Abstract
PEN5 is a sulfated polylactosamine carbohydrate epitope first described in a subpopulation of mature natural killer cells. Here we report that it is also expressed in a developmentally regulated fashion in human and rat central nervous systems and that its protein carrier is P-selectin glycoprotein ligand-1 (PSGL-1), a ligand for selectins. In rat neural primary cultures, PEN5 is transiently and selectively expressed by oligodendrocyte precursor cells and marks the transition from proliferative to postmitotic stages. In concordance, in human central nervous system tumors, PEN5 is observed in a subset of oligodendrogliomas and in all pilocytic astrocytomas, a class of tumor of uncertain histogenesis. These data suggest that PEN5-PSGL-1 plays a role in the differentiation of oligodendrocytes and that pilocytic astrocytomas are likely to result from a dysregulation occurring in oligodendrocyte precursor cells at the crucial stage of exit from the cell cycle.
Many developmentally important cell-cell interaction and cell differentiation processes are shared by the immune and nervous systems. Indeed, several cell surface molecules are expressed by subpopulations of both neural and immune cells. This is the case of growth factors, interleukins and their receptors, 1,2 class I MHC 3 and cell adhesion molecules. 4 For example, among the latter, the heavily glycosylated CD24 antigen or the polysialylated isoforms of NCAM/CD56 have been shown to be transiently expressed by developing neural cells 5,6 as well as by subsets of cells from the hematopoietic lineage. 7-9 Interestingly, these molecules can be considered as oncofetal antigens with diagnostic and prognostic values for neuroectoderm-derived tumors 10,11 and leukemias. 12
As for polysialic acid (PSA) on NCAM/CD565, PEN5 is a carbohydrate whose expression is developmentally regulated. 13,14 In the immune system, PEN5 is expressed onto a subpopulation of cells of the natural killer (NK) lineage. 15 Recently, its protein carrier in these cells has been identified as PSGL-1 (André P, Spertini D, Vivier E, in preparation), the best characterized selectin ligand to date. 16 PSGL-1 is required for recognition of P- and E-selectins for all classes of leukocytes 17 and is expressed as an homodimer of 120 kd. To be recognized by selectins it requires several post-translational modifications including sulfatation and modifications of O-glycans. 18-20
To define further commonalities between the immune and neural cells, we have analyzed the expression of the PEN5 epitope in developing brain and central nervous system (CNS) tumors. We observed PEN5 selective expression by a subset of human CNS tumors, the pilocytic astrocytomas, and discovered that in rat neural primary cultures it is selectively expressed on the surface of a subpopulation of oligodendrocytes precursor cells (OPCs).
During mammalian development, OPCs undergo an orderly pattern of cell proliferation and differentiation during which they change their cellular architecture dramatically, culminating in the ability of oligodendrocytes to myelinate axons. These successive stages of oligodendrocyte development have been delineated in the brain and optic nerve with surface epitopes identifying several cell phenotypes emerging in sequence. 21 OPCs are a highly mobile population of cells, they migrate long distances from proliferative zones, from which they originate on axons, which they will subsequently myelinate. For example, in rodents, the migration of OPCs through the optic chiasm into the optic nerve begins at about embryonic day 16 (E16). Despite this early appearance in the nerve, the first oligodendrocyte is not generated until one week later at about postnatal day 1 (P1) and it is not until P6 that rapid generation of oligodendrocytes begins, concurrent with the onset of myelination. 22 In human, the timing of specific CNS pathways is so precise that the age of fetuses can be determined accurately simply by assessing which pathways are myelinated. 23
These observations indicate that highly localized and precise mechanisms regulate the timing of OPCs proliferation and migration. Our present data suggest that as PSA-NCAM/CD56, PEN5-PSGL1 can be a component of the molecular machinery that regulates differentiation and/or migration of subpopulations of cells, both in the immune and nervous system.
Materials and Methods
Antibodies and Reagents
The anti-PEN5 monoclonal antibody (MAb) (5H10, mouse IgM) has been previously described. 13 The hybridoma supernatant was used for immunohistochemistry and 1:10 diluted for Western blot analyses.
The anti-PSGL1 MAb (5D8–8-12, PL2; mouse IgG) was from Immunotech (Marseilles, France) The rabbit anti-neurofilament Ab was a kind gift of Dr. Leterrier (Angers, France), and the rabbit anti-GFAP Ab was purchased from Sigma (Saint Louis, MO). The anti-KI-67 MAb was from Novocastra Laboratories (Newcastle on Tyne, UK). A2B5, O4, and GalC hybridoma cell lines were from ATTC (Rockville, MD), and ascites were prepared in nude mice. Fluorescent FITC anti-mouse IgG, anti-rabbit Ig and Cy3 anti-mouse IgM were purchased from Immunotech (Marseilles, France) and were diluted 1:200 in Dulbecco’s modified Eagle’s medium (DMEM)/fetal calf serum (FCS).
For Western blotting, rabbit anti-mouse IgM was purchased from Jackson (Immunotech) and used at 1:2000 dilution. Peroxidase-conjugated goat anti-rabbit IgG was purchased from Pierce (Beijerland, The Netherlands) and used at 1:5000 dilution.
All of the cell culture media, FCS, were purchased from Gibco BRL (Cergy-Pontoise, France).
