Abstract
Atypical adenomatous hyperplasia (AAH) has recently been implicated as a precursor to lung adenocarcinoma. We previously reported loss of heterozygosity (LOH) in tuberous sclerosis (TSC) gene-associated regions to frequently be observed in lung adenocarcinoma with multiple AAHs. In this study, we analyzed LOH in four microsatellite loci on 9q, including the TSC1 gene-associated region, and four loci on 16p, including the TSC2 gene-associated region, in both 18 AAHs and 17 concomitant lung adenocarcinomas from 11 patients. Seven of 18 (39%) AAHs and 9 of 17 (53%) adenocarcinomas displayed LOH on 9q. Five (28%) AAHs and seven (41%) adenocarcinomas harbored LOH at loci adjacent to the TSC1 gene. Four of 18 (22%) AAHs and 6 of 17 (35%) adenocarcinomas displayed LOH on 16p. One (6%) AAH and five (29%) adenocarcinomas harbored LOH at loci adjacent to the TSC2 gene. These findings may indicate a causal relationship of LOH on 9q and 16p in a fraction of AAH lesions and adenocarcinomas of the lung. Especially, the frequencies of LOH on 9q and at the TSC1 gene-associated region were high. The TSC1 gene or another neighboring tumor suppressor gene on 9q might be involved in an early stage of the pathogenesis of lung adenocarcinoma.
Carcinogenesis is a multistep process that results from an accumulation of genetic alterations in oncogenes and tumor suppressor genes. It is reasonable to regard each preneoplastic lesion as possibly having a characteristic genetic change and it is essential to investigate the biological features of preneoplastic lesions to elucidate the pathogenesis of carcinomas. In lung cancers, squamous dysplasia has long been recognized as a preneoplastic lesion of squamous cell carcinoma. 1,2 However, the etiology of adenocarcinoma, one of the major histological types of lung cancer, is not well understood. Several genetic alterations in atypical adenomatous hyperplasia (AAH), such as K-ras or p53 mutations or loss of heterozygosity (LOH) on chromosomes 3p, 9p, or 17p, have been reported. 3-10 These genetic abnormalities and other immunohistochemical or morphometric abnormalities in AAH overlap with those of adenocarcinomas. 11-13 Thus, AAH has been implicated as a preneoplastic lesion of lung adenocarcinoma, and listed as a precursor lesion in the World Health Organization 1999 classification of lung tumors. 14
Tuberous sclerosis (TSC) is a relatively common autosomal-dominant disease that causes mental retardation, seizures, and multiple hamartomas in many organs including the brain, eyes, kidney, skin, and heart. Mutations of either the TSC1 or the TSC2 gene are responsible for this disease. 15,16 The lesion most commonly described in the lung is lymphangioleiomyomatosis, which occurs in 1% of patients with TSC and affects only females. 17 Multifocal micronodular pneumocyte hyperplasia (MMPH) has also been described as a rare pulmonary manifestation of TSC. 18 MMPH is so similar to AAH morphologically that histological distinction between MMPH and AAH is difficult. 19 We previously reported that LOH in the TSC gene-associated regions was frequently observed in lung adenocarcinoma with multiple AAHs. 20 In this study, we analyzed microsatellite alterations at several microsatellite loci including the TSC gene-associated regions in both AAH lesions and concomitant adenocarcinomas to clarify the stage of lung adenocarcinoma pathogenesis in which these genetic alterations are involved.
Materials and Methods
From November 1997 through June 1998, 126 patients underwent surgical resection of lung cancer at our hospital. AAH was found in 22 patients with adenocarcinoma, 2 patients with adenosquamous carcinoma, 1 with squamous cell carcinoma, and 1 with large cell carcinoma. AAH is much more frequently found in patients with adenocarcinoma than in those with other histological subtypes.
We analyzed LOH on chromosomes 9q and 16p in 18 AAHs and 17 adenocarcinomas from 11 patients. The patients’ characteristics are summarized in Table 1 ▶ . There were four males and seven females, and their ages ranged from 47 to 74 years. Seven of the 11 (64%) patients were non-smokers. Two patients had a past history of malignancy. Three patients had a family history of malignancy in first-degree relatives; two were lung cancers, one a gastric cancer. There were three patients with multiple adenocarcinomas, and nine with multiple AAHs. All patients with multiple adenocarcinomas had concomitant multiple AAHs.
Table 1.
