Abstract
The subset of myoid cells is a normal component of the thymic stroma. To characterize these cells, we immortalized stromal cells from human thymus by using a plasmid vector encoding the SV40 T oncogene. Among the eight cell lines obtained, one had myoid characteristics including desmin and troponin antigens. This new line was designated MITC (myoid immortalized thymic cells). These cells expressed both the fetal and adult forms of muscle acetylcholine receptor (AChR) at the mRNA level, as well as the myogenic transcription factor MyoD1. α-Subunit AChR protein expression was detected by flow cytometry and the AChR was functional in patch-clamp studies. In addition, AChR expression was down-modulated by myasthenia gravis sera or by monoclonal antibody anti-AChR on MITC line similarly to TE671 rhabdomyosarcoma cells, making the MITC line an interesting tool for AChR antigenic modulation experiments. Finally, the MITC line expressed LFA-3, produced several cytokines able to act on T cells, and protected total thymocytes from spontaneous apoptosis in vitro. These results are compatible with a role of thymic myoid cells in some steps of thymocyte development. Therefore MITC line appears to be a useful tool to investigate the physiological role of thymic myoid cells.
The thymus is the central organ for differentiation of bone marrow-derived precursor T lymphocytes into functionally and phenotypically discrete mature T cells. 1 Analytical studies of clonal and highly purified thymic stromal components indicate that epithelial cells, including nurse cells, provide fundamental signals for many different steps of T cell maturation by producing hormones, humoral factors, and the necessary microenvironment for lymphocytes to learn to discriminate between self and non-self. 2-3 Thus, thymic lymphocytes depend on the cellular and humoral environment of non lymphoid thymic compartments for full proliferation and maturation. The precise mechanisms underlying these events are largely unknown. The contribution of individual thymic stromal components to T cell development has been difficult to study, particularly in humans. Thymic myoid cells are one such cell type. They have been described as a regular constituent of the thymus of embryonic and young vertebrates, 4 but the role and characteristics of these cells remain to be determined.
Myoid cells share some characteristics with thymic epithelial cells 5 and it has been suggested that myoid cells may derive from myoepithelial cells within the thymus. 6 Other reports have suggested that myoid cells come from pluripotent stem cells 7 or from endodermal reticular cells. It has also been postulated that they are of extrathymic origin, arising during embryogenesis from muscle precursor cells of the surrounding mesoderm. 5 Experiments with chick/quail chimeras do not support the notion that myoid cells arise from transdifferentiation of thymic epithelial cells but are rather of neuroectodermal origin. 8 Thymic myoid cells express several muscle-specific proteins including troponin T, desmin, 9 and the acetylcholine receptor (AChR). 9-10 They have therefore the antigenic characteristics of the skeletal muscle cells within the thymus. 11 Their biological role is unclear, but their involvement in human myasthenia gravis (MG) has been suggested. 7 Van de velde and Friedman reported that myoid cells were present in thymus and in thymoma from both young and adult patients with MG. 4
Myasthenia gravis is an autoimmune disease in which anti-AChR autoantibodies impair neuromuscular transmission. The thymus plays a pivotal role in the pathogenesis of MG. It frequently shows abnormalities (hyperplasia in 50 to 60% of cases, thymoma in 10 to 15%) and thymectomy is clinically beneficial. 12-13 The hyperplastic thymus is a site of T and B cell hyperactivation and autoreactivity to AChR. 14-15 Autosensitization to AChR thus appears to take place in the thymus. The presence of AChR in the thymus has been clearly demonstrated both on myoid cells 9,16 and on thymic epithelial cells, 17 indicating that these cells could be involved in the primary autosensitization step.
No immortalized human thymic myoid cell line has so far been described. We transfected postnatal thymic stromal cultures from a normal human thymus with a plasmid recombined with the SV40LT oncogene and isolated such a cell line. Here we describe the establishment, analysis, and possible biological relevance of this new cell line designated MITC (myoid immortalized thymic cells).
Materials and Methods
Transfection of Thymic Stromal Cells
Fresh thymic fragments (discarded tissue) were obtained from children undergoing corrective cardiovascular surgery at Hôpital Marie Lannelongue (Le Plessis Robinson, France) and were used to culture primary thymic stromal cells. 18
After 12 days of culture, the confluent monolayers were treated with 0.25% trypsin-EDTA (Gibco BRL, Life Technologies, France) for 5 minutes at room temperature. The cells were collected, washed in 10 mmol/L Na2HPO4/Na2H2PO4, 250 mmol/L sucrose, 1 mmol/L MgCl2, and incubated for 10 minutes at 4°C in the same buffer (pH 7.45) in the presence of 10 μg of plasmid pMK16 obtained after insertion of the origin-defective mutant of simian virus 40 large T antigen. 19
Cells were transiently permeabilized by eight square electric pulses generated by an electropulsator (100 μs, 1350 V/cm, 1 Hz, Bioblock, Paris), as previously described. 19 After 4 weeks in culture, highly proliferative clones of cells were isolated with a cloning ring from a series of foci and amplified.
Cultures of Cells
The human rhabdomyosarcoma cell line TE671, a gift from Dr. Bloc (Geneva, Switzerland), was used as a positive control for AChR expression. It was grown in modified Dulbecco’s medium supplemented with 2 mmol/L L-glutamine, 100 U penicillin/ml, 100 μg/ml streptomycin (Gibco BRL), 1 mmol/L Na pyruvate, and 10% FCS (Eurobio, Les Ulis, France). SV40-transformed COS-7 monkey kidney fibroblasts were maintained in DMEM (Gibco BRL) containing 2 mmol/L L-glutamine, 100 U penicillin/ml, 100 μg/ml streptomycin (Gibco BRL), 1 mmol/L Na pyruvate, and 10% FCS (Eurobio, Les Ulis, France). The human MITC line was maintained in RPMI 1664 (Gibco BRL) containing 100 U penicillin/ml, 100 μg/ml streptomycin (Gibco BRL), and 10% FCS (Eurobio, Les Ulis, France). All cells were grown at 37°C in 5% CO2. To induce differentiation and fusion of MITC, cultures were switched to a medium consisting of MEM (Gibco BRL) containing 10% horse serum, 2 mmol/L L-glutamine, 100 U penicillin/ml, 100 μg/ml streptomycin (Gibco BRL) to which 10−4 mol/L cytosine arabinoside was added after 24 hours.
Monoclonal Antibodies and Immunofluorescence Studies
Anti-desmin monoclonal antibody (MAb) (Organon Teknika), anti-troponin T MAb (gift from Dr. L. Mesnard, CNRS-URA 1159), anti-keratin MAb (Dako, Denmark), and anti-SV40 large T MAb (Pharmingen) were used. Immunofluorescence staining was performed using cells grown on slide culture chambers (2-well Labtek chambers, Nunc) precoated with 10 μg/ml laminin (Gibco BRL, Life Technologies). After washing with PBS (Gibco BRL), cells were immediately fixed with ice-cold acetone for 10 minutes and incubated with mAbs for 1 hour at room temperature, then washed with PBS, and incubated with fluorescein (FITC)-conjugated goat anti-mouse Ig for 1 hour at room temperature.
Immunofluorescence studies were also performed on thymic sections. Briefly, 5-μm frozen thymic sections were fixed in 4% paraformaldehyde in PBS (Gibco BRL, Life Technologies). Subsequently, the sections were stained with anti-desmin monoclonal antibody and revealed with a goat anti-mouse (GAM) coupled to tetramethyl rhodamine (TRITC) (Organon Teknika). After subsequent saturation with uncoupled goat anti-mouse immunoglobulins the sections were labeled with anti-keratin monoclonal antibodies (mix of MNF116 and CK1, DAKO Corp.), MAb anti-ICAM-1, MAb anti-LFA-3, or MAb anti-MHC class I (Immunotech, Marseilles, France) and revealed with GAM coupled to fluorescein (FITC) (Silenus, Eurobio, France). The control sections were performed by omitting either the first layer (anti-desmin antibody) or the third layer (MAbs anti-keratin, anti-ICAM-1, anti-LFA-3, anti-MHC class I), or both.
Flow Cytometry Analysis
We used an anti-HLA ABC MAb (Immunotech, France), anti-HLA DR MAb (Immunotech, France), anti-ICAM-1 MAb (Immunotech, France), and LFA-3 MAb (Immunotech, France). The cells were tested for their responsiveness to IFN-γ (Genzyme), as follows: 72 hours after the addition of IFN-γ, adherent cells were trypsinized and stained with the mAbs indicated above for 30 minutes at 4°C, followed by washing and labeling with FITC-conjugated goat anti-mouse Ig. Flow cytometry was performed on a FACSCalibur apparatus (Becton Dickinson Immunocytometry Systems, Mountain View, CA). To assess cell surface AChR expression, we used two mAbs. MAb 35 recognizes the main immunogenic (MIR) determinant, and MAb 155 recognizes a cytoplasmic region of AChR. 20 The second layer was performed with anti-rat Ig FITC-labeled (Valbiotech, France). Paraformaldehyde solution (2%) was added to the cell suspension to permeabilize cells for cytoplasmic AChR labeling by MAb 155.