Human Tissues
The present study was undertaken after informed consent from each patient (or their relatives). Autopsy or surgically removed control human tissues sent to the Pathology Department for diagnosis purposes were studied. These samples included brain tissues (frontal lobes) collected and pooled from two fetuses (17 and 20 weeks of gestation) obtained after spontaneous termination for Duchenne muscular dystrophy and normal peripheral nerves pooled from six normal sural nerve biopsies. All neuropathological examinations were normal. Normal adult frontal lobes were also pooled from two lobectomies for epilepsy. Neuropathological examination was normal. Forty-one surgically removed CNS tumors were also studied. In accordance with the WHO international histological classification of tumors, 24 they were classified into pilocytic astrocytomas (10 cases), glioblastomas (5 cases), oligodendrogliomas (6 cases), anaplastic oligodendrogliomas (5 cases), ependymomas (2 cases), choroid plexus papilloma (1 case), medulloblastomas (4 cases), meningiomas (4 cases), and schwannomas (4 cases). Samples from all these tissues were both flash-frozen in liquid nitrogen and stored at −80°C until use and fixed in 10% formalin and embedded in paraffin for routine histology.
Other Cells and Tissues
Embryonic (E16), neonatal, P7, and adult mouse and rat forebrains were dissected and stored at −80°C.
Cell Cultures
Primary cultures enriched in neurons were obtained from the hemispheres of embryonic day 17 or cerebellum of 5-day-old rat and Swiss mice 25 and maintained in DMEM/Ham’s F12 (3 vol/1 vol) containing 10% FCS, allowing development of glial cells.
Primary glial cultures were established from newborn rat cerebral hemispheres as described 26 with some modifications. Briefly, cells were dissociated in culture medium by passage through a 2 mm diameter needle, plated in 100-mm Falcon Petri dishes or on glass coverslips (12 mm diameter) placed in 35-mm dishes, precoated with poly(L)-lysine (Sigma) (10 μg/ml), and maintained in DMEM (Gibco BRL) supplemented with 10% FCS. After 10 days, the primary culture consists of an astroglial cell layer on which OPCs (antigenically A2B5+) are dispersed and differentiate. To obtain secondary cultures of oligodendroglial cells, OPCs present in the primary culture were detached from the astroglial underlayer by gently syringing culture medium onto the cell layer. The detached cells were sedimented by centrifugation and the cell pellet dissociated by 10 passages through a 2 mm diameter needle. The cells (3.5 × 105/ml) were replated onto poly(L)-lysine precoated glass coverslips, and maintained in DMEM containing 10% FCS for 1 hour then in defined medium without FCS.
Tissue Extracts, Western Blotting, and Immunoprecipitation
For human samples, microsomes were prepared from normal brain tissues, adult peripheral nerves and from all of the 42 CNS tumor specimens as previously described. 8 Microsomes were prepared in the same way from developing and adult mouse and rat brains. Samples were boiled for 3 minutes in reducing electrophoresis sample buffer. Proteins (20 μg) were separated on 7% sodium dodecyl sulfate (SDS)-polyacrylamide gels and blotted onto Hybond C-super nitrocellulose sheets (Amersham, Les Ulys, France). After saturation (1.5 hours) in phosphate-buffered saline (PBS) containing 3% (weight/volume) defatted milk, the sheets were treated with anti-PEN5 MAb. Incubation (12 hours at 4°C) was performed under shaking. The rinsed blots were then incubated (2 hours at room temperature) with rabbit anti-mouse IgM, rinsed, and incubated for 2 hours at room temperature with peroxidase-conjugated goat anti-rabbit IgG. The enhancement chemiluminescence system (ECL, Amersham) was used for detection of Abs.
In addition, for the preparation of embryonic human brain NP-40 lysates, microsomes were lysed in NP-40 lysis buffer. The lysates were separated on a 6% SDS-PAGE gel and probed with the anti-PSGL-1 MAb or submitted to immunoprecipitation with either the anti-PEN5 or anti-PSGL-1 mAbs as previously described. 13 The immunoprecipitate was separated on gel and revealed with anti-PSGL-1.
Immunohistochemistry
Immunoperoxidase
Three serial-frozen 5-μm-thick sections were obtained from 10 tumors (including 5 pilocytic astrocytomas, 2 oligodendrogliomas, and 3 glioblastomas). The first sections were treated with anti-PEN5 MAb and the others used as controls (omission of primary Ab and irrelevant immunoglobulin). Immunoperoxidase staining was performed using avidin-biotin peroxidase complex (ABC Kits, Vector, Burlingame, VT) as previously described. 8 Peroxidase was visualized using 3-aminoethyl carbazole as a substrate. In addition, serial sections from developing human brain (17th week of gestation) were probed with anti-PEN5, anti-PSGL-1, and anti-Ki67 mAbs, respectively.
Indirect Immunofluorescence
For the visualization of PEN5 antigen, live cells were incubated (1 hour, 25°C) with the hybridoma culture supernatant. After washing, cells were incubated (30 minutes, 25°C) with TRITC-conjugated donkey anti-mouse IgM (1:50) diluted in PBS/3% bovine serum albumin. Fixation with 4% paraformaldehyde (10 minutes) was performed after incubation with the secondary Ab. Coverslips were then mounted in Mowiol (Calbiochem).
