Abstract
Megalin is an endocytic receptor expressed on the luminal surface of the renal proximal tubules. The receptor is believed to play an important role in the tubular uptake of macromolecules filtered through the glomerulus. To elucidate the role of megalin in vivo and to identify its endogenous ligands, we analyzed the proximal tubular function in mice genetically deficient for the receptor. We demonstrate that megalin-deficient mice exhibit a tubular resorption deficiency and excrete low molecular weight plasma proteins in the urine (low molecular weight proteinuria). Proteins excreted include small plasma proteins that carry lipophilic compounds including vitamin D-binding protein, retinol-binding protein, α1-microglobulin and odorant-binding protein. Megalin binds these proteins and mediates their cellular uptake. Urinary loss of carrier proteins in megalin-deficient mice results in concomitant loss of lipophilic vitamins bound to the carriers. Similar to megalin knockout mice, patients with low molecular weight proteinuria as in Fanconi syndrome are also shown to excrete vitamin/carrier complexes. Thus, these results identify a crucial role of the proximal tubule in retrieval of filtered vitamin/carrier complexes and the central role played by megalin in this process.
The proximal tubules in the kidney are responsible for the retrieval of solutes that have been filtered through the glomerulus. This reuptake pathway selectively captures essential low molecular weight metabolites which pass freely through the glomerular membranes and which would otherwise be lost in the urine. Besides water, the main blood constituents taken up in the proximal tubules are ions, glucose, and amino acids. In addition, small plasma proteins (<70 kd) are also filtered through the glomerulus and reabsorbed in the proximal tubules. These include plasma carrier proteins (eg, retinol-binding protein), peptide hormones (eg, insulin, parathyroid hormone), and lysozyme. 1,2 The tubular uptake of water, electrolytes, and metabolites plays a central role in the regulation of volume and composition of body fluids. The reasons for uptake of filtered plasma proteins are less obvious as these proteins are not transferred back into the circulation but degraded in lysosomes. 3
The cell type mainly responsible for the uptake of filtered macromolecules are the epithelial cells of the proximal convoluted tubules. These cells exhibit a number of structural features indicative of a tissue involved in resorption processes. 4 Their apical (luminal) plasma membranes form extensive microvilli creating a brush border surface. This enlargement of the apical membranes facilitates endocytosis of macromolecules from the lumen of the tubules via clathrin-coated pits. Furthermore, the cytoplasm of the cells is highly enriched in components of the endocytic apparatus including endosomes, lysosomes, and dense apical tubules (recycling membrane vesicles). Endosomes and lysosomes contain proteins absorbed from the glomerular filtrate. 5
The physiological importance of tubular uptake processes is underscored by the pathological changes observed in patients with a resorption deficiency of the proximal tubules. This defect is referred to as the Fanconi syndrome (Lignac-de Toni-Debré-Fanconi syndrome). 6-8 In adults, the most frequent cause of Fanconi syndrome is a myeloma-like proliferation of plasma cells secreting immunoglobulin light chains of peculiar toxicity for the proximal tubule epithelium. 6 In addition, Fanconi syndrome is acquired by toxification of the proximal tubules by heavy metals and certain therapeutics such as aminoglycosides, anti-tumor drugs (ifosfamide), and anticonvulsants (valproate). 6 As a consequence of tubular dysfunction, essential metabolites and proteins cannot be retrieved from the glomerular filtrate but are excreted in the urine instead. One of the earliest symptoms of acquired Fanconi syndrome is the urinary loss of small plasma proteins. Marker proteins of such low molecular weight (LMW) proteinuria are retinol-binding protein, β2-microglobulin, and lysozyme. 9 After prolonged exposure to nephrotoxic compounds, patients also excrete serum potassium and phosphorus, urate, glucose, and amino acids. As a consequence of urinary loss of these solutes, individuals suffer from polyuria, hypokalemia, acidosis, impaired growth, and osteomalacia. 6,8
A receptor that might play an important role in uptake processes in the proximal tubules is megalin, also known as gp330 or LRP2. 10 Megalin is a 600-kd endocytic receptor and one of the most abundant membrane proteins on the brush border surface of the proximal tubular epithelium. 4 This receptor binds several proteins known to be reabsorbed in the proximal tubules (eg, insulin, β2-glycoprotein, retinol-binding protein). 5,11,12 Therefore, it was postulated that megalin might be involved in the clearance of filtered plasma proteins. Alternatively, a role for the receptor in tubular uptake of calcium has been suggested. 13,14
To reveal the significance of megalin for tubular uptake processes in vivo, we recently have generated mice carrying a disruption of the receptor genes. Unexpectedly, megalin-deficient mice exhibit severe malformations of the forebrain, and most of them die within minutes after birth. 15 These findings emphasize the importance of the receptor for development of the central nervous system. However, the exact role of megalin in embryonic development remains to be elucidated. Fortunately, the severity of forebrain malformations in megalin knockout mice is variable and approximately 2% of the animals survive to adulthood. 5 These mice enabled us to study the consequence of receptor deficiency for renal function.
Our in vivo studies confirm that megalin is the major endocytic receptor for the tubular retrieval of filtered plasma proteins. In particular, it mediates the uptake of small plasma carrier proteins including vitamin D-binding protein, retinol-binding protein, α1-microglobulin, and odorant-binding protein. Absence of the receptor in megalin knockout mice results in low molecular weight proteinuria and urinary loss of lipophilic vitamins bound to the carrier proteins.
Materials and Methods
Materials
Midstream urine samples were obtained from five patients with myeloma-associated Fanconi syndrome. These patients have been described previously. 16 Antisera against human urinary proteins were purchased from DAKO (Copenhagen, Denmark) or The Binding Site (Birmingham, AL). Purified lysozyme and β2-microglobulin were purchased from Sigma (Deisenhofen, Germany) or Calbiochem (Bad Soden, Germany), respectively. Vitamin D-binding protein and retinol-binding protein were purified from human serum as published. 17,18
Immunohistochemistry
Wild-type and megalin-deficient mice were perfusion-fixed through the heart with 4% paraformaldehyde in 0.1 mol/L sodium cacodylated buffer. The tissues were trimmed into smaller blocks, further fixed by immersion for 1 hour in 1% glutaraldehyde, and processed for conventional Epon embedding (for electron microscopy). Alternatively, the tissue blocks were immersion-fixed for 1 hour in 1% paraformaldehyde, infiltrated with 2.3 mol/L sucrose containing 2% paraformaldehyde for 30 minutes, and frozen in liquid nitrogen. For immunolight microscopy, 0.8-μm cryosections were incubated with rabbit anti-human lysozyme antibody (DAKO, 1:1000 dilution) for 1 hour at room temperature, followed by peroxidase-conjugated anti-rabbit IgG (DAKO). Bound IgG was visualized with diaminobenzidine. The sections were counterstained with Meier’s stain for 2 minutes. For immunoelectron microscopy, ultrathin kidney sections (70–90 nm) were incubated sequentially with sheep anti-rat megalin antibody (kindly provided by P. Verroust, Hôpital Tenon, Paris; 1:10 6 dilution), with rabbit anti-sheep IgG (1:20,000 dilution) and with 10 nm goat anti-rabbit gold particles (BioCell, Cardiff, UK).
