings support a role for p27Kip1 and cyclin E as strong candidate markers for predicting the biological potential of bladder tumors and identifying those tumors most likely to progress to muscle invasive disease.
Acknowledgments
We thank Dr. Charles Suter of the Department of Surgery for his assistance with constructing the Kaplan-Meier survival curves and Mr. Richard Milanich and Mr. Jordan Denner at Veterans Affairs Medical Media for their help in preparation of the color photography.
Footnotes
Address reprint requests to Natasha Kyprianou, Ph.D., Division of Urology, University of Maryland Medical Systems, 22 South Greene Street, Room S8D18, Baltimore, MD 21201. E-mail: nkyprianou@smail.umaryland.edu.
Supported in part by a National Institute of Diabetes and Digestive and Kidney Diseases ROI grant (DK 53525-02).
References
- 1.Messing EM, Catalona W: Urothelial tumors of the urinary tract. Campbell’s Urology, ed 7, vol 3. Edited by PC Walsh, AB Retik, ED Vaughan, AJ Wein. Philadelphia, WB Saunders, 1998, pp 2327–2410
- 2.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 3.Cordon-Cardo C: Mutations of cell cycle regulators: biological and clinical implications for human neoplasia. Am J Pathol 1995, 147:545-560 [PMC free article] [PubMed] [Google Scholar]
- 4.Del Sal G, Loda M, Pagano M: Cell cycle and cancer: critical events at the G1 restriction point. Crit Rev Oncol 1996, 7:127-142 [DOI] [PubMed] [Google Scholar]
- 5.Hartwell LH, Kastan MB: Cell cycle control and cancer. Science 1994, 266:1821-1828 [DOI] [PubMed] [Google Scholar]
- 6.Clurman B, Roberts J: Cell cycle and cancer. J Natl Cancer Inst 1995, 87:1499-1501 [DOI] [PubMed] [Google Scholar]
- 7.Weinstein IB, Zhou P: Cell cycle control gene defects and human cancer. Bertino JR eds. Encyclopedia of Cancer, 1996, vol 1.:pp 256-267 Academic Press, New York [Google Scholar]
- 8.Jacks T, Weinberg RA: The expanding role of cell cycle regulators. Science 1998, 280:1035-1036 [DOI] [PubMed] [Google Scholar]
- 9.Hussain SP, Harris CC: Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res 1998, 58:4023-4037 [PubMed] [Google Scholar]
- 10.Hall M, Peters G: Genetic alterations of cyclins, cyclin dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res 1996, 68:67-108 [DOI] [PubMed] [Google Scholar]
- 11.Oyama T, Kashiwabara K, Yoshimoto K, Arnold A, Koerner F: Frequent overexpression of the cyclin D1 oncogene in invasive lobular carcinoma of the breast. Cancer Res 1998, 58:2876-2880 [PubMed] [Google Scholar]
- 12.Sgambato A, Zhang YJ, Ciaparrone M, Soh JW, Cittadini A, Santella R, Weinstein IB: Overexpression of p27Kip1 inhibits the growth of both normal and transformed human mammary epithelial cells. Cancer Res 1998, 58:3448-3454 [PubMed] [Google Scholar]
- 13.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 14.Keyomarsi K, Conte D, Toyofuku W, Fox MP: Deregulation of cyclin E in breast cancer. Oncogene 1995, 11:941-950 [PubMed] [Google Scholar]
- 15.Thomas GV, Szigeti K, Murphy M, Draetta G, Pagano M, Loda M: Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol 1998, 153:681-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, Wang S, Weinstein IB, Holt PR: Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 1996, 110:669-674 [DOI] [PubMed] [Google Scholar]
- 17.Ciaparrone M, Yamamoto H, Yao Y, Sgambata A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB: Localization and expression of p27Kip1 in multistage colorectal carcinogenesis. Cancer Res 1998, 58:114-122 [PubMed] [Google Scholar]
- 18.Cote RJ, Shi Y, Groshen S, Feng AC, Cardon-Cardo C, Skinner D, Lieskovosky G: Association of p27Kip1 levels with recurrence and survival in patients with stage C prostate carcinoma. J Natl Cancer Inst 1998, 90:916-920 [DOI] [PubMed] [Google Scholar]