For double detection of PEN5/GalC or Pen5/MBP the two mAbs were incubated together on live cells as above. GalC was revealed with FITC conjugated donkey anti-mouse IgG (1:300). For double detection of PEN5/GFAP or neurofilament, application of Abs was performed sequentially with PEN5 MAb applied first. The cells were then permeabilized with PBS containing 0.1% Triton X-100 (10 minutes) and incubated with rabbit anti-GFAP (1:1000) or anti-neurofilament Abs (1:1000). Fixation was then performed after incubation with the relevant secondary Abs.
Results
The PEN5 Epitope Is Present in Developing CNS
PEN5 epitope is detected in the human CNS and its expression is developmentally regulated. Western blotting of human developing brain tissue extracts separated onto 7% SDS-PAGE and probed with anti-PEN5 MAb revealed a broad and fuzzy band with an apparent molecular mass (Mr) of 250 to −180 kd (Figure 1A ▶ , lane 2). Adult human brain expressed a much lower amount of the PEN5 epitope and in such extracts the MAb revealed a more discrete band migrating at 220 kd (Figure 1A ▶ , lane 1). Importantly, the PEN5 epitope was undetectable in human peripheral nerve. Altogether, these data strongly suggest that within the nervous system, PEN5 expression is temporally regulated on cells and/or specific to cell populations mostly present during development of CNS.
Figure 1.
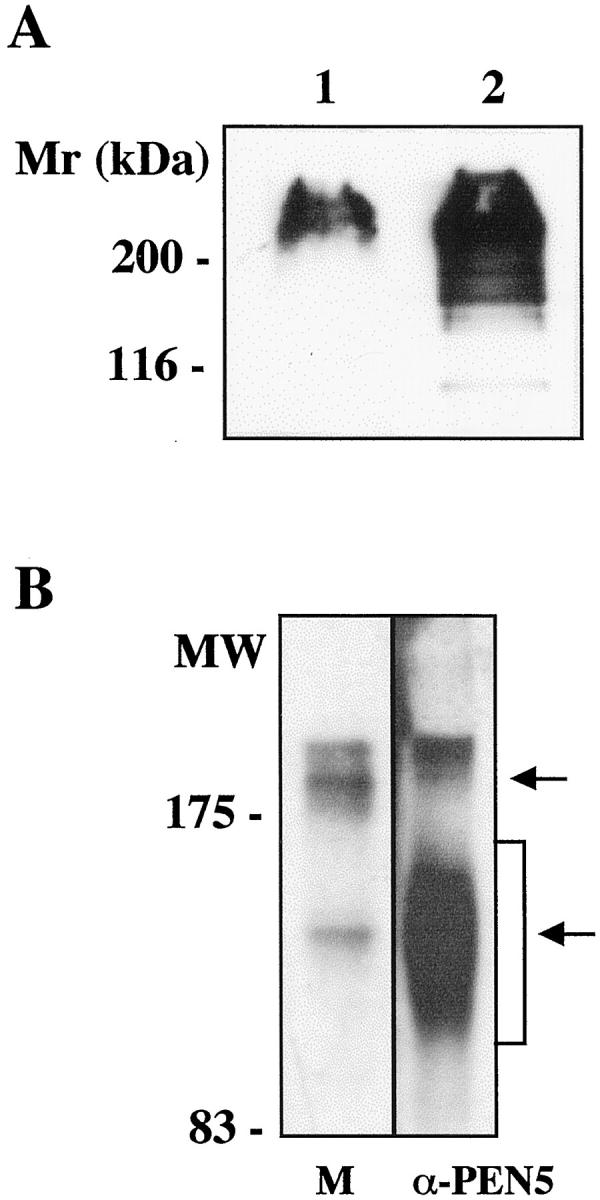
A: Western blot for PEN5 in adult (lane 1) and developing human brain (lane 2). The same amount of protein was analyzed for each sample (20 μg) and separated on 7% SDS-PAGE gel. B: NP-40 human embryonic brain lysates were separated on a 6% SDS-PAGE gel and probed with anti-PSGL-1 MAb (lane 1) or submitted to immunoprecipitation with the anti-PEN5 Ab. The immunoprecipitate was separated on gel and revealed with anti-PSGL-1 (lane 2). Note that the PEN5 immunoprecipitate countains PSGL-1 molecules found in two areas corresponding to the monomeric and dimeric forms.
PSGL-1 Is a Protein Carrier of PEN5 in CNS
We then investigated whether in human brain, as on NK cells (André P, Spertini D, Vivier E, in preparation), PEN5 can be linked to the PSGL-1 protein. NP-40 protein lysate was prepared from human embryonic brain (17th week of gestation), an aliquot was separated on 6% SDS-PAGE and Western blotted with anti-PSGL-1 MAb. The anti-PSGL-1 MAb revealed discrete bands migrating as a doublet in the 200 to 250 kd area and as a single band at 140 kd (Figure 1B ▶ , lane 1). This is in agreement with the reported migration profile of PSGL-1, which is expressed both under monomeric (140 kd) and dimeric (240 kd) forms. 27 Thus PSGL-1 is expressed in developing human CNS. To assess whether PEN5 is carried by PSGL-1, an aliquot of the extract was also submitted to immunoprecipitation with the anti-PEN5 MAb. The PEN5 immunoprecipitate was separated onto gel and subsequently probed in Western blotting with the anti-PSGL-1 MAb. Two broad bands were detected, migrating at 200 to 250 kd and 90 to 160 kd, respectively (Figure 1B ▶ , lane 2). The data unambiguously indicates that PSGL-1 is present in the PEN5 immunoprecipitate, demonstrating that in the CNS PSGL-1 is a protein carrier for PEN5. Moreover, brain extract submitted to sequential immunoprecipitation with anti-PSGL1 to totally deplete the antigen still exhibited some PEN5 immunoreactivity although at a very low level when compared to control extract (not shown). Thus, in the human developing CNS PSGL-1 appears to be a major carrier for PEN5.