Co-Culture of Thymocytes with MITC or Human Thymic Epithelial Cell (HTEC) Line
MITC and HTEC lines were cultured to confluence in a six-well culture. 5 × 10 6 of total human thymocytes were incubated in the presence or absence of adherent cells for 24 hours. The whole cell population was harvested by 0.25% trypsin-EDTA (Gibco BRL, Life Technologies) and stained with FITC-annexin V according to Boehringer Mannheim protocol. 21 Thymocytes were then analyzed in the appropriate gate on a FACSCalibur apparatus. The treatment with trypsin does not affect the staining with annexin which binds to phosphatidylserine on the surface of apoptotic cells (data not shown).
Total RNA Preparation
RNA was extracted from the TE671, COS-7, thymic epithelial cells (TEC), and MITC cell lines using a slight modification of the method described by Chomczynski, 22 using 100 μl of denaturing solution (4 mol/L guanidinium thiocyanate, 25 mmol/L sodium citrate, pH 7, 0.5 N lauryl sarcosyl, and 0.1 mol/L 2-mercaptoethanol), 30 μl of sodium acetate, 100 μl of phenol, and 40 μl of chloroform per 10 7 cells, without the need for homogenization. After extraction, total RNA was purified with 0.5 volumes of 7.5 mol/L ammonium acetate and 2.5 volumes of 100% ethanol, and then centrifuged at 15,000 rpm for 30 minutes at 4°C. The pellet was washed twice in 75% ethanol, dried under vacuum, and stored at −80°C after dissolution in diethylpyrocarbonate-treated water. The total RNA concentration was determined by measuring absorbance at 260 nm on a Gene Quant II spectrophotometer (Pharmacia Biotech, Uppsala, Sweden). Purity was checked by measuring the 260 nm/280 nm absorbance ratio.
Oligonucleotides Used for Reverse Transcription Polymerase Chain Reaction Amplification
On the basis of the published nucleotide sequences of the α-, β-, δ-, ε-, and γ-subunit genes of the human skeletal muscle AChR, oligonucleotide primers for polymerase chain reaction (PCR) were designed by using Oligo software (Med Probe, Oslo, Norway), a computer program used to optimize the annealing temperature and sequence specificity and to limit self-complementarity. The oligonucleotide primers were purchased from Eurogentec (Seraing, Belgium) and the sequences are indicated in Table 1 ▶ . Primers, used to amplify a 573-bp fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, are also described in Table 1 ▶ .
Table 1.
Characteristics of the Oligonucleotides Used in This Study
| Oligonucleotide | Name | Strand | Sequence 5′ 3′ | Hybridization temperature (°C) | Expected size (bp) |
|---|---|---|---|---|---|
| α-chain | A1 | − | GGCTCCGAACATGAGACCCG | 57 | 704 and 629* |
| A2 | + | GAAGCAGTACGTCGCGGACG | 57 | ||
| β-chain | B1 | − | GTGTCAGGGTCAGCGTTGGT | 60 | 579 |
| B2 | + | TGCGGCGGATGATGAGGTAG | 60 | ||
| δ-chain | D1 | − | GCCCTCACACTCTCCAACCT | 62 | 468 |
| D2 | + | TCTCCCACTCCCCGTTCTCT | 62 | ||
| γ-chain | G1 | − | GAAGCCCTCACCACCAATGT | 62 | 513 |
| G2 | + | GTAGGTGAAGGAAGGACGGT | 62 | ||
| ε-chain | E1 | − | CGAGGAACTGCGTCTTTATC | 56 | 644 |
| E2 | + | CGGATGATGAGCGAGTAGAT | 56 | ||
| GAPDH | − | ATCACCATCTTCCAGGAGCG | 62 | 574 | |
| + | CCTGCTTCACCACCTTCTTG | 62 |
*Expected size for P3A+ and P3A− α-subunit isoforms, respectively.
RT-PCR
Total RNA from normal muscle and the MITC cell line were reverse transcribed into a total volume of 50 μl containing 2 μg of total RNA, 5 μl of 10× RT buffer (500 mmol/L Tris-HCl, pH 8.3, 60 mmol/L MgCl2, 400 mmol/L KCl, 40 mmol/L dithiothreitol, 1.5 mmol/L dNTPs (Eurobio, Les Ulis, France), 40 units of RNAsin inhibitor of ribonuclease (Promega, Madison, WI), 50 pmol of 3′ primer and 5 units of avian myeloblastosis virus reverse transcriptase (Eurobio, Les Ulis, France). The mixture was then incubated at 42°C for 60 minutes and then quickly chilled on ice. PCR was carried out in a total volume of 100 μl containing 10 μl of RT reaction mixture, 10 μl of PCR buffer (50 mmol/L KCl, 10 mmol/L Tris-HCl, 1.5 mmol/L MgCl2, 0.1% gelatin, 1% Triton X-100), 0.5 μmol/L of each primer, 200 μmol/L of each dNTP, and 2.5 units of Taq polymerase (Eurobio). The reaction mixture was overlaid with mineral oil and then amplified in a PHC3 thermal cycler (Techne, Cambridge, UK) as follows: denaturing step, 94°C for 1 minute; annealing step at the indicated hybridization temperature (Table 1) ▶ for 1 minute; extension step, 72°C for 2 minutes. The final elongation step lasted 10 minutes at 72°C. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide.
Northern Blot Analysis
Total RNA was isolated by guanidinium isothiocyanate extraction as described above. After denaturation, RNA samples were electrophoresed in 1% agarose, 2.2 mol/L formaldehyde gel and then transferred to nylon membranes (Hybond N+, Amersham, Buckinghamshire, UK) and hybridized with 32P-labeled probe (see below) using the Rediprime kit from Amersham. The SV40 large T probe consisted of the 5.2-kb BamHI fragment of pSV40 containing the viral sequence without the replication origin (ori−). 19 The Myf3 (myoD1) probe was derived from a human full-length cDNA clone ligated into the EcoRI site of the pEMSV-scribe vector (a generous gift from Dr. Hans Henning Arnold, Institute of Biochemistry and Biotechnology, Technical University of Braunschweig, Germany). To normalize the amount of RNA present on the filters, we used a 20-mer oligonucleotide complementary to part of the sequence of rat 18S ribosomal RNA labeled with [γ-32P]dATP by T4 polynucleotide kinase (Promega). Blots were autoradiographed at −80°C with intensifying screens (Appligene, Illkirch-Graffenstaden, France).
Cytokine Assays
Cytokine concentrations in HTEC and MITC (two cell lines obtained from stroma cells transfected by SV40) supernatants were assayed by enzyme-linked immunosorbent assays (ELISA). Human IL-6, IL-8, RANTES, MIP-1, MCP-1, and TNF-α ELISA kits were purchased from Genzyme. Undiluted samples were assayed in duplicate and cytokine concentrations were read from calibration curves constructed with serial dilutions of the respective recombinant cytokines.
Electrophysiological Analysis
Cell Culture
In brief, cells were grown to confluence in RPMI containing penicillin-streptomycin (Gibco BRL) supplemented with 10% (v/v) fetal calf serum (Boehringer). On passaging, cells were treated with trypsin (1.5% ethylene glycol) for 10 minutes at room temperature and then gently dissociated. For electrophysiological experiments, cells from different passages were plated at 5 × 10 4 per 35-mm Petri dish (Nunc) and were used between days 2 and 4 after plating.
Solutions
To record the ACh-activated current, the bath solution contained (in mmol/L): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 N-(2-hydroxyethyl)piperazine-N′-(2-ethane sulfonic acid) (HEPES), 10 glucose; pH was adjusted to 7.3 with NaOH. The different cell superfusion media were the bath solution with the appropriate concentration of ACh, nicotine, atropine or α-bungarotoxin (αBgT) as indicated in the Results section. The intracellular pipette medium contained (in mmol/L): 115 K-aspartate, 25 KCl, 5 NaCl, 4 MgATP, 2 MgCl2, 0.1 ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 HEPES; pH was adjusted to 7.2 with KOH. Chemicals were purchased from Sigma Chemical Co. (St, Louis, MO). All experiments were conducted at room temperature (20 to 22°C).
