Abstract
Eosinophils, basophils, and Th2 cells express the chemokine receptor CCR3, which binds eotaxin, RANTES, and some other chemokines. Using immunohistochemistry and flow cytometry, we demonstrate that CCR3 is also expressed by a variable proportion of human mast cells in gut, skin, and lung tissue. By contrast, with the same anti-CCR3 antibody (B711), CCR3 was poorly if at all detectable on human Th2 cells in vitro and in vivo. Eotaxin neither induced histamine release from purified human mast cells nor increased anti-IgE-stimulated histamine secretion. However, both eotaxin and RANTES elicited mast cell migration in vitro with a similar efficacy. High percentages of CCR3-expressing mast cells were present in the skin and in the intestinal submucosa; much lower percentages were found in the intestinal mucosa and in lung interstitium. Double immunostaining with anti-CCR3 and anti-chymase antibody showed that the vast majority of CCR3-expressing mast cells in the various tissues examined were tryptase-chymase double-positive. Therefore, tryptase-chymase double-positive mast cells express CCR3 and are attracted by CCR3-binding chemokines, eotaxin, and RANTES. Our findings indicate that these chemokines may play an important role in the differentiation and/or migration of this mast cell subset in connective tissues, as well as in sites of allergic inflammation.
The attraction of leukocytes to tissues is a basic mechanism for inflammation and for the host response to infections. This process is controlled by a large number of chemokines, which are chemotactic cytokines. 1,2 Chemokines are 8- to 10-kd proteins with 20 to 70 percent homology in amino acid sequences. They have been subdivided into families on the basis of the relative position of their cysteine residues. 1,3 There are at least four families of chemokines, but only two have been extensively characterized. The α and β chemokines, which contain four cysteines, appear to be the largest families. In the α-chemokines, one amino acid separates the first two cysteines residues (CXC), whereas in the β-chemokines, the first two cysteine residues are adjacent to each other (CC). The α-chemokines containing the sequence glutamic acid-leucine-arginine near the N terminal (preceding the CXC sequence) are chemotactic for neutrophils; those not containing the sequence act on lymphocytes. The β-chemokines, in general, do not act on neutrophils but attract monocytes, eosinophils, basophils, and lymphocytes with variable selectivity. 1,3
Chemokines induce cell migration and activation by binding to specific G protein-coupled cell-surface CXCR and CCR receptors on target cells. 1,3 Four human CXC chemokine receptors, CXCR1 through CXCR4, and nine human CC chemokine receptors, CCR1 through CCR9, have been identified. Chemokine receptors are expressed on different types of leukocytes, some being restricted to certain cells, others being more widely expressed not only on leukocytes but also on nonhemopoietic cells. Although most chemokine receptors bind more than one chemokine, CC receptors bind only CC chemokines and CXC receptors bind only CXC chemokines. 1,3 The chemokine receptor CCR3, which binds eotaxin, RANTES, monocyte chemotactic protein (MCP)-3, MCP-4, and some other chemokines, is expressed in human eosinophils, 4-6 basophils, 7 and type 2 T helper (Th2) cells. 8,9
In this study, by using immunohistochemistry and flow cytometry, we did not detect CCR3 expression on human Th2 cells in vitro or in vivo, whereas we found that CCR3 is expressed by basophils and, at the tissue level, by eosinophils. In addition, we demonstrate CCR3 expression on remarkable proportions of mast cells (MC) in different human tissues and on isolated cells. Although eotaxin apparently did not induce histamine release by purified human MC, both eotaxin and the CCR3 ligand, RANTES, exerted chemotactic activity on these cells. Interestingly, CCR3 expression was virtually limited to tryptase-chymase double-positive mast cells (MCTC), suggesting that migration and persistence of this MC subset into non-inflamed and inflamed tissues may depend largely on its CCR3 expression.
Methods
Reagents
Anti-CCR3 monoclonal antibody (mAb) (7B11; IgG2a) was kindly provided by Leukosite (Boston, MA); anti-human eosinophil cationic protein (ECP; clone EG2) and anti-tryptase mAb 10 by Pharmacia & Upjohn (Uppsala, Sweden); anti-macrophage associated antigen mannose receptor (anti-PAM-1) mAb 11 by A. Mantovani (Istituto Mario Negri, Milano, Italy); anti-FcɛRI α chain mAb (IgG1) by J. Hakimi (Hoffman-La Roche Inc., Nutley, NJ); rabbit anti-human-Fcɛ Ab by T. and K. Ishizaka (La Jolla, CA). Anti-CD3 mAb was purchased from Ancell (Bayport, MN); anti-chymase mAb from Chemicon International Inc. (Temecula, CA); eotaxin, RANTES, and MIP-1α from PeproTech EC LTD (London, UK); irrelevant anti-E-selectin mAb from R & D Systems (Minneapolis, MN); BSA, α-chymotrypsin, piperazine-N,N′-bis (2-ethanesulfonic acid) hyaluronidase, chymopapain, collagenase, elastase type I, and rabbit and sheep polyclonal nonimmune IgG from Sigma Chemical Co. (St. Louis, MO); FCS from Gibco (Grand Island, NY); deoxyribonuclease I and pronase from Calbiochem (La Jolla, CA); 60% HClO4 from Baker Chemical Co. (Deventer, The Netherlands); RPMI 1640 with 25 mmol/L Hepes buffer, Eagle’s minimum essential medium (MEM) from Flow Laboratories (Irvine, Scotland); Dextran 70 and Percoll from Pharmacia Fine Chemicals (Uppsala, Sweden); Alcian Blue 8GX from Carlo Erba (Milan, Italy); recombinant human stem cell factor (SCF) from Amgen (Thousand Oaks, CA). PMA, ionomycin, brefeldin A, anti-glycophorin A and B mAbs, and saponin were purchased from Sigma. The PE-conjugated anti-IL-4 (3010.211, IgG1), FITC-conjugated anti-IFN-γ (25723.11, IgG2b), PerCP- and PE-conjugated anti-CD4 as well as the purified anti-CD8, anti-CD14, anti-CD20, anti-CD56 and anti-CD45Ro mAbs were purchased from Becton Dickinson (San Jose, CA). The purified IgG1 and IgG2a isotype control mAbs, as well as the FITC-conjugated anti-IgG2a and PE-conjugated IgG1 Abs, were purchased from Southern Biotechnology Associates (Birmingham, AL). The goat anti-mouse IgG Abs conjugated with magnetic beads (MACS) were purchased from Miltenyi Biotec (Bisley, Germany).
