Abstract
Bovine thrombin is used as an aid to hemostasis in medical and surgical procedures. At least 500,000 Americans are exposed to this therapeutic annually and reports suggest that exposure is associated with the development of autoreactive antibodies. To determine whether bovine thrombin can induce pathological autoimmunity we exposed nonautoimmune-prone galactose-α1-3-galactose-deficient mice to the two bovine thrombin preparations currently approved for use in the United States. We found that, like humans exposed to bovine thrombin, mice developed an immune response against the therapeutic and the xenogeneic carbohydrate galactose-α1-3-galactose, and some mice developed autoantibodies against clotting factors. Further, unexpectedly, a single exposure to this therapeutic also induced autoimmunity with features characteristic of systemic lupus erythematosus including antibodies against nuclear antigens, native DNA, double-stranded DNA, and cardiolipin. High levels of these autoantibodies correlated with glomerulonephritis in all mice evaluated. This autoimmune syndrome was detected in mice 15 weeks after a secondary exposure to bovine thrombin and female mice were found to develop the syndrome at a significantly greater frequency than males. Thus, these studies indicate that exposure to bovine thrombin preparations can induce a pathological systemic autoimmune syndrome with lupus-like serology.
Bovine thrombin is derived from bovine plasma and is used as an aid to hemostasis. This biological therapeutic is widely used in a variety of surgical procedures in the areas of, but not limited to, thoracic, cardiac, vascular, neurological, orthopedic, general, transplant, plastic, urological, otolaryngological, maxiofacial, gynecological, and eye surgeries, 1-3 and is also used in endoscopy, interventional radiology, and tooth extraction. 4,5 Considering the widespread use of bovine thrombin and that it is not only sold as a stand alone hemostatic agent, but is often included in other hemostatic therapeutics, 4-6 it is conservatively estimated that at least 500,000 Americans are exposed to a bovine thrombin preparation each year. 3
Although bovine thrombin continues to be used clinically, reports have suggested that its use may result in development of autoreactive antibodies, 2,7-27 at least some of which are pathogenic. 1,2,8,10-16,19-22,25,26,28-31 Most of these reports suggest that exposure to bovine thrombin leads to the development of autoantibodies that cross-react with human coagulation proteins and with the corresponding bovine coagulation proteins found in the therapeutic. In addition, it was recently reported in a prospective study that, out of 151 patients exposed to bovine thrombin during cardiac surgery, 77 (51%) developed autoantibodies against at least one human coagulation factor. 1 Furthermore, other case reports have identified increases in anti-phospholipid antibodies after exposure to bovine thrombin. 32
Although the evidence described above has cast considerable doubt on the safety of bovine thrombin, no unequivocal proof that exposure to these reagents can cause the development of pathological autoreactive antibodies has been offered. Indeed, neither randomized nor controlled clinical trials have been conducted. Such studies are important because several factors other than exposure to bovine thrombin might account for the observations of autoreactive antibodies found in the literature. For example, many surgical patients may have undocumented autoimmune diseases before surgery. 33,34 Further, in patients with or without underlying autoimmune disease, the general inflammation associated with surgery alone might result in the increased production of polyreactive and autoreactive antibodies. 35 Yet another complicating issue is that even if autoantibodies are produced in response to a bovine thrombin preparation, it remains to be determined what percentage of those antibodies are pathogenic. 2
Although properly randomized and controlled studies are still needed, we and others have serious concerns regarding potential dangers of these therapeutics 1,2,13,15,32 and believe that clinical studies involving further exposure of humans to bovine thrombin should be avoided if alternatives to such studies are available. These concerns arise not only because of potential induction of autoimmunity, but also because of the potential for acute adverse reactions in the patient because, perhaps, of interaction of the bovine reagents with xenoreactive antibodies. 3 Thus, as an alternative to studies in humans, we have developed a model in which we exposed nonautoimmune-prone mice to bovine thrombin.
This study shows that exposure of mice to bovine thrombin resulted in an immunological response similar to that observed in humans, including development of antibodies against the therapeutic, against the xenogeneic carbohydrate galactose-α1-3-galactose (αGal) and, in some cases, against autologous clotting factors. Unexpectedly these mice also developed systemic autoimmunity with lupus-like serology, including anti-nuclear antibodies (ANA), anti-native DNA (nDNA) antibodies, anti-double-stranded DNA (dsDNA) antibodies, and anti-cardiolipin (aCL) antibodies.
Materials and Methods
Materials
Thrombogen was obtained from Johnson and Johnson (Somerville, NJ) and Thrombin-JMI was obtained from Jones Pharma Incorporated (St. Louis, MO). Slides containing human laryngeal epithelioma cancer cells (HEp-2) and Crithidia lucialiae were purchased from Scimedx Corporation (Denville, NJ). An assay kit designed to measure murine anti-dsDNA IgG was obtained from Alpha Diagnostic International (San Antonio, TX). An ImmunoPure ABC Peroxidase Mouse IgG staining kit was obtained from Pierce (Rockford, IL). Galactose(α1-3)galactose (galactobiose) and human serum albumin (HSA) conjugated with galactose(α1-3)galactose(β1-4)N-acetylglucosamine (HSA-αGal) were obtained from V-Labs (Covington, LA). Spectrozyme TH and Russel’s viper venom were obtained from American Diagnostica, Inc. (Greenwich, CT). HSA, bovine serum albumin, fetal bovine serum, S-Sepharose, d-Sepharose, calf thymus DNA, cardiolipin, fluorescein isothiocyanate-conjugated goat anti-mouse IgG, and alkaline phosphatase-conjugated goat anti-mouse IgG were obtained from Sigma (St. Louis, MO). A nucleic acid detection kit, DNAeasy, was obtained from Qiagen Inc. (Valencia, CA).
Mice
Mice with a BDF-1 background and deficient in the α-galactosyl transferase gene (GalT−/− mice) were obtained from Dr. John Lowe (University of Michigan, Ann Arbor, MI-Howard Hughes Medical Institute). 36 These mice, unlike wild-type mice, do not synthesize terminal galactose(α1-3)galactose (αGal) and are able to mount an immune response against αGal. GalT−/− mice were selected for these studies because a reaction against αGal is a major feature of the human immune response after exposure to bovine thrombin. 3 Further, the immune recognition of αGal may be important in the development of autoimmunity in humans after exposure to bovine thrombin. 3
Serum from a 14-week-old MRL/MpJ-Tnfrsf6lpr mouse was generously provided by Dr. David S. Pisetsky, Department of Rheumatology, Duke University Medical Center. MRL/MpJ-Tnfrsf6lpr mice are homozygous for the lymphoproliferation spontaneous mutation (Faslpr) and are used as a model for systemic lupus erythematosus (SLE)-like autoimmune syndromes. 37 Onset of symptoms in the MRL/MpJ background begins at ∼8 weeks of age, and females and males die at an average age of 17 and 22 weeks, respectively. Animal studies were approved by the Duke University Institutional Animal Care and Use Committee.
Mouse Study I—Exposure of GalT−/− Mice to Thrombogen during Surgery
Ten GalT−/− mice (1 to 1.5 years old) were anesthetized with isofluorane and a midline laparotomy was performed using a 3- to 5-mm incision. One ml of phosphate-buffered saline (PBS) with (n = 5 mice) or without (n = 5 mice) 5000 U of Thrombogen was sprayed into the peritoneal cavity and the incision was closed with 4-0 vicryl sutures. Each mouse was housed separately and observed daily for gross appearance and behavioral changes. Blood was obtained before and at 3 weeks after exposure to antigen using a tail-bleed method as previously described. 38 Four weeks after exposure, citrated and noncitrated blood was drawn through a pericardial puncture and the mice were sacrificed. Biopsies were taken, fixed in 10% formalin, cut and stained with hematoxylin and eosin or stained for IgG deposition as previously described. 39 Renal sections were prepared for electron microscopy as follows: tissue was retrieved from paraffin blocks, deparaffinized in three changes of xylene, rehydrated through a series of graded ethanols, postfixed in 3% glutaraldehyde, stained with 2% osmium tetroxide followed by 1% uranyl acetate, and then infiltrated with epoxy resin. After polymerization of the resin, semi-thin survey sections were stained with toluidine blue and examined by light microscopy for the presence of glomeruli. Thin sections prepared from representative blocks were poststained sequentially with uranyl acetate and lead citrate. The stained sections were examined using a JEOL 1200EXII (Tokyo, Japan) electron microscope.
Mouse Study II—Exposure of GalT−/− Mice to Thrombogen and Thrombin-JMI During Surgery and by Intraperitoneal Injection
Before exposure to bovine thrombin preparations, 58 GalT−/− mice (0.25- to 0.6-years-old) were sensitized to αGal by subcutaneous injection of 50 μg each of HSA-αGal. This sensitization was performed so that anti-αGal antibodies would be present at the time of exposure to the bovine thrombin preparation. Anti-αGal antibodies are present naturally in the sera of humans 40 and of older GalT−/− mice, 41 and may affect the immune response to the bovine thrombin preparation. 3 Thrombogen and Thrombin-JMI were dialyzed at 4°C against three changes of PBS, pH 7.4, using a 3000 molecular weight cutoff membrane. The specific activity of each bovine thrombin preparation was calculated using the manufacturer’s reported units of thrombin activity and the results of a Lowry protein assay. Thirty-six GalT−/− mice were exposed to 100 U/g Thrombogen, by either surgery (n = 6) or intraperitoneal injection (n = 30). Seventeen GalT−/− mice were exposed to 100 U/g Thrombin-JMI, by either surgery (n = 9) or intraperitoneal injection (n = 8). Five GalT−/− mice were injected with an equal volume of PBS. All surgery was performed as described in study I, with the exception that surgical incisions were closed with 9-mm surgical clips (MikRon Autoclip; Becton Dickinson, Sparks, MD) instead of sutures. Clips were removed after the wound was healed (typically 1 to 2 weeks after surgery). Intraperitoneal injections were made into the intraperitoneal cavity using a 26-gauge needle. Before exposure to bovine thrombin, 22 mice from this study were bled using a tail-bleed method. 38 The samples thus obtained were used to generate normal values in all serology studies. Each mouse was housed, observed, and bled as described above.
Behavioral Studies
Behavior was measured in a quantitative and blinded manner according to the method described by Holland. 42 Briefly, each mouse was placed in an unfamiliar environment at the same time of the day (to avoid differences based on circadian rhythms) and behavior was documented at 0.67- to 1.5-minute intervals. Under these conditions, the mice normally exhibit exploratory behavior, obtaining familiarity with the new environment (sitting, standing, walking, running, hanging on cage, and digging). Nonexploratory behavior (grooming, scratching, biting tail, twitching, and licking wounds) was considered to be abnormal.
Determination of Anti-Thrombogen and Anti-Thrombin-JMI Antibodies by Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of IgG against the bovine thrombin used in these studies were determined by ELISA. In brief, 50 μl per well of Thrombogen or Thrombin-JMI (10 μg protein/ml in PBS) was added to a Nunc Maxisorp 96-well plate (Nunc AIS, Roskilde, Denmark) and incubated for 3 hours at 24°C. A portion of the plate was coated with 10 μg/ml of bovine serum albumin as a control. The wells were washed three times with PBS, blocked with blocking buffer (0.1% bovine serum albumin and 0.05% Tween 20 in PBS) for 1 hour at 24°C and washed three times in PBS. Mouse sera (diluted 1:100 in PBS) were added and incubated for 1 hour at 24°C. Next, the wells were washed three times with PBS and then incubated for 1 hour at 24°C with goat anti-mouse IgG conjugated with alkaline phosphatase. The plates were developed using 1.0 mg/ml of p-nitrophenyl phosphate (chromogenic substrate) in 100 mmol/L of diethanolamine, 0.5 mmol/L MgCl2, 0.2% (w/v) NaN3, pH 9.5. The change in absorbance at 405 nm as a function of time was then measured on an EL 340 microplate reader from Bio Kinetics (Winooski, VT). The rate of change in absorbance in wells coated with bovine serum albumin only was taken to be background and subtracted from the rate in wells coated with bovine thrombin. All experiments were conducted in duplicate.
Determination of Anti-αGal Antibodies by ELISA
Levels of anti-αGal IgG were detected by ELISA as described previously. 3,38 Briefly, 50 μl per well of HSA-αGal (10 μg/ml in PBS) was added to a Nunc Maxisorp 96-well plate and incubated for 3 hours at 24°C. A portion of the plate was coated as described above with 10 μg/ml of HSA as a control. The wells were blocked with blocking buffer (0.1% HSA and 0.05% Tween 20 in PBS) and then incubated with mouse serum (diluted 1:100 in PBS) for 1 hour at 24°C. Next, the ELISA plates were washed, incubated with anti-mouse IgG conjugated to alkaline phosphatase, developed, and measured as described above. The rate of change in absorbance in wells coated with HSA only was taken to be background and subtracted from the rate in wells coated with HSA-αGal. All experiments were conducted in duplicate.
Purification of GalT−/− Mouse Thrombin Preparation and GalT−/− Mouse Albumin
Mouse thrombin was partially purified from GalT−/− mouse plasma. In brief, 1 ml of citrated plasma was diluted 1:5 with 20 mmol/L of sodium citrate, 50 mmol/L of NaCl, and 1 mmol/L of benzamidine, pH 6.0. One ml of d-Sepharose was added to the diluted plasma, the sample was rotated for 1 hour at 4°C, and centrifuged for 1 minute at 2000 × g. The d-Sepharose was washed three times with 20 mmol/L of sodium citrate, 50 mmol/L of NaCl, and 1 mmol/L of benzamidine, pH 6.0, and then batch eluted using 20 mmol/L of citrate, 500 mmol/L of NaCl and 1.0 mmol/L of benzamidine, pH 6.0. The eluant was dialyzed against Tris-buffered saline and then activated by the addition of 250 μl of GalT−/− mouse thromboplastin and 600 μmol/L of Russel’s viper venom in 5 mmol/L of CaCl2 for 3 hours at 37°C. The sample was then diluted with 25 mmol/L of sodium phosphate, pH 6.0, followed by the addition of 1.0 ml of S-Sepharose. This mixture was rotated for 1 hour at 24°C and then centrifuged for 1 minute at 2000 × g. The S-Sepharose was washed two times with 25 mmol/L of sodium phosphate, pH 6.0, and then eluted with 250 mmol/L of sodium phosphate. Spectrozyme TH was used to confirm thrombin activity. GalT−/− mouse albumin was purified using a previously described protocol for the purification of HSA. 3
Determination of Anti-Clotting Factor Autoantibody Levels by ELISA
Levels of autoantibodies against coagulation factors were determined by measuring IgG levels against a murine thrombin preparation isolated from GalT−/− mice. In brief, 50 μl of the murine thrombin preparation (isolation described above) was coated onto a Nunc Maxisorp 96-well plate at 20 μg/ml in PBS and was incubated overnight at room temperature. A portion of the plate was coated with 10 μg/ml of GalT−/− mouse albumin as a control. After this overnight incubation, the wells were blocked with blocking buffer (0.1% GalT−/− mouse albumin and 0.05% Tween 20 in PBS) for 1 hour at room temperature. Next, 50 μl of mouse sera (diluted 1:200 in PBS) were added and the plates incubated for 1 hour at room temperature. The ELISA plates were then washed, incubated with goat anti-mouse IgG, and developed as described above. The absorbance at 405 nm was then measured on an EL 340 microplate reader from Bio Kinetics. Absorbance in wells coated with GalT−/− mouse albumin only was taken to be background and subtracted from the absorbance in wells coated with GalT−/− mouse thrombin preparation. All experiments were conducted in duplicate.
Determination of Anti-dsDNA Antibody Levels by ELISA
Levels of anti-dsDNA IgG were measured according to the manufacturer’s instructions using a commercially available ELISA assay kit described in Materials and Methods. Serum was diluted 1:100 in the buffer provided. Two mmol/L of galactobiose was added to serum samples to prevent any anti-αGal antibodies from binding to the assay plate, because plates may contain αGal if antigen is derived from a non-human or non-primate source. 43
Determination of Anti-ssDNA Antibody Levels by ELISA
Levels of anti-ssDNA IgG were measured by ELISA as described previously. 44 To prepare ssDNA, dsDNA (4 mg/ml) was boiled in water for 10 minutes and diluted in ice-cold SSC buffer to 2 μg/ml. Fifty μl per well of ssDNA was added to a Dynatech Microtiter 96-well plate (Dynatech, Chantilly, VA) and incubated for 2 hours at 24°C. The wells were washed three times with PBS and 100 μl per well of mouse serum diluted 1:100 in a PBS-Tween buffer (0.5% Tween 20) was added and the plates were incubated for 1 hour at 24°C. The ELISA plates were then washed, incubated with goat anti-mouse IgG conjugated to alkaline phosphatase, and developed as described above. Finally, the rate of change in absorbance at 405 nm was measured as described above.
Determination of aCL Antibody Levels by ELISA
Levels of aCL IgG were measured by ELISA. Briefly, 50 μl/well of cardiolipin (1.35 μg/well) diluted in absolute EtOH was added to a Becton Dickinson (San Diego, CA) Probind 96-well microtiter plate and incubated for 2 hours in a fume hood. A portion of the plate was coated with blocking buffer (10% fetal calf serum in PBS). The wells were washed three times with PBS and blocked with 200 μl per well of blocking buffer for 1 hour at 24°C. Next, the plates were washed three times with PBS and 50 μl per well of mouse serum diluted 1:100 in PBS was added and the plates were incubated for 1 hour at 24°C. The ELISA plates were then washed, incubated with goat anti-mouse IgG conjugated to alkaline phosphatase, developed, and measured as described above.
Determination of ANA and Antibodies Specific for nDNA by Indirect Fluorescent Antibody
Titers of IgG against nuclear antigens and nDNA were determined by indirect fluorescent antibody assay. Briefly, serum samples were diluted in PBS and prepared at dilutions ranging from 1:40 to 1:160 for the detection of ANA on HEp-2 slides and at dilutions ranging from 1:10 to 1:80 for the detection of nDNA on C. lucilaiae slides. Thirty μl of serum was added per well and incubated for 30 minutes at 24°C in a wet chamber. Each well was washed with PBS, 30 μl of PBS was added to each well, slides were incubated for 5 minutes in a wet chamber, and then washed with PBS. Next, 30 μl of fluorescein isothiocyanate-conjugated goat anti-mouse IgG diluted 1:40 in PBS was added to each well and slides were incubated for 30 minutes in a wet chamber. Slides were washed as described above, coated with mounting medium (Cytoseal; Richard-Allan Scientific, Kalamazoo, MI) and examined with an Olympus IX50 fluorescent microscope (Melville, NY).
Determination of Relative Purity of Thrombogen and Thrombin-JMI
Equal amounts (25 mU) of Thrombogen and Thrombin-JMI were reduced using 5% (v/v) 2-mercaptoethanol in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, separated by SDS-PAGE 45 on a 5 to 15% gradient, and stained with Coomassie blue. A nucleic acid detection kit was used according to the manufacturer’s protocol to determine whether nucleic acid contaminants were present in these preparations.
Statistical Analysis
Data were analyzed with Graph Pad Prism Version 3.00 for Windows, Graph Pad Software (San Diego, California; www.graphpad.com). Comparison of serology from different study populations was analyzed by an unpaired t-test. All P values are two-tailed and α = 0.05.
Results
Mouse Study I
Development of Anti-Thrombogen and Anti-αGal Antibodies
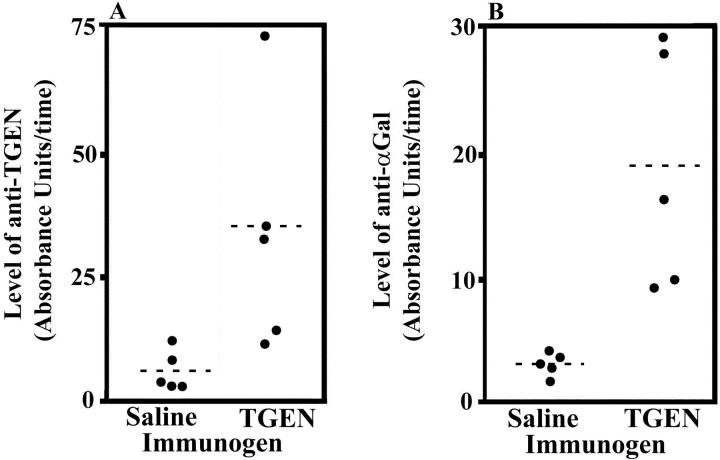
Four weeks after exposure to Thrombogen during surgery, mice had a significantly (P = 0.0356) greater level of antibodies that bound to Thrombogen as compared to mice undergoing the same procedure and exposed to saline (Table 1 ▶ and Figure 1A ▶ ). Further, mice also had significantly (P = 0.0101) greater amounts of antibodies that bound to αGal compared to mice exposed to saline (Table 1 ▶ and Figure 1B ▶ ).
Table 1.
Study I—GalT−/− Mice Exposed to Thrombogen or to Saline during Surgery
| Immunogen Mouse number | TGEN* | Saline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2† | 3† | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Sex (M/F) | F | M | M | M | M | F | F | M | M | M |
| Age‡ (years) | 1 | 1 | 1 | 1.5 | 1 | 1 | 1 | 1 | 1.5 | 1 |
| ANA§¶ (titer/pattern) | 160/p | 160/p | 160/p | 0 | 40/p | 0 | 0 | 80/s | 0 | 0 |
| Anti-nDNA¶∥ (titer) | 80 | 80 | 40 | 20 | 20 | 0 | 20 | 10 | 10 | 20 |
| Anti-dsDNA** | + | + | + | − | + | − | − | − | − | − |
| Anti-ssDNA** | + | + | − | − | − | − | − | − | − | − |
| Anti-cardiolipin** | + | + | + | + | − | − | − | − | − | − |
| Anti-Murine thrombin** | + | + | − | − | − | − | − | − | − | − |
| Anti-TGEN** | + | − | + | + | + | + | − | − | − | − |
| Anti-αGal** | + | + | + | + | + | − | − | − | − | − |
| Percent glomeruli stained¶†† | 100 | 91 | 83 | 68 | 67 | 43 | 53 | 65 | 52 | 59 |
| Percent abnormal behavior¶ | 0 | 1 | 74 | 39 | 1 | 0 | 4 | 3 | 4 | 0 |
*Thrombogen.
†Lesions in the facial area and skin were observed in these mice.
‡Age at time of exposure. Data were collected 4 weeks after exposure.
§Serum titer >1:40 was considered positive for ANA. Patterns observed were peripheral (p) or speckled (s).
¶The individual making the measurement was blinded to the type of antigen exposure.
∥Serum titer >1:10 was considered positive for anti-nDNA antibodies.
**A “+” value represents a result at least double the average of negative controls.
††Glomeruli were stained with anti-mouse IgG.
Figure 1.
Increased levels of serum anti-Thrombogen and anti-αGal antibodies 4 weeks after exposure to Thrombogen during surgery in study I. Levels of anti-Thrombogen (TGEN) antibodies (A) and anti-αGal antibodies (B) in serum obtained from mice exposed to saline or Thrombogen in study I (see Materials and Methods). The dashed lines indicate the means.
Development of Anti-Murine Coagulation Factor Antibodies
Four weeks after exposure to Thrombogen during surgery, increased levels of autoantibodies that bound to a murine thrombin preparation were found in the serum of two mice (Table 1) ▶ . Importantly, the murine thrombin preparation isolated from GalT−/− mice used in this assay is a partially pure α-thrombin preparation, as is the bovine preparation used clinically and in this study (Thrombogen). For this reason, the autoantibodies found using this assay are autoantibodies against one or more of the plasma proteins in this preparation, not necessarily autoantibodies against murine thrombin.
Development of aCL Antibodies
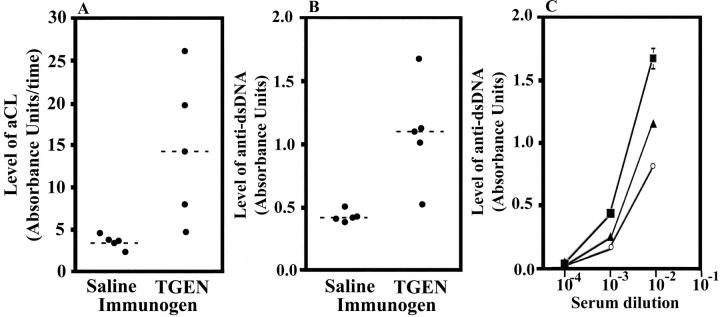
As shown in Figure 2A, 4 ▶ ▶ weeks after exposure to Thrombogen during surgery, mice had significantly higher levels of aCL antibodies than those exposed to saline (P = 0.033). This finding indicates that the measurement of aCL antibodies might serve as a rapid measure of autoimmune processes in mice exposed to bovine thrombin. No difference in binding to cardiolipin was detected between wells with and wells without 2 mmol/L of galactobiose, indicating that these results do not simply represent binding of anti-αGal antibodies to the xenogeneic carbohydrate αGal.
Figure 2.
Increased levels of serum aCL and anti-dsDNA antibodies 4 weeks after exposure to Thrombogen during surgery in study I. Levels of aCL antibodies (A) and anti-dsDNA antibodies (B) in serum obtained from mice exposed to saline or Thrombogen (TGEN) in study I (see Materials and Methods). The dashed lines indicate the means. C: The level of serum anti-dsDNA antibodies of mouse 1 (filled square) and mouse 2 (filled triangle) exposed to Thrombogen relative to those in the serum of a 14-week-old MRL/MpJ-Tnfrsf6lpr mouse (open circle).
Figure 4.

Relative purity of Thrombogen and Thrombin-JMI at therapeutically equivalent doses. Coomassie blue staining of biologically equivalent amounts (25 mU thrombin) of Thrombin-JMI (lane 1) and of Thrombogen (lane 2) separated by electrophoresis under reducing conditions reveals a relatively greater amount of impurities in Thrombogen. The migration of molecular weight markers and chain A and chain B of α-thrombin are indicated.
Development of Anti-dsDNA and Anti-ssDNA Antibodies
As shown in Figure 2B, 4 ▶ ▶ weeks after exposure to Thrombogen during surgery, mice had significantly (P = 0.0063) higher levels of anti-dsDNA antibodies than those exposed to saline. Binding of anti-dsDNA antibodies was linearly related to serum concentrations under the conditions used. As shown in Figure 2C ▶ , the levels of anti-dsDNA antibodies from mice exposed to bovine thrombin during surgery were higher than those in the serum of a 14-week-old MRL/MpJ-Tnfrsf6lpr mouse. These observations indicate that the measurement of anti-dsDNA antibodies might also serve as a rapid measure of autoimmune processes in mice exposed to bovine thrombin. No difference in binding to dsDNA was detected between wells with and wells without 2 mmol/L of galactobiose, indicating that these results do not simply represent binding of anti-αGal antibodies to the xenogeneic carbohydrate αGal. As shown in Table 1 ▶ , 4 weeks after exposure to Thrombogen during surgery, the two mice who developed the highest levels of anti-dsDNA antibodies also developed anti-ssDNA antibodies.
Development of ANA and Anti-nDNA Antibodies
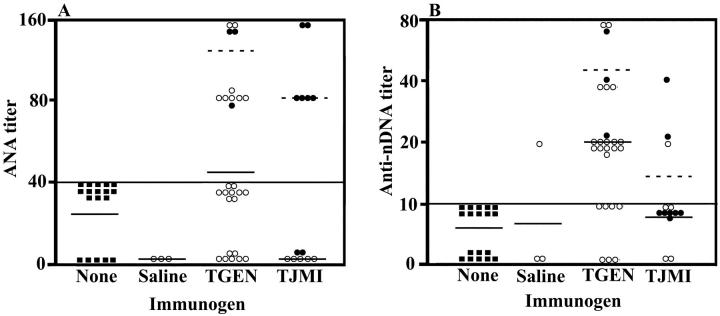
As shown in Table 1 ▶ , 4 weeks after exposure to Thrombogen during surgery, mice tended to have higher titers of ANA (P = 0.0510) than those exposed to saline. The characteristic ANA-staining pattern of nuclei observed in mice exposed to Thrombogen was peripheral (Figure 3, A and B) ▶ . This staining pattern is considered specific for ANA associated with SLE. 46,47 One mouse stained positive for ANA from the group exposed to saline. The ANA staining pattern observed in this mouse was speckled, which is not considered specific for ANA associated with SLE. 46,47 As shown in Table 1 ▶ and Figure 3 ▶ , C and D, 4 weeks after exposure to Thrombogen during surgery mice had significantly higher titers of anti-nDNA antibodies (P = 0.0337) than those exposed to saline.
Figure 3.
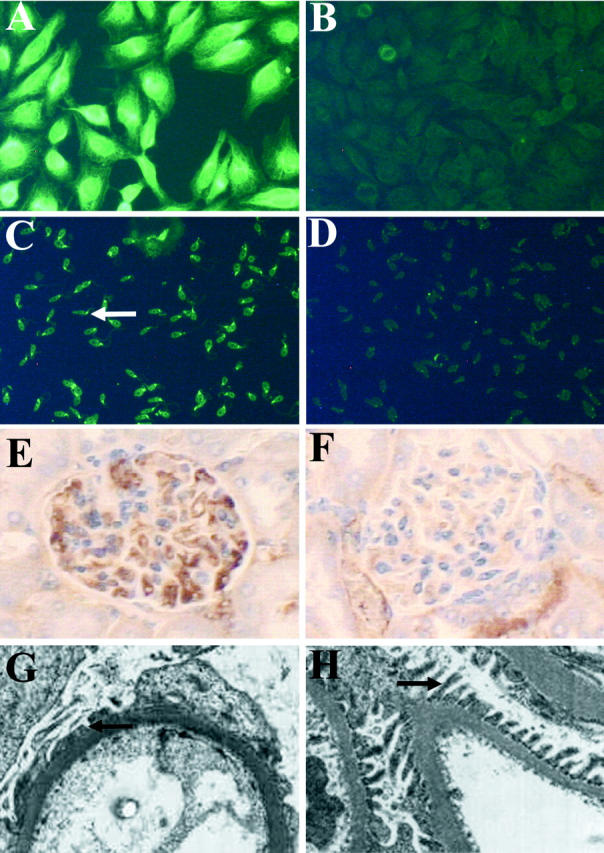
Representative staining for serum ANA and antibodies against nDNA and evidence of glomerulonephritis in mice 4 weeks after exposure to Thrombogen during surgery in study I. Representative staining (original magnification, ×200) for ANA in serum obtained from mice exposed to Thrombogen (A) and saline (B). Representative staining (original magnification, ×200) for anti-nDNA antibodies in serum obtained from mice exposed to Thrombogen (C, arrow indicates IgG binding to the kinetoplast of C. luciliae) or saline (D). Renal sections stained for IgG with peroxidase-conjugated goat anti-mouse IgG (1:40) reveal increased IgG deposition in mice exposed to Thrombogen (E) as compared to mice exposed to saline (F). Electron micrographs (original magnifications, ×2500) of renal sections reveal the breakdown of podocyte foot processes in mice exposed to Thrombogen (G) as compared to mice exposed to saline (H). Podocyte foot processes are indicated by the arrows.
Immunopathology, Histology, and Electron Microscope Studies
Three out of five mice exposed to Thrombogen had a larger amount of glomerular IgG deposition as compared to mice exposed to saline (Figure 3, E and F) ▶ . The level of IgG staining in the glomeruli of mice exposed to Thrombogen was significantly (P = 0.006) higher than levels in the glomeruli of mice exposed to saline only (Table 1) ▶ . It was not determined whether the immune complexes found in the glomeruli contained self-antigens, bovine antigens, or both. Further evaluation of renal sections by electron microscopy revealed podocyte foot process simplification and increased mesangium cellularity in the glomeruli of mice exposed to Thrombogen but not in mice exposed to saline (Figure 3, G and H) ▶ .
Behavioral Changes
When behavior was measured in a quantitative manner, two mice exposed to Thrombogen showed signs of abnormal behavior (Table 1) ▶ , including excessive grooming and scratching. Both mice demonstrating abnormal behavior had substantial and chronic bleeding in the facial area, presumably because of excessive scratching. One mouse was considered to be in sufficient distress (74% abnormal behavior) to warrant early termination of the study and was sacrificed at 3.5 weeks after exposure to antigen. The facial bleeding observed in the two GalT−/− mice has not been observed in any of the other hundreds of GalT−/− mice we have housed since first obtaining the mouse line.
Mouse Study II
Although the data described above show that exposure to Thrombogen during surgery can result in autoimmune disease, it does not address whether this autoimmune syndrome would only develop in older mice or if the development of autoimmunity observed might be because of some unknown factors only present in those mice. To shed further light on these issues and to evaluate the other bovine thrombin preparation available in the United States, additional GalT−/− mice were exposed to either Thrombogen or to Thrombin-JMI (see Materials and Methods). The development of antibodies against nuclear antigens, nDNA, dsDNA, and cardiolipin were used as indicators for the development of autoimmunity. Normal levels of serum anti-nuclear antigen, anti-nDNA, anti-dsDNA, aCL, anti-Thrombogen, anti-Thrombin-JMI, and anti-αGal IgG was established from 22 GalT−/− mice and are presented in Table 2 ▶ and relevant figures.
Table 2.
Study II—GalT−/− Mice Exposed to Thrombogen or to Thrombin-JMI during Surgery or during Intraperitoneal Injection
| Immunogen Route of exposure | None | Saline | TGEN* | TJMI† | ||||
|---|---|---|---|---|---|---|---|---|
| – | IP‡ | Surgery | IP | Either | Surgery | IP | Either | |
| n | 22 | 5 | 6 | 30 | 36 | 9 | 8 | 17 |
| Sex (M/F) | (12/10) | (3/2) | (3/3) | (19/11) | (22/14) | (6/3) | (7/1) | (13/4) |
| Average age§ (years) | 0.35 | 0.35 | 0.42 | 0.42 | 0.42 | 0.5 | 0.32 | 0.41 |
| ANA | ||||||||
| n¶ | 18 | 3 | 3 | 24 | 27 | 8 | 5 | 13 |
| Percent positive∥ | 0 | 0 | 100 | 33 | 41 | 75 | 0 | 46 |
| P** | – | ns†† | <0.0001 | ns | 0.0303 | 0.0028 | ns | ns |
| Anti-nDNA | ||||||||
| n | 18 | 3 | 3 | 24 | 27 | 8 | 5 | 13 |
| Percent positive | 0 | 33 | 100 | 67 | 70 | 25 | 20 | 23 |
| P | – | ns | <0.0001 | 0.0019 | 0.0009 | 0.005 | ns | 0.021 |
| Anti-dsDNA | ||||||||
| n | 18 | 5 | 6 | 28 | 34 | 9 | 8 | 17 |
| Percent positive | 0 | 0 | 67 | 68 | 68 | 33 | 0 | 18 |
| P | – | ns | <0.0001 | <0.0001 | <0.0001 | 0.0087 | 0.0055 | 0.0019 |
| aCL | ||||||||
| n | 22 | 5 | 6 | 22 | 28 | 9 | 8 | 17 |
| Percent positive | 0 | 0 | 67 | 73 | 72 | 11 | 38 | 24 |
| P | – | ns | 0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0002 | 0.0002 |
| Anti-αGal | ||||||||
| n | 18 | 5 | 6 | 29 | 35 | 9 | 5 | 14 |
| Percent positive | 0 | 20 | 33 | 55 | 51 | 33 | 60 | 43 |
| P | – | ns | 0.0058 | 0.0048 | 0.0041 | ns | 0.0008 | 0.0254 |
| Anti-TGEN | ||||||||
| n | 18 | 5 | 6 | 22 | 28 | 9 | 8 | 17 |
| Percent positive | 6 | 20 | 100 | 100 | 100 | 100 | 100 | 100 |
| P | – | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Anti-TJMI | ||||||||
| n | 18 | nd‡‡ | 6 | nd | 6 | 9 | nd | 9 |
| Percent positive | 0 | nd | 33 | nd | 33 | 100 | nd | 100 |
| P | – | nd | ns | nd | ns | <0.0001 | nd | <0.0001 |
*Thrombogen.
†Thrombin-JMI.
‡Intraperitoneal injection.
§Average age at time of exposure. Data were collected 4 weeks after exposure.
¶All tests were not run on some samples because of limited amounts of those samples.
∥Values at least double the average of normal values established from GalT−/− mice not exposed to an immunogen were considered to be positive. Serum titer >1:40 and serum titer >1:10 were considered positive for ANA and anti-nDNA antibodies, respectively.
**All statistical analysis was calculated using an unpaired t-test.
††Not significant.
‡‡Not determined.
Relative Purity of Thrombogen and Thrombin-JMI