Clinicopathological Characteristics of Patients
| Patient | Age | Gender | Smoking (pack-years) | Past history of malignancy | Family history of malignancy | Number of adenocarcinomas | Number of AAH |
|---|---|---|---|---|---|---|---|
| 1 | 74 | M | 41 | − | − | 4 | 2 |
| 2 | 72 | F | 0 | − | + (lung, sister)† | 1 | 1 |
| 3 | 60 | F | 0 | + (thyroid)* | − | 4 | 3 |
| 4 | 51 | F | 0 | − | + (lung, mother) | 1 | 3 |
| 5 | 47 | M | 4 | − | − | 1 | 2 |
| 6 | 62 | F | 0 | − | − | 3 | 6 |
| 7 | 75 | F | 0 | − | − | 1 | 1 |
| 8 | 65 | M | 45 | − | − | 1 | 2 |
| 9 | 65 | M | 70 | + (stomach) | − | 1 | 5 |
| 10 | 61 | F | 0 | − | − | 1 | 2 |
| 11 | 69 | F | 0 | − | + (stomach, father) | 1 | 2 |
*Organs affected by a past malignancy.
†Affected organ and family member with history of malignancy.
All resected specimens prefixed with 100% methanol were sliced at 5-mm intervals and examined macroscopically. Appropriate tissue sections were fixed with 100% methanol and embedded in paraffin. It is difficult to extract enough amount of DNA for molecular analysis from a tiny lesion, such as AAH. DNA extracted from formalin-fixed materials is often fragmented artificially. Using methanol fixation, even relatively higher molecular weight DNA was preserved well. 21
Primary lung adenocarcinomas and AAHs were evaluated microscopically by conventional hematoxylin and eosin (H&E) staining. The pathological characteristics of the 17 adenocarcinomas and 18 AAHs were assessed by two pathologists (AO and TY).
Histological Evaluation of Adenocarcinoma
Histological typing of adenocarcinomas was performed according to the World Health Organization classification of lung tumors. 14 Nuclear atypia was categorized into three grades: 1, nuclei that were uniform in size and slightly larger than those of reactive type II alveolar epithelial cells; 2, nuclei that were in uniform size and up to twice the size of reactive type II alveolar epithelial cells; 3, nuclei of various sizes and more than twice the size of reactive type II alveolar epithelial cells. The mitotic indices were divided into three grades: 1, ≤5 mitotic cells/10 high-power fields; 2, 6 to 15/10 high-power fields; 3, ≥16/10 high-power fields. The scar grades were classified into four grades based on fibrotic foci in the tumors: 1, no or minimal desmoplasia; 2, fibroblastic tissue with a small amount of collagen; 3, fibroblastic tissue with moderate to abundant collagen; 4, hyalinized tissue. 22
Histological Criteria of AAH
We analyzed solitary AAHs in this study, AAHs continuous with or directly adjacent to a primary adenocarcinoma were not included. The histological diagnosis of AAH was based on the following criteria, as previously described. 23 1) The lesion had well-defined boundaries and consisted of proliferation of single-layered atypical epithelial cells without central scar formation or collapse (Figure 1A) ▶ . 2) The cytoplasm was eosinophilic, and the cells often had a rounded or domed appearance resembling either type II pneumocytes or Clara cells. 3) The atypical cells in AAH had usually hyperchromatic nuclei and inconspicuous nucleoli, but the atypia was less marked than that of adenocarcinoma cells. AAHs were classified into two grades: 24 1) Low-grade AAH; the cell density was low to moderate, and the cells were arranged in a single layer, intermittently or focally and continuously, on the alveolar septa (Figure 1B) ▶ . Their nuclei were mostly small, but occasionally large, and exhibited lesser degrees of variation in size, shape, and hyperchromasia than high-grade AAH. 2) High-grade AAH; the cells were continuously and densely arranged in a single layer, but did not exhibit the piled-up structure often observed in adenocarcinoma (Figure 1C) ▶ . Their nuclei showed significant atypia, but lacked the margin irregularity and eosinophilic nucleoli observed in frank adenocarcinoma.
Figure 1.