Current Recording and Analysis
Currents were recorded with the classical whole-cell voltage-clamp method with a 500 MΩ feedback resistor and a patch-clamp amplifier (Axopatch 200A, Axon Instruments Inc., Foster City, CA) and filtered through an eight-pole Bessel 920 LPF low-pass filter (Frequency Devices) set at 20 kHz (−3 dB point). Patch pipettes (0.5 to 2 MΩ when filled with experimental solutions) were pulled from Pyrex capillaries (Corning code 7740, Corning Glass Inc., Corning, NY) and were not fire-polished before use. Resistance in series with the cell membrane was compensated, whereas neither cell membrane capacitive current nor leakage current was compensated. A flow of solution from one of a series of five piped outlets continuously superfused the cell from which the recording was being made. The flow rate of perfusion solutions was 50 to 100 μl/minute. Steady-state currents were elicited from a holding potential of −60 mV by a voltage ramp of 20 seconds, from −80 mV to +60 mV at a frequency of 0.04 Hz. Whole-cell currents were recorded and digitized at 6 kHz and analyzed with a microcomputer (Tandon, MCS 486) using a Digidata 1200 interface (Axon Instruments Inc.). They were printed with an HP Laserjet 4MP (Hewlett-Packard Co., San Diego, CA). Cell capacitance was used as an index of cell size 23 and was measured as follows: after electronic canceling of pipette capacitance and before compensation of series resistance, the membrane was perforated and cell membrane capacitance was measured. A sequence of 10 hyperpolarizing pulses of 10 mV amplitude and 10 ms duration was imposed on the cell membrane from −70 mV, at a frequency of 10 Hz. The capacitive currents produced by these pulses were averaged and cell membrane capacitance (Cm) was calculated as the ratio of the numerical integration of the averaged current transient (total charge) to the magnitude of the hyperpolarizing pulse. ACh-activated current (IACh) was measured as peak inward current amplitude with reference to the current before drug application. The current density (IACh/Cm) was calculated by dividing current amplitudes by membrane capacitance. When appropriate, data are given as means ± SD of n determinations. Statistical significance was determined by one-way analysis of variance. P values lower than 0.05 were considered significant. All experiments were conducted at room temperature (20 to 22°C).
Antigenic Modulation of AChR Expression
Serum from MG patients and control subjects were stored at −40°C until use. Their anti-AChR antibody titer was determined using human muscle AChR complexed to 125I-labeled α-bungarotoxin (125I-α-BgT) as antigen. 24 TE671 and MITC lines were plated in 35-mm Petri dishes at a density of 0.2 × 10 6 per plate. Three days after plating the culture medium was replaced by fresh culture medium containing an optimal concentration of anti-α-subunit 35 and 155 as described (usually 1:1000), 25 and MG or normal human sera (at 1:100 dilution). After overnight incubation at 37°C with the antibodies, the medium was replaced by fresh medium containing 10 nmol/L 125I-α-BgT and cultures were maintained for another 20 minutes at room temperature. Subsequently, the cells were processed as described above for surface AChR evaluation. Background radioactivity was estimated by incubating cells with a 100-fold excess of unlabeled α-BgT for 1 hour before adding 10 nmol/L 125I-α-BgT.
Percentage of surface AChR loss was estimated from the equation:
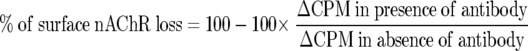 |
Results
Establishment of the MITC Line
Adherent primary epithelial cell-enriched cultures were obtained from a postnatal normal thymus. Cells with the morphology of packed polygonal epithelial cells were subcultured and subjected to electropermeabilization in the presence of plasmid pMK16 recombined with the origin-defective (ori−) SV40 genome. The resulting transfected thymic cells led to the establishment of seven epithelial cell lines and one thymic myoid cell line designated MITC. After 4 weeks in culture, a highly proliferative clone of cells was isolated with a cloning ring from a series of foci and amplified.
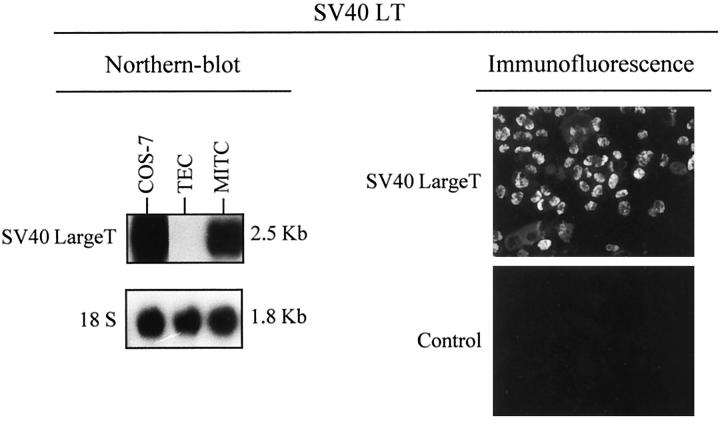
Northern blot and immunofluorescence analysis indicated that the large T oncogene of SV40 was functionally inserted into MITC line (Figure 1) ▶ . The SV40 LT transcript was identified as a main band of 2.5 kb in MITC cells and in the COS-7 cell line immortalized with SV40 LT (positive control). No hybridization was detected in the primary epithelial cell-enriched culture. The expression of the SV40 LT oncogene was also observed in these cells by means of immunofluorescence with a MAb to the SV40 LT antigen, and was detected within their nuclei (Figure 1) ▶ .
Figure 1.
SV40 large T antigen expression in MITC. Northern blot revealed a 2.5-kb mRNA band corresponding to the SV40 large T antigen in MITC and Cos7 cells (positive control). An 18 S probe was used to check the quality of the RNA. Indirect immunofluorescence using anti-SV40 large T antibody was clearly positive in MITC cells. These experiments were repeated three times at different subcultures.
Morphological Analysis of the MITC Line
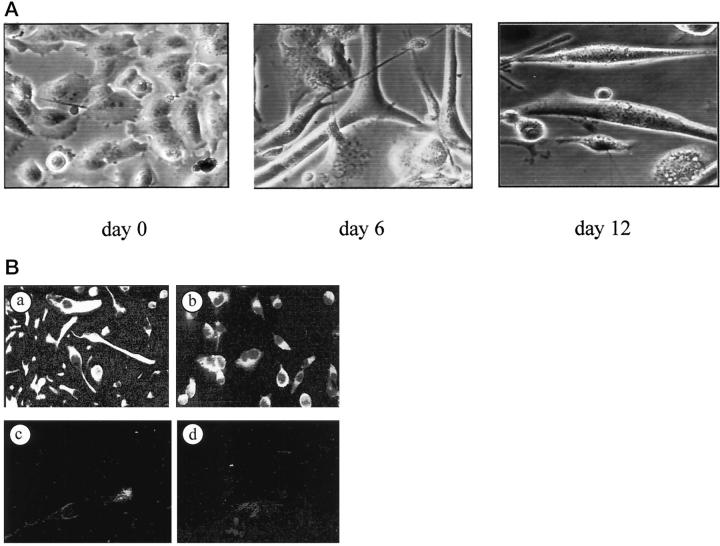
Morphological features of the MITC line after 10 passages in vitro are shown in Figure 2A ▶ . The structural appearance indicated that these cells were undifferentiated. Indeed, after treatment with cytosine arabinose, which is known to induce differentiation and fusion of myoblasts, 26 some cells presented multiple nuclei, some fusing cells were seen at day 6, and small myotubes at day 12. The myoid nature of the MITC line was evidenced by using anti-desmin (Figure 2B ▶ (a) and anti-troponin T antibodies (Figure 2B ▶ (b) in immunofluorescence studies. The cells were reactive to anti-desmin and anti-troponin T antibodies, while they were unreactive to the anti-keratin MAb (Figure 2B ▶ (c). The control antibody was negative (Figure 2B ▶ (d). These experiments were repeated after 3, 12, and 25 passages, with similar results.
Figure 2.
Morphological and immunohistological analysis of MITC cells. Phase-contrast microscopy of MITC cells at passage 8 (A). After treatment with cytosine-arabinose the cells presented multiple nuclei, some fusing cells were seen at day 6, and small myotubes at day 12 were observed. B: In another cell preparation anti-desmin (a) and anti-troponin T (b) antibodies were clearly reactive with MITC, while anti-keratin antibodies were not (c). The control antibody was consistently negative (d).