One- and Two-Dimensional Polyacrylamide Gel Electrophoresis
For one-dimensional SDS polyacrylamide gel electrophoresis (SDS-PAGE), mouse urine samples were separated on 4 to 15% nonreducing polyacrylamide gels. Where indicated, the separated proteins were transferred to nitrocellulose filters and incubated with primary followed by peroxidase-coupled secondary antibodies as described. 17 Bound IgG was detected by the enhanced chemiluminescence system (ECL, Amersham, Braunschweig, Germany). For two-dimensional PAGE, 600 μg of mouse urinary proteins were separated in the first dimension on a nonlinear pH 3 to pH 10 gradient (Immobiline Dry Strip, Pharmacia, Uppsala, Sweden), and in the second dimension on a 15% SDS polyacrylamide gel as published. 19
Edman Sequencing
Coomassie Blue-stained protein bands were cut from the gels and digested in the gel with sequencing-grade modified trypsin (Promega). 20 Tryptic peptide maps were obtained by reverse-phase high performance liquid chromatography on a μRPC C2/C18 SC2.1/10 column using the Smart system (Pharmacia Biotech). The flow rate was 100 μl/minute at 25°C employing a linear gradient of acetonitrile in 0.1% trifluoroacetic acid. Peptides of interest were loaded onto a Biobrene-coated glass filter fiber of a Procise sequencer (Perkin Elmer/Applied Biosystems), and sequenced according to standard protocols.
Receptor Binding Assay
Megalin was purified by receptor-associated protein (RAP) affinity chromatography from rabbit renal cortex according to standard protocols. The preparation was essentially pure as seen by SDS-PAGE. No contaminations with other receptors of the low density lipoprotein receptor gene family or with the RAP were detected by Western blot analysis (not shown). For megalin affinity chromatography, 4.5 mg of purified rabbit megalin were coupled to 200 mg CNBr-activated Sepharose 4B (Pharmacia). Two milliliters of mouse urine were dialyzed against 10 mmol/L Tris-HCl, pH 7.5, lyophilized, and resuspended in 200 μl of H2O. Then, 100 μl of the concentrated urine sample was radiolabeled with 125I using the IODOGEN method 21 and circulated over the megalin Sepharose column for 16 hours at 4°C in incubation buffer (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 2 mmol/L CaCl2, 2 mmol/L MgCl2, pH 7.6). The resin was washed with 20 ml of incubation buffer and specifically bound proteins were eluted in 0.1 mol/L glycin, 10 mmol/L EDTA, pH 2.8. Binding of ligands to megalin was quantified by BIAcore analysis (Biosensor, Sweden) as described previously. 5 A continuous flow of HBS buffer (10 mmol/L HEPES, 3.4 mmol/L EDTA, 150 mmol/L NaCl, 0.005% surfactant P20, pH 7.4) passing over the sensor surface was maintained at 5 μl/minute. The carboxylated dextran matrix of the sensor chip flow cell was activated by injection of 60 μl of a solution containing 0.2 mol/L N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide and 0.05 mol/L N-hydroxysuccimide in H2O. Then, 180 μl of 10 mmol/L sodium acetate, pH 4.5, containing 10 μg/ml purified rabbit megalin was injected. The remaining binding sites were blocked by subsequent injection of 35 μl of 1 mol/L ethanolamine, pH 8.5. The surface plasmon resonance signal from immobilized megalin generated 22,000 BIAcore response units equivalent to 34 fmol of megalin/mm. 2 To test ligand binding, rabbit megalin immobilized on the CM5 sensor chip was incubated with the ligands (0.1–5 μmol/L) in 10 mmol/L HEPES, 150 mmol/L NaCl, 1.5 mmol/L CaCl2, 1 mmol/L EGTA, pH 7.4, and the relative increase in response between megalin and control flow channels was determined. For determination of binding affinities, 6 to 10 different concentrations of each ligand were subjected to BIAcore analysis on the megalin sensor chips (as shown in Figure 8A ▶ ). The kinetic parameters were determined by using the BIAevaluation 3.0 software.
Figure 8.
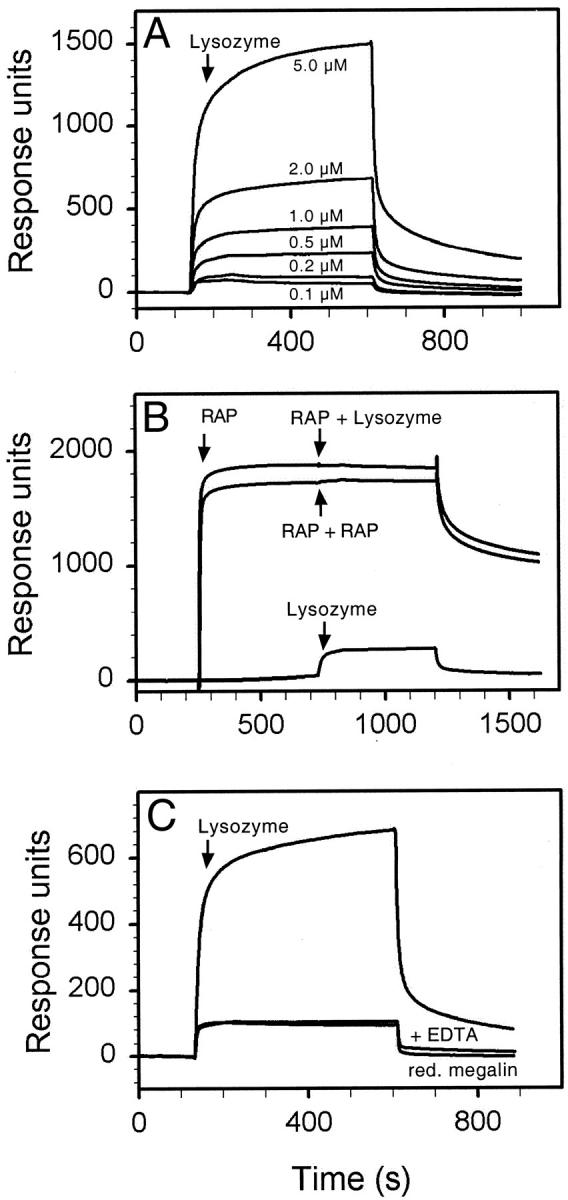
Binding of lysozyme to megalin. In A, megalin immobilized on a BIA sensor chip was incubated with the indicated concentrations of lysozyme. From the kinetic parameters, a Kd value of 0.32 μmol/L was calculated (see under Materials and Methods). In B, the receptor was preincubated with 5 μmol/L RAP resulting in 1800 response units. No further increase in response was achieved by subsequent addition of 0.5 μmol/L lysozyme (or RAP) indicating complete inhibition of ligand binding by RAP. As a control, 0.5 μmol/L lysozyme were added to the receptor chip without prior addition of RAP (200 response units). In C, binding of 2 μmol/L lysozyme to native megalin was tested in the presence or absence of EDTA, or to megalin reduced by 0.5% dithiothreitol, 6 mol/L guanidine hydrochloride. Adding EDTA or reducing the receptor changed the Kd to 39 μmol/L (as compared to 0.32 μmol/L for functional megalin). Similar results were obtained with DBP, RBP, and β2-M (not shown). The arrows indicate time points of ligand addition to the sensor chip.