Buffers
The PIPES buffer used in the histamine release and chemotaxis experiments was made up of 24 mmol/L PIPES, pH 7.37, 110 mmol/L NaCl, 5 mmol/L KCl (P). The mixture which is referred to as P2CG contains in addition to P, human serum albumin (HSA) 3%, 2 mmol/L CaCl2, 1 g/l dextrose. pH was titrated to 7.4 with sodium bicarbonate. PACGM contains in addition to P, HSA 3%, 1 mmol/L CaCl2, 1 g/l dextrose and 0.25 g/l MgCl2×6H2O, pH 7.4; PGMD contains 0.25 g/l MgCl2×6H2O, 10 mg/l DNase, and 1 g/l gelatin in addition to P, pH 7.37. 12,13
Immunohistochemical Analysis
Immunohistochemical analysis was performed on skin, gut, and lung tissues. Skin specimens were obtained from four patients with systemic sclerosis, one with lichen ruber planus and one with systemic mastocytosis, undergoing skin biopsy for diagnostic purpose. Gut specimens were obtained from biopsies of four patients with ulcerative colitis and from fragments of normal gut tissue of three patients undergoing colectomy because of colon cancer. Lung specimens were obtained from fragments of normal lung of three patients subjected to pneumectomy because of lung cancer. The procedures used in this study were in accordance with the criteria of the regional ethical committee on human experimentation.
Immunohistochemical staining was performed according to a technique previously described. 14 To this end, 10-μm cryostat sections were fixed in 4% paraformaldehyde for 20 minutes and subsequently exposed to 0.3% hydrogen peroxide-methanol solution to quench endogenous peroxidase activity. After a 30-minute preincubation with normal horse serum (Vectastain ABC kit; Vector Laboratories, DBA, Milan, Italy), sections were layered for 30 minutes with anti-CCR3 (0.2 μg/ml), anti-CD3, (5 μg/ml), anti-PAM-1 (5 μg/ml), anti-ECP (2 μg/ml), anti-tryptase (0.4 μg/ml) or anti-chymase (0.5 μg/ml) Abs, followed by biotinylated anti-mouse IgG Ab, and the avidin-biotin-peroxidase complex (Vectastain ABC kit), as described. 13 3-amino-9-ethylcarbazole (AEC; Vector) or Vector SG were used as peroxidase substrates. Finally, sections were counterstained with Gill’s hematoxylin or Methyl Green and mounted with Kaiser’s glycerol gelatin. All incubations were performed at room temperature. As negative control, primary mAb was replaced with an isotype-matched antibody with irrelevant specificity or mouse ascites fluid.
Double immunostaining was performed with anti-CCR3 and anti-CD3, anti-CCR3 and anti-ECP, anti-CCR3 and anti-tryptase, and anti-CCR3 and anti-chymase Ab by using the avidin-biotin-peroxidase system with two different substrates, as described. 14 To identify the two molecules on the same specimen, the Vector SG (bluish-grey color) and the AEC (red color) substrates were used, respectively.
Generation of Th2-Oriented T Cell Lines from Umbilical Cord Blood (UCB) Lymphocytes
Polyclonal CD4+ T cell lines were generated from UCB mononuclear cell (MNC) suspensions of four donors, as described. 15 Briefly, CD4+ CD45RA+ T cells were purified by negative magnetic selection using the MACS system after a two-step incubation with a mixture of anti-CD8, anti-CD14, anti-CD20, anti-CD56, antiCD45R0, anti-glycophorin A and B mAbs, followed by goat anti-mouse IgG conjugated with magnetic beads. Recovered cells (>99% CD3+ CD4+ CD45RA+) were then stimulated with PHA (0.1% vol/vol) and IL-2 (20 U/ml) in the presence of IL-4 (100 U/ml) in RPMI medium containing 10% heat-inactivated FCS (primary stimulation). On day 7, cells were washed and restimulated with PHA (0.1% vol/vol), IL-2 (20 U/ml), and IL-4 (100 U/ml) for 4 more days (secondary stimulation).
Detection of Intracellular IL-4 and IFN-γ Production by T Cell Lines
Intracytofluorimetric analysis of IFN-γ and IL-4 synthesis at the single-cell level was performed as described elsewhere. 15 Briefly, T cell blasts were stimulated with PMA (10 ng/ml) plus ionomycin (1 μmol/L) for 4 hours, the last 2 of which were in the presence of brefeldin A (5 μg/ml). After incubation, cells were washed twice with PBS, pH 7.2, fixed 15 minutes with formaldehyde (2% in PBS, pH 7.2), washed twice with PBS, pH 7.2, permeabilized with PBS, pH 7.2, containing 0.5% BSA and 0.5% saponin, then incubated with the appropriate mAb. Cells were analyzed on a FACSCalibur cytofluorimeter using CellQuest software (Becton Dickinson). The area of positivity was determined using an isotype-matched Ab. A total of 10 4 events for each sample were acquired in all cytofluorimetric analyses.
Purification of Peripheral Blood (PB) Basophils
Basophils were purified from PB cells of normal subjects undergoing hemapheresis. “Buffy coat” cell packs from healthy volunteers, provided by the Immunohematology Service at the University of Naples Federico II, were reconstituted in PBS containing 0.5 g/l HSA and 3.42 g/l sodium citrate, and loaded onto a countercurrent elutriator (model J2-21, Beckman Instruments, Fullerton, CA). Several fractions were collected, and fractions containing basophils in large numbers (>20 × 10 6 basophils) and of good purity (>15%) were further enriched by discontinuous Percoll gradients. 16 Yields ranged from 3 to 10 × 10 6 basophils with purity usually higher than 65%, as assessed by basophil staining with Alcian Blue and counting in a Spiers-Levy eosinophil counter. 16
Isolation and Purification of Lung MC
Lung tissue was obtained from 12 patients undergoing thoracotomy and lung resection. These samples were different from those used for immunohistochemical analysis. Macroscopically normal parenchyma was dissected free from pleura, bronchi, and blood vessels, and minced into a single-cell suspension, as previously described. 12 Yields with this technique ranged between 3 × 10 6 and 18 × 10 6 MC, and purities were between 1% and 8%. Human lung MC were purified by countercurrent elutriation (J2-21, Beckman Instruments) and then by discontinuous Percoll density gradient, as previously described. 12 The final preparations contained >95% viable cells, as assessed by trypan blue exclusion method, and consisted of 25 to 96% MC.