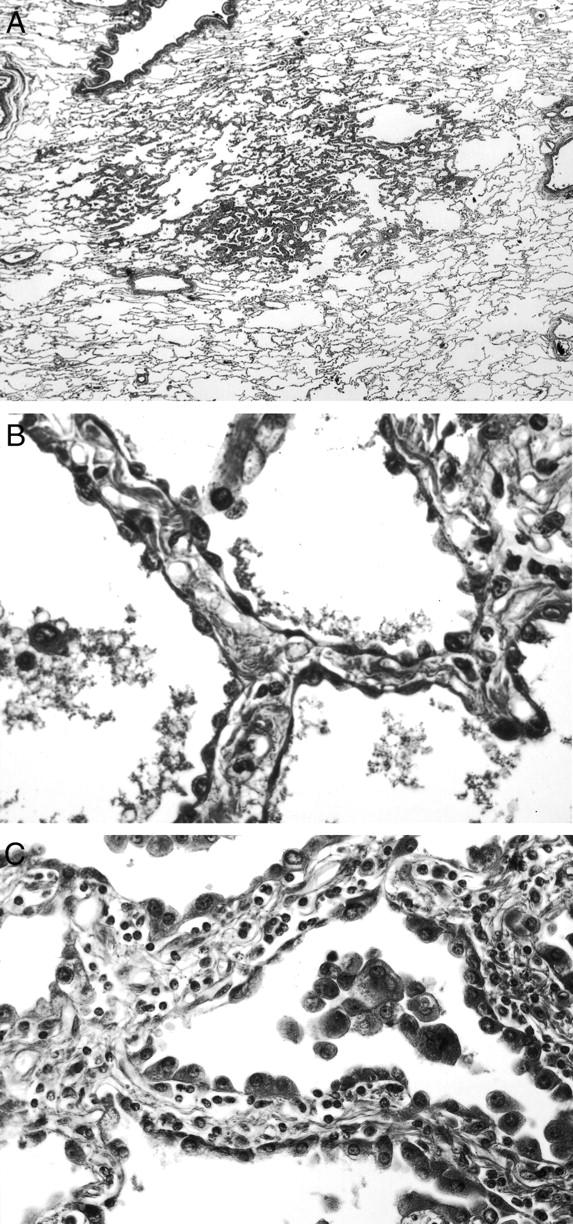
Histological features of AAH (H&E staining). A: The lesion has well-defined boundaries and no central scar formation or collapse. B: Low-grade AAH: the cell density is low to moderate, with the cells arranged in a single layer, intermittently or focally and continuously, on the alveolar septa. C: High-grade AAH: the cells are continuously and densely arranged in a single layer, but do not exhibit the piled-up structure often observed in adenocarcinoma. Original magnifications: ×2 (A), ×150 (B), ×100 (C).
Microdissection and DNA Extraction
Serial 5-μm slices were made from each block for microdissection. The slides were dewaxed with xylene and rehydrated in a graded alcohol series, then stained with H&E to define the location of AAHs or adenocarcinomas. Microdissection was performed under a microscope (BX50W1; Olympus, Tokyo, Japan) by using a microcapillary tube drawn to a thin tip with a micropipette puller (PC-10; Narishige, Tokyo, Japan) and a joystick-operated hydraulic micromanipulator (ONO-125; Olympus-Narishige, Tokyo, Japan). The microdissected cells were allowed to adhere to Parafilm (American Can, Greenwich, CT) and placed in 500-μl microcentrifuge tubes. Normal lymph node tissue or normal lung tissue was scraped with a 27-gauge needle to provide a normal control. DNA was extracted with a DNA extractor WB kit (Wako Pure Chemicals, Osaka, Japan). The DNA concentration was adjusted to ∼50 cell equivalents per μl.
Polymerase Chain Reaction (PCR)-Based LOH Analysis
We analyzed LOH on chromosome 9q (including the TSC1 gene-associated region) and 16p (including the TSC2 gene-associated region), using the following microsatellite markers. Four markers on 9q from centromere to telomere, D9S146 (9q13), D9S149 (9q34), D9S150 (9q34), and DBH (9q34); 25 and four markers on 16p from centromere to telomere, D16S300 (16p11.1-11.2), D16S292 (16p13.12-13.13), D16S291 (16p13.1), and D16S525 (16p13.3). 26,27 PCR was performed in a 20-μl volume of a mixture containing 10 mmol/L Tris (pH 8.3), 50 mmol/L KCl, 1.0 to 1.5 mmol/L MgCl2, 200 μmol/L of each Cy 5′-end labeled primer (Pharmacia Biotech, Tokyo, Japan), 0.25 U of Taq polymerase (TaKaRa Biomedicals, Shiga, Japan) and then cycled 36 to 38 times in a GeneAmp PCR System 9600 (Perkin Elmer, Foster City, CA); each cycle consisted of 1 minute at 94°C for denaturation, 2 minutes at 55 to 62°C for annealing, 1 minute at 72°C for strand elongation, and 7 minutes at 72°C for final elongation. The PCR products were diluted with a loading buffer consisting of 95% formamide, 20 mmol/L ethylenediaminetetraacetic acid (pH 8.0), and Dextran blue, and denatured for 5 minutes at 98°C. The samples were electrophoresed on 5% polyacrylamide gels containing 8.3 mol/L urea for 3 hours at 34 W using an ALFred DNA sequencer (Pharmacia Biotech). To confirm the reproducibility of the experiment, all cases were examined at least twice by independent PCR and electrophoresis. LOH was considered to be present if the reduction rate of the height of the allele in the tumor was >40%, as previously defined. 28
Statistical Analyses
The two-sided Fisher’s exact test was used for statistical analysis (Stat View-J 5.0, Macintosh). A P value <0.05 was considered significant.