Urine Analysis
A total of 10 megalin-deficient mice and their control litter mates were available for the studies presented here. For urine collection, these mice were placed in metabolic cages for 16 hours and given 10% sucrose in drinking water. Urine samples (approximately 5 ml/16 hours) were collected on ice and were qualitatively indistinguishable from samples collected without sucrose load. Urine volume per hour and creatinine levels were identical in megalin −/− and in control mice (approximately 0.5 mmol creatinine/L). Creatinine, sodium, potassium, chloride, total calcium, phosphate, urea, uric acid, and glucose were determined on a random access automated clinical chemistry analyzer (Synchron CX5, Beckman, Munich, Germany) by standard clinical chemistry procedures. For amino acid analysis, free amino acids in the urine samples were separated and quantified by ion exchange chromatography followed by ninhydrin reaction on a Biotronik LC3000 amino acid analyzer (Eppendorf, Hamburg, Germany). Retinol and tocopherol were determined by high performance liquid chromatography after solvent extraction (Chromsystem, Munich, Germany). For determination of 25-OH vitamin D3, urine samples were concentrated 30-fold by freeze-drying and analyzed by competitive protein binding assay (Immundiagnostik, Bensheim, Germany).
Results
Initially, we examined wild-type and megalin −/− kidneys by electron microscopy to identify potential changes in the ultrastructure of the receptor-deficient tissue. As shown for rat kidneys previously, 4 in wild-type mouse kidneys megalin is expressed in abundance in the epithelial cells of the proximal tubules. In particular, the receptor is present at the brush border surface and in all parts of the endocytic apparatus, including coated pits, coated vesicles, endosomes and dense apical tubules (Figure 1B) ▶ . No receptor protein is detectable in megalin −/− kidneys (Figure 1A) ▶ . Megalin deficiency does not affect the overall architecture of the epithelial cells or the appearance of the brush border surface (Figure 2A) ▶ . However, absence of megalin results in a significant reduction in the number of coated pits, endosomes, and lysosomes as compared to wild-type cells (Figure 2B) ▶ . The histological appearance of other renal tissues such as glomeruli (Figure 3) ▶ , distal tubules and collecting ducts (not shown) is indistinguishable from wild-type controls.
Figure 1.
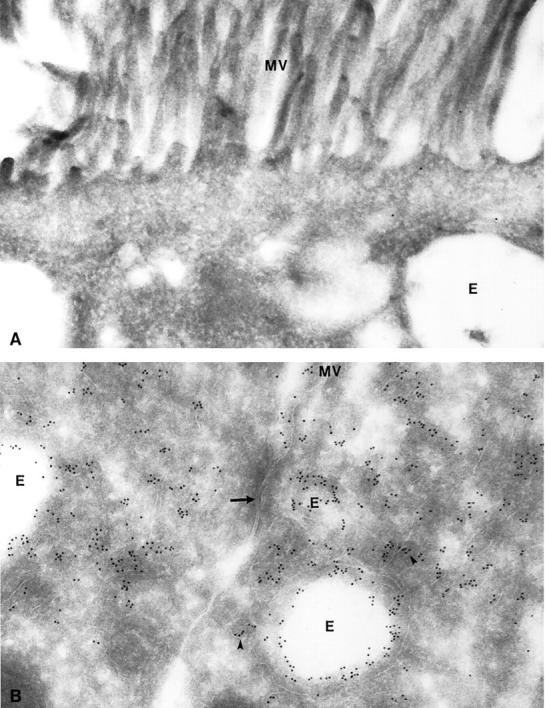
Immunocytochemical detection of megalin in mouse proximal tubules. Ultrathin cryosections from megalin-deficient (A) and control kidneys (B) were incubated with an anti-rat megalin antibody visualized by 10 nm gold particles. No labeling is seen in receptor-deficient tubules (A), whereas an abundant signal is observed in endosomes (E) and dense apical tubules (arrowhead) of control tissue (B). The arrow indicates tight junctions between two proximal tubule cells. MV, microvilli. Magnification, ×63,000.
Figure 2.
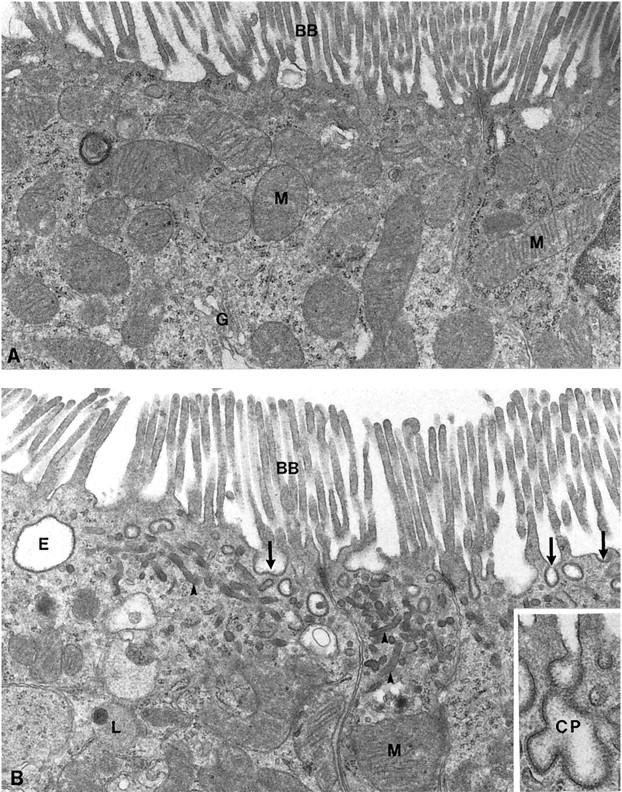
Electron micrographs of apical parts of proximal tubular cells from megalin-deficient (A) and control mice (B). Cells from megalin −/− mice exhibit a significant reduction of the endocytic apparatus including coated pits (arrows), endosomes (E), and dense apical tubules (arrowheads). The brush border (BB) and mitochondria (M) appear normal. The inset highlights a coated pit (CP) in the wild-type kidney section. G, Golgi apparatus; L, lysosome. A and B, ×20,000; inset, ×47,000.
Figure 3.
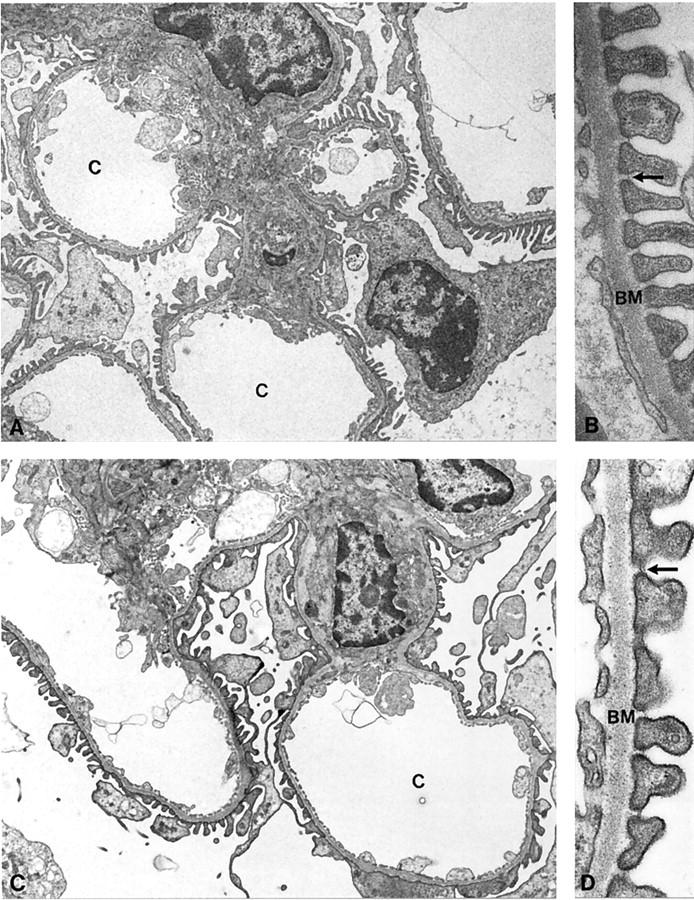
Electron micrographs of glomeruli from megalin-deficient (A and B) and wild-type mice (C and D). No obvious differences in the ultrastructure of the glomeruli is seen in knockout versus wild-type kidneys. Arrows indicate the split diaphragm. BM, basement membrane; C, capillary loops. A and C, ×5,000; B and D, ×47,000.