Flow Cytometric Analysis of Surface Molecules
Flow cytometric analysis of cell surface molecules was performed as described elsewhere. 15 Briefly, after saturation of nonspecific binding sites with total rabbit IgG, cells were incubated for 20 minutes on ice with specific or isotype control Abs. For indirect staining this step was followed by a second incubation on ice with an appropriate anti-isotype-conjugated Ab. Finally, cells were washed and analyzed on a FACSCalibur cytofluorimeter using CellQuest software (Becton Dickinson). A total of 10 4 events for each sample were acquired in all cytofluorimetric analyses.
Histamine Release
Cells (∼3 × 10 4 MC per tube) were resuspended in P2CG, and 0.3 ml of the cell suspensions were placed in 12 × 75 mm polyethylene tubes (Sarstadt Inc., Princeton, NJ) and warmed to 37°C; 0.2 ml of each prewarmed releasing stimulus was added, and incubation was continued at 37°C. 16 The P2CG and PGMD buffers 12,13 were used in these experiments. At the end of this step, the reaction was stopped by centrifugation (1,000 × g, 22°C, 1 minute), and the cell-free supernatants were stored at −20°C for subsequent assay of histamine content, with an automated fluorimetric technique. 17 Total histamine content was assessed by lysis induced by incubating the cells with 2% HClO4 before centrifugation. To calculate histamine release as a percentage of total cellular histamine, the spontaneous release of histamine from mast cells (2% to 14% of the total cellular histamine) was subtracted from both numerator and denominator. 13 The percentage of histamine release was calculated according to the following equation:
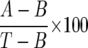 |
where A is the sample, B is the spontaneous histamine release, and T is the total histamine content. All values are based on means of duplicate or triplicate determinations. Replicated differed in histamine content by less than 10%.
Chemotactic Assay
MC chemotaxis was performed using a modified Boyden chamber technique, as described previously. 18 Briefly, 25 μl of PACGM buffer or various concentrations of the stimuli in the same buffer were placed in triplicate in the lower compartment of a 48-well micro-chemotaxis chamber (Neuroprobe, Cabin John, MD). The lower compartments were covered with a two-filter sandwich, a lower 5-μm pore size and an upper 8-μm pore size polycarbonate membranes (Nucleopore Corp., Pleasanton, CA), and then 50 μl of the cell suspension (5 × 104/well) resuspended in PACGM were pipetted into the upper compartments. The chemotactic chamber was then incubated for 3 hours at 37°C in a humified incubator with 5% CO2 (Automatic CO2 Incubator, Model 160 IR, ICN Flow). After the incubation period, the upper polycarbonate filter was discarded, while the lower nitrate cellulose filters were fixed in methanol, stained with Alcian Blue, 19 and then mounted on a microscope slide with Cytoseal (Stephen Scientific, Springfield, NJ). MC chemotaxis was quantitated microscopically by counting the number of cells that had traversed the upper 8-μm polycarbonate filter and were attached to the surface of the 5-μm cellulose nitrate filter. In each experiment, ten fields per triplicate filter were measured at 40× magnification. The results were compared with buffer controls.
To discriminate between chemotaxis and nondirected migration (chemokinesis) of MC, checkerboard analyses were performed. In the experiments, MC were placed in the upper chambers and various concentrations of SCF (10 to 100 ng/ml), eotaxin (10 to 100 ng/ml), RANTES (10 to 100 ng/ml) or PACGM buffer was added into either the upper or lower wells or into both. Spontaneous migration (chemochinesis) was determined in the absence of chemoattractant or when stimuli were added into either the lower and upper chambers. The MC migratory response to SCF, RANTES, and eotaxin was largely due to chemotaxis and not to chemokinesis. Indeed, a checkerboard analysis, in which chemoattractants above and below the filter were varied, resulted in significant migration only when there was a gradient of the factor below the filters (data not shown).
Statistical Analysis
The results are expressed as means ± SE. Statistical significance was analyzed by one-way analysis of variance and, when the F value was significant, by Duncan’s multiple range test. Differences were considered significant when P < 0.05.
Results
CCR3 Expression by Human MC
We used immunohistochemistry to investigate CCR3 expression in human skin, gut, and lung tissues. Several CCR3+ cells were detected in all tissues, but were more abundant in the skin and in the intestinal submucosa than in intestinal mucosa or in the lung (Figure 1 A–D) ▶ . To identify the nature of CCR3+ cells in the various tissues, we used double immunostaining with anti-CCR3 and anti-CD3 Ab (for T cells) or CCR3 and anti-ECP Ab (for eosinophils). Cells staining for both CCR3 and ECP were absent from normal tissues, but they were clearly detectable in the gut of patients with ulcerative colitis. However, several CCR3+ cells in the gut of these patients, as well as the vast majority of those present in the other tissues tested, were ECP-negative (Figure 1E) ▶ . Cells staining for both CCR3 and CD3 were virtually absent from every tissue examined, including the gut of patients with ulcerative colitis (data not shown), where CCR3+ T cells have been reported, 9 and the skin of patients with systemic sclerosis (Figure 1F) ▶ , where the majority of infiltrating T cells express a Th2-type cytokine profile. 20 Morphologically, the CCR3+ ECP− CD3− cells present in all tissues examined were usually larger than lymphocytes and eosinophils. In an attempt to identify these cells, we double-stained them with CCR3 and PAM-1, which is expressed by macrophages and dendritic cells. 11 As shown in Figure 1G ▶ , CCR3 and PAM-1 staining was clearly distinct, indicating that these cells do not belong to the macrophage lineage.
Figure 1.

Detection of CCR3-expressing cells in various human tissues. A: High numbers of CCR3+ cells (blue color, ×100) in the intestinal submucosa of normal gut. B: A few CCR3+ cells (blue, ×100) in the interstitium from normal lung. C: High numbers of CCR3+ cells (blue, ×100) in the derma of a skin biopsy specimen from a patient with lichen ruber planus. D: Absence of immunoreactivity in the same section stained with an isotype control Ab (×100). E: Double immunostaining for CCR3 (blue) and ECP (eosinophil, red), showing colocalization of CCR3 and ECP in one of the three cells (×1000). F: Double immunostaining for CCR3 (blue) and CD3 (pan-T, red) and (G) for CCR3 (blue) and PAM-1 (macrophage, red), showing a distinct separation of the two stainings in cells (×1000).