Results
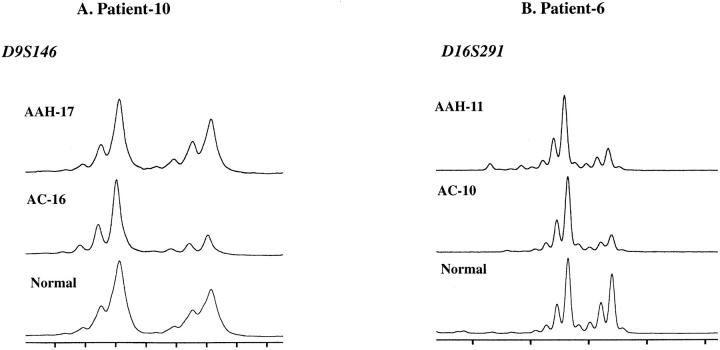
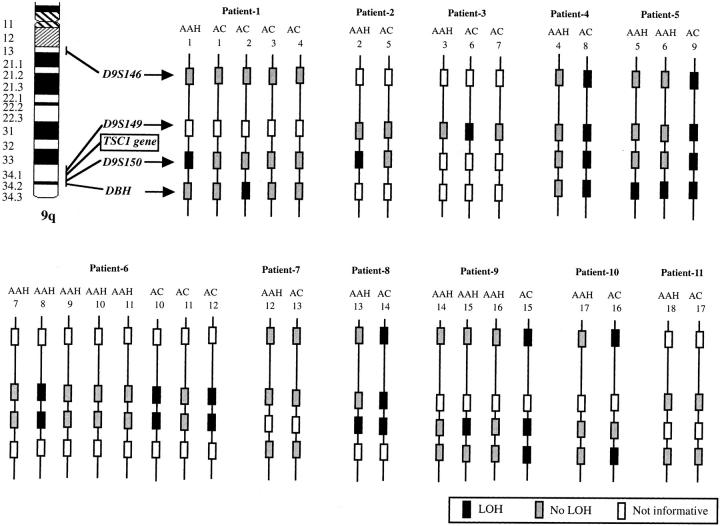
We examined 18 AAHs and 17 adenocarcinomas of the lung from 11 patients for LOH on chromosome 9q, including the TSC1 gene-associated region (9q34), and chromosome16p, including the TSC2 gene-associated region (16p13.3), by using two sets of four microsatellite markers, respectively. Representative examples are shown in Figure 2 ▶ . The distributions of 9q region deletions in AAHs and adenocarcinomas are shown in Figure 3 ▶ . Seven of 18 AAHs (39%) and 9 of 17 adenocarcinomas (53%) showed LOH at one or more loci on chromosome 9q. Seven of nine adenocarcinomas with LOH on 9q (78%) harbored LOH at all informative loci on 9q (complete LOH). However, only one AAH showed complete LOH on 9q. Five of seven AAHs with LOH on 9q harbored LOH at the same locus as the concomitant adenocarcinomas.
Figure 2.
Representative examples of microsatellite analyses of chromosomes 9q and 16p in AAHs and adenocarcinomas (AC). A: AAH-17 retained heterozygosity at D9S146. AC-16 showed LOH at D9S146. B: Both AAH-11 and AC-10 showed LOH at D16S291.
Figure 3.
The distributions of deletions on 9q in AAHs and adenocarcinomas. AC, primary lung adenocarcinoma.
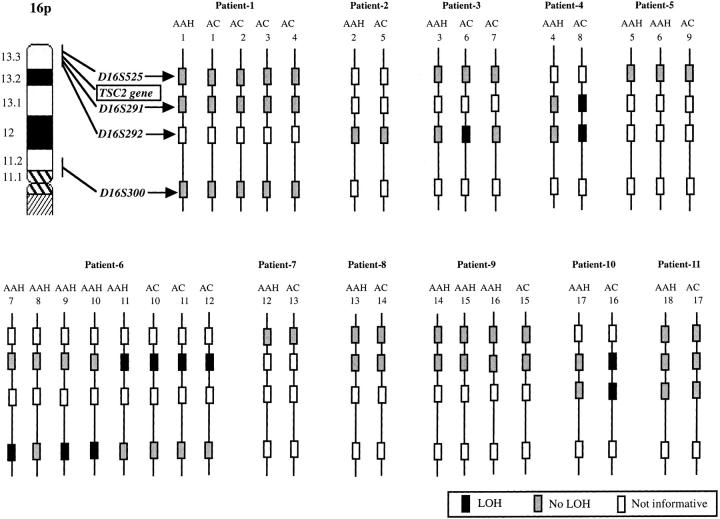
The distributions of deletions on 16p in AAHs and adenocarcinomas are shown in Figure 4 ▶ . Four of 18 AAHs (22%) and 6 of 17 adenocarcinomas (35%) showed LOH at one or more loci on chromosome 16p. These four AAHs were from the same patient (patient 6). Two of six adenocarcinomas with LOH on 16p (33%) showed complete LOH on 16p, but all AAHs harbored LOH at only one locus among all of the informative loci. One AAH harbored LOH at the same locus as the concomitant adenocarcinomas.