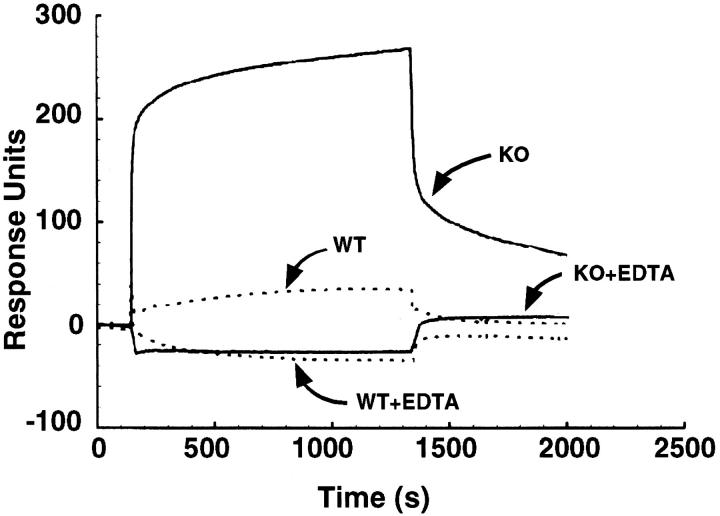
The histological analysis of renal tissues suggested that megalin deficiency might specifically impair endocytosis of macromolecules from the lumen of the proximal tubules. As a consequence, ligands for this receptor should be excreted in the urine of knockout mice. To test this hypothesis, we analyzed binding of mouse urine samples to purified megalin immobilized on a BIA sensor chip (BIAcore analysis). As shown in Figure 4 ▶ , urine samples from megalin −/− animals specifically interacted with the receptor. The interaction was reversible and dependent on calcium, a characteristic feature of ligand binding to the receptor. In contrast, little if any interaction was observed in urine samples obtained from wild-type animals. These findings demonstrated that normal urine was virtually devoid of any receptor ligands and that absence of megalin resulted in a state of renal resorption deficiency.
Figure 4.
Binding of mouse urine samples to megalin. Urine samples from wild-type (WT) and megalin-deficient mice (KO) were incubated with immobilized megalin in the presence or absence of 20 mmol/L EDTA. Binding to the receptor was detected by surface plasmon resonance signal (BIAcore) as described under Materials and Methods, and is indicated in response units.
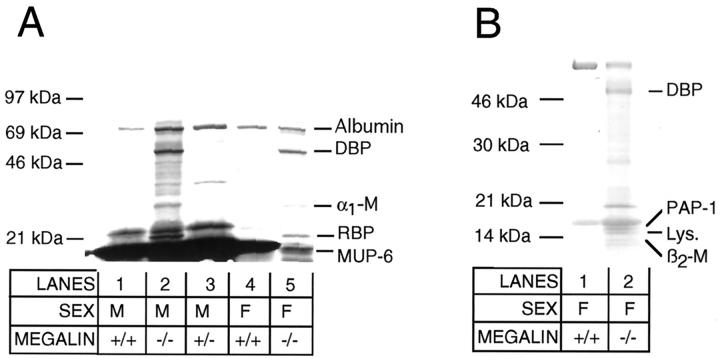
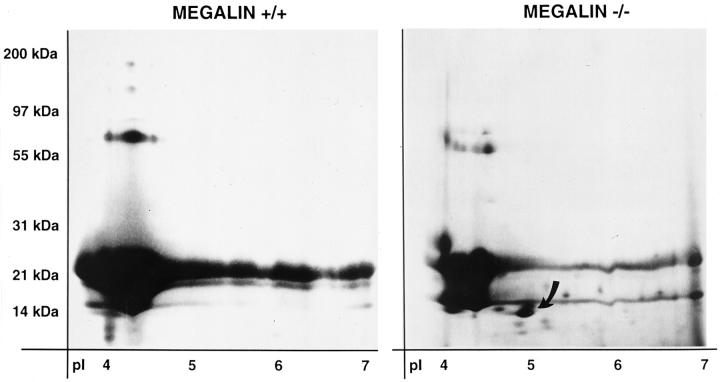
Next, we analyzed the protein profile in urine from knockout and control mice by SDS polyacrylamide gel electrophoresis (SDS-PAGE, Figure 5A ▶ ). No proteins larger than approximately 70 kd were present in the urine of control and megalin −/− animals indicating intact glomerular filtration. However, knockout mice specifically excreted several low molecular weight (LMW) proteins that were not seen in control samples. Potentially, these proteins represented previously unknown ligands for the receptor. To characterize some of the excreted proteins, we isolated individual protein bands from the gel and obtained peptide sequences by Edman degradation (Figure 5 ▶ ; Table 1 ▶ ). The 68-kd protein band that was present in both control and knockout urine was identified as mouse serum albumin. Apparently, albumin represented the largest plasma protein filtered in large quantities through the glomerular membranes. No major difference in the amount of albumin excretion was observed in knockout compared to control samples by SDS-PAGE. The second protein band that was present in all samples was the 21-kd major urinary protein 6 (MUP-6), a pheromone carrier that is abundant in urine from male rodents (Figure 5A ▶ , lanes 1–3). Besides MUP-6 and serum albumin, the urine of knockout mice displayed three major protein bands that were not seen in control urine. These proteins were identified by peptide sequencing as the vitamin D-binding protein (DBP), α1-microglobulin (α1-M), and the retinol-binding protein (RBP) (Figure 5A ▶ ; Table 1 ▶ ). Because massive amounts of MUP-6 in the urine of adult male mice obscured analysis of additional protein bands in the same molecular weight range, we further examined urine samples from wild-type and knockout females (Figure 5B) ▶ . In these samples, pancreatitis-associated protein (PAP-1), lysozyme, and β2-microglobulin (β2-M) were identified as novel proteins in urine from megalin −/− mice (Table 1) ▶ . Finally, we resolved mouse urine samples by two-dimensional SDS-PAGE and discovered another major protein spot present in knockout urine exclusively (Figure 6) ▶ . This protein was identified as the odorant-binding protein IA (OBPIA, Table 1 ▶ ).
Figure 5.