By using immunohistochemistry on serial frozen sections, we observed in developing human brain at week 17 of gestation a strong PSGL-1 expression in the subventricular, intermediate zones (Figure 2C) ▶ and the cortical plate whereas PEN5 expression was observed in the intermediate zone (Figure 2, A and B) ▶ . Moreover, cells immunoreactive with anti-PEN5 MAb did not expressed Ki67 indicating that they were not in the cell cycle (Figure 2D) ▶ .
Figure 2.
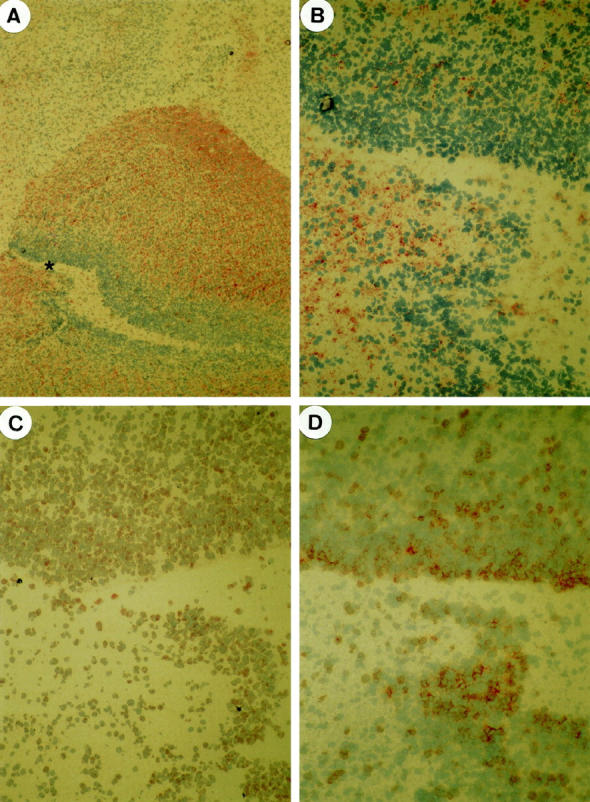
In developing human brain at week 17 of gestation, PEN5 expression is observed in the intermediate zone only (A, B). Serial sections at higher magnification of the area marked * in (A) showing diffuse PSGL-1 expression in the subventricular and intermediate zones (C) and mutually exclusive pattern of immunostaining with anti-Ki67 (D) and anti-PEN5 mAbs (B). A, ×30; B–D, ×180.
In Rat Neural Primary Cultures PEN5 Is Expressed by a Subpopulation of OPCs
The mouse anti-PEN5 IgM MAb used in this study revealed in developing rat brain extracts a band similar to that observed for human brain (not shown). We thus used rat neural cultures to identify the phenotype of cells bearing the PEN5 epitope. In primary cultures prepared from newborn hemispheres which contained a mixed population of neural cells including neurons, astrocytes, and OPCs, immunofluorescence staining with anti-PEN5 MAb revealed a population of cells with a morphology reminiscent of OPCs (Figure 3) ▶ .
Figure 3.