Phenotype of the MITC Line
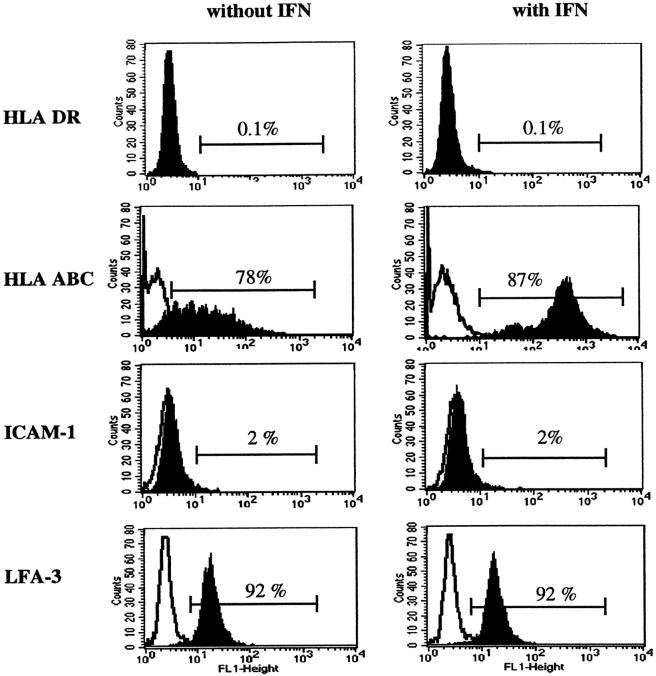
Since most stromal cells express the major histocompatibility complex (MHC) antigens, we examined the expression of MHC molecules by cell-surface staining with mAbs reactive with monomorphic determinants on class I or class II antigens. The MITC line expressed low levels of MHC class I molecules (HLA-ABC), which increased after treatment with human IFN-γ (Figure 3) ▶ . In contrast, MHC class II molecules (HLA-DR) were not present on the MITC line, even after treatment by IFN-γ (Figure 3) ▶ . In addition to MHC molecules, we investigated the expression of other cell-surface molecules involved in thymocytes-thymic stroma interactions that lead to mature thymocyte activation as ICAM-1 (CD54) and LFA-3 (CD58). 27 MITC cells did not express ICAM-1(CD54) even after treatment with IFN-γ. By contrast, LFA-3 (CD58) antigen was present in MITC cells, indicating possible interactions between thymocytes and myoid cells, and suggesting a possible role for these cells in thymocyte development (Figure 3) ▶ . This experience was reproduced three times with similar results.
Figure 3.
Analysis of MHC class I, MHC class II, ICAM-1, and LFA-3 antigens on MITC and effect of IFN-γ. Representative experiment: MITC cells express MHC class I and LFA-3 but not MHC class II antigens or ICAM-1. IFN-γ had no effect on HLA-DR expression, while it up-regulated HLA-ABC antigen expression.
This phenotype is similar to that observed ex vivo. Double staining experiments using anti-desmin antibody, and anti-CD54, or anti-CD58, or and anti-MHC class I molecule antibodies indicate that myoid cells on thymic section are essentially MHC class I-positive, LFA-3-positive and mainly ICAM-1 and MHC class II-negative. Thus the MITC line in culture has a similar phenotype as the myoid cells ex vivo according to these markers (Figure 4) ▶ . Finally, as shown in Figure 4 ▶ at low magnification, the myoid cells were mainly located in the medulla, and in the corticomedullary junction.
Figure 4.
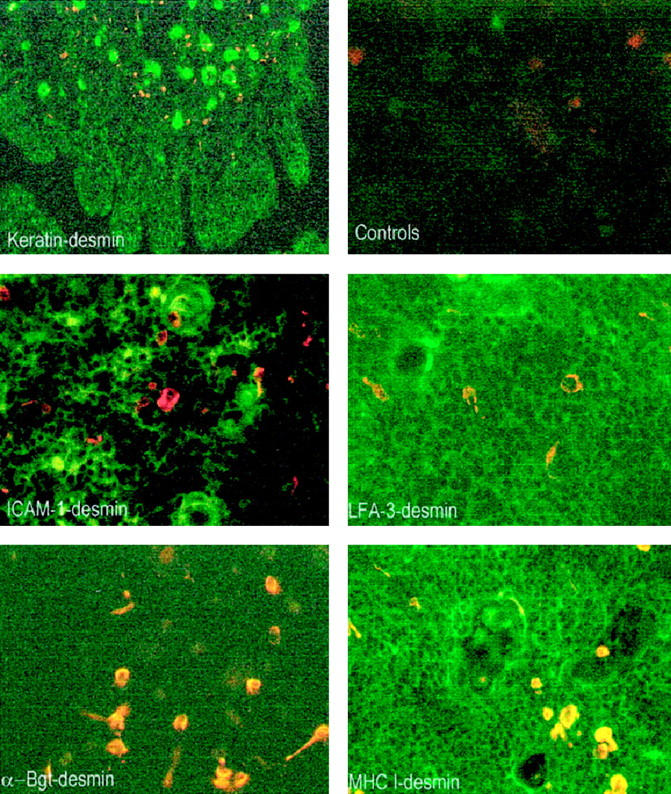
Analysis of myoid cells by double immunofluorescence on thymic sections. All microphotographs show superimposed staining of anti-desmin antibody (red staining) and one of the other antibodies (green staining). Double staining with anti-keratin antibody at a low magnification indicates that myoid cells (orange staining) are essentially located in the medulla and in the corticomedullary junction (magnification, ×40). Double staining with anti-ICAM-1 antibody indicates that myoid cells are mainly negative (magnification, ×200). Double staining with LFA-3 antibody shows a staining of the thymic network and indicates that myoid cells are positive (magnification, ×200). Double staining with α-Bgt indicates that myoid cells are the only cells that express α-Bgt binding sites (magnification, ×180). Double staining with anti-MHC class I antibody shows a strong staining of the thymic cells and indicates that myoid cells express MHC class I antigen (magnification, ×180).
Expression of AChR in MITC Line
mRNA Level
To examine the expression of AChR on MITC line (passage 9), we performed RT-PCR on total RNA. We used primers specific for the α, β, δ, γ, and ε subunits. The results are shown in Figure 5A ▶ . Primers specific for the extracellular regions (Table 1) ▶ of the α-subunit yielded two amplification products of 704 and 629 bp for P3A+ and P3A− isoforms, respectively. The two isoforms were equally expressed. A muscle sample was used as a positive control. With primers specific for the β-subunit, a single cDNA fragment of 579 bp was amplified in both MITC line and human muscle sample. Using primers specific for the δ-subunit, a single cDNA fragment was amplified in both the MITC line and muscle. The ε- and γ- subunits were also amplified in the MITC line, while the γ-subunit was not expressed in muscle as previously described 28 (Figure 5A) ▶ . The MITC line expressed both adult and fetal muscle AChR forms. These experiments were repeated after passage 25 and gave similar results.
Figure 5.
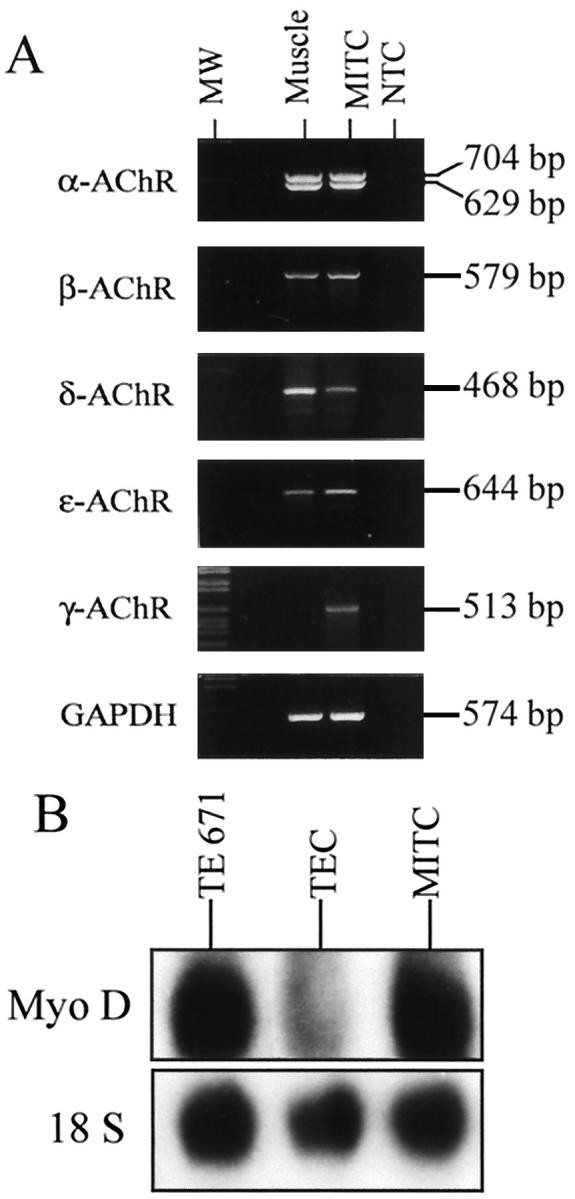
Expression of AChR subunits and MyoD factor in MITC cells. A: Amplification of the extracellular region of the α-subunit with primers A1 and A2 (704 and 629 bp for the P3A+ and P3A− isoforms, respectively), the β-subunit with primers D1 and D2 (579 bp), the γ-subunit with primers E1 and E2 (513 bp), and the ε-subunit with primers F1 and F2 (644 bp); GADPH was used to check RNA quality (574 bp). Muscle was used as a positive control. B: Expression of myogenic MyoD factor in MITC analyzed by Northern blotting.