Urinary protein profile of wild-type and megalin −/− mice. Fifteen microliters of urine from adult (A) or juvenile mice (B) of the indicated genotypes were subjected to 4 to 15% nonreducing SDS-PAGE and staining with Coomassie Blue. The position of migration of marker proteins in the gel is shown. Protein bands that were identified by peptide sequencing are indicated. α1-M, α1-microglobulin; β2-M, β2-microglobulin; DBP, vitamin D-binding protein; F, female; Lys, lysozyme c; M, male; MUP-6, major urinary protein 6; PAP-1, pancreatitis-associated protein 1; RBP, retinol-binding protein.
Table 1.
Sequence Analysis of Mouse Urinary Proteins
| Sequence | Protein | MW (kd) | Reference |
|---|---|---|---|
| EIAHRYNDLGEQHFK | Serum albumin (aa 6–20*) | 68 | Swiss-Prot. P07724 |
| LERGRDYE | Vitamin D-binding protein (DBP, aa 13–20) | 53 | Swiss-Prot. P21614 |
| DPASTLPDIQVQENF | α1-Microglobulin precursor (α1-M, aa 20–34) | 30 | Swiss-Prot. Q07456 |
| LLSNWEVCADMVGTF | Retinol-binding protein (RBP, aa 81–95) | 20 | Swiss-Prot. Q00724 |
| NFNVEKINGEWHTIILASDKR | Major urinary protein 6 (MUP 6, aa 27–48) | 21 | Swiss-Prot. P02762 |
| LVDESPENLTFYSEN | Odorant-binding protein IA (OBPIA, aa 81–95) | 17 | EMBL Y10971 |
| RPGGHLVSVLNSAEA | Pancreatitis-associated protein 1 (PAP-1, aa 71–85) | 19 | Swiss-Prot. P35230 |
| GDQSTDYGIFQINSR | Lysozyme (aa 66–80) | 17 | Swiss-Prot. P08905 |
| TPQIQVYSR | β2-Microglobulin (β2-M, aa 24–32) | 14 | Swiss-Prot. P01887 |
Several peptide sequences were obtained from each protein by Edman sequencing. One representative peptide sequence is shown for each of the proteins. aa, amino acid position of the peptide sequence; MW, molecular weight in kd.
*The amino acid numbers refer to the sequence of the published murine serum albumin fragment.
Figure 6.
Two-dimensional SDS-PAGE analysis of mouse urinary proteins. Mouse urinary proteins (600 μg) from wild-type (+/+) and megalin-deficient animals (−/−) were subjected to two-dimensional SDS-PAGE analysis and stained with Coomassie Blue. Staining time was kept at a minimum to avoid overstaining in the molecular weight range of MUP-6 proteins (21 kd). Additional proteins present in the knockout urine samples (eg, DBP, RBP) are not seen under these conditions. The arrow denotes the odorant-binding protein IA (OBPIA) in knockout urine.
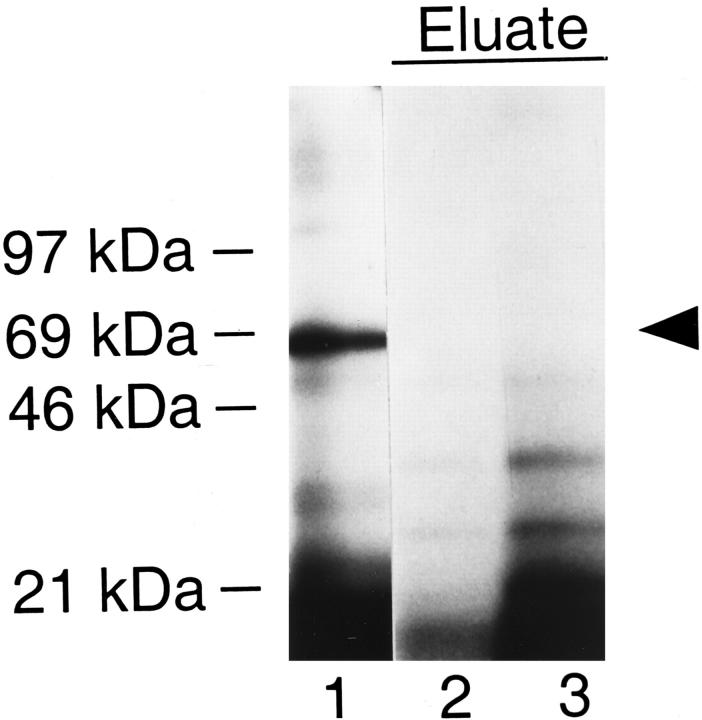
Because knockout but not wild-type urine samples strongly reacted with megalin in BIAcore analysis (Figure 4) ▶ , we hypothesized that proteins exclusively present in knockout samples represented receptor ligands. This hypothesis was confirmed when iodinated urinary proteins were incubated with megalin coupled to Sepharose resin (Figure 7) ▶ . Specifically bound proteins were eluted from the receptor column and analyzed by SDS-PAGE and autoradiography. Albumin, which was abundantly present in the urine sample (lane 1), did not bind to the megalin column to any major extent, while a number of smaller proteins interacted with the receptor and were enriched in the column eluate (lanes 2 and 3). No specific binding was seen with wild-type urine samples (not shown). To investigate whether these megalin-binding proteins represented some of the LMW proteins exclusively seen in knockout urine, we directly tested receptor binding by BIAcore analysis. Similar to the findings obtained with megalin affinity chromatography (Figure 7) ▶ , serum albumin did not bind to the receptor whereas DBP, RBP, lysozyme, and β2-M interacted with the receptor with a Kd of 0.1 to 1.8 μmol/L (Table 2) ▶ . The binding of ligands was concentration dependent (Figure 8A) ▶ and blocked completely by the receptor-associated protein, a receptor antagonist (Figure 8B) ▶ . Furthermore, ligand binding was abolished by adding EDTA or by reducing the receptor (Figure 8C) ▶ . Taken together, these results demonstrated specificity of ligand-receptor interaction.
Figure 7.
Purification of megalin-binding proteins from mouse urine by megalin affinity chromatography. One milliliter of knockout mouse urine was iodinated and subjected to megalin affinity chromatography as described under Materials and Methods. Proteins bound to the megalin column were eluted and 20 μl of the eluate (total of 1 ml) were subjected to 4 to 15% SDS-PAGE and autoradiography on X-ray film for 16 hours at −80°C (lanes 2 and 3). As reference sample, total urinary proteins applied to the column are shown in lane 1. The arrowhead indicates mouse serum albumin.
Table 2.
Affinities of Binding of Ligands to Megalin
| Ligand | Kd (μmol/L) |
|---|---|
| Albumin | n.d. |
| DBP | 0.11 |
| RBP | 1.8 |
| Lys. | 0.32 |
| β2-M | 0.42 |
Binding of purified proteins to immobilized rabbit megalin was quantified by BIAcore analysis (as described under Materials and Methods). No significant binding was detected for albumin. n.d., binding not detectable.
To test whether megalin constituted the only receptor for tubular uptake of the identified proteins, cryosections of mouse kidneys were incubated with antibodies directed against three of the identified receptor ligands. As shown for lysozyme, this protein was detected in endosomes and lysosomes of wild-type kidney sections, indicating uptake from the glomerular filtrate (Figure 9B) ▶ . In contrast, no uptake of lysozyme was detected in knockout kidneys (Figure 9A) ▶ . Similar results were obtained with antibodies directed against DBP and RBP (not shown). No murine specific antibodies against other identified proteins were available to extend these investigations.