Figure 4.
The distributions of deletions on 16q in AAHs and adenocarcinomas. AC, primary lung adenocarcinoma.
The TSC1 gene is located between D9S149 and D9S150. Five of seven AAHs with LOH on 9q (71%) and seven of nine adenocarcinomas with LOH on 9q (78%) showed LOH at loci adjacent to the TSC1 gene, ie, loci including D9S149 or D9S150. The TSC2 gene is located between D16S291 and D16S525. None of the AAHs or adenocarcinomas harbored LOH at D16S525. One of four AAHs with LOH on 16p (25%) and five of six adenocarcinomas with LOH on 16p (83%) showed LOH at loci adjacent to the TSC2 gene, ie, at D16S291.
The frequencies of LOH at all loci examined were as follows: 5 of 20 informative samples (25%) at D9S146, 7 of 29 (29%) at D9S149, 11 of 28 (39%) at D9S150, 7 of 20 (35%) at DBH, 3 of 13 (23%) at D16S300, 3 of 11 (27%) at D16S292, 6 of 25 (24%) at D16S29, and none (0%) at D16S525.
We analyzed the relationships between LOH on chromosome 9q or 16p, or LOH at microsatellite loci adjacent to the TSC1 or TSC2 gene, and the following histological characteristics of adenocarcinoma and AAH: size, histological subtype (bronchioloalveolar carcinoma versus other subtypes), vascular invasion, lymphatic permeation, nuclear atypia, mitotic index, or scar grade of adenocarcinoma, and the size or histological grade of AAH. No histological characteristic was found to be associated with LOH on 9q or 16p, or with LOH at microsatellite loci adjacent to the TSC1 or TSC2 gene (Tables 2 and 3) ▶ ▶ .
Table 2.
Relationships between Histological Characteristics of Primary Adenocarcinoma and LOH on Chromosomes 9q and 16p
| n | LOH on 9q | LOH on 16p | TSC1LOH | TSC2LOH | |
|---|---|---|---|---|---|
| Tumor size | |||||
| ≤2 cm | 9 | 4 | 3 | 3 | 2 |
| >2 cm | 8 | 5 (0.64)* | 3 (1.0) | 4 (0.64) | 3 (0.62) |
| Histological subtype | |||||
| BAC | 8 | 4 | 2 | 3 | 1 |
| Other than BAC | 9 | 5 (1.0) | 4 (0.62) | 4 (1.0) | 4 (0.29) |
| Vascular invasion | |||||
| Negative | 10 | 5 | 4 | 4 | 3 |
| Positive | 7 | 4 (1.0) | 2 (1.0) | 3 (1.0) | 2 (1.0) |
| Lymphatic permeation | |||||
| Negative | 11 | 5 | 3 | 4 | 2 |
| Positive | 6 | 4 (0.62) | 3 (0.60) | 3 (0.64) | 3 (0.28) |
| Nuclear atypia | |||||
| 1 or 2 | 6 | 2 | 1 | 1 | 0 |
| 3 | 11 | 7 (0.34) | 5 (0.33) | 6 (0.30) | 5 (0.10) |
| Mitotic index | |||||
| 1 | 12 | 5 | 5 | 4 | 4 |
| 2 or 3 | 5 | 4 (0.29) | 1 (0.60) | 3 (0.59) | 1 (1.0) |
| Scar grade | |||||
| 1 or 2 | 9 | 4 | 3 | 3 | 2 |
| 3 or 4 | 8 | 5 (0.64) | 3 (1.0) | 4 (0.64) | 3 (0.62) |
BAC, bronchioloalveolar carcinoma.
*Numbers in parentheses are P values (Fisher’s exact test).
Table 3.
Relationships between Histological Characteristics of AAH and LOH on Chromosomes 9q and 16p
| n | LOH on 9q | LOH on 16p | TSC1LOH | TSC2LOH | |
|---|---|---|---|---|---|
| Size | |||||
| <5 mm | 10 | 6 | 1 | 4 | 0 |
| ≥5 mm | 8 | 1 (0.07)* | 3 (0.27) | 1 (0.31) | 1 (0.44) |
| Grade | |||||
| Low | 10 | 4 | 2 | 4 | 0 |
| High | 8 | 3 (1.0) | 2 (1.0) | 1 (0.31) | 1 (0.44) |
Low, low-grade AAH; high, high-grade AAH.
*Numbers in parentheses are P values (Fisher’s exact test).