Protein Level
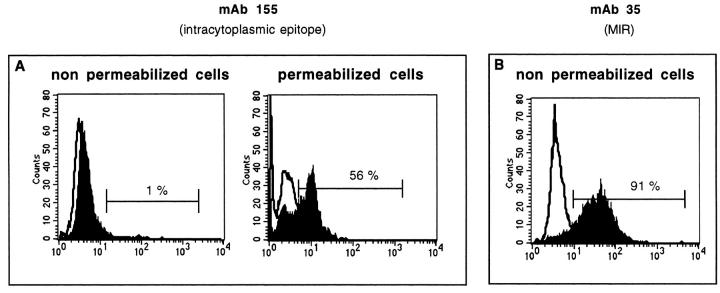
Cell suspensions obtained by trypsinization were stained with MAb 35, which targets an extracellular region of the AChR α-subunit, and MAb 155, which reacts with the cytoplasmic domain. The cells were analyzed by flow cytometry. As shown in Figure 6B ▶ , AChRs were present on the surface of MITC cells stained with MAb 35. Using MAb 155, the cells were unreactive. However, when MITC line was permeabilized and fixed with paraformaldehyde (2%) and then labeled with MAb 155, AChR was clearly expressed (Figure 6A) ▶ . The mean values ± SD for three different experiments are 91 ± 1% and 62 ± 5% using MAb 35 and MAb 155, respectively.
Figure 6.
Detection of AChR expression on MITC cells by flow cytometry in one representative experiment. Monoclonal antibody 155 directed to a cytoplasmic region was unreactive, unless cells were permeabilized (A), while MAb 35, which recognizes the MIR determinant in the extracellular domain, was reactive (B).
The expression of AChR on thymic myoid cells was previously shown on the thymic sections 9 and was confirmed in this study using fluorescent α-bungarotoxin (Figure 4) ▶ .
Expression of the MyoD1 Myogenic Factor in MITC Cells
The myoblast gene (MyoD1) is important for differentiation into skeletal rather than smooth or cardiac muscle cells. In two different cultures, we analyzed the expression of the MyoD1 transcript in MITC line by Northern blot analysis. Total RNA from MITC and TE671 lines were hybridized with a 32P-labeled cDNA MyoD1 (Myf3) probe (Figure 5B) ▶ . The MyoD1 transcript was clearly identified as a main band of 1.8 kb in MITC cells and TE671 cells (positive control) and faintly observed in TEC cells.
Evidence of ACh-Activated Current: Electrophysiological Measurements
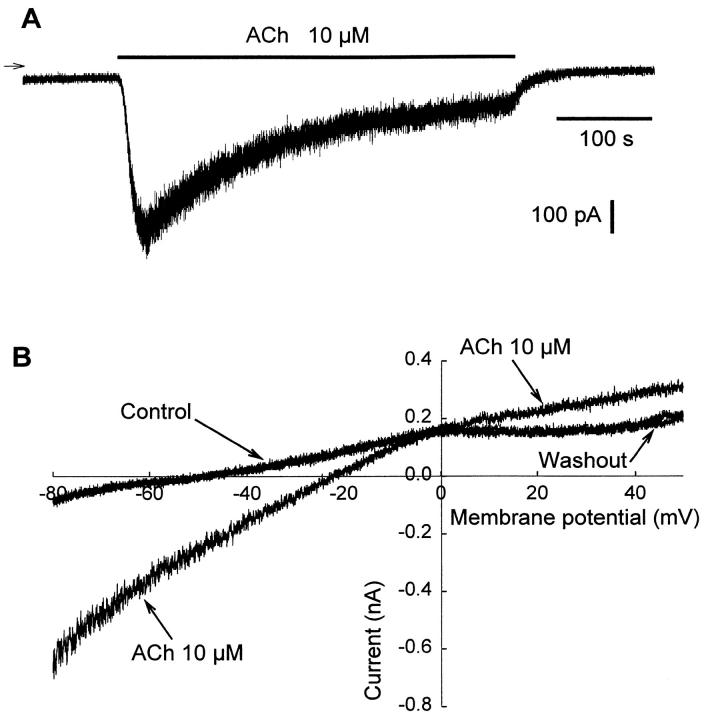
The response of AChRs in the MITC line was tested in patch-clamp measurements. On application of superfusion medium containing 10 μmol/L ACh to MITC line maintained at a holding potential of −80 mV, a transient inward current (IACh) surged rapidly to a peak value of approximately 400 to 600 pA, decayed as a result of desensitization, and stabilized after 3 to 4 minutes at about 30% of the peak value (Figure 7A) ▶ . During washout, the base line value was recovered within a few tens of seconds, but the sensitivity of the AChRs never returned to 100% even after 10 minutes of washout. The mean current density was 5.1 ± 0.2 pA/pF for 12 cells issuing from different subcultures (n = 12). Carbamylcholine mimicked ACh at the same concentration. As observed by Siara et al, 29 the rate of desensitization increased with the ACh concentration, and desensitization reached 100% with 100 μmol/L ACh (data not shown). Steady-state current/voltage relationships obtained before and during ACh superfusion (Figure 7B) ▶ showed a sensitive ACh current (IACh) with a reversal potential (EACh) of about −2 mV (−1.6 ± 2.1 mV, determined from six measurements). The activating effect of ACh on the steady-state current was completely reversible, as indicated by the current obtained during washout (Figure 7B) ▶ .
Figure 7.
Existence of an ACh-induced current in MITC cells. A: Representative whole-cell recording of ACh-induced inward current at a holding potential of −80 mV. Arrow indicates zero current. This experiment was repeated on 12 cells. B: Typical effect of ACh on the steady-state current-voltage relationship. Current traces obtained in control conditions, during application of external medium containing ACh to the cell, and after washout. The intersection of the control trace with the x axis gives a resting membrane potential of about −50 mV, whereas the intersection of the control trace with the ACh trace gives a reversal potential (EACh) of the ACh-induced current of about −2 to −4 mV. This experiment was performed on six cells from different subcultures.
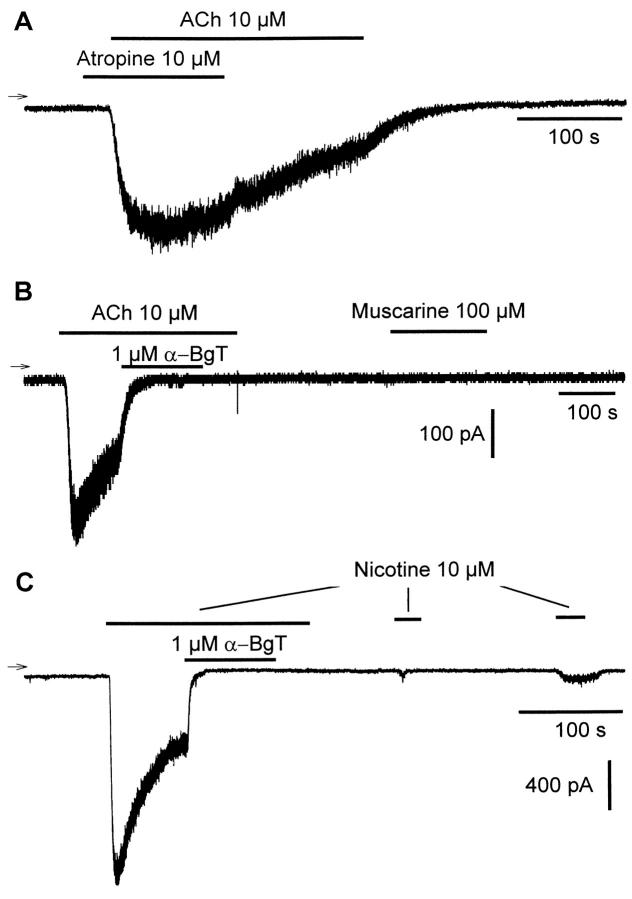
To determine the nicotinic or muscarinic nature of AChRs responsible to the activation of IACh, we performed experiments showing the following. 1) In the presence of 10 μmol/L atropine (Figure 8A) ▶ , 10 μmol/L ACh was still able to induce an inward current, whereas no particular change in the time course of desensitization was observed when application of atropine was stopped in the presence of ACh (these observations were consistently observed with 9 cells). 2) Application of 1 μmol/L α-BgT completely abolished the ACh-induced current (Figure 8B) ▶ . 3) Whereas consecutive application of 100 μmol/L muscarine (Figure 8B) ▶ failed to activate an inward current (n = 9), in 5 other cells to which short application of 10 μmol/L ACh alone gave rise to the usually observed inward current, subsequent application of 10 μmol/L muscarine was also ineffective. 4) Only partial recovery of the inward current was observed (Figure 8C) ▶ when successive applications of 10 μmol/L nicotine were made to the cell after complete blockade of the nicotine-activated current by short application of 1 μmol/L α-BgT (n = 3). The above results strongly support the notion that only the nicotinic AChRs are functionally expressed in MITC line.
Figure 8.
Only nAChRs are functional in MITC cells. A: Representative whole-cell ACh-induced inward current in the presence of atropine. B: Complete blockade of ACh-induced current by α-bungarotoxin (α-BgT) and total absence of muscarin-induced current. C: Partial recovery of a completely blocked nicotine-activated current by α-BgT during successive short applications of nicotine. Holding potential: −80 mV. Three different cells were used.