Figure 9.
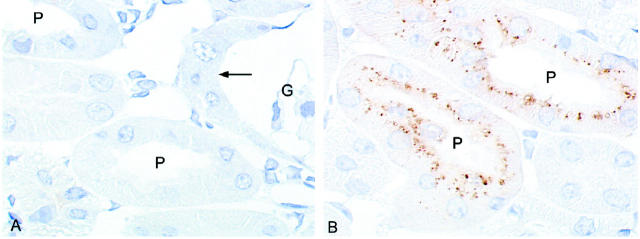
Immunohistochemical analysis of lysozyme in megalin-deficient (A) and control kidney cortex (B). Cryosections from kidney cortex were incubated with rabbit anti-human lysozyme IgG followed by peroxidase-conjugated anti-rabbit IgG. Bound IgG was visualized by diaminobenzidine. In wild-type tissue (B), strong labeling for lysozyme is seen in apical endosomes and lysosomes of the proximal tubules (P). No staining is seen in megalin-deficient proximal tubules (A) including the very initial part of the tubule (arrow) that is connected to Bowman′s capsule of the glomerulus (G). Magnification, ×900.
So far, our studies had established that megalin was involved in the retrieval of plasma proteins from the glomerular filtrate. Besides plasma proteins, other metabolites are also taken up from the lumen of the proximal tubules. To investigate whether megalin might also be responsible for the clearance of these compounds, we determined electrolyte and endocrine parameters in knockout urine samples and compared them to controls (Table 3) ▶ . No significant differences were observed in the concentrations of glucose, electrolytes, urea, uric acid, and amino acids. Furthermore, the total amount of protein excreted was also unchanged. The latter finding reflects the fact that mice excrete massive amounts of MUP-6 and that the additional loss of LMW proteins in the knockout mice does not increase the total urinary protein concentration significantly.
Table 3.
Electrolyte and Endocrine Parameters in Mouse Urine Samples
| Parameter | Genotypes | ||
|---|---|---|---|
| +/+; +/− | −/− | Unit | |
| Glucose | 4.1 ± 2.7 | 2.9 ± 1.5 | mmol/mmol creatinine |
| Electrolytes | |||
| Na | 79.2 ± 32.1 | 144 ± 89.1 | mmol/mmol creatinine |
| K | 79.3 ± 13.9 | 84.9 ± 13.1 | mmol/mmol creatinine |
| Cl | 60.3 ± 8.1 | 69.2 ± 5.2 | mmol/mmol creatinine |
| Ca | 2.8 ± 0.6 | 3.3 ± 1.6 | mmol/mmol creatinine |
| Phosphate | 10.5 ± 2.0 | 14.4 ± 7.0 | mmol/mmol creatinine |
| Amino acids (sum) | 0.1 | 0.1 | mmol/mmol creatinine |
| Urea | 755.3 ± 59.3 | 835 ± 80.9 | mmol/mmol creatinine |
| Uric acid | 173.7 ± 29.1 | 130 ± 40.9 | mmol/mmol creatinine |
| Total protein | 50.0 ± 7.8 | 52.4 ± 2.4 | mg/mmol creatinine |
| Vitamins | |||
| Retinol | n.d. | 0.4 ± 0.14 | mg/mmol creatinine |
| 25-OH vitamin D3 | n.d. | 1.6 ± 0.44 | nmol/mmol creatinine |
| Tocopherol | n.d. | n.d. |
A total of 4 (−/−) or 6 (+/+; +/−) animals were analyzed. Data points are given as mean ± SEM. Because urine samples were obtained on sucrose load, the concentration of metabolites may be more dilute than in untreated animals (see under Materials and Methods). All amino acids were determined individually. No statistically significant differences were observed for individual (not shown) or the total amount of amino acids excreted. n.d., not detectable.
We observed that many of the proteins excreted by megalin knockout mice were plasma carriers for lipophilic compounds (eg, fatty acids, odors, vitamin A and D metabolites). This finding suggested that megalin-mediated uptake of plasma proteins might be essential to prevent urinary loss of lipids bound to small carrier proteins. Consistent with our previous observations, 12,17 urine samples from knockout mice contained significant amounts of vitamin A (retinol) and 25-OH vitamin D3, which were not present in control urine (Table 3) ▶ . In contrast, vitamin E (tocopherol) was not detectable in any of the urine samples. This vitamin is transported in lipoprotein particles which are too large to be filtered through the glomerulus. Therefore, tocopherol was not expected to be present in the glomerular filtrate.
Finally, we wanted to test whether phenotypic similarities exist between megalin-deficient mice and patients with Fanconi syndrome. We focused our analysis on individuals with multiple myeloma, a malignant proliferation of immunoglobulin producing plasma cells. Multiple myeloma is characterized in a majority of cases by the appearance of massive amounts of immunoglobulin light chains in the glomerular filtrate (Bence-Jones protein). 6 By yet unknown mechanisms, certain light chain molecules impair proximal tubular function causing Fanconi syndrome. 6,16 We obtained urine samples from five patients with multiple myeloma-associated Fanconi syndrome. As described previously, these patients presented with a low tumor mass myeloma (2 to 9% marrow plasma cells) and mild but progressive renal insufficiency. Renal biopsies showed epithelial lesions, some with crystal formation in the cytoplasm of proximal tubular cells. No glomerular lesions were observed. 16 No difference in the urinary excretion of megalin fragments was seen when comparing these patients with control subjects (unpublished observations). By analysis of their urinary protein profiles, these patients were shown to exhibit low molecular weight proteinuria (Figure 10 ▶ , lanes 3 and 4) and to excrete the characteristic pattern of small plasma proteins 9 including DBP, α1-M, RBP, and β2-M (lanes 7 and 8). Similar to megalin-deficient mice, excretion of RBP resulted in concomitant loss of retinol in the urine. All 5 patients excreted between 0.08 and 0.18 mg retinol/mmol creatinine (mean, 0.132 mg retinol/mmol creatinine). No retinol was detectable in control urine samples (n = 9). In 2 of the patients, we also determined the levels of 25-OH vitamin D3. Significantly more 25-OH vitamin D3 (0.2–0.3 nmol/mmol creatinine) was detected in patients urine as compared to control samples (0.01–0.06 nmol/mmol creatinine). The three other patients received vitamin D supplementation and could not be used for analysis of the vitamin D metabolism. Tocopherol was not present in any of the urine samples.
Figure 10.