We then verified whether CCR3-expressing cells present in different tissues were MC. To this end, we first examined a skin biopsy specimen from a patient with systemic mastocytosis for CCR3-positive cells. CCR3 expression was found in a remarkable proportion of tryptase-positive cells in the skin of this patient (Figure 2, A and B) ▶ . Therefore, we performed double immunostaining for tryptase and CCR3 on normal lung, skin, and gut specimens. In all tissues examined, variable proportions of tryptase-positive cells showed costaining for CCR3. A representative experiment showing double immunostaining for tryptase and CCR3 in the submucosa of normal gut is shown in Figures 2, C and D ▶ .
Figure 2.
CCR3 expression by human MC. A: Large numbers of tryptase-positive cells (red) throughout the derma of a skin biopsy specimen from a patient with systemic mastocytosis (×250). B: CCR3+ cells (red) in a consecutive section from the same specimen (×250). C: Double immunostaining for tryptase (blue) and CCR3 (red) in normal gut; low numbers in the mucosa and large numbers in the submucosa of tryptase-positive cells (blue) also exhibit CCR3 reactivity (red), resulting in a brown color (×100). D: high power magnification (×1000) of two cells in the intestinal mucosa, one showing single staining for tryptase (blue) and the other costaining for both tryptase and CCR3 (brown) (×100).
MC-enriched suspensions were then prepared from normal lung tissue and assessed for CCR3 expression by flow cytometry. As controls, CCR3 expression was also assessed in basophil-enriched suspensions obtained from normal PB and in Th2-polarized short-term T cell lines generated from normal UCB CD4+ T lymphocytes. CCR3 expression occurred in the vast majority of FcɛRI+ cells in basophil-enriched PB suspensions (Figure 3A) ▶ and in a remarkable proportion of FcRɛl+ cells (25%) present in MC-enriched lung suspensions (Figure 3B) ▶ . By contrast, despite their clearly polarized Th2 profile of cytokine production, short-term T cell lines generated from UCB T lymphocytes did not show perceptible reactivity with the same anti-CCR3 mAb (Figure 3C) ▶ .
Figure 3.
Cytofluorimetric analysis of CCR3 expression by circulating basophils, lung MC, and Th2-polarized CD4+ UCB T lymphocytes. Basophil-enriched suspensions (A) and MC-enriched suspensions (B) were incubated with anti-CCR3 mAb and anti-FcɛRI mAb or isotype-matched control mAbs, followed by PE- or FITC-conjugated goat anti-mouse isotype. IL-4-conditioned CD4+ T cell lines (C) were assessed on day 11 for intracellular IL-4 and IFN-γ expression, as described in Methods, and for CD4 and CCR3 expression, as reported above. One representative of three performed experiments for each cell type preparation (basophil, MC, and Th2 cell) is reported.
CCR3 on Human MC Is Functionally Active and Is Involved in their Chemotaxis
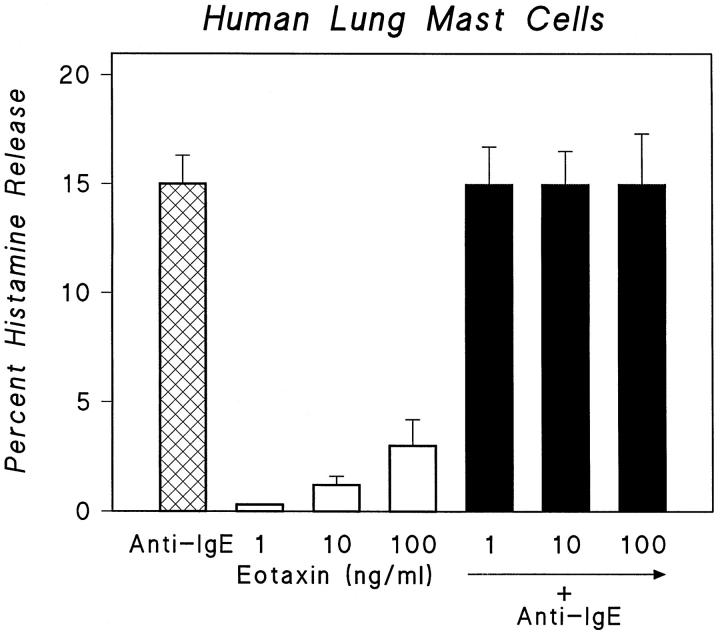
To establish whether CCR3 expressed by a percentage of MC was functionally active, MC-enriched suspensions obtained from fragments of lung tissues were tested for their ability to release histamine in response to eotaxin, which selectively binds to CCR3. The effect of eotaxin on histamine release from MC-enriched suspensions was compared with the effect induced by an anti-IgE Ab, the most potent immunological stimulus of human lung MC mediator release. Eotaxin alone elicited poor or no response and did not enhance the histamine release induced by anti-IgE Ab (Figure 4) ▶ .
Figure 4.
Effects of anti-IgE (1 μg/ml) and increasing concentrations of eotaxin (1 to 100 ng/ml) alone and in combination on histamine release from MC. Cells were preincubated for 30 minutes at 37°C with the indicated concentration of eotaxin and then challenged (45 minutes at 37°C) with anti-IgE. Results are the mean (±SE) of four experiments. Error bars are not shown when graphically too small.
Because eotaxin is a weak inducer of histamine release, but a potent chemoattractant for basophils, 7 we compared the ability of eotaxin and other chemokines, such as RANTES and MIP-1α, to act as chemoattractants for MC-enriched preparations with that of SCF. Eotaxin induced strong attraction of MC and its chemoattractant activity, as expressed by the number of migrating MC at optimal concentration, was similar to that of RANTES and SCF (Figure 5) ▶ . By contrast, MIP-1α was ineffective (data not shown).
Figure 5.
Effects of increasing concentrations of SCF (□), eotaxin (⊠), and RANTES (▪) on MC chemotaxis. MC-enriched suspensions obtained from human lung were allowed to migrate toward the indicated concentrations of SCF, eotaxin, and RANTES for 3 hours at 37°C in a humidified incubator with 5% CO2. Values are the mean (±SE) of four distinct experiments with different human lung MC-enriched preparations. P < 0.01 when compared with control.