Antigenic Modulation of AChR by MG Sera on MITC Cells
TE671 human rhabdomyosarcoma cells, which express an AChR apparently identical to the extrajunctional AChR of human muscle, are appropriate for antigenic modulation experiments. As MITC line expresses both adult and fetal forms of AChR, we wondered whether MG sera would induce antigenic modulation on MITC cells, as is the case with TE671 cells. The effects of MG sera were analyzed in terms of α-BgT binding (Figure 9) ▶ . Incubation with three seropositive MG sera led to clear reduction of the 125I-α-BgT binding sites in both cell lines (40.5% ± 3.3). In contrast to seropositive MG sera, seronegative MG sera behaved similarly to control sera with both cell lines. In addition, MAb 155 (against a cytoplasmic epitope) had no effect on surface AChR expression, whereas a marked loss of AChR α-BgT binding sites was observed with MAb 35 (against an extracellular epitope). This effect had the same order of magnitude as that observed with seropositive MG sera. Therefore, the MG patient sera induce similar effects on MITC and TE671 cell lines.
Figure 9.
Comparative antigenic modulation of AChR on MITC and TE671 cells by MG sera. The effects of MG sera (three seropositive and three seronegative), control sera and anti-AChR mAbs (MAb 35 directed to the MIR region, and MAb 155 directed to a cytoplasmic domain) were tested on MITC and TE671 cells on the number of α-bungarotoxin binding sites. Very similar data were obtained in MITC and TE671 cells.
Cytokine Production by MITC Cells
The spontaneous production of cytokines was measured by ELISA in the supernatants of MITC line at the passage 9. Previous studies indicate that rat thymic myoid 871207B cells produce IL-1-α, IL-6, and IL-7. 30 In MITC line several cytokines and chemokines were detected: TNF-α, IL-6, IL-8, IL-10, RANTES, MIP-1α, and MCP-1 (Table 2) ▶ . The cytokine profile of MITC cells is different from that of the epithelial cell lines (obtained from the same stroma cells, as MITC, transfected by SV40), indicating that the two components of the thymic stroma have distinct roles. MITC cells produced higher levels of IL-8 and TNF-α but lower level of IL-6 and RANTES, compared to human thymic epithelial cell lines.
Table 2.
Cytokine and Chemokine Production in Supernatants
| Cell line | IL-6 | TNF-α | MCP-1 | IL-8 | RANTES | MIP-1α |
|---|---|---|---|---|---|---|
| HTEC-1 | 100 | 117 | 11923 | 797 | 6463 | 130 |
| HTEC-2 | 522 | 118 | 14594 | 640 | 5350 | 196 |
| HTEC-4 | 254 | 110 | 13573 | nd | 5975 | 178 |
| MITC | 16 | 370 | 14588 | 4793 | 326 | 79 |
Cytokine and chemokine concentrations (pg/ml) in the supernatants of three epithelial cell lines (HTEC-1, -2, and -4) and in the MITC line were assayed by ELISA. Results are expressed as means of duplicate measures.
Evidence of a Protective Effect of MITC Line on Thymocyte Apoptosis
To explore the possible role of the MITC line on thymocyte development, thymocytes were co-cultured with MITC or HTEC line for 24 hours, and we analyzed the apoptosis state of the total human thymocytes. We have first verified that trypsin treatment does not affect the staining with annexin which binds to phosphatidylserine on the surface of apoptotic cells (data not shown). Three criteria were analyzed. First, the absolute number of living thymocytes was 88 ± 3% when they were co-cultured with myoid cells, 73 ± 2% when they were co-cultured with HTEC, and 69 ± 1% when thymocytes were cultured alone. Second, FSC/SSC parameters showed two distinct gates, gate R1 containing mainly apoptotic cells and gate R2 containing mainly living cells. 31 The ratio of the numbers of cells included in gate R1/gate R2 was clearly modified when thymocytes were co-cultured with MITC (0.20 versus 0.32 in presence of HTEC, and 0.37 in control thymocytes cultured alone), indicating that the number of apoptotic cells was lower when thymocytes were co-cultured in presence of MITC, but not of HTEC (Figure 10A) ▶ . The third parameter analyzed was annexin V staining. Among cells included in the R2 gate (mainly living cells), the percentage of annexin V staining was decreased when cells were co-cultured with MITC line (1.6% versus 12.5% in control, and 13% in presence of HTEC). The values of annexin V-positive cells in the gate R2 obtained from three different experiments are shown in Figure 10B ▶ .
Figure 10.
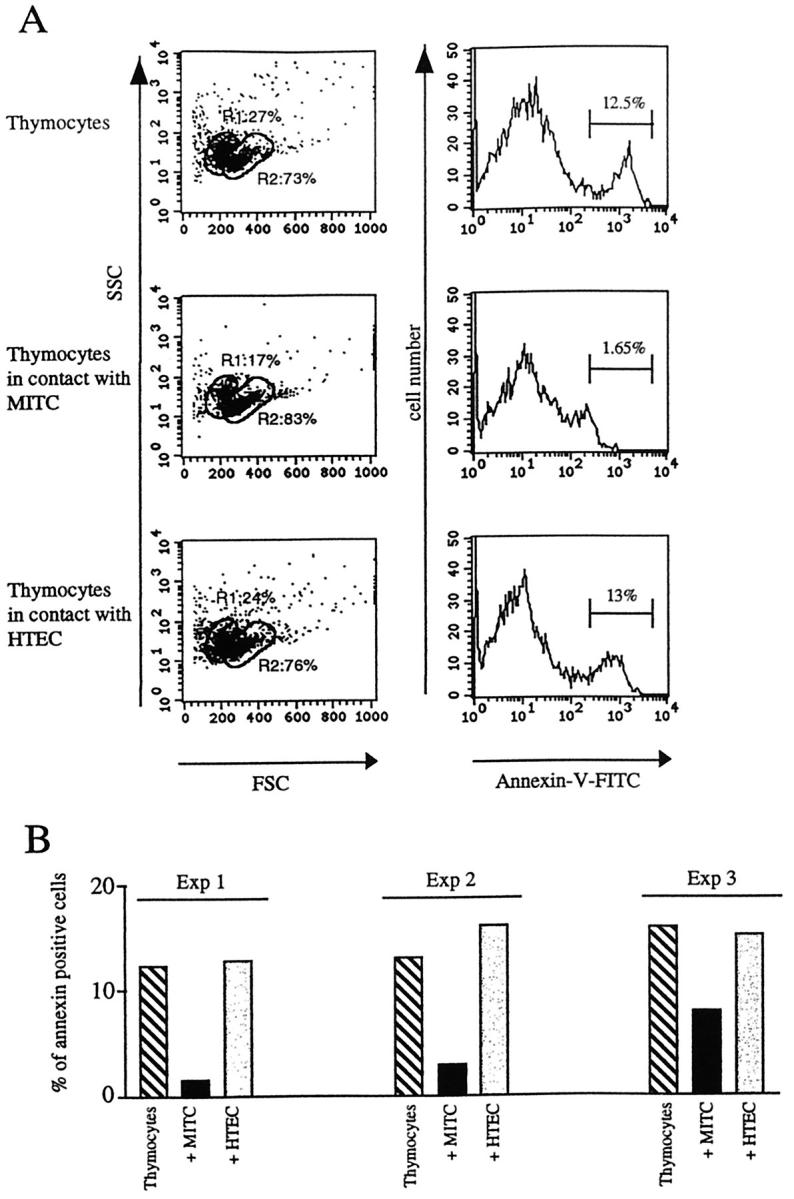
Analysis of apoptotic thymocytes. FCS/SSC parameters define two gates: R1, which contains cells that are mainly apoptotic cells, and R2 which contains cells that are mainly living cells. When thymocytes are co-cultured with MITC, the ratio R1/R2 is decreased. Annexin V FITC staining in thymocytes alone or co-cultured with MITC or HTEC indicates the percentage of apoptotic thymocytes. In the presence of MITC the percentage of annexin V-positive cells is reduced. A: One representative experiment. B: Results obtained from three independent experiments.
Together these three parameters indicated that the MITC line had a protective effect on thymocyte apoptosis.
Discussion
The main findings in this study are the following. 1) Human thymic myoid cells can be immortalized from thymic explant cultures. 2) These cells express both the fetal and adult forms of muscle AChR at the mRNA level. 3) α-Subunit AChR protein expression is detectable in MITC line by flow cytometry. 4) MITC line express a functional AChR, as shown by patch-clamp analysis. 5) AChR expression on MITC line is down-modulated by MG sera, as on TE671 rhabdomyosarcoma cells, making MITC line an interesting tool for AChR antigenic modulation experiments. 6) MITC line produces high levels of TNF-α and IL-8 and protects thymocytes from apoptosis, indicating that thymic myoid cells could play a role in thymocyte differentiation.