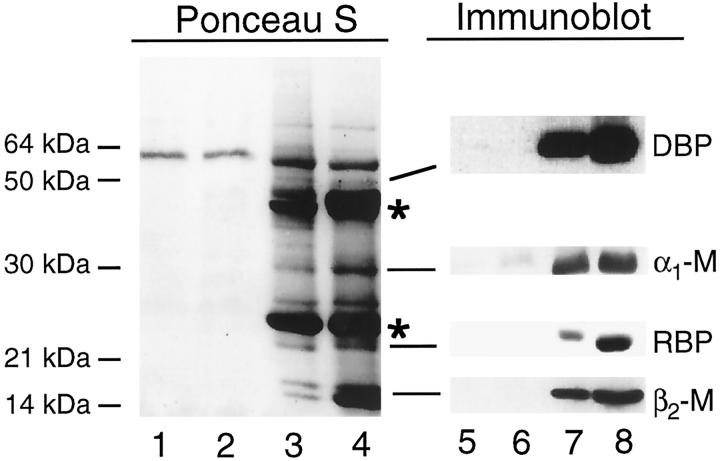
Identification of proteins in urine from Fanconi patients. Twenty microliters of urine samples from two healthy subjects (lanes 1, 2, 5, 6) and 5 μl of urine samples from two patients with multiple myeloma (lanes 3, 4, 7, 8 ) were subjected to 4 to 15% nonreducing SDS-PAGE and transfer to nitrocellulose membranes. Replicate filters were either stained with Ponceau S (lanes 1–4) or analyzed by immunoblot analysis using polyclonal antisera against the indicated proteins (lanes 5–8). The asterisks denote immunoglobulin light chain monomers and dimers present in urine from patients with multiple myeloma. Similar results were seen in three additional patients with multiple myeloma (not shown).
Discussion
In the present study, we demonstrate that megalin is involved in the tubular uptake of several plasma carrier proteins. Megalin deficiency results in LMW proteinuria and urinary loss of lipophilic vitamins bound to the carriers. Similar defects are observed in patients with acquired Fanconi syndrome.
A number of animal models are available to study the consequence of proximal tubular dysfunction in vivo. For example, intravenous injection of maleic acid into rats results in severe renal toxicity and Fanconi syndrome. 22,23 Furthermore, several genetic disease models have been reported including Basenji dogs 24 and mice carrying a targeted disruption of the hepatocyte nuclear factor 1 (HNF1) genes. 25 Similar to patients with acquired Fanconi syndrome, these animal models are characterized by combined defects in several tubular uptake processes resulting in urinary excretion of proteins, electrolytes, and metabolites. Basenji dogs exhibit an altered brush border membrane fluidity, which apparently affects transport across the plasma membrane. 24 HNF1 knockout mice are characterized by glucosuria, polyuria, amino aciduria, and phosphaturia. 25 Detailed studies suggest that these animals have an impaired function of the Na+/K+-ATPase and fail to maintain a proper Na+ electrochemical gradient across the apical brush border surface. As a consequence, cellular uptake processes dependent on this electrochemical gradient are defective. In contrast to aforementioned animal models, megalin-deficient mice exhibit a specific defect in one tubular uptake pathway only, the endocytosis of filtered plasma proteins. Uptake of electrolytes, amino acids, and glucose is not affected by the receptor gene defect. Thus, these mice constitute an important animal model with which to dissect the various tubular uptake pathways and to elucidate the significance of tubular uptake of LMW proteins.
In humans, hundreds of milligrams of plasma proteins are filtered daily through the glomerulus into the lumen of the proximal tubules. 1,2 Despite this massive influx of protein, human urine is virtually devoid of significant amounts of protein. The reasons for such massive uptake of proteins in the proximal tubules and the receptors involved in this process remain unclear. Because internalized proteins are subjected to lysosomal degradation, it is hypothesized that protein uptake is essential to salvage amino acids. Alternatively, the clearance of proteins from the glomerular filtrate is believed to remove metabolites that might serve as food source for bacteria and hence increase the risk of bacterial infections of the urogenital tract. 2,4
Our studies now have identified megalin as a central receptor pathway for tubular retrieval of small plasma proteins. The pattern of proteins excreted in the urine of megalin-deficient mice is similar to the pattern in patients with general tubular resorption deficiency. 9 DBP, RBP, lysozyme, and β2-M bind to the receptor with similar affinities as previously identified ligands (Kd of 0.1–1.8 μmol/L). 26 No purified α1-M and OBPIA were available to test their binding. Taken together, our findings suggest that megalin constitutes a low affinity but high capacity receptor pathway for scavenging proteins from the glomerular filtrate. Besides direct ligand binding, megalin may alternatively mediate the endocytic uptake of some of the identified proteins via co-receptor systems. Studies by Moestrup and colleagues have identified a 460-kd receptor in the epithelium of the proximal tubules called intrinsic factor/vitamin B12 receptor or cubilin. 27 This receptor mediates the tubular uptake of intrinsic factor/vitamin B12 complexes from the glomerular filtrate. Interestingly, cubilin does not contain a transmembrane domain like other endocytic receptors. Because it exhibits high affinity for megalin and co-localizes with this receptor in the endocytic pathway of proximal tubular cells, cubilin is believed to mediate ligand uptake by binding to megalin. 27 Such a co-receptor system would also be defective in megalin knockout kidneys. Thus, megalin could act as a central receptor pathway in the kidney either by direct ligand binding or via co-receptors.
In a previous study, uptake of albumin microinfused into rat proximal tubules was blocked by the addition of RAP, a megalin antagonist. 28 These results suggested a prominent role of megalin as a receptor for tubular clearance of albumin. In experiments presented here, albumin did not bind to megalin to any significant extend and no major difference in albumin clearance was seen in megalin-deficient as compared to control mice. Taken together both studies suggest that an alternative RAP-sensitive receptor may be mainly responsible for the tubular uptake of albumin.
The identification of ligands internalized via megalin (or co-receptors) has uncovered the importance of tubular protein uptake for homeostasis of lipophilic compounds. Many of the ligands taken up via megalin-dependent pathways are plasma carriers for lipophilic substances. 29 DBP is the transporter for vitamin D3 metabolites; RBP and α1-M carry retinoids; and OBPIA binds lipophilic odors. Excretion of these carrier proteins results in concomitant loss of lipids (eg, 25-OH vitamin D3, retinol) in the urine of megalin −/− mice. Additional studies demonstrate that the urinary loss of 25-OH vitamin D3 in megalin knockout mice results in plasma vitamin D deficiency and impaired bone formation. 17 Similar to megalin knockout mice, humans with multiple myeloma also excrete complexes of retinol and RBP in their urine (Figure 10) ▶ . In addition, enhanced urinary loss of 25-OH vitamin D3 was seen in two patients not receiving vitamin D supplementation. If confirmed in a larger number of patients, these findings might have important implications for our understanding of the pathology of the Fanconi syndrome. Individuals with this disease are characterized by osteomalacia and vitamin D-resistant rickets. 6 Such defects might be explained in part by the enhanced urinary loss of vitamin D3 metabolites bound to DBP. Also, urinary loss of retinoids and other essential lipids yet to be identified may contribute to the multiple defects in Fanconi patients. 6
One protein found in the urine of megalin knockout mice does not represent a normal plasma protein. This is the pancreatitis-associated protein 1. PAP-1 is a secretory protein that is induced in the acute phase of pancreatitis. 30 Because PAP-1 is not present in the circulation of healthy mice, it is not likely to be a physiological ligand for megalin. Instead, the presence of PAP-1 may indicate pancreatitis in receptor-deficient animals and point to additional roles of the receptor in other tissues than the kidney. Due to the poor viability and the complex phenotype of the knockout mice, such investigations are difficult to perform at present. The generation of mice carrying tissue-specific receptor gene defects will be helpful in the future in dissecting the multiple functions of the receptor in various tissues.
In conclusion, our studies have identified megalin as the central receptor for the tubular retrieval of several low molecular weight plasma proteins. Megalin-mediated uptake of plasma carrier proteins is required for reuptake of filtered lipid/carrier complexes. These findings have uncovered a crucial role of tubular protein uptake for systemic vitamin homeostasis.