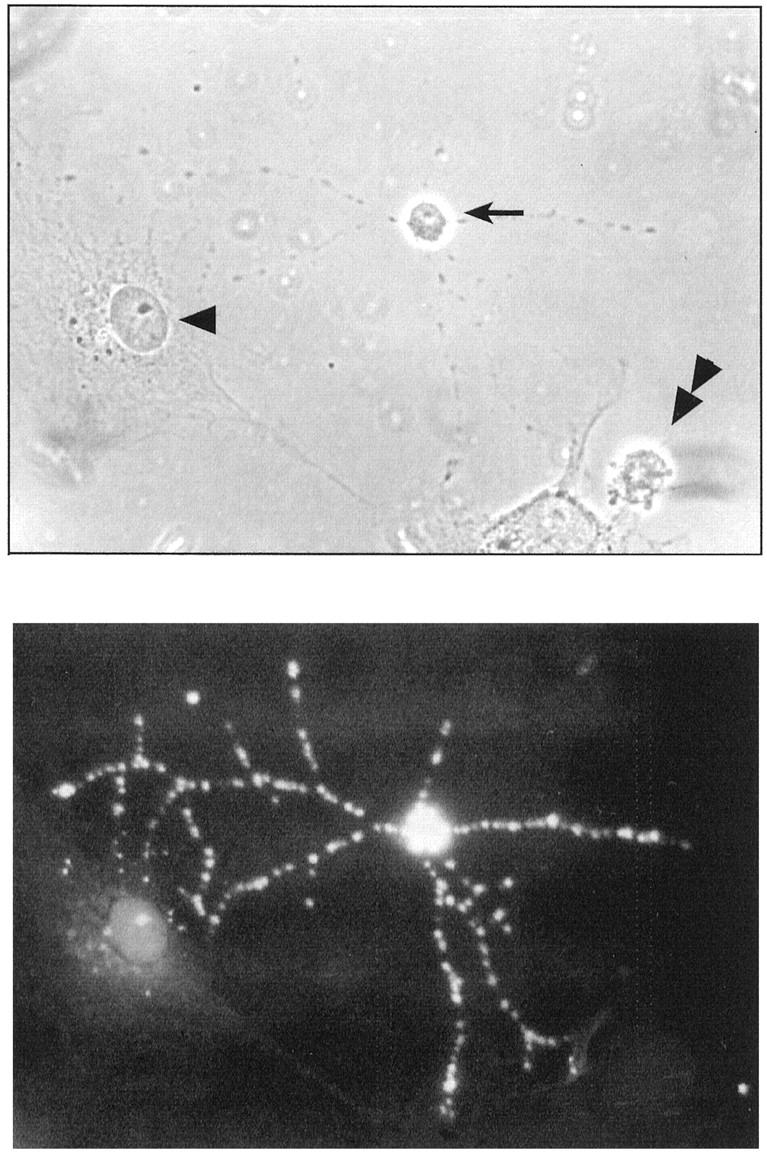
Strong PEN5 expression on the membrane of a cell with an OPC morphology (arrow) in a rat hemisphere primary culture. Note that neighboring cells with astrocyte (arrowhead) and neuronal (double arrowhead) morphologies are negative.
The labeling observed on live, unfixed cells was bright and very characteristic, with patches on the cell body and the multipolar processes (Figure 3) ▶ . To define the phenotype of this PEN5-positive cell population, we performed double labeling with cell type-specific markers. Under our culture conditions, the neurofilament-positive neurons (Figure 4C) ▶ as well as the GFAP-positive astrocytes (Figure 4F) ▶ were negative for PEN5 (Figure 4, B and E) ▶ . In some experiments, very rare cells (less than 0.5%) with a neuronal morphology were PEN5-positive (not shown). In contrast, a subpopulation of GalC-positive cells (Figure 4, G and I) ▶ was also PEN5-positive (Figure 4, G and H) ▶ . PEN5-positive as well as PEN5/GalC double-labeled cells were often found in groups, close to each other, suggesting that they may have derived from a common progenitor. These data indicate that PEN5 is transiently expressed during oligodendrocyte differentiation. To further address this issue we then prepared primary cultures of glial cells derived from newborn hemispheres. After 10 days, such cultures are composed of an underlayer of type 1 astrocytes and of smaller cells with various morphologies dispersed on top of this layer, these are developing oligodendrocytes (Figure 4J) ▶ . Different stages of differentiation can be distinguished by the expression of stage-specific markers. The ordered and partially overlapping expression of A2B5, PSA-NCAM, O4, GalC, and MBP distinguishes mainly four consecutive, phenotypically defined stages of the oligodendrocyte lineage 28 (Figure 5) ▶ . Early OPCs with a bipolar morphology are bipotential precursors of oligodendrocytes and type 2 astrocytes, they are characterized by the expression of the A2B5 ganglioside and PSA-NCAM at their cell surface. In our cultures, A2B5 and PSA-NCAM-positive cells did not appear to express PEN5 (not shown).
Figure 4.

A–C: Primary neuronal culture of rat brain hemisphere double-labeled with PEN5 (B) and neurofilament (C) which revealed neurons. D–F: Primary neuronal culture of rat cerebellum double-labeled with PEN5 (E) and GFAP (F) which revealed astrocytes. G, H: Primary neuronal culture of rat cerebellum double-labeled with PEN5 (H) and GalC (I), which reveals cells from oligodendrocyte lineage. Note that two OPCs bearing processes are co-expressing these molecules. J–L: Glial primary cultures from rat hemispheres double-labeled with PEN5 and GalC. Two process-bearing cells with an OPC morphology are expressing PEN5 (K) but not GalC (L). A, D, G, J: Phase contrasts.
Figure 5.
Schematic developmental time-course labeling in oligodendrocyte lineage cell type. Results are summarized by solid lines which designate the presence of each marker listed on the left for each stage shown. Our study showed that PEN5 labeling is evident in OPCs after the appearance of the epitopes recognized by A2B5 and PSA-NCAM but before GalC, although they can be transiently co-expressed. GalC, galactocerebroside C; MBP, myelin basic protein.
In addition, some (less than 1%) of GFAP-positive cells had a stellate appearance corresponding to type 2 astrocytes and were also PEN5 negative (not shown). As O4, the PEN5 MAb stained OPCs which have developed multipolar processes but appear still immature (Figure 4K) ▶ . Although it was impossible to precisely determine the onset of the expression of PEN5, double labeling with anti-GalC/PEN5 mAbs indicates that PEN5 epitope is expressed somewhat earlier than GalC (Figure 4, J and L) ▶ , since double-labeled cells were more numerous in older cultures (10% versus 30%) when staining was performed on day 10 and 12 in vitro, respectively. It also appeared that GalC expression was maintained whereas PEN5 expression was transient. Our data are schematized in Figure 5 ▶ .