As far as we know, this is the first successful immortalization of human thymic myoid cells obtained by functional insertion of the SV40 oncogene. After transfection, the cell line was maintained in culture for up to 25 passages. Injection into nude mice induced palpable tumors after 40 to 50 days. Consequently, we may properly conclude that MITC line is engaged in the immortalization processus to the permanent cell lines.
Myoid Nature of MITC Line
The following experimental arguments support the myoid nature of MITC line.
1. In the postnatal thymus, thymic myoid cells express several striated-muscle-specific proteins, including myosin, 32 desmin, and troponin T. 9 Accordingly, we found that MITC line was troponin T-positive and desmin-positive, but keratin-negative. In addition, the MITC line has similar phenotype characteristics as myoid cells ex vivo, according to AChR, MHC class I and class II, LFA-3, and ICAM-1 antigens.
2. Our data clearly indicate the presence of MyoD transcripts in MITC cells. MyoD is expressed only in skeletal muscle, and activates myogenesis by directly binding to the control regions of muscle-specific genes. 33 Pluripotential stem cells in the thymus might express such a gene and develop into immature skeletal muscle cells in certain conditions. This is supported by the fact that cell types as different as osteocytes and chondrocytes can differentiate from thymus cells in appropriate conditions in vitro. 34
3. Human MITC line expressed AChR in both adult and fetal forms. In addition, anti-AChR autoantibodies induced a loss of AChR on MITC cells in vitro, similarly to TE671 cells; this mechanism was also observed using the anti-MIR monoclonal antibody MAb 35. These results indicate that AChR present on these cells is recognized by antibodies found in MG sera.
Some of MG patients’ antibodies may show a marked preference for adult or fetal AChR, and some patients (up to 7%) are negative in diagnostic assays using only fetal AChR. That could explain why 10 to 15% of patients with clinical MG have very low titers of antibodies (0.2 to 2 nmol/L). 35 To enhance the sensitivity of the diagnostic assay for low-titer sera, Beeson et al 36 used TE671 cells transfected with the epsilon subunit of AChR (TE671-ε) as a source of AChR antigen. Thus a mix of AChR extracts from TE671-ε and TE671 cells, in which adult and fetal AChR are present, was as sensitive as AChR from amputated leg muscle in MG diagnostic assay. Our data show that MITC line expresses constitutively the adult and the fetal forms of the AChR, thus MITC line might be an appropriate source of AChR for titrating anti-AChR antibodies in MG sera, as well as for studies of their functional effects.
MITC Cells Express Functional Nicotinic AChR Receptors
Furuya et al found that cultured human thymic myoid cells were excitable after electrical stimulation, like very immature skeletal muscle cells, and that some of the cultured cells had fast overshot action potentials without slow repolarizing calcium components, like primary cultured skeletal muscle cells, 16 but the response of AChR was never tested. The response of AChR of MITC line was tested here by patch-clamp measurements on application of ACh. We observed an ACh-induced inward current with systematic desensitization, similar to that observed by Siara et al 29 on the TE671 cell line. To determine the nicotinic or muscarinic nature of the AChRs responsible for the activation of IACh, we used atropine and α-BgT, which are antagonists specific for muscarinic and nicotinic receptors, respectively. 37 Application of atropine during the desensitization phase of ACh-induced current, or application of ACh to the cell already in the presence of atropine, induced no change in the characteristics of the ACh-induced current. Moreover, application of α-BgT completely abolished the ACh-induced current, and no current was observed when muscarin was applied to the cell. These results demonstrate that nicotinic, not muscarinic, AChRs are functionally expressed on MITC line.
This functional receptor on myoid cells contrasts with the nonfunctional receptors on TEC. Indeed, AChR is expressed on TEC in mice, 38 and humans, 17 but several reports indicate that AChR expressed on TEC is non functional. 39 In our experience, no ACh-induced inward current can be detected by stimulating TEC lines (data not shown). This is a major difference between AChR expressed on myoid and epithelial cells.
It is tempting to speculate that thymic AChR on myoid cells has a role in the primary autosensitization underlying myasthenia gravis. Myoid cells are rare in the thymus of MG and control patients, 9 and are located mainly in the medulla. Myoid cells have a high level of mRNA for the different AChR subunits relative to TEC (data not shown). However, and by contrast to TEC, myoid cells are consistently HLA-DR-negative. In addition, there is no major difference in myoid-cell frequency or aspect between MG patients and control subjects, 9 which argues against a primary role of myoid cells in the autosensitization against AChR. However, in pathological circumstances, myoid cells might be degraded and AChR fragments might be captured and presented by professional antigen-presenting cells, inducing the activation of thymic autoreactive cells. Thus, AChR on both TEC and myoid cells might participate in the autosensitization of thymic lymphocytes to AChR.
Role of Myoid Cells in the Thymus
Thymic stromal cells are thought to play a critical role in the proliferation, differentiation, and selection of precursor cells in the T cell lineage, but the precise mechanisms by which these events occur, and the particular contribution of individual thymic stromal components, are largely unknown. Most cells of the thymic stroma are of epithelial origin. Human thymic epithelial cells have been shown to produce numerous cytokines including IL-1, IL-6, granulocyte colony-stimulating factor (G-CSF), and macrophage CSF (M-CSF), that are important in various stages of thymocyte differentiation. 40
We observed that human MITC line was capable of producing biologically active cytokines, and chemokines including IL-6, IL-8, IL-10, TNF-α, RANTES, MIP-1, and MCP-1. Compared to human thymic epithelial cell line (HTEC), we found that MITC line produced high levels of IL-8 and TNF-α, but low levels of RANTES and IL-6, indicating that these two components of the thymic stroma are likely to play distinct roles during thymocyte development.
The role of myoid cells has been explored by co-culturing the myoid cells with thymocytes. Interestingly, the myoid cell line appears to protect thymic cells from spontaneous apoptosis, while the human HTEC line has no effect. The mechanism of the protective effect needs further investigation. It could be mediated by soluble factors or by direct cell contacts. The presence of the adhesion molecule LFA-3 (CD58) on MITC makes possible interactions between myoid cells and most thymocytes that constitutively express CD2. Indeed LFA-3 is important in the interaction with thymocytes at both immature and mature stages of development. 27
Expression of MHC molecules by components of the thymic microenvironment is required for normal T cell development 41 and has been implicated in the selection of the emerging T cell repertoire. MITC cells maintained the expression of HLA class I antigens, which increased after treatment with IFN-γ, indicating that MITC cells remain responsive to IFN-γ in vitro. In contrast, HLA class II antigens were not present on the cell surface, possibly owing to the absence of the class II transactivator (CIITA) necessary for both constitutive and IFN-γ-induced MHC class II expression. 42
Taken together, our data indicate that myoid cells are an original thymic cell compartment. They may have a role in thymic physiology, as they produce cytokines involved in thymic development, and chemokines, as they protect thymocytes from apoptosis and as they express LFA-3 and HLA class I antigens. They may also have a role in myasthenia gravis, as they express a high level of AChR, in both the mature and embryonic forms.
Acknowledgments
We thank Dominique Emilie and Alain Portier for the determination of cytokine and chemokine concentrations.
Footnotes
Address reprint requests to Sonia Berrih-Aknin, CNRS ESA 8078, Hôpital Marie Lannelongue, 133 Avenue de la Résistance, 92350 Le Plessis-Robinson, France. E-mail: berrih@wanadoo.fr.
Supported by grants from AFM (Association Française contre les Myopathies), CNRS (Centre National de la Recherche Scientifique), CNAMTS (Caisse Nationale d’Assurance Maladie des Travailleurs Salariés), and the BIOMED program of EU (CT93–1100). A.W. received a doctoral fellowship from AFM.