Acknowledgments
We thank C. Räder, H. Schulz, and H. Sidelmann for expert technical assistance, I. Kappler and A. Nasswetter from Chromsystems for help with retinol/tocopherol analysis, and Christian Bönsch for critical reading of the manuscript.
Footnotes
Address reprint requests to Thomas E. Willnow, Max-Delbrueck-Center for Molecular Medicine, R.-Roessle-Strasse 10, D-13125 Berlin, Germany. E-mail: willnow@mdc-berlin.de.
Supported by Grant Wi 1158/3–1 and a Heisenberg fellowship (to TEW) from the Deutsche Forschungsgemeinschaft and by the Danish Medical Research Council and the Novo Nordic Foundation.
References
- 1.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D: Renal filtration, transport and metabolism of low-molecular-weight proteins: a review. Kidney Int 1979, 16:251-270 [DOI] [PubMed] [Google Scholar]
- 2.Maack T, Park CH, Camargo MJF: Renal filtration, transport and metabolism of proteins. Seldin DW Giebisch G eds. The Kidney: Physiology and Pathophysiology. 1985, :pp 1773-1803 Raven Press, New York [Google Scholar]
- 3.Christensen EI, Nielsen S: Structural and functional features of protein handling in renal proximal tubule. Seminars in Nephrology. Edited by Tisher CC, Madsen WB. Philadelphia, WB Saunders Co, 1991, 11:414–439 [PubMed]
- 4.Christensen EI, Birn H, Verroust P, Moestrup SK: Membrane receptors for endocytosis in the renal proximal tubule. Int Rev Cytol 1998, 180:237-284 [DOI] [PubMed] [Google Scholar]
- 5.Moestrup SK, Schousboe I, Jacobsen C, Leheste JR, Christensen EI, Willnow TE: β-2 glycoprotein-I and β-2 glycoprotein-I-phospholipid complex harbor a recognition site for the endocytic receptor megalin. J Clin Invest 1998, 102:902-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron M, Gougoux A, Vinay P: The renal Fanconi syndrome. The Metabolic and Molecular Basis of Inherited Disease. Edited by Scriver CR, Beudet AL, Sly WS, Valle D. New York, McGraw-Hill, 1995, 7:3691–3704
- 7.Harrison HE: The Fanconi syndrome. J Chron Dis 1953, 7:346-355 [DOI] [PubMed] [Google Scholar]
- 8.van’t Hoff WG: Biology and genetics of inherited renal tubular disorders. Exp Nephrol 1996, 4:253-262 [PubMed] [Google Scholar]
- 9.Pergande M, Jung K, Precht S, Fels LM, Herbort C, Stolte H: Changed excretion of urinary proteins and enzymes by chronic exposure to lead. Nephrol Dial Transplant 1994, 9:613-618 [DOI] [PubMed] [Google Scholar]
- 10.Saito A, Pietromonaco S, Loo AK-C, Farquhar MG: Complete cloning and sequencing of rat gp330/“megalin”: a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci USA 1994, 91:9725-9729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlando RA, Rader K, Authier F, Yamazaki H, Posner BI, Bergeron JJ, Farquhar MG: Megalin is an endocytic receptor for insulin. J Am Soc Nephrol 1998, 9:1759-1766 [DOI] [PubMed] [Google Scholar]
- 12.Christensen EI, Moskaug JO, Vorum H, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK: Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 1999, 10:685-695 [DOI] [PubMed] [Google Scholar]
- 13.Farquhar MG, Saito A, Kerjaschki D, Orlando RA: The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol 1995, 6:35-47 [DOI] [PubMed] [Google Scholar]
- 14.Christensen EI, Gliemann J, Moestrup SK: Renal tubule gp330 is a calcium-binding receptor for endocytic uptake of protein. J Am Soc Nephrol 1992, 40:1481-1490 [DOI] [PubMed] [Google Scholar]
- 15.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J: Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA 1996, 93:8460-8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leboulleux M, Lelongt B, Mougenot B, Touchard G, Makdassi R, Rocca A, Noel L-H, Ronco PM, Aucouturier P: Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int 1995, 48:72-79 [DOI] [PubMed] [Google Scholar]
- 17.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE: An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96:507-515 [DOI] [PubMed] [Google Scholar]
- 18.Gundersen TE, Lundanes E, Blomhoff R: Qunatitative high-performance liquid chromatographic determination of retinoids in human serum using on-line solid-phase extraction and column switching. J Chromatogr B Biomed Sci Appl 1997, 691:43-58 [DOI] [PubMed] [Google Scholar]
- 19.Vorum H, Liu X, Madsen P, Rasmussen HH, Honore B: Molecular cloning of a cDNA encoding human calumenin, expression in Escherichia coli and analysis of its calcium-binding activity. Biochim Biophys Acta 1998, 1386:121-131 [DOI] [PubMed] [Google Scholar]
- 20.Otto A, Thiede B, Müller EC, Scheler C, Wittmann-Liebold B, Jungblut P: Identification of human myocardial proteins separated by two-dimensional electrophoresis using an effective sample preparation for mass spectrometry. Electrophoresis 1996, 17:1643-1650 [DOI] [PubMed] [Google Scholar]
- 21.Fraker PJ, Speck JC: Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem Biophys Res Commun 1978, 80:849-857 [DOI] [PubMed] [Google Scholar]
- 22.Christensen EI, Maunsbach AB: Proteinuria induced by sodium maleate in rats: effects on ultrastructure and protein handling in renal proximal tubule. Kidney Int 1980, 17:771-787 [DOI] [PubMed] [Google Scholar]
- 23.Bergeron M, Mayers P, Brown D: Specific effect of maleate on an apical membrane glycoprotein (gp330) in proximal tubule of rat kidneys. Am J Physiol 1996, 271:F908-F916 [DOI] [PubMed] [Google Scholar]
- 24.Hsu BY, McNamara PD, Mahoney SG, Fenstemacher EA, Rea CT, Bovee KC, Segal S: Membrane fluidity and sodium transport by renal membranes from dogs with spontaneous idiopathic Fanconi syndrome. Metabolism 1992, 41:253-259 [DOI] [PubMed] [Google Scholar]
- 25.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M: Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 1996, 84:575-585 [DOI] [PubMed] [Google Scholar]
- 26.Moestrup SK, Cui S, Vorum H, Bregengard C, Bjorn SE, Norris K, Gliemann J, Christensen EI: Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest 1995, 96:1404-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moestrup SK, Kozyraki R, Kristiansen M, Kaysen JH, Rasmussen HH, Brault D, Pontillon F, Goda FO, Christensen EI, Hammond TG, Verroust PJ: The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J Biol Chem 1998, 273:5235-5242 [DOI] [PubMed] [Google Scholar]
- 28.Cui SC, Verroust PJ, Moestrup SK, Christensen EI: Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol 1996, 271:F900-F907 [DOI] [PubMed] [Google Scholar]
- 29.Flower DR: The lipocalin protein family: a role in cell regulation. FEBS Lett 1994, 354:7-11 [DOI] [PubMed] [Google Scholar]
- 30.Dusetti NJ, Ortiz EM, Mallo GV, Dagorn JC, Iovanna JL: Pancreatitis-associated protein I (PAP I), an acute phase protein induced by cytokines. J Biol Chem 1995, 270:22417-22421 [DOI] [PubMed] [Google Scholar]