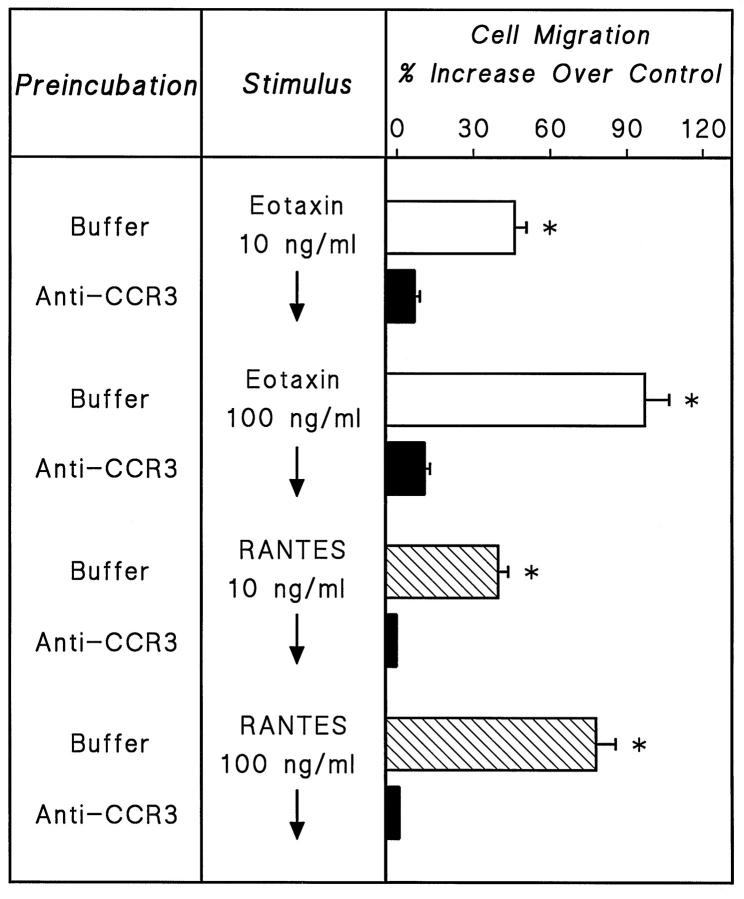
To obtain direct evidence that the chemoattractant effects of eotaxin and RANTES on human MC were mediated by their interaction with CCR3, human lung MC-enriched preparations were pre-incubated with anti-CCR3 Ab and then assessed for their ability to migrate in the presence of eotaxin. Figure 6 ▶ shows that pre-incubation of MC with anti-CCR3 Ab completely blocked the chemoattractant effects of both eotaxin and RANTES.
Figure 6.
Chemotactic responses of human lung MC to eotaxin and RANTES with (closed bars) and without (open bars) 5 μg/ml anti-CCR3 added to the cells 10 minutes before loading into the chemotaxis chamber. Values are the mean (±SE) of three distinct experiments with human lung MC-enriched suspensions, obtained from different donors. P < 0.01 when compared with MC preincubated with anti-CCR3 Ab.
CCR3-Expressing MC are MCTC
Because the percentage of CCR3-expressing cells in normal tissues was usually lower than that of tryptase-positive cells, we investigated whether CCR3 was expressed by a subset of MC, and examined the nature of this MC subset. To this end, the percentages of tryptase-positive cells showing CCR3 expression in biopsy specimens from gut, lung, and skin were evaluated. High percentages (>70%) of tryptase-positive cells showing CCR3 expression were found in the skin and in the intestinal submucosa, whereas much lower percentages (≤20%) were found in the intestinal mucosa and in lung interstitium (Table 1) ▶ . This suggests that CCR3 is preferentially expressed by MC present in connective tissue rather than in mucosal MC. Because tryptase is present in the vast majority of human MC, whereas chymase is localized to MC predominantly associated with connective tissues, 21,22 we asked whether CCR3 expression by human MC was a selective property of MCTC. To test directly this possibility, we double stained gut, lung, and skin specimens with anti-CCR3 and anti-chymase Ab. The vast majority of CCR3+ MC in the tissues examined also stained positive for chymase, the proportion of those reactive with chymase, but not with CCR3, being lower than 1%. A representative experiment, showing double immunostaining for chymase and CCR3 in the intestinal submucosa, is depicted in Figure 7 ▶ .
Table 1.
Distribution of Tryptase-Positive Cells Showing CCR3 Expression in Different Tissues
| Tissue | No. of cells/field (×250) | |
|---|---|---|
| Tryptase+ | Tryptase+/CCR3+ | |
| Gut | ||
| Mucosa | 25.5 ± 1.5 | 4.4 ± 0.5 (17%) |
| Submucosa | 22.7 ± 3.1 | 19.7 ± 1.2 (87%) |
| Ulcerative colitis | 46.2 ± 9.8 | 30.2 ± 9.7 (65%) |
| Lung | 19.4 ± 4.0 | 2.8 ± 0.5 (14%) |
| Skin | ||
| Systemic sclerosis | 16.6 ± 4.3 | 13.0 ± 3.5 (72%) |
Cells were costained for tryptase and CCR3 as described in Methods. Mean values (±SE) of absolute numbers of cells staining for tryptase (tryptase+) or for both (tryptase+/CCR3+), found by counting five consecutive microscopic fields (×250) for each section, are reported in the first and second columns, respectively. Values in parentheses express percentages of tryptase+/CCR3+ versus tryptase+ cells found in every tissue.
Figure 7.