The PEN5 Epitope Is Expressed by a Subset of CNS Tumors
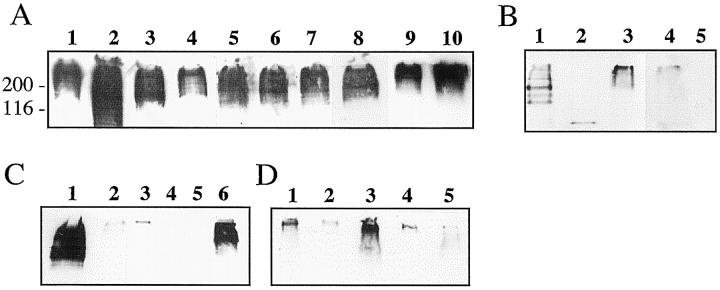
These data prompted us to search for PEN5 expression in glial tumors. Particular attention was paid to oligodendroglioma and to pilocytic astrocytomas, a highly heterogeneous tumor exhibiting features of astrocytic cells together with round cells with a morphology reminiscent of oligodendrocytes. In addition, pattern of PEN5 expression was analyzed in other CNS tumors including medulloblastomas, ependymal and choroid plexus tumors, meningiomas, and schwannomas. Western blotting analyses indicated that 10 of the 10 pilocytic astrocytomas examined strongly expressed the PEN5 epitope (Figure 6A) ▶ . Molecular weight of the revealed band was similar to that observed in embryonic human CNS (Figure 1A) ▶ . Two of six oligodendrogliomas (Figure 6C) ▶ and one of five anaplastic oligodendrogliomas (Figure 6D) ▶ tested also expressed PEN5, with a low immunoreactivity for the later. In contrast, glioblastomas (Figure 6B) ▶ and all of the other types of CNS tumors tested were almost negative. Immunohistochemistry performed on tissue sections of the tumors confirmed these data (Figure 7) ▶ . All pilocytic astrocytomas (Figure 7A) ▶ and some oligodendrogliomas expressed PEN5 (Figure 7B) ▶ . For these tumors, all tumor cells were diffusely stained on their cell surface. In contrast, PEN5 expression was absent in glioblastomas (Figure 7C) ▶ .
Figure 6.
A: strong PEN5 expression in all pilocytic astrocytomas (lanes 1–10 ). PEN5 is absent or very weakly revealed in glioblastomas (B, lanes 1–5 ). Two of six oligodendrogliomas strongly express PEN5 (C, lane 1 and 6), whereas one anaplastic oligodendroglioma (D, lane 3) expresses a moderate amount of PEN5.
Figure 7.

Strong PEN5 expression in a pilocytic astrocytoma (A) and in an oligodendroglioma (B). Glioblastoma cells are negative (C). Note that vessels are negative (arrows). A–C, ×300
Discussion
In this report, we show that PEN5 epitope is present in the developing mammalian CNS and that in rat neural cultures it is expressed by OPCs, a transient and highly mobile cell population in developing brain. These results extend the number of molecules which are shared by the immune and nervous systems. We showed for the first time that PSGL-1 is a carrier for the PEN5 carbohydrate epitope in human CNS. We also observed that all pilocytic astrocytomas and some oligodendrogliomas expressed PEN5.
This is in agreement with the fact that tumors often re-expressed carbohydrate antigens transiently expressed during the development of cells from which they derive. 10
PEN5 is a carbohydrate whose expression in the immune system is differently regulated from its protein carrier PSGL-1. Our immunohistochemical data (Figure 2) ▶ indicate that it is also the case in developing human brain. Similarly, PSA, which we extensively studied previously, is transiently carried by the adhesion molecule NCAM/CD56. In mammals, NCAM/CD56 is the only protein carrier for PSA. 5,29 From our present study, we cannot exclude that PEN5 can be carried by other protein backbones and this issue deserves to be further investigated.
In the immune system, PEN5 is selectively expressed onto NK cells and more precisely within the CD56dim/CD16+ population. In rat glial cultures, PEN5 expression is transient and specific for a subset of OPCs.
The appearance of PEN5 allows a specific step in OPCs differentiation in vitro to be distinguished, defining a distinct transient intermediate stage between the A2B5+/GalC− and A2B5−/GalC+ in which the expression of A2B5 has ended but the expression of GalC has not yet begun. Cells with the phenotype A2B5+/GalC− are proliferative and respond to mitogenic signals, 28 they retain some developmental plasticity, as shown by their ability to differentiate into either oligodendrocytes or stellate type 2 astrocytes on culture conditions. 21 In contrast, those with A2B5−/GalC+ phenotype are postmitotic and committed to an oligodendrocyte phenotype. PEN5 expression appears to coincide with the withdrawal of OPCs from the cell cycle. This growth arrest state is a very critical state which also results in changes of expression of many gene products. A recent study 30 reports that the inability of OPCs to differentiate in the cyclin inhibitor p27 knockout mice correlates with their continued proliferation. Further studies should allow precision as to whether PEN5 expression precedes or is concomitant with cycle withdrawal and how it could be related to this complex process.