References
- 1.Kisielow P, von Boehmer H: Development and selection of T cells: facts and puzzles. Adv Immunol 1995, 58:87-209 [DOI] [PubMed] [Google Scholar]
- 2.Boehmer V: Positive selection of lymphocytes. Cell 1994, 76:219-228 [DOI] [PubMed] [Google Scholar]
- 3.Anderson G, Moore NC, Owen JJT, Jenkinson EJ: Cellular interactions in thymocyte development. Annu Rev Immunol 1996, 14:73-99 [DOI] [PubMed] [Google Scholar]
- 4.Van de Velde RL, Friedman NB: Thymic myoid cells and myasthenia gravis. Am J Pathol 1970, 59:347-368 [PMC free article] [PubMed] [Google Scholar]
- 5.Puchtler H, Meloan SN, Branch BW, Gropp S: Myoepithelial cells in human thymus: staining, polarization and fluorescence microscopic studies. Histochemistry 1975, 45:163-176 [DOI] [PubMed] [Google Scholar]
- 6.Dardenne M, Savino W, Bach JF: Thymomatous epithelial cells and skeletal muscle share a common epitope defined by a monoclonal antibody. Am J Pathol 1987, 126:194-198 [PMC free article] [PubMed] [Google Scholar]
- 7.Wekerle H, Hohlfeld R, Ketelsen UP, Kalden JR, Kalies I: Thymic myogenesis, T-lymphocytes and the pathogenesis of myasthenia gravis. Myasthenia Gravis. Edited by Drachman DB. Ann NY Acad Sci 1981, pp 455–476 [DOI] [PubMed]
- 8.Nakamura H, Ayer Le Lievre C: Neural crest and thymic myoid cells. Curr Top Dev Biol 1986, 20:111-115 [DOI] [PubMed] [Google Scholar]
- 9.Schluep M, Willcox N, Vincent A, Dhoot GK, Newsom Davis J: Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol 1987, 22:212-222 [DOI] [PubMed] [Google Scholar]
- 10.Kao I, Drachman DB: Thymic muscle cells bear acetylcholine receptors: possible relation to myasthenia gravis. Science 1977, 195:74-75 [DOI] [PubMed] [Google Scholar]
- 11.Seifert R, Christ B: On the differentiation and origin of myoid cells in the avian thymus. Anat Embryol (Berl) 1990, 181:287-298 [DOI] [PubMed] [Google Scholar]
- 12.Le Brigand H: Evolution of the indications and results of treatment of myasthenia by thymectomy. Int Surg 1982, 67:9-11 [PubMed] [Google Scholar]
- 13.Berrih-Aknin S, Morel E, Raimond F, Safar D, Gaud C, Binet JP, Levasseur P, Bach JF: The role of the thymus in myasthenia gravis: immunohistological and immunological studies in 115 cases. Myasthenia Gravis: Biology and Treatment. Edited by Drachman DB. Ann NY Acad Sci 1987, pp 50–70 [DOI] [PubMed]
- 14.Cohen Kaminsky S, Levasseur P, Binet JP, Berrih Aknin S: Evidence of enhanced recombinant interleukin-2 sensitivity in thymic lymphocytes from patients with myasthenia gravis: possible role in autoimmune pathogenesis. J Neuroimmunol 1989, 24:75-85 [DOI] [PubMed] [Google Scholar]
- 15.Leprince C, Cohen Kaminsky S, Berrih Aknin S, Vernet Der Garabedian B, Treton D, Galanaud P, Richard Y: Thymic B cells from myasthenia gravis patients are activated B cells: phenotypic and functional analysis. J Immunol 1990, 145:2115-2122 [PubMed] [Google Scholar]
- 16.Furuya A, Kobayashi T, Kameda N, Tsukagoshi H: Human myasthenia gravis thymic myoid cells: de novo immunohistochemical and intracellular electrophysiological studies. J Neurol Sci 1991, 101:208-220 [DOI] [PubMed] [Google Scholar]
- 17.Wakkach A, Guyon T, Bruand C, Tzartos S, Cohen Kaminsky S, Berrih Aknin S: Expression of acetylcholine receptor genes in human thymic epithelial cells: implications for myasthenia gravis. J Immunol 1996, 157:3752-3760 [PubMed] [Google Scholar]
- 18.Berrih S, Arenzana Seisdedos F, Cohen S, Devos R, Charron D, Virelizier JL: Interferon-γ modulates HLA class II antigen expression on cultured human thymic epithelial cells. J Immunol 1985, 135:1165-1171 [PubMed] [Google Scholar]
- 19.Chastre E, Di Gioia Y, Barbry P, Simon Bouy B, Mornet E, Fanen P, Champigny G, Emami S, Gespach C: Functional insertion of the SV40 large T oncogene in cystic fibrosis intestinal epithelium. Characterization of CFI-3 cells. J Biol Chem 1991, 266:21239-21246 [PubMed] [Google Scholar]
- 20.Tzartos SJ, Kokla A, Walgrave SL, Conti Tronconi BM: Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67–76 of the α subunit. Proc Natl Acad Sci USA 1988, 85:2899-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermes I, Haanen C, Steffens-Nakken H, Reutlingsperger C: A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods 1995, 184:39-51 [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 23.Isenberg G, Klockner U: Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch 1982, 395:30-41 [DOI] [PubMed] [Google Scholar]
- 24.Morel E, Raimond F, Goulon Goeau C, Berrih S, Vernet Der Garabedian B, Harb J, Bach JF: Assay of acetylcholine receptor antibodies in myasthenia gravis: a study of 329 sera. Nouv Presse Med 1982, 11:1849-1854 [PubMed] [Google Scholar]
- 25.Sophianos D, Tzartos SJ: Fab fragments of monoclonal antibodies protect the human acetylcholine receptor against antigenic modulation caused by myasthenic sera. J Autoimmun 1989, 2:777-789 [DOI] [PubMed] [Google Scholar]
- 26.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD: Agrin acts via a MuSK receptor complex. Cell 1996, 85:513-523 [DOI] [PubMed] [Google Scholar]
- 27.Singer KM, Denning SM, Whichard LP, Haynes BF: Thymocyte LFA-1, and thymic epithelial cell ICAM-1 molecules mediate binding of active human thymocytes to thymic epithelial cells. J Immunol 1990, 144:2931-2939 [PubMed] [Google Scholar]
- 28.Guyon T, Wakkach A, Poea S, Mouly V, Klingel-Schmitt I, Levasseur P, Beeson D, Asher O, Tzartos S, Berrih-Aknin S: Regulation of acetylcholine receptor gene expression in human myasthenia gravis muscles. J Clin Invest 1998, 102:249-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siara J, Ruppersberg JP, Rudel R: Human nicotinic acetylcholine receptor: the influence of second messengers on activation and desensitization. Pflugers Arch 1990, 415:701-706 [DOI] [PubMed] [Google Scholar]
- 30.Iwakami N, Kunishita KA, Yamamoto T, Nonaka H, Kamo I: Analysis of lymphoproliferative cytokines produced by thymic myoid cells. Immunology 1996, 87:108-112 [PMC free article] [PubMed] [Google Scholar]
- 31.Swat W, Ignatowicz L, Kisielow P: Detection of apoptosis of CD4+CD8+ thymocytes by flow cytometry. J Immunol Methods 1991, 137:79-87 [DOI] [PubMed] [Google Scholar]
- 32.Drenckhahn D, von Gaudecker B, Muller Hermelink HK, Unsicker K, Groschel Stewart U: Myosin and actin containing cells in the human postnatal thymus: ultrastructural and immunohistochemical findings in normal thymus and in myasthenia gravis. Virchows Arch B Cell Pathol 1979, 32:33-45 [DOI] [PubMed] [Google Scholar]
- 33.Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, Weintraub H: MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 1989, 58:823-831 [DOI] [PubMed] [Google Scholar]
- 34.Friedenstein AJ, Lalykina KS: Thymus cells are inducible to osteogenesis. Eur J Immunol 1972, 2:602-603 [DOI] [PubMed] [Google Scholar]
- 35.Vincent A, Newsom Davis J: Acetylcholine receptor antibody characteristics in myasthenia gravis. III. Patients with low anti-AChR antibody levels. Clin Exp Immunol 1985, 60:631-636 [PMC free article] [PubMed] [Google Scholar]
- 36.Beeson D, Jacobson L, Newsom Davis J, Vincent A: A transfected human muscle cell line expressing the adult subtype of the human muscle acetylcholine receptor for diagnostic assays in myasthenia gravis. Neurology 1996, 47:1552-1555 [DOI] [PubMed] [Google Scholar]
- 37.Grassi F, Giovannelli A, Fucile S, Mattei E, Eusebi F: Cholinergic responses in cloned human TE671/RD tumour cells. Pflugers Arch 1993, 425:117-125 [DOI] [PubMed] [Google Scholar]
- 38.Wheatley LM, Urso D, Tumas K, Maltzman J, Loh E, Levinson AI: Molecular evidence for the expression of nicotinic acetylcholine receptor α-chain in mouse thymus. J Immunol 1992, 148:3105-3109 [PubMed] [Google Scholar]
- 39.Siara J, Rudel R, Marx A: Absence of acetylcholine-induced current in epithelial cells from thymus glands and thymomas of myasthenia gravis patients. Neurology 1991, 41:128-131 [DOI] [PubMed] [Google Scholar]
- 40.Meilin A, Shoham J, Sharabi Y: Analysis of thymic cell subpopulation grown in vitro on extracellular in defined medium. IV. Cytokines secreted by human thymic epithelial cells in culture and their activities on murine thymocytes and bone marrow cells. Immunology 1992, 77:208-213 [PMC free article] [PubMed] [Google Scholar]
- 41.Kruisbeek AM, Mond JJ, Fowlkes BJ, Carmen JA, Bridges S, Longo DL: Absence of the Lyt-2-,L3T4+ lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A-bearing antigen-presenting cell function. J Exp Med 1985, 161:1029-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reith W, Steimle V, Mach B: Molecular defects in the bare lymphocyte syndrome and regulation of MHC class II genes. Immunol Today 1995, 16:539-546 [DOI] [PubMed] [Google Scholar]