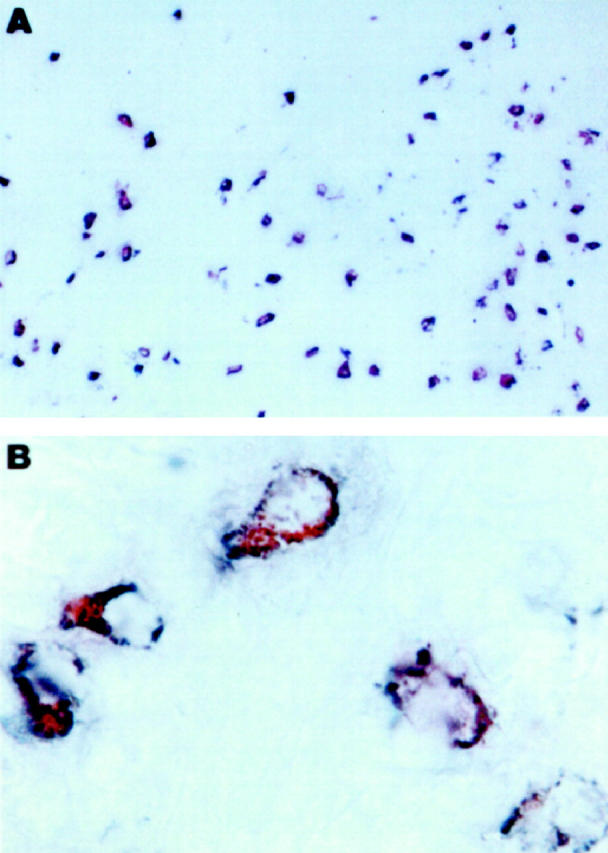
Immunohistochemical localization of CCR3 in MCTC. A: Double immunostaining for chymase (blue) and CCR3 (red) in normal gut. Virtually all chymase-positive cells also stained for CCR3 (brown). B: High power magnification (×1000) of five cells in the intestinal submucosa, all showing costaining for chymase and CCR3 (brown).
Discussion
The chemokine receptor CCR3 is expressed on human eosinophils 4-6 and basophils. 7 CCR3 expression has also been reported on a subset of T cells that have a Th2 type of cytokine secretion. 8,9 Eotaxin is a selective chemoattractant for these cell types. 4-9 Our study confirms that CCR3 is expressed by the great majority of human circulating basophils, whereas it was poorly (if at all) expressed by T lymphocytes activated under conditions that are known to favor strong Th2 polarization. 8,13 Moreover, remarkable percentages of CCR3+ eosinophils were found only in the gut of patients with ulcerative colitis. However, in normal gut, lung, and skin, as well as in the skin of patients with systemic sclerosis, the great majority of CCR3-expressing cells were neither eosinophils nor T lymphocytes. Moreover, CCR3+ cells in the same tissues were not costained by the anti-PAM-1 Ab, which identifies the antigen mannose receptor expressed by macrophages and dendritic cells. 11
Our finding that polyclonally activated Th2-polarized naive T cells, as well as Th2 cells present in the skin of patients with systemic sclerosis, 20 do not express CCR3 is apparently at variance with reports of CCR3 expression by human Th2 cells in vitro and in vivo. 8,9 The reason for this discrepancy is presently unclear, since in our experiments the same anti-CCR3 Ab (7B11) already reported to be reactive with Th2 cells 8,9 was used. However, the present finding is consistent with recent data showing poor or no CCR3 expression by human Th2-polarized T lymphocytes, 23-25 as well as with the demonstration that there are virtually no CCR3-expressing T cells in bronchial biopsy specimens of patients with atopic asthma, 26 where Th2 cells have been shown to play a pathogenic role. 27 Thus, it can be concluded that CCR3 expression by Th2 cells both in vitro and in vivo is too faint to be revealed by either flow cytometry or immunocytochemistry.
The results of our study suggest, rather, that the other cell type expressing CCR3 in human tissues in addition to eosinophils (and possibly basophils) is not a Th2 cell, but a MC. First, the assessment of skin biopsy specimens from one patient with systemic mastocytosis, showing diffuse MC dermal infiltration, revealed CCR3 expression in a remarkable proportion of these cells. More importantly, double immunostaining for CCR3 and tryptase in skin, gut, and lung, as well as double labeling for FcɛRI and CCR3 on MC-enriched suspensions obtained from normal lung, confirmed the MC nature of the majority of CCR3-expressing cells in different tissues.
MC are not a homogeneous population, but show marked inter- and intraspecies differences. 28-31 In rodents, metachromatic staining differentiated mucosal MC (MMC) from connective tissue MC (CTMC). 30,31 Although a similar subdivision has been suggested in humans, the histochemical subtype appears to be unrelated with anatomical site because both subtypes are found in the skin 32,33 and in the gut. 34-36 An immunohistochemical subtyping of human MC has been proposed based on the presence of neutral proteases (tryptase and chymase) in these cells. Tryptase is present, in different concentrations, in the vast majority of human MC from different tissues, 21,22 whereas it was reported that chymase is present only in MC associated with connective tissue. 33 Thus, an immunocytochemical subtyping of MC into MCT (MC containing only tryptase) and MCTC (MC containing both tryptase and chymase) has . 21,37 MCT are preferentially located at mucosal surfaces, whereas MCTC are found predominantly in submucosal and connective tissues. 38 The results of our study suggest that CCR3 expression is mainly a property of MCTC. First, CCR3+ MC were more abundant in skin derma and intestinal submucosa than in intestinal mucosa and lung interstitium and their proportions in these tissues roughly approximated those of MCTC. More importantly, double immunostaining for CCR3 and chymase showed that the vast majority of chymase-positive MC also stained for CCR3.