The persistence of some PEN5 expression in adult human brain as shown by Western blotting is in agreement with studies showing that a few number of OPCs is still present in adult CNS. 31 Indeed, all OPCs do not differentiate, preserving a reservoir of precursor cells that might be able to generate new oligodendrocytes after injury or disease. 32 It will be interesting to examine PEN5 expression under such pathological conditions.
OPCs must be able to respond and to interact with their environment. From our data on PEN5 expression, we can infer that this determinant can mediate either negative or positive signal. In vitro observations indicated that OPCs differentiate into oligodendrocytes in the absence of inducing signals on withdrawal from the cell cycle. 33 PEN5 can be part of the molecular machinery preventing OPCs from responding to environmental signals that affect their ability to divide. By analogy, PSA determinant on NCAM/CD56 can possibly affect the effect of growth factors acting on neuronal differentiation. 6 Alternatively, it could play a role as a positive signal regulating migration.
OPCs migrate long distances from their germinal zones to enter white matter throughout the CNS. PEN5 might be crucial for ensuring that they successfully make it to their final destination. Recent studies on the optic nerve have shown that OPCs migrate along axons. 34 If, in the CNS, PSGL-1, which is highly expressed, also functions as an adhesion molecule, 35 the PEN5 determinant might be part of a selective recognition process guiding migrating OPCs along axons. Future work will focus on the various function of this determinant which might be critical to ensure that the process of myelination occurs correctly.
In a large population of tumors classified as oligodendrogliomas, PEN5 expression is observed in a fraction of them (Figure 6) ▶ . A simple explanation is that tumors can develop at different stages of the oligodendrocyte lineage, some of them outside of the time window of PEN5 expression. It is conceivable that oncogenic processes particularly occur in cells at stages when they withdraw from the cell cycle. These processes are complex and involve regulatory networks coordinating the expression and activity of several gene products such as cyclin components and their regulators. 36 It is therefore not surprising to find tumors expressing antigens, characteristic of this stage. Pilocytic astrocytomas, a class of tumor so far poorly defined, expressed PEN5 in all of the tested samples. We propose that these tumors result from a dysregulation occurring at a stage corresponding to the transition of OPCs from mitotic to nonmitotic states. To our knowledge, at present they are no markers to define these tumors, which in some instances are mistaken for malignant glioblastomas, leading to overgrading and inaccurate chemotherapy in infants. 24 The availability of the PEN5 marker should help their diagnosis.
Acknowledgments
We thank neurosurgeons (Drs. F. Grisoli, J.C. Beragot, and M. Choux) for providing tumor samples.
Footnotes
Address reprint requests to G. Rougon, Laboratoire de Génétique et de Physiologie du Développement, UMR 6545 CNRS, IBDM, Case 907 Parc Scientifique de Luminy, 13288 Marseilles, France. E-mail: rougon@ibdm.univ-mrs.fr.
Supported by institutional grants from CNRS and INSERM (GR, EV), by the Programme Hospitalier de Recherche Clinique and the GEFLUC (DFB) and the Association de la Recherche contre le Cancer (GR). PA was supported by Zeneca.
References
- 1.Straub RH, Westermann J, Scholmerich J, Falk W: Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today 1998, 19:409-413 [DOI] [PubMed] [Google Scholar]
- 2.Jiang CL, Lu CL: Interleukin-2 and its effects in the central nervous system. Biol Signals Recept 1998, 7:148-156 [DOI] [PubMed] [Google Scholar]
- 3.Corriveau RA, Huh GS, Shatz CJ: Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 1998, 21:505-520 [DOI] [PubMed] [Google Scholar]
- 4.Di Sciullo G, Donahue T, Schachner M, Bogen SA: L1 antibodies block lymph node fibroblastic reticular matrix remodeling in vivo. J Exp Med 1998, 187:1953-1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rougon G, Dubois C, Buckley N, Magnani J, Zollinger W: A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult NCAM. J Cell Biol 1986, 103:2429-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss JZ, Rougon G: Cell biology of polysialic acid. Curr Opin Neurobiol 1997, 7:640-646 [DOI] [PubMed] [Google Scholar]
- 7.Rougon G, Alterman LA, Dennis K, Guo XJ, Kinnon C: The murine heat-stable antigen: a differentiation antigen expressed in both the hemato-lymphoid and neural cell lineages. Eur J Immunol 1991, 21:1397-1402 [DOI] [PubMed] [Google Scholar]
- 8.Figarella-Branger D, Moreau H, Pellissier JF, Bianco N, Rougon G: CD24, a signal-transducing molecule expressed on human B lymphocytes, is a marker for human regenerating muscle. Acta Neuropathol 1993, 86:275-284 [DOI] [PubMed] [Google Scholar]
- 9.Robertson MJ, Ritz J: Biology and clinical relevance of human natural killer cells. Blood 1990, 12:2421-2438 [PubMed] [Google Scholar]
- 10.Figarella-Branger D, Durbec C, Rougon G: Differential spectrum of expression of NCAM isoforms and L1 adhesion molecules on human neuroectodermal tumors. Cancer Res 1990, 50:6364-6370 [PubMed] [Google Scholar]