- 19.Salem CE, Tomasic NA, Elmajian DA, Esrig D, Nichols PW, Taylor CR: p53 protein and gene alterations in pathological stage C prostate carcinoma. J Urol 1997, 158:510-514 [PubMed] [Google Scholar]
- 20.Guo YP, Sklar GN, Borkowski A, Kyprianou N: Loss of the cyclin-dependent kinase inhibitor p27Kip1 protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 1997, 3:2269-2274 [PubMed] [Google Scholar]
- 21.Jiang W, Zhang YJ, Kahn SM, Hollenstein MC, Santella RM, Lu SH, Harris CC, Montesano R, Weinstein IB: Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci USA 1993, 90:9026-9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SP, Lipman J, Goldman H, Ellis FH, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagnano M, Loda M: Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res 1998, 58:1730-1735 [PubMed] [Google Scholar]
- 23.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T: p27 expression in gastric carcinoma. Nat Med 1997, 3:593-597 [DOI] [PubMed] [Google Scholar]
- 24.Esposito V, Baldi A, DeLuca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi B, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 3381–3385 [PubMed]
- 25.Del Sal G, Loda M, Pagano M: Cell cycle and cancer: critical events at the G1 restriction point. Crit Rev Oncol 1996, 7:127-142 [DOI] [PubMed] [Google Scholar]
- 26.Hunter T, Pines J: Cyclins and cancer II: cyclin D and Cdk inhibitors come of age. Cell 1994, 79:573-582 [DOI] [PubMed] [Google Scholar]
- 27.Rao P, Wilson T: G1 events, and regulation of cell proliferation. Science 1989, 246:603-608 [DOI] [PubMed] [Google Scholar]
- 28.Sgambato A, Han E, Zhou P, Schieren I, Weinstein IB: Overexpression of cyclin E in the HC11 mammary cell line is associated with growth inhibition and increased expression of p27Kip1. Cancer Res 1996, 56:1389-1399 [PubMed] [Google Scholar]
- 29.Sgambato A, Doki Y, Schieren I, Weinstein IB: Effects of cyclin E overexpression on cell growth and response to transforming growth factor β depend on cell context and p27Kip1 expression. Cell Growth Differ 1997, 8:393-405 [PubMed] [Google Scholar]
- 30.Keyomarsi K, O’Leary N, Molner G, Lees E, Fingert HJ, Pardee A: Cyclin E, a potential prognostic marker for breast cancer. Cancer Res 1994, 54:380-385 [PubMed] [Google Scholar]
- 31.Ohtsubo M, Theodoras A, Shumacher J, Roberts J, Pagano M: Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol 1995, 15:2612-2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polyak K, Kato J, Solomon J, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 33.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI: Distinct altered patterns of p27Kip1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst 1998, 90:1284-1291 [DOI] [PubMed] [Google Scholar]
- 34.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 35.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteosome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 36.Kaplan EL, Meier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958, 53:457-481 [Google Scholar]
- 37.Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fuks Z, Reuter VE: Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst 1992, 84:1251-1261 [DOI] [PubMed] [Google Scholar]
- 38.Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA, Cote RJ: Accumulation of p53 and tumor progression in bladder cancer. N Engl J Med 1994, 331:1259-1255 [DOI] [PubMed] [Google Scholar]
- 39.Stein JP, Grossfeld GD, Ginsberg DA, Esrig D, Freeman JA, Figueroa AJ, Skinner DG, Cote RJ: Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol 1998, 160:645-659 [DOI] [PubMed] [Google Scholar]