In this study, chemotaxis assay yielded essential information about the functional role of CCR3 in MC. First, eotaxin (a selective ligand for CCR3) 1-3,39 and RANTES (which also binds to CCR1, CCR5 and CCR9) 1-3,39 acted as chemoattractants for human MC as SCF, whereas MIP-1α (which binds to CCR1, CCR5, and CCR9, but not to CCR3) 1-3,39 was inactive. This suggests that MC, like human basophils and eosinophils, express functional CCR3. More importantly, in vitro, anti-CCR3 Ab abrogated chemotaxis elicited by eotaxin. Therefore, eotaxin appears to be a selective agonist for CCR3 on human MC, as well as on eosinophils 4-6 and basophils. 7 The inhibitory effect of the anti-CCR3 antibody indicates that CCR3 is the predominant receptor for MC migration in response not only to eotaxin, but also to RANTES. In contrast to the effects exerted on chemotaxis, histamine release was poorly or not at all sensitive to eotaxin, suggesting that CCR3 may primarily mediate migration rather than mediator secretion by human MC. These results are in agreement with the observation that eotaxin is a much better stimulus of human basophil chemotaxis than of histamine and leukotriene release. 7 Mediator release was induced by eotaxin only in IL-3-primed basophils and at concentrations 10 to 100 times greater than those used here. Thus, from this study CCR3 emerges as a major receptor for the eotaxin- and RANTES-mediated recruitment not only of eosinophils and basophils, but also of human MC. Interestingly, in vivo injection of human recombinant RANTES in mice has been shown to cause MC recruitment. 40
Why CCR3 is primarily expressed by MCTC, which predominate in connective tissues rather than in the mucosa, is unknown, also because the mechanisms that regulate the in situ differentiation of human MC are largely unclear. It has been proposed that MC subtypes represent different stages of differentiation of a single cell line, 41 whereas others suggest that they derive from distinct precursors. 38 In rodents, Kitamura and coworkers demonstrated that the development of various phenotypes depends on the anatomical microenvironment where the final differentiation takes place. 42,43 It is noteworthy that in mice both eotaxin and CCR3 are expressed by embryonic tissues responsible for blood development, eg, fetal liver, yolk sac, and peripheral blood. 44 Moreover, in combination with SCF, eotaxin promotes the growth and differentiation of MC progenitors. 44 Finally, eotaxin increased significantly during fibroblast-MC interaction, which appeared to be dependent upon SCF production. 45
Some years ago, Furitsu et al showed that prolonged coculture of human UCB nucleated cells with skin fibroblasts results in the development of mature MC, mostly MCTC. 46 This suggests that fibroblasts not only facilitate the differentiation of MC precursors to mature MC, but also contribute to the determination of the MCTC phenotype. 46 Interestingly, murine MC, which develop in response to the combined action of SCF and eotaxin, also exhibit the phenotype of connective-tissue-type MC. 47 Because eotaxin is constitutively expressed even in nonimmune tissues, such as intestine, skin, and mammary gland, 48 where the vast majority of MC are MCTC, 29 it is tempting to speculate that this chemokine not only plays an important role in the preferential differentiation of MC precursors into the MCTC phenotype, but also favors their migration into the connective tissues and helps maintaining their differentiation pathway and/or their survival. Furthermore, the demonstration that, like basophils and eosinophils, a proportion of human MC express CCR3 and therefore can respond to eotaxin, as well as other agonistic cytokines, may account for their up-regulation in tissues known to be sites of allergic reactions such as the airways. Indeed, in vivo administration of RANTES induced MC hyperplasia 40 and high eotaxin expression occurs in the epithelium and submucosa of bronchial biopsies from patients with atopic asthma. 26 Thus, the finding reported here may open new avenues for the identification of mechanisms involved in MCTC differentiation and homing, and possibly for a better understanding of their pathophysiological significance.
Acknowledgments
We thank Leukosite (Boston, MA) for the generous supply of anti-CCR3 antibody (7B11) used throughout the study.
Footnotes
Address reprint requests to Sergio Romagnani, Sezione Di Immunoallergologia E Malattie Respiratorie, Dipartimento Di Medicina Interna, Viale Morgagni 85, Firenze 50134 Italy. E-mail: s.romagnani@dfc.unifi.it.
Supported by grants provided by Istituto Superiore Di Sanita (AIDS Project to S. R. and G. M.) and by C. N. R. (Target Project Biotechnology Nos. 97.01140.PF49 and 98.00085.PF31) to G. M.
References
- 1.Baggiolini MB, Dewald B, Moser B: Human chemokines: an update. Ann Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Vecchi A, Sozzani S: Chemokines and chemokine receptors during activation and deactivation of monocytes and dendritic cells and in amplification of Th1 versus Th2 responses. Int J Clin Lab Res 1998, 28:77-82 [DOI] [PubMed] [Google Scholar]
- 3.Luster AD: Chemokines: chemotactic cytokines that mediate inflammation. New Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 4.Kitamura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O: Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine and identification of a specific eosinophil eotaxin receptor. J Biol Chem 1996, 271:7725-7730 [DOI] [PubMed] [Google Scholar]
- 5.Ponath PD, Qin SX, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR: Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 1997, 183:2437-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS: Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med 1996, 183:2349-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA: High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4 and other chemokines. J Clin Invest 1997, 100:1137-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallusto F, Lanzavecchia A: Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997, 277:2005-2007 [DOI] [PubMed] [Google Scholar]
- 9.Gerber BO, Zanni MP, Uguccioni M, Loetscher P, Mackay CR, Pichler WJ, Yawalkar N, Baggiolini B, Moser B: Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol 1997, 7:836-843 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, Metcalfe DD: The α form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest 1995, 96:2702-2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uccini S, Sirianni MC, Vincenzi V, Stopacciaro A, Lesnoni LA, Parola L, Capuana M, Cerimele D, Cells M, Lanzavecchia A, Allavena P, Mantovani A, Baroni CD, Ruco LP: Kaposi’s sarcoma cells express the macrophage associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi’s sarcoma patients. Am J Pathol 1997, 150:929-938 [PMC free article] [PubMed] [Google Scholar]
- 12.De Paulis A, Marinò I, Ciccarelli A, de Crescenzo G, Concardi M, Verga L, Arbustini E, Marone G: Human synovial mast cells. I. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum 1996, 39:1222-1233 [DOI] [PubMed] [Google Scholar]
- 13.Patella V, Casolaro V, Ciccarelli A, Petit GR, Columbo M, Marone G: The antineoplastic bryostatins affect differently human basophils and mast cells. Blood 1995, 85:1272-1281 [PubMed] [Google Scholar]
- 14.Romagnani P, Annunziato F, Manetti R, Mavilia C, Lasagni L, Manuelli C, Vannelli GB, Maggi E, Pupilli C, Romagnani S: High CD30 ligand expression by epithelial cells and Hassal’s corpuscles in the medulla of human thymus. Blood 1998, 91:3323-3332 [PubMed] [Google Scholar]
- 15.Galli G, Annunziato F, Mavilia C, Romagnani P, Cosmi L, Manetti R, Pupilli C, Maggi E, Romagnani S: Enhanced HIV expression during Th2-oriented responses explained by the opposite regulatory effect of IL-4 and IFN-γ on fusin/CXCR4. Eur J Immunol 1998, 28:3280-3290 [DOI] [PubMed] [Google Scholar]
- 16.de Paulis A, Ciccarelli A, de Crescenzo G, Cirillo R, Patella V, Marone G: Cyclosporin H is a potent and selective competitive antagonist of human basophil activation by FMLP. J Allergy Clin Immunol 1996, 98:152-164 [DOI] [PubMed] [Google Scholar]
- 17.Siraganian RP: An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem 1974, 57:383-394 [DOI] [PubMed] [Google Scholar]
- 18.Schleimer RP, Freeland HS, Peters SP, Brown KE, Perse CP: An assessment of the effects of glucocorticoids on degranulation, chemotaxis to vascular endothelial cells and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther 1992, 250:598-604 [PubMed] [Google Scholar]
- 19.Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J: Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA 1987, 84:2975-2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavilia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, Emmi L, Pupilli C, Pizzolo G, Maggi E, Romagnani S: Type 2 helper T (Th2) cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol 1997, 151:1751-1758 [PMC free article] [PubMed] [Google Scholar]
- 21.Irani AM, Schechter NM, Craig SS, DeBlois G, Schwartz LB: Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA 1986, 83:4464-4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patella V, Marinò I, Lamparter B, Arbustini E, Adt M, Marone G: Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J Immunol 1995, 154:2855-2865 [PubMed] [Google Scholar]
- 23.Annunziato F, Cosmi L, Galli G, Beltrame C, Romagnani P, Manetti R, Romagnani S, Maggi E: Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leukoc Biol 1999, 65:691-699 [DOI] [PubMed] [Google Scholar]
- 24.Bonecchi R, Bianchi G, Panina-Bordignon P, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F: Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 1998, 187:129-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PA, Matsushima K, Yoshie O: Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999, 11:81-86 [DOI] [PubMed] [Google Scholar]
- 26.Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB: Enhanced expression of eotaxin and CCR mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant colocalization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol 1997, 27:3507-3516 [DOI] [PubMed] [Google Scholar]
- 27.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB: Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992, 326:298-304 [DOI] [PubMed] [Google Scholar]
- 28.Galli SJ: New concepts about the mast cell. N Engl J Med 1993, 328:257-265 [DOI] [PubMed] [Google Scholar]
- 29.Weidner N, Austen KF: Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase and carboxypeptidase content. Pathol Res Pract 1993, 189:156-162 [DOI] [PubMed] [Google Scholar]
- 30.Welle M: Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol 1997, 61:233-245 [DOI] [PubMed] [Google Scholar]
- 31.Patella V, de Crescenzo G, Ciccarelli A, Marinò I, Adt M, Marone G: Human heart mast cells: a definitive case of mast cell heterogeneity. Int Arch Allergy Immunol 1995, 106:386-393 [DOI] [PubMed] [Google Scholar]
- 32.Befus AD, Goodacre R, Dick N, Bienenstock J: Mast cell heterogeneity in man. I. Histologic studies of the intestine. Int Arch Allergy Appl Immunol 1985, 76:232-236 [DOI] [PubMed] [Google Scholar]
- 33.Marshall JS, Ford GP, Bell EB: Formalin sensitivity and differential staining of mast cells in human dermis. Br J Dermatol 1987, 117:29-36 [DOI] [PubMed] [Google Scholar]
- 34.Enerback L: Mast cells in the gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand 1996, 67:365-379 [DOI] [PubMed] [Google Scholar]
- 35.Enerback L: Mast cells in the gastrointestinal mucosa. II. Dye binding and metachromatic properties. Acta Pathol Microbiol Scand 1996, 66:303-312 [DOI] [PubMed] [Google Scholar]
- 36.Aldenborg F, Enerback L: The immunohistochemical demonstration of chymase and tryptase in human intestinal mast cells. Histochem J 1994, 26:587-596 [DOI] [PubMed] [Google Scholar]
- 37.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB: Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem 1989, 37:1509-1515 [DOI] [PubMed] [Google Scholar]
- 38.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB: Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol 1987, 138:4381-4386 [PubMed] [Google Scholar]
- 39.Rollins BJ: Chemokines. Blood 1987, 90:909-928 [PubMed] [Google Scholar]
- 40.Conti P, Reale M, Barbacane RC, Letourneau R, Theoharides TC: Intramuscular injection of hRANTES causes mast cell recruitment and increased transcription of histidine decarboxylase in mice: lack of effects in genetically mast cell-deficient W/Wv mice. FASEB J 1998, 12:1693-1700 [DOI] [PubMed] [Google Scholar]
- 41.Caughey GH, Viro NF, Calonico LD, McDonald DM, Lazarus SC, Gold WM: Chymase and tryptase in dog mastocytoma cells: asynchronous expression as revealed by enzyme cytochemical staining. J Histochem Cytochem 1988, 36:1053-1060 [DOI] [PubMed] [Google Scholar]
- 42.Kitamura Y, Sonoda S, Nakano T, Yanayama Y: Probable dedifferentiation of mast cells in mouse connective tissues. Curr Top Devel Biol 1986, 20:325-332 [PubMed] [Google Scholar]
- 43.Kitamura Y, Kanakura Y, Sonoda S, Asai H, Nakano T: Mutual phenotype changes between connective tissue type and mucosal mast cells. Int Arch Allergy Appl Immunol 1987, 82:244-248 [DOI] [PubMed] [Google Scholar]
- 44.Quackenbush EJ, Wershil BK, Aguirre V, Gutierrez-Ramos J-C: Eotaxin modulates myelopoiesis and mast cell development from embryonic hematopoietic progenitors. Blood 1998, 92:1887-1897 [PubMed] [Google Scholar]
- 45.Hogaboam C, Kunkel SL, Strieter RM, Taub DT, Lincoln P, Standiford TJ, Lukacs NW: Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol 1998, 160:6166-6171 [PubMed] [Google Scholar]
- 46.Furitsu T, Saito H, Dvorak AM, Schwartz LB, Irani AM, Burdick JF, Ishizaka K, Ishizaka T: Development of human mast cells in vitro. Proc Natl Acad Sci USA 1989, 86:10039-10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jippo T, Tsujino K, Kim H-M, Kim D-K, Lee Y-M, Nawa Y, Kitamura Y: Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. Am J Pathol 1997, 150:1373-1382 [PMC free article] [PubMed] [Google Scholar]
- 48.Rothenberg ME, Luster AD, Leder P: Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin-4-induced tumor suppression. Proc Natl Acad Sci USA 1995, 92:8960-8964 [DOI] [PMC free article] [PubMed] [Google Scholar]