- 11.Figarella-Branger D, Dubois C, Chauvin P, De Victor B, Gentet JC, Rougon G: Correlating PSA-NCAM levels in the cerebrospinal fluid with medulloblastoma outcomes. J Clin Oncol 1996, 14:2066-2072 [DOI] [PubMed] [Google Scholar]
- 12.Kimura N, Yoshida T, Nagano M, Tamura K: Importance of T-cell receptor delta-chain gene analysis on CD7+ and CD56+ myeloid/natural killer cell precursor acute leukemia. Blood 1998, 91:2622-2624 [PubMed] [Google Scholar]
- 13.Vivier E, Sorell JM, Ackerly M, Robertson MJ, Rasmussen RA, Levine H, Anderson P: Developmental regulation of a mucinlike glycoprotein selectively expressed on natural killer cells. J Exp Med 1993, 178:2023-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivier E, Munroe M, Ariniello P, Anderson P: Identification of tissue-infiltrating lymphocytes expressing PEN5, a mucin-like glycoprotein selectively expressed on natural killer cells. Am J Pathol 1995, 146:409-418 [PMC free article] [PubMed] [Google Scholar]
- 15.Calatayud S, Vivier E, Bernaud J, Merieux Y, Rigal D: Expression of a NK cell restricted epitope on decidual large granular lymphocytes. Int Immunol 1996, 8:1637-1642 [DOI] [PubMed] [Google Scholar]
- 16.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KB, Ahern TJ, Furie B, Cumming DA, Larsen GR: Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 1993, 75:1179-1186 [DOI] [PubMed] [Google Scholar]
- 17.Snapp KR, Wagers AJ, Craig R, Stoolman LM, Kansas GS: P-selectin glycoprotein ligand-1 (PSGL-1) is essential for adhesion to P-selectin but not E-selectin in stably transfected hematopoietic cell lines. Blood 1996, 89:896-901 [PubMed] [Google Scholar]
- 18.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD: A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell 1995, 83:323-331 [DOI] [PubMed] [Google Scholar]
- 19.Wilkins PP, Moore KL, McEver RP, Cummings RD: Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem 1995, 270:22677-22680 [DOI] [PubMed] [Google Scholar]
- 20.McEver RP: Leukocyte adhesion through selectins under flow. Immunologist 1998, 6:61-67 [Google Scholar]
- 21.Temple S, Raff MC: Differentiation of a bipotential glial progenitor cell in a single cell microculture. Nature 1985, 313:223-225 [DOI] [PubMed] [Google Scholar]
- 22.Barres BA, Raff MC: Control of oligodendrocyte number in the developing rat optic nerve. Neuron 1994, 12:935-942 [DOI] [PubMed] [Google Scholar]
- 23.Friede RL: Dating the development of human cerebellum. Acta Neuropathol 1973, 23:48-58 [DOI] [PubMed] [Google Scholar]
- 24.Kleihues P, Burger PC, Scheithauer BW: Histological Typing of Tumours of the Central Nervous System. World Health Organization International Histological Classification of Tumours, Ed 2. 1993, Springer, Berlin-Heidelberg-New York
- 25.Buttiglione M, Revest JM, Rougon G, Faivre-Sarrailh C: F3 neuronal adhesion molecule controls outgrowth, and fasciculation of cerebellar granule cell neurites: a cell type-specific effect mediated by the Ig-like domains. Mol Cell Neurosci 1996, 8:53-69 [DOI] [PubMed] [Google Scholar]
- 26.Deloume JC, Laeng P, Jauet T, Sensenbrenner M, Baudier J: Expression of neuromodulin (GAP 43) and its regulation by β-FGF during the differentiation of O-2A progenitor cells. J Neurosci Res 1993, 36:147-162 [DOI] [PubMed] [Google Scholar]
- 27.Snapp KR, Craig R, Herron M, Nelson RD, Stoolman LM, Kansas GS: Dimerization of P-selectin glycoprotein ligand-1 (PSGL-1) required for optimal recognition of P-selectin. J Cell Biol 1998, 142:263-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gard AL, Pfeiffer SE: Two proliferative stages of the oligodendrocyte lineage (A2B5+O4- and O4+GalC-) under different mitogenic control. Neuron 1990, 5:615-625 [DOI] [PubMed] [Google Scholar]
- 29.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff P, Barthels D, Rajewsky K, Wille W: Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994, 367:455-459 [DOI] [PubMed] [Google Scholar]
- 30.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich VJ, Chao MV, Koff A: Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev 1997, 11:2335-2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME: Pre-oligodendrocytes from adult human CNS. J Neurosci 1992, 12:1538-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Marinovich A, Barres BA: Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci 1998, 1:4627-4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barres BA, Hart IK, Coles HS, Burnes JF, Voyvodic JT, Richardson WD, Raff MC: Cell death and control of cell survival in the oligodendrocyte lineage. Cell 1992, 70:31-46 [DOI] [PubMed] [Google Scholar]
- 34.Ono K, Yasui Y, Rutishauser U, Miller RH: Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron 1997, 19:283-292 [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Galipeau J, Kozak CA, Furie BC, Furie B: Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization and expression of a functional P-selectin receptor. Blood 1996, 87:4176-4186 [PubMed] [Google Scholar]
- 36.Russo AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP: Structural basis for inhibition of the cyclin-dependent kinase of cdK 6 by the tumour suppressor p16INK4a. Nature 1998, 395:237-243 [DOI] [PubMed] [Google Scholar]