Abstract
The mechanisms of pancreatic fibrosis are poorly understood. In the liver, stellate cells play an important role in fibrogenesis. Similar cells have recently been isolated from the pancreas and are termed pancreatic stellate cells. The aim of this study was to determine whether pancreatic stellate cell activation occurs during experimental and human pancreatic fibrosis. Pancreatic fibrosis was induced in rats (n = 24) by infusion of trinitrobenzene sulfonic acid (TNBS) into the pancreatic duct. Surgical specimens were obtained from patients with chronic pancreatitis (n = 6). Pancreatic fibrosis was assessed using the Sirius Red stain and immunohistochemistry for collagen type I. Pancreatic stellate cell activation was assessed by staining for α-smooth muscle actin (αSMA), desmin, and platelet-derived growth factor receptor type β (PDGFRβ). The relationship of fibrosis to stellate cell activation was studied by staining of serial sections for αSMA, desmin, PDGFRβ, and collagen, and by dual-staining for αSMA plus either Sirius Red or in situ hybridization for procollagen α1 (I) mRNA. The cellular source of TGFβ was examined by immunohistochemistry. The histological appearances in the TNBS model resembled those found in human chronic pancreatitis. Areas of pancreatic fibrosis stained positively for Sirius Red and collagen type I. Sirius Red staining was associated with αSMA-positive cells. αSMA staining colocalized with procollagen α1 (I) mRNA expression. In the rat model, desmin staining was associated with PDGFRβ in areas of fibrosis. TGFβ was maximal in acinar cells adjacent to areas of fibrosis and spindle cells within fibrotic bands. Pancreatic stellate cell activation is associated with fibrosis in both human pancreas and in an animal model. These cells appear to play an important role in pancreatic fibrogenesis.
Progressive fibrosis is a characteristic feature of chronic pancreatitis of various etiologies. The cellular and molecular mechanisms leading to pancreatic fibrosis are poorly understood and, until recently, have received little attention.
In contrast to the pancreas, the process of fibrogenesis has been closely studied in the liver. Stellate cells (previously known as Ito cells, vitamin A storing cells, or lipocytes 1 ) are now known to play a major role in the production of hepatic fibrosis and are the major source of collagen and other extracellular matrix proteins in liver disease. 2 In the normal liver, stellate cells may be identified by the presence of fat droplets containing vitamin A 3 and by positive staining for desmin, a cytoskeletal intermediate filament protein. 4 When activated during liver injury, stellate cells undergo both morphological and functional changes. The cells enlarge, proliferate, and lose the vitamin A-containing lipid droplets. 3 Activated stellate cells exhibit positive staining for the cytoskeletal protein alpha smooth muscle actin (αSMA) and become responsive to cytokines such as platelet-derived growth factor (PDGF) 3 and transforming growth factor-β (TGFβ). 5 Expression of extracellular matrix proteins, especially collagen types I and III, 2 is increased.
Similar cells have recently been identified and isolated from the pancreas, and have been termed pancreatic stellate cells (PSCs). 6,7 In the normal rat pancreas, stellate cells stain positively for desmin but do not stain for αSMA, indicating a quiescent, nonactivated state. 6 Recent in vitro studies of cultured pancreatic stellate cells have demonstrated that these cells exhibit morphological and functional features similar to cultured hepatic stellate cells, including positive αSMA staining after a period of time in culture, increased proliferation in response to PDGF, and increased collagen synthesis in response to TGFβ. 8
The relationship of PSCs to pancreatic fibrosis in vivo remains to be established. Wells and Crawford have recently highlighted the need for morphological studies in pancreatic fibrosis to examine the role of the PSCs in fibrosis and their relationship to the profibrogenic cytokines PDGF and TGFβ. 9 Therefore, the aim of this study was to determine whether pancreatic stellate cell activation occurs during pancreatic fibrogenesis in vivo. This has been studied using an animal model of pancreatic fibrosis and the findings have been compared with those in human pancreatic fibrosis.
Methods
Animal Model
Pancreatic fibrosis was induced in male Sprague-Dawley rats by infusion of trinitrobenzene sulfonic acid (TNBS) into the pancreatic duct by a modification of the method described by Puig-Divi et al. 10 Male Sprague-Dawley rats (350–500 g, n = 24) were fed standard rodent chow and tap water ad libitum until the day before surgery. Animals were fasted overnight but given free access to water. Anesthesia was induced by inhalation of 4% halothane in 100% O2 for 10 minutes and maintained with 2% halothane. During closure of the abdominal wound, halothane was ceased and O2 was given alone. Heat loss was prevented during the operation by placing the animals on a warming tray and using a hot incandescent light, and postoperatively by using an infrared warming lamp until the animals were awake. A single subcutaneous dose of ceftriaxone 10 mg (Roche Products Pty Ltd., Dee Why, NSW, Australia) was given. The abdomen was shaved, prepared with povidone-iodine, and covered with a fenestrated sterile drape. Using sterile technique, a midline upper abdominal incision was made. The entry point of the pancreatic-biliary duct into the duodenum was identified and the duodenum was opened through a horizontal 0.5-cm antimesenteric incision. The ampulla of Vater was cannulated with polyethylene tubing (O.D. 0.62 mm; Dural Plastics, Auburn, NSW, Australia) and sutured so that the tip lay 3–5 mm within the pancreatobiliary duct. The pancreato-biliary duct was occluded with a vascular clamp at the hilum of the liver to prevent entry of the infusate into the liver. 2% TNBS (Sigma Chemical Co., St Louis, MO) solution in phosphate-buffered saline (PBS, pH 8.0) with 10% ethanol was infused for 60 minutes to a total volume of 0.4 ml (Ohmeda 9000 syringe pump). Control rats (n = 4) were infused with the same volume of 10% ethanol in PBS without TNBS. The hilar clamp was removed and, after a 5-minute washout period, the cannula was removed. The duodenotomy was closed with a single interrupted layer of absorbable sutures and the abdominal wound was closed in two layers. Bupivocaine (0.25%, 1.5 ml) was infiltrated into the abdominal wound for postoperative analgesia and normal saline (30 ml/kg body weight) given by subcutaneous injection to maintain hydration.
Postoperatively, rats were transferred to individual cages and were given O2 by mask until awake and mobile. Rats were fasted for 72 hours, but allowed free access to water after 48 hours. Hydration was maintained by twice daily subcutaneous injections of sterile fluid (60 ml/kg/day; normal saline alternating with 4% dextrose containing 0.18% saline) until the animal began drinking voluntarily. Analgesia with subcutaneous buprenorphine 0.1 ml was given when necessary for postoperative analgesia. Standard rat chow and water were freely available from the third day and weight gain was recorded weekly.
Animals were killed by carbon dioxide inhalation at 4 weeks. The pancreas was removed and portions were fixed in 10% neutral buffered formalin for histology and embedded in OCT compound for frozen sections.
Human Specimens
Paraffin-embedded surgical specimens were obtained from six patients who had undergone pancreatic resection for chronic alcoholic pancreatitis.
Histological Studies
Rat Pancreas
Paraffin sections of rat pancreatic tissue were stained by hematoxylin and eosin and for collagen using Sirius Red. 11 Immunohistochemistry was performed for collagen type I, α-smooth muscle actin (αSMA), and TGFβ using paraffin sections, and for desmin, glial fibrillary acidic protein (GFAP), and platelet-derived growth factor receptor types α and β (PDGFRα and PDGFRβ) using frozen sections. The relationship between pancreatic fibrosis and pancreatic stellate cell activation was studied in rats by staining of serial sections for αSMA, desmin, and collagen and by dual-staining of sections for αSMA plus Sirius Red for collagen protein or αSMA plus in situ hybridization for procollagen α1 (I) mRNA.
Human Pancreas
Paraffin sections obtained from human alcoholic pancreatitis were stained with hematoxylin and eosin and Sirius Red. Immunohistochemistry was performed for αSMA and for the profibrogenic cytokine transforming growth factor β (TGFβ). Serial sections were stained for αSMA and collagen and dual staining was performed as for rat tissues.
Immunohistochemistry
In preparation for immunostaining, paraffin sections of the pancreas were rehydrated and washed in TBS for 5 minutes three times, and frozen sections were fixed with acetone at 4°C for 10 minutes, air-dried for 10 minutes, and washed in TBS as above. Sections were incubated with 1% H2O2 for 30 minutes to block endogenous peroxidases and washed. To prevent nonspecific binding of antibody, sections were incubated for 30 minutes at room temperature with a blocking solution containing TBS, 1% bovine serum albumin (BSA), and 10% goat serum.
αSMA
Paraffin sections of the pancreas were prepared for immunostaining as described above and then incubated at room temperature for 30 minutes with the anti-αSMA primary antibody (mouse monoclonal antibody, clone 1A4; Sigma), diluted 1:100 in the blocking solution. After further washes, the secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG; Sigma) was applied in a dilution of 1:100 for 30 minutes at room temperature. After further washes, the color was developed using the DAKO liquid with 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate chromogen system (DAKO, Botany, Australia). Sections were counterstained with Mayer’s hematoxylin (Sigma) for 5 minutes. Sections incubated with an isotype control monoclonal IgG or without primary antibody were included in each staining experiment as negative controls.
Desmin
Immunohistochemistry for desmin was performed using frozen sections of the pancreas. The primary antibody used was a mouse monoclonal antibody (clone de-u-10; Sigma) and the same types of negative controls were used as described for αSMA above. Colour was developed using the DAKO liquid with 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate-chromogen system (DAKO, Botany, Australia) and sections were subsequently counterstained with Mayer’s hematoxylin (Sigma).
PDGFRβ
Frozen sections of the pancreas were used for PDGFRβ immunostaining. The primary anti-PDGFRβ and anti-PDGFRα antibodies were rabbit polyclonal antibodies (1:50; Santa Cruz Biotechnology, Santa Cruz, CA). The antibodies were prepared in 10% swine serum in 1% BSA and TBS and applied overnight at 4°C. The secondary antibody was alkaline phosphatase-conjugated swine anti-rabbit IgG diluted 1:100 in the appropriate blocking solution. After TBS washes, the colour was developed using the Sigma Fast Red naphthol substrate system according to the manufacturer’s instructions. Sections incubated with preimmune host serum were used as negative controls.
Collagen Type I
The collagen type I antibody was a rabbit polyclonal antibody (specific for rat collagen type I, no cross-reactivity with collagen types III or IV; Amrad Pharmacia Biotech, Victoria, Australia) which was diluted 1:50 in 10% swine serum in TBS containing 1% BSA and incubated overnight at 4°C. The secondary antibody was alkaline phosphatase-conjugated swine anti-rabbit IgG and the chromogen was the Sigma Fast Red naphthol substrate system.
TGFβ
Paraffin sections of the pancreas were subjected to antigen retrieval by heating in a microwave oven on high power for 8 minutes in 0.01 M citrate buffer (pH 6.0), then incubated with a mouse monoclonal anti-TGFβ1,2,3 primary antibody to active TGFβ (150 μg/mL; Genzyme Diagnostics, Cambridge, MA) for the cellular localization of TGFβ protein as previously described. 12 The DAKO StreptAB Complex/horseradish peroxidase kit was used as the detection system with DAB as the chromogenic substrate.
GFAP
GFAP staining was performed using a rabbit polyclonal anti-GFAP primary antibody as previously described. 6
In Situ Hybridization for Detection of Procollagen a1 (I) mRNA
In situ hybridization for procollagen α1 (I) mRNA was performed on both rat and human pancreas sections using previously described methods. 12,13 Briefly, a 1500-bp fragment of human procollagen α1 (I) cDNA was subcloned into pGEM 11Z vector and then subjected to alkaline hydrolysis to produce a 300-bp fragment for use in in situ hybridization. Digoxigenin-labeled riboprobes, for sense (control) and antisense, were produced by in vitro transcription with SP6 and T7 polymerases. In situ hybridization was performed on 5-μm rat and human paraffin-embedded pancreas sections. After hybridization, sections were washed to remove unbound probe and incubated with alkaline phosphatase conjugated anti-digoxigenin polyclonal sera (1:200) at room temperature for 2 hours. Unbound antibody was removed by washes prior to color visualization with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate in the dark at room temperature for 16 hours. Unbound complex was removed by washing and sections were subjected to immunohistochemistry for αSMA 12 to determine whether procollagen α1 (I) mRNA colocalized with αSMA-positive PSCs.
Ethics Approval
These studies were approved by the Animal Care and Ethics Committee of the University of New South Wales, Australia and the Human Research Ethics Committee of the South Eastern Sydney Area Health Service.
Results
Histopathology
TNBS-Treated Rats
All 24 rats treated with TNBS developed chronic pancreatitis with fibrosis (Figure 1, A and B ▶ , is representative of the changes seen in all animals). The lesions were focal and, as reported by Puig-Divi et al, 10 the foci varied with respect to size and number. The major histopathological features included periductal and intralobular fibrosis associated with a mononuclear inflammatory infiltrate and segmental glandular atrophy. Where periductal disease extended into pancreatic lobules, fibrosis and inflammatory cells were typically seen in the peri-acinar region (Figure 1B) ▶ . In severe cases, the enclosed acini were atrophic and surrounded by fibrotic bands. Areas of fibrosis stained strongly for the collagen stain Sirius Red (Figure 1C) ▶ .
Figure 1.
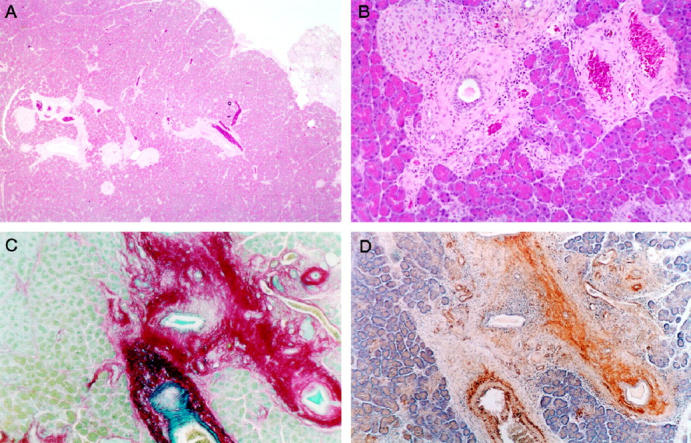
Histological appearance of experimental rat pancreatic fibrosis. A: Hematoxylin and eosin stain demonstrating focal periductal fibrosis with inflammatory infiltration. An area of lobular atrophy is seen at the right edge of the field. Original magnification, ×40. B: Higher power view of an area of focal fibrosis showing periductal changes that extend into the lobule with associated acinar atrophy. Original magnification, ×200. C: Sirius Red collagen stain demonstrating marked periductal fibrosis with focal extension of collagen fibrils into peri-acinar regions of adjacent parenchyma. Original magnification, ×100. D: Serial section of the fibrotic area shown in C stained for αSMA (brown). There is co-localization of αSMA positive cells with areas of fibrosis. Original magnification, ×100.
Human Alcoholic Pancreatitis
The histological appearance of the pancreas from these subjects was typical of the well characterized features of alcoholic pancreatitis. The main characteristics were acinar atrophy associated with intralobular, interlobular, and periductular fibrosis (Figure 2A) ▶ . Intralobular fibrosis was most evident in the peri-acinar regions and was associated with atrophy of adjacent acinar tissue. These histological features, particularly the periductular and periacinar distribution of fibrosis, resembled those found in TNBS-induced pancreatitis in rats. As with rat pancreatic fibrosis, areas of fibrosis in human tissue stained strongly for Sirius Red (Figure 2B) ▶ and also stained strongly for collagen type I by immunohistochemistry (Figure 2C) ▶ .
Figure 2.
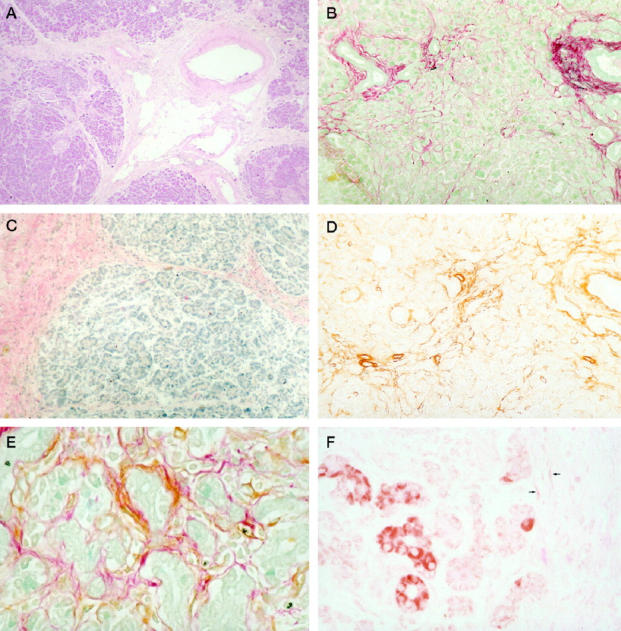
Histological appearance of human chronic pancreatitis. A: Hematoxylin and eosin stain demonstrating periductal fibrosis similar to the rat model (see Figure 1A ▶ ). Original magnification, ×100. B: Sirius Red stain shows collagen deposition in the peri-acinar areas. Original magnification, ×400. C: Immunohistochemistry demonstrating positive staining (pink) for collagen type I in bands of fibrosis. Original magnification, ×100. D: αSMA positive stellate cells are found in peri-acinar areas. Original magnification, ×100. E: Co-localization of collagen (red) and αSMA (brown) in cells. Original magnification, ×400. F: TGFβ expression (brown) in acinar cells adjacent to a fibrotic band; some weakly positive spindle-shaped cells are also observed within the fibrotic band (arrows). Original magnification, ×400.
Immunohistochemistry for Stellate Cell Markers
αSMA
αSMA has recently been shown to be an excellent marker for the activated PSC phenotype in vitro. 6 In this study, activated stellate cells were demonstrated both by the stellate morphology and by the expression of αSMA. This marker was strongly positive for stellate cells in pancreas of both rats and humans (Figures 1D and 2D) ▶ ▶ . The peri-acinar distribution of αSMA-positive PSCs is clearly demonstrated at higher magnification (Figure 2E) ▶ .
Desmin
This study confirmed our recent observations that desmin is a useful marker for stellate cells of rat pancreas both in vitro and in vivo. 6 Cells that were strongly positive for desmin were observed in fibrotic areas of rat pancreas (Figure 4B) ▶ . At higher magnification, the stellate morphology of these desmin-positive cells was evident (data not shown). In contrast to the rat pancreas, no desmin staining was observed in human pancreas, using either formalin-fixed, paraffin-embedded tissue or frozen sections of unfixed tissue.
Figure 4.
The relationship between areas of rat pancreatic fibrosis, desmin, and PDGFRβ using serial sections. A: Sirius Red collagen stain. Original magnification, ×40. B: Desmin stain of the same area showing accumulation of desmin-positive cells in fibrotic area. Original magnification, ×40. C: The fibrotic area is also PDGFRβ-positive. Original magnification, ×40.
GFAP
No staining was seen for GFAP in pancreatic fibrosis tissues. The methods used were validated by using a control positive tissue. Staining rat liver tissue for GFAP yielded the expected pattern of stellate cell staining.
Relationship between Activated Stellate Cells and Pancreatic Fibrosis
Dual staining techniques and staining of serial sections revealed a strong association between activated stellate cells and fibrosis. In the rat pancreas, Sirius Red collagen staining was closely associated with αSMA-positive cells in serial sections (Figure 1, C and D) ▶ . Desmin-positive cells were also closely associated with areas of pancreatic fibrosis in serial sections (Figure 4, A and B) ▶ . The relationship between pancreatic fibrosis and stellate cells was most clearly demonstrated in human pancreas by dual staining for collagen and αSMA (Figure 2E) ▶ .
Identification of Activated PSCs as the Cellular Source of Procollagen α1 (I) mRNA Expression
In both rat and human pancreas, blue staining for procollagen mRNA was restricted to spindle-shaped cells in areas of fibrosis (Figure 3, A–C) ▶ . No such staining was observed in acinar and ductular cells. Using the in situ hybridization technique in combination with immunostaining for αSMA, a striking colocalization of procollagen mRNA and αSMA staining could be observed in the majority of stellate cells, although some cells appeared to stain positively for only one or other of the two markers. There are two possible explanations for the latter observation:
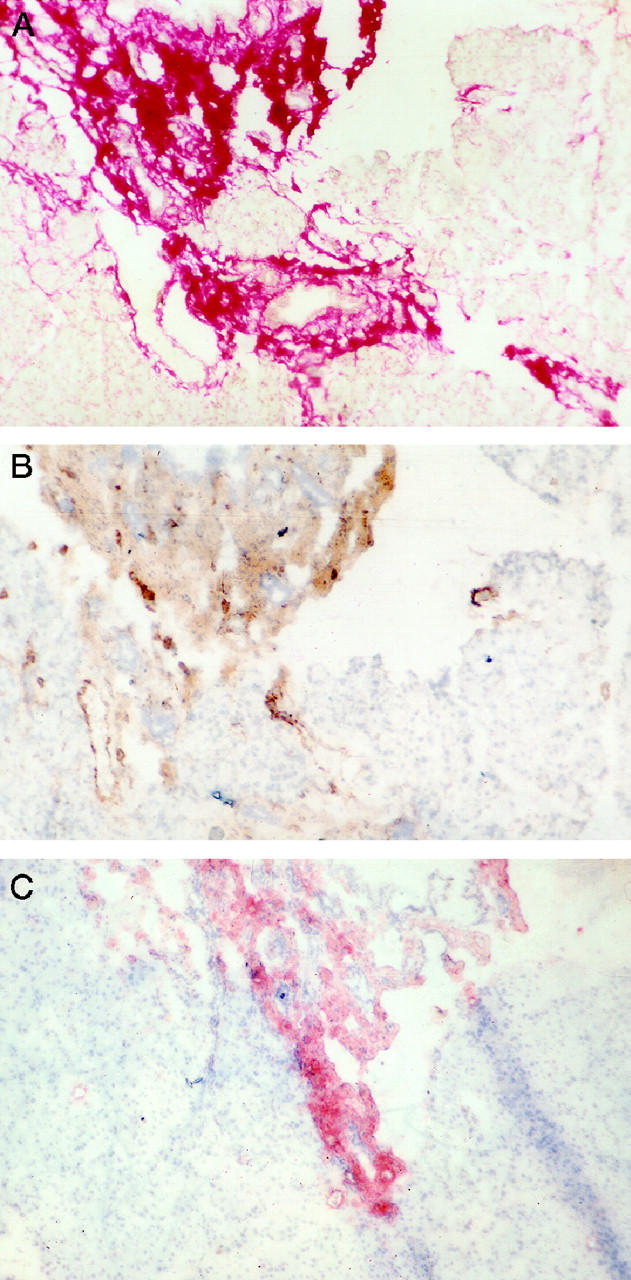
Figure 3.

Identification of activated PSCs as the predominant cellular source of collagen production in pancreatic fibrosis. These images show colocalization of αSMA (brown) and procollagen α1 (I) mRNA expression (blue) A: Rat pancreatic fibrosis. Original magnification, ×200. B. Human chronic pancreatitis showing a band of fibrosis with adjacent acinar tissue. Original magnification, ×100. C: Human chronic pancreatitis at higher magnification. Original magnification, ×400.
1) Stellate cell activation and fibrogenesis (at least in the liver, where the process has been best studied) is a dynamic process. Indeed, it is well established in hepatic stellate cells that the expression of αSMA is one of the earliest events in stellate cell activation, occurring before the deposition of collagen. 2 Thus at any point in time during fibrogenesis, there will be some stellate cells in the early phase of activation (expressing only αSMA) while others will be in the late phase of activation expressing marked levels of both αSMA and collagen mRNA. 12,14,15 It is possible that a similar dynamic process occurs during pancreatic fibrogenesis, accounting for the lack of collagen mRNA staining in some cells.
2) The star-shaped morphology of pancreatic stellate cells (a central cell body containing the nucleus and numerous cytoplasmic processes extending outward from the cell body) means that in any one plane of sectioning, there will be some cells that are represented only by their cytoplasmic processes (where αSMA staining is maximal) and others that show only the cell body and nucleus (where staining for collagen mRNA is maximal). This would explain why some cells appear to be positive for collagen mRNA and not αSMA and vice versa.
Relationship of Cytokine Staining to Fibrosis
PDGFRβ
Positive PDGFRβ staining was closely associated with areas of fibrosis in rat pancreas as evident from serial sections (Figure 4 ▶ A and C) but was absent in nonfibrotic areas of the pancreas. PDGFRβ staining was also closely associated with desmin staining in fibrotic areas (Figure 4, B and C) ▶ , suggesting that PSCs express the PGDFRβ receptor. No staining for PDGFRα was observed (data not shown) in the pancreas.
TGFβ
Significant expression of TGFβ was observed in fibrotic human pancreas, particularly within acinar cells adjacent to fibrotic bands (Figure 2F) ▶ . Acinar cells remote from fibrosis did not stain positively. In addition, there was positive, albeit weak, TGFβ staining in spindle-shaped cells within the bands of pancreatic fibrosis (Figure 2F ▶ , arrows). TGFβ expression in rat pancreas was also observed to a lesser extent.
Discussion
The major findings of this study are that PSCs are activated in both experimental and human pancreatic fibrosis and that these activated PSCs are the main cellular source of collagen in chronic pancreatitis. These results suggest that PSCs play an important role in pancreatic fibrogenesis. The present study also indicates that the cytokines PDGF and TGFβ may be involved in pancreatic fibrogenesis. A major source of TGFβ in the pancreas appears to be pancreatic acinar cells. It is possible that TGFβ released by acinar cells secondary to cell injury may be one of the predominant factors promoting a fibrotic response in pancreatic stellate cells.
Recent studies in the liver have established that stellate cells, when activated by profibrogenic mediators, play a key role in hepatic fibrogenesis by synthesizing and secreting increased amounts of extracellular matrix proteins. 2,16,17 The findings of the present study provide, for the first time, strong in vivo evidence in support of a similar process in pancreatic fibrogenesis. First, positive staining for the cytoskeletal protein desmin, a stellate cell marker, was found to be concentrated within areas of fibrosis in the pancreas, suggesting increased replication of stellate cells during fibrogenesis. Second, dual staining techniques demonstrated colocalization of αSMA staining (indicating activated stellate cells) and Sirius Red staining for collagen protein; this observation provides circumstantial evidence to support the concept that activated stellate cells may be involved in collagen production during pancreatic fibrogenesis. Third and perhaps most important, a combination of immunostaining for αSMA and in situ hybridization for procollagen mRNA (Figure 3, A–C) ▶ demonstrated conclusively that it was activated stellate cells that were the principal source of collagen production in the fibrotic pancreas.
The advantage of the rat model of pancreatic fibrosis used in this study was that a substantial degree of pancreatic fibrosis was induced within a relatively short period of 4 weeks. Furthermore, the peri-acinar pattern of fibrosis produced in this model was very similar to that seen in human chronic pancreatitis. The presence of fibrosis in the peri-acinar region is in keeping with the concept that PSCs make an important contribution to fibrogenesis, since these cells have been shown to be situated in the peri-acinar region both in rat pancreas (by desmin staining) 6 and in human pancreas (by electron microscopy). 18 Although desmin is a reliable marker of quiescent stellate cells in rat pancreas, no such immunohistochemical marker has yet been identified for quiescent human pancreatic stellate cells. Therefore, the rat model provides a useful tool for future in vivo studies of the biology of stellate cells, including characterization of the process of transformation from a quiescent to an activated state, identification of factors that may activate or inactivate these cells, and delineation of the temporal relationship between stellate cell activation and production of fibrosis in the pancreas.
Immunohistochemical studies demonstrated that the fibrotic bands in both rat and human pancreatic tissue contained type I collagen. This observation may be of particular relevance to fibrogenesis, because previous studies have reported that the change in collagen synthesis from nonfibrillar collagens to fibril-forming collagens, notably type I, is an important step in hepatic fibrosis 2 as well as in experimental and human pancreatic fibrosis. 19,20
This study also evaluated the role of two profibrogenic cytokines in pancreatic fibrosis. PDGF is a dimer of two types of peptide chains, named A and B peptides, resulting in the isoforms PDGF AA, PDGF BB, and PDGF AB. 21 The PDGF receptor is also composed of two types of peptides (α and β) assembled into a dimer (αα, αβ, or ββ). The α receptor recognizes both A and B chains of PDGF, whereas the β receptor recognizes only the B chain of PDGF. 21 PDGF BB is known to stimulate hepatic stellate cells to proliferate, resulting in increased responsiveness to other proinflammatory cytokines. 22 The proliferative effect of PDGF BB has also recently been demonstrated in an in vitro study using cultured pancreatic stellate cells. 8 The present study has demonstrated that the expression of PDGFRβ, but not PDGFRα, is closely associated with areas of pancreatic fibrosis. This finding is consistent with reports demonstrating increased expression of PDGF ligand type BB and receptor type ββ in chronic pancreatitis 23 and selective up-regulation of the type β receptor in activated hepatic stellate cells. 24
The other profibrogenic cytokine examined in this study was TGFβ. A number of studies have previously demonstrated increased expression of TGFβ in human chronic pancreatitis. 25,26 van Laethem et al have reported positive TGFβ staining of acinar cells and spindle-shaped cells within fibrotic bands. 25 In contrast, Slater reported maximal TGFβ staining in ductal and ductular epithelial cells with no staining in spindle-shaped cells. 26 Up-regulation of TGFβ mRNA levels has also been reported in caerulein-induced pancreatitis in rats. 27-29 The importance of TGFβ in pancreatic fibrosis is shown by studies in which collagen synthesis in a rat model of recurrent pancreatitis was stimulated by exogenously administered TGFβ 30 and inhibited by administration of a TGFβ-neutralizing antibody. 31 Our laboratory has recently reported that cultured PSCs respond to exogenous TGFβ with increased collagen synthesis. 8 In the present study, increased TGFβ expression was demonstrated within acinar cells adjacent to areas of fibrosis. This observation suggests that acinar cells may be a significant cellular source of TGFβ in pancreatic fibrogenesis. We propose that TGFβ is an important profibrogenic cytokine in vivo that appears to act by stimulating collagen production by pancreatic stellate cells close to TGFβ-positive acinar cells.
In conclusion, this study has provided significant in vivo evidence in support of a major role for pancreatic stellate cells in pancreatic fibrogenesis. PSCs were found to be the principal cellular source of type I collagen in pancreatic fibrosis in both humans and in an experimental animal model. A potential mechanism for the observed stellate cell activation may involve TGFβ released from injured acinar cells leading to increased expression of procollagen α1 (I) mRNA in PSCs, and ultimately increased collagen protein synthesis by these cells.
Acknowledgments
Professor Margaret Rose gave helpful advice regarding the care of experimental animals used for these studies. Mr. Steven Collins assisted with animal care. Dr. Roger Crouch and colleagues at the Department of Anatomical Pathology, Prince of Wales Hospital, Randwick, Australia, assisted with preparation of histological sections. Mr. Mike Oakey of the Medical Illustrations Unit, University of New South Wales, Sydney, Australia, assisted with preparation of the figures. AstraZeneca provided generous support toward the cost of color reproduction of photomicrographs.
Footnotes
Address reprint requests to Assoc. Prof. J.S. Wilson, Department of Gastroenterology, Prince of Wales Hospital, Randwick, NSW 2031, Australia. E-mail: js.wilson@unsw.edu.au.
Supported by research grants from the Gastroenterological Society of Australia, the National Health and Medical Research Council of Australia, the Department of Veterans’ Affairs, and the Clive and Vera Ramaciotti Foundation.
References
- 1.Ahern M, Hall P, Halliday, Liddle C, Olynyk J, Ramm G, Denk H: Hepatic stellate cell nomenclature (letter). Hepatology 1996, 23:193. [PubMed] [Google Scholar]
- 2.Friedman SD: The cellular basis of hepatic fibrosis. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL: Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis 1990, 10:20-29 [DOI] [PubMed] [Google Scholar]
- 4.Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K: Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology 1984, 4:709-714 [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Yamasaki G, Wong L: Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response: enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem 1994, 269:10551-10558 [PubMed] [Google Scholar]
- 6.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS: Periacinar stellate-shaped cells in rat pancreas: identification, isolation and culture. Gut 1998, 42:128-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G: Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115:421-432 [DOI] [PubMed] [Google Scholar]
- 8.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS: Pancreatic stellate cells are activated by proinflammatory cytokines: implications for fibrogenesis. Gut 1999, 44:534-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells RG, Crawford JM: Pancreatic stellate cells: the new stars of chronic pancreatitis? Gastroenterology 1998, 115:491-493 [DOI] [PubMed] [Google Scholar]
- 10.Puig-Divi V, Molero X, Salas A, Guarner F, Guarner L, Malagelada JR: Induction of chronic pancreatic disease by trinitrobenzene sulfonic acid infusion into rat pancreatic ducts. Pancreas 1996, 13:417-424 [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Zhao J, Lieber CS: Polyenylphosphatidylcholine attenuates non-alcoholic hepatic fibrosis and accelerates its regression. J Hepatol 1996, 24:604-613 [DOI] [PubMed] [Google Scholar]
- 12.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH: Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol 1998, 153:527-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex M, Scotting PJ: Simultaneous detection of RNA and protein in tissue sections by nonradioactive in situ hybridisation followed by immunohistochemistry. Biochemica 1994, 3:24-26 [Google Scholar]
- 14.Ooi LP, Crawford DH, Gotley DC, Clouston AD, Strong RW, Gobe GC, Halliday JW, Bridle KR, Ramm GA: Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol 1997, 26:798-807 [DOI] [PubMed] [Google Scholar]
- 15.Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW: Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol 1997, 26:584-592 [DOI] [PubMed] [Google Scholar]
- 16.Gressner AM, Bachem MG: Molecular mechanisms of liver fibrogenesis: a homage to the role of activated fat-storing cells. Digestion 1995, 56:335-346 [DOI] [PubMed] [Google Scholar]
- 17.Day CP: Is necroinflammation a prerequisite for fibrogenesis? Hepato-Gastroenterology 1996, 43:104-120 [PubMed] [Google Scholar]
- 18.Ikejiri N: The vitamin-A storing cells in the human and rat pancreas. Kurume Med J 1990, 37:67-81 [DOI] [PubMed] [Google Scholar]
- 19.Sparmann G, Merkord J, Jaschke A, Nizze H, Jonas L, Lohr M, Liebe S, Emmrich J: Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride. Gastroenterology 1997, 112:1664-1672 [DOI] [PubMed] [Google Scholar]
- 20.Vyas SK: Growth factors and cytokines in chronic pancreatitis. Johnson CD Imrie CW eds. Pancreatic Disease: Towards the Year 2000. 1999, :pp 155-165 Springer, London [Google Scholar]
- 21.Ross R: Platelet-derived growth factor. Lancet 1989, 6:1179-1182 [DOI] [PubMed] [Google Scholar]
- 22.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE: Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest 1989, 84:1786-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert M, Kasper HU, Hernberg S, Friess H, Buchler MW, Roessner A, Korc M, Malfertheiner P: Overexpression of platelet-derived growth factor (PDGF) B chain and type beta PDGF receptor in human chronic pancreatitis. Dig Dis Sci 1998, 43:567-574 [DOI] [PubMed] [Google Scholar]
- 24.Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE: Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol 1991, 260:C485-C491 [DOI] [PubMed] [Google Scholar]
- 25.Van Laethem J-L, Deviere J, Resibois A, Rickaert F, Vertongen P, Ohtani H, Cremer M, Miyazono K, Robberecht P: Localization of transforming growth factor β1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 1995, 108:1873-1881 [DOI] [PubMed] [Google Scholar]
- 26.Slater SD, Williamson RC, Foster CS: Expression of transforming growth factor-beta 1 in chronic pancreatitis. Digestion 1995, 56:237-241 [DOI] [PubMed] [Google Scholar]
- 27.Gress T, Muller-Pillasch F, Elsasser HP, Bachem M, Ferrara C, Weidenbach H, Lerch M, Adler G: Enhancement of transforming growth factor β1 expression in the rat pancreas during regeneration from cerulein-induced pancreatitis. Eur J Clin Invest 1994, 24:679-685 [DOI] [PubMed] [Google Scholar]
- 28.Riesle E, Friess H, Zhao L, Wagner M, Uhl W, Baczako K, Gold LI, Korc M, Buchler MW: Increased expression of transforming growth factor beta s after acute oedematous pancreatitis in rats suggests a role in pancreatic repair. Gut 1997, 40:73-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konturek PC, Dembinski A, Warzecha Z, Ihlm A, Ceranowicz P, Konturek SJ, Stachura J, Hahn EG: Comparison of epidermal growth factor and transforming growth factor-beta1 expression in hormone-induced acute pancreatitis in rats. Digestion 1998, 59:110-119 [DOI] [PubMed] [Google Scholar]
- 30.Van Laethem J-L, Robberecht P, Resibois A, Deviere J: Transforming growth factor β promotes development of fibrosis after repeated courses of acute pancreatitis. Gastroenterology 1996, 110:576-582 [DOI] [PubMed] [Google Scholar]
- 31.Menke A, Yamaguchi H, Gress TM, Adler G: Extracellular matrix is reduced by inhibition of transforming growth factor beta1 in pancreatitis in the rat. Gastroenterology 1997, 113:295-303 [DOI] [PubMed] [Google Scholar]