Discussion
Little is known about the etiology of lung adenocarcinoma, as compared with squamous cell carcinoma. AAH has recently been implicated as a preneoplastic lesion of lung adenocarcinoma. Miller 29 initially proposed a pulmonary adenoma-carcinoma sequence analogous to that of the colon. AAH is much more frequently associated with adenocarcinoma than other histological subtypes of lung cancer. 30 Multiple AAHs are occasionally detected in patients with multiple lung adenocarcinomas. 29 In our series, the histological subtype was adenocarcinoma in 22 of 26 (85%) lung cancer patients with AAH, and all patients with multiple adenocarcinomas had concomitant multiple AAHs. These findings suggest that AAH and adenocarcinoma might be induced by common etiological factors.
AAH has been reported to have several genetic alterations in common with lung adenocarcinoma. Mutations of K-ras codon 12 have variously been detected in 15 to 50% of AAHs and 22 to 42% of adenocarcinomas. 7-10 LOH on chromosome 3p was detected in 10 to 18% of AAHs and 12 to 67% of adenocarcinomas. 4-6 LOH on chromosome 9p was detected in 5 to 13% of AAHs and 19 to 50% of adenocarcinomas. 4-6 We demonstrated AAHs to harbor LOH on chromosomes 9q and 16p, LOHs that are also found in lung adenocarcinomas. The frequency of LOH on 9q, especially in the TSC1 gene associated regions, was high. Petersen and colleagues 31 reported LOH on 9q34 to be significantly associated with lung adenocarcinoma. Our results were also consistent with this observation. The relatively high frequency of LOH on 9q34 in AAH lesions and adenocarcinomas suggests that a novel tumor suppressor gene for lung adenocarcinomas may exist in this region, and the TSC1 gene is one candidate.
Hung and colleagues 3 and Kishimoto and colleagues 4 detected more frequent and extensive 3p and 9p losses in carcinomas than in corresponding preneoplastic lesions, and the identical allele was lost among them, an allele-specific loss. Their findings were consistent with our results. Six of seven AAHs with LOH on 9q and all AAHs with LOH on 16p showed LOH at only one microsatellite locus. However, the allelic losses on 9q and 16p were more extensive in adenocarcinomas than in AAHs. In particular, the rate of complete LOH on 9q was high (78%) in adenocarcinomas. This progressive loss on 9q during progression might suggest existence of more than one tumor suppressor gene on 9q for lung adenocarcinoma. In addition, the identical allele was lost among multiple AAHs and concomitant adenocarcinomas in the same patients. These observations indicate that one allele might be inactivated congenitally because of mutation or epigenetically, and the remaining allelic loss would then be acquired. This suggests a possible role of genetic predisposition in the pathogenesis of lung adenocarcinoma with AAH. In our series, 7 of 11 (64%) patients with lung adenocarcinoma and corresponding AAH were non-smokers and 5 of 11 (45%) had either a past or family history of malignancy. These findings might also indicate genetic predisposition.
Muir and colleagues 18 reported that MMPH is distinguishable from AAH by the following features: AAH cells have a greater degree of nuclear-to-cytoplasmic ratio than MMPH, less well circumscribed with peripheral lepidic spread, less prominent interstitial reticuln, and fewer air space macrophages than MMPH. However, some investigators have reported that MMPH is so similar to AAH morphologically that histological distinction between MMPH and AAH is difficult. 19 Thus, we supposed that these two conditions might share some molecular mechanisms of pathogenesis. Genetic alteration in TSC1 gene-associated regions is a candidate.
If we postulate that the TSC1 gene itself is responsible for early development of lung adenocarcinoma, there would be an obvious discrepancy. We would more frequently encounter TSC patients with MMPH. However, MMPH is believed to be a rare pulmonary manifestation of TSC, and only a few such cases have been reported. 18,19 How can we explain this discrepancy? Because MMPH is a subtle pulmonary lesion, underestimation of the incidence of MMPH might be one explanation. The life expectancy of patients with TSC is relatively short, 32 and this is another possible reason. We do not understand how many genetic alterations are required for formations of MMPH and AAH. If several genetic alterations are required, low incidence of MMPH association in TSC patients might be explained. This is the third possible reason. The forth and most probable explanation is that TSC1 itself is not the responsible gene but rather that some novel tumor suppressor gene for lung adenocarcinoma exists very close to the TSC1 gene.
In our present study, we analyzed only a limited number of lesions. Further study of a large number of adenocarcinomas and AAH lesions is clearly required. We did not analyzed LOH in AAH adjacent to adenocarcinoma, because it was difficult to convincingly differentiate the border between AAH and adenocarcinoma. If we could analyze several adenocarcinomas obviously associated with AAH adjacently, it would become more convincing. In addition, an extensive mutation analysis or demonstrating the suppressed expression of the TSC1 gene in lung adenocarcinoma might be helpful in answering the above question.
Footnotes
Address reprint requests to Hiroyasu Esumi, Director of the National Cancer Center Research Institute East, 6-5-1, Kashiwanoha, Kashiwa, Chiba, 277-8577, Japan. E-mail: hesumi@east.ncc.go.jp.
Supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare of Japan, and a Smoking Research Foundation Grant for Biomedical Research.
References
- 1.Sozzi G, Miozzo M, Donghi R, Pilotti S, Cariani CT, Pastorino U, Della Porta G, Pierotti MA: Deletions of 17p and p53 mutations in preneoplastic lesions of the lung. Cancer Res 1992, 52:6079-6082 [PubMed] [Google Scholar]
- 2.Bennett WP, Colby TV, Travis WD, Borkowski A, Jones RT, Lane DP, Metcalf RA, Samet JM, Takeshima Y, Gu JR, Vähäkangas KH, Soini Y, Pääkkö P, Welsh JA, Trump BF, Harris CC: p53 protein accumulates frequently in early bronchial neoplasia. Cancer Res 1993, 53:4817-4822 [PubMed] [Google Scholar]
- 3.Hung J, Kishimoto Y, Sugio K, Virmani A, McIntire DD, Minna JD, Gazdar AF: Allele-specific chromosome 3p deletions occur at an early stage in the pathogenesis of lung carcinoma. JAMA 1995, 273:558-563 [PubMed] [Google Scholar]
- 4.Kishimoto Y, Sugio K, Hung JY, Virmani AK, McIntire DD, Minna JD, Gazdar AF: Allele-specific loss in chromosome 9p loci in preneoplastic lesions accompanying non-small-cell lung cancers. J Natl Cancer Inst 1995, 87:1224-1229 [DOI] [PubMed] [Google Scholar]
- 5.Kitaguchi S, Takeshima Y, Nishisaka T, Inai K: Proliferative activity, p53 expression and loss of heterozygosity on 3p, 9p and 17p in atypical adenomatous hyperplasia of the lung. Hiroshima J Med Sci 1998, 47:17-25 [PubMed] [Google Scholar]
- 6.Kohno H, Hiroshima K, Toyozaki T, Fujisawa T, Ohwada H: p53 mutation and allelic loss of chromosome 3p, 9p of preneoplastic lesions in patients with nonsmall cell lung carcinoma. Cancer 1999, 85:341-347 [PubMed] [Google Scholar]
- 7.Cooper CA, Carby FA, Bubb VJ, Lamb D, Kerr KM, Wyllie AH: The pattern of K-ras mutation in pulmonary adenocarcinoma defines a new pathway of tumour development in the human lung. J Pathol 1997, 181:401-404 [DOI] [PubMed] [Google Scholar]
- 8.Westra WH, Baas IO, Hruban RH, Askin FB, Wilson K, Offerhaus GJ, Slebos RJ: K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res 1996, 56:2224-2228 [PubMed] [Google Scholar]
- 9.Sagawa M, Saito Y, Fujimura S, Linnoila RI: K-ras point mutation occurs in the early stage of carcinogenesis in lung cancer. Br J Cancer 1998, 77:720-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohshima S, Shimizu Y, Takahama M: Detection of c-Ki-ras gene mutation in paraffin sections of adenocarcinoma and atypical bronchioloalveolar cell hyperplasia of human lung. Virchows Arch 1994, 424:129-134 [DOI] [PubMed] [Google Scholar]
- 11.Kitamura H, Kameda Y, Ito T, Hayashi H, Nakamura N, Nakatani Y, Inayama Y, Kanisawa M: Cytodifferentiation of atypical adenomatous hyperplasia and bronchioloalveolar lung carcinoma: immunohistochemical and ultrastructural studies. Virchows Arch 1997, 431:415-424 [DOI] [PubMed] [Google Scholar]
- 12.Kitamura H, Kameda Y, Nakamura N, Inayama Y, Nakatani Y, Shibagaki T, Ito T, Hayashi H, Kimura H, Kanisawa M: Atypical adenomatous hyperplasia and bronchoalveolar lung carcinoma. Analysis by morphometry and the expressions of p53 and carcinoembryonic antigen. Am J Surg Pathol 1996, 20:553-562 [DOI] [PubMed] [Google Scholar]
- 13.Kodama T, Biyajima S, Watanabe S, Shimosato Y: Morphometric study of adenocarcinomas and hyperplastic epithelial lesions in the peripheral lung. Am J Clin Pathol 1986, 85:146-151 [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Corrin B, Shimosato Y, Brambilla E (Eds): World Health Organization: Histological Typing of Lung and Pleural Tumours, ed 3. Berlin, Springer-Verlag, 1999
- 15.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JBM, Ward S, Green AJ, Yates JRW, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ: Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997, 277:805-808 [DOI] [PubMed] [Google Scholar]
- 16.: : Identification and characterization of the tuberous sclerosis gene on chromosome 16: The European Chromosome 16 Tuberous Sclerosis Consortium. Cell 1993, 75:1305-1315 [DOI] [PubMed] [Google Scholar]
- 17.Capron F, Ameille J, Leclerc P, Mornet P, Barbagellata M, Reynes M, Rochemaure J: Pulmonary lymphangioleiomyomatosis and Bourneville’s tuberous sclerosis with pulmonary involvement: the same disease? Cancer 1983, 52:851-855 [DOI] [PubMed] [Google Scholar]
- 18.Muir TE, Leslie KO, Popper H, Kitaichi M, Gagne E, Emelin JK, Vinters HV, Colby TV: Micronodular pneumocyte hyperplasia. Am J Surg Pathol 1998, 22:465-472 [DOI] [PubMed] [Google Scholar]
- 19.Guinee D, Singh R, Azumi N, Singh G, Przygodzki RM, Travis W, Koss M: Multifocal micronodular pneumocyte hyperplasia: a distinctive pulmonary manifestation of tuberous sclerosis. Mod Pathol 1995, 8:902-906 [PubMed] [Google Scholar]
- 20.Suzuki K, Ogura T, Yokose T, Nagai K, Mukai K, Kodama T, Nishiwaki Y, Esumi H: Loss of heterozygosity in the tuberous sclerosis gene associated regions in adenocarcinoma of the lung accompanied by multiple atypical adenomatous hyperplasia. Int J Cancer 1998, 79:384-389 [DOI] [PubMed] [Google Scholar]
- 21.Noguchi M, Furuya S, Takeuchi T, Hirohashi S: Modified formalin and methanol fixation methods for molecular biological and morphological analyses. Pathol Int 1997, 47:685-691 [DOI] [PubMed] [Google Scholar]
- 22.Shimosato Y, Suzuki A, Hashimoto T, Nishiwaki Y, Kodama T, Yoneyama T, Kameya T: Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol 1980, 4:365-373 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K, Nagai K, Yoshida J, Yokose T, Kodama T, Takahashi K, Nishimura M, Kawasaki H, Yokozaki M, Nishiwaki Y: The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastasis. Cancer 1997, 79:1521-1526 [DOI] [PubMed] [Google Scholar]
- 24.Yokose T, Yuji I, Atushi O: High prevalence of atypical adenomatous hyperplasia of the lung in autopsy specimens from elderly patients with malignant neoplasms. Lung Cancer 2000, 29:125-130 [DOI] [PubMed] [Google Scholar]
- 25.Henske EP, Ozelius L, Gusella JF, Haines JL, Kwiatkowski DJ: A high-resolution linkage map of human 9q34.1. Genomics 1993, 17:587-591 [DOI] [PubMed] [Google Scholar]
- 26.Thompson AD, Shen Y, Holman K, Sutherland GR, Callen DF, Richards RI: Isolation and characterisation of (AC)n microsatellite genetic markers from human chromosome 16. Genomics 1992, 13:402-408 [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Holman K, Doggett NA, Callen DF, Sutherland GR, Richards RI: Dinucleotide repeat polymorphisms at the D16S525, D16S359, D16S531 and D16S522 loci. Hum Mol Genet 1994, 3:210. [DOI] [PubMed] [Google Scholar]
- 28.Shiseki M, Kohno T, Nishikawa R, Sameshima Y, Mizoguchi H, Yokota J: Frequent allelic losses on chromosomes 2q, 18q, and 22q in advanced non-small cell lung carcinoma. Cancer Res 1994, 54:5643-5648 [PubMed] [Google Scholar]
- 29.Miller RR: Bronchioloalveolar cell adenomas. Am J Surg Pathol 1990, 14:904-912 [DOI] [PubMed] [Google Scholar]
- 30.Colby TV, Wistuba II, Gazdar A: Precursors to pulmonary neoplasia. Adv Anat Pathol 1998, 5:205-215 [DOI] [PubMed] [Google Scholar]
- 31.Petersen I, Bujard M, Petersen S, Wolf G, Goeze A, Schwendel A, Langreck H, Gellert K, Reichel M, Just K, du Manoir S, Cremer T, Dietel M, Ried T: Patterns of chromosomal imbalances in adenocarcinoma and squamous cell carcinoma of the lung. Cancer Res 1997, 57:2331-2335 [PubMed] [Google Scholar]
- 32.Shepherd CW, Gomez MR, Lie JT, Crowson CS: Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991, 66:792-796 [DOI] [PubMed] [Google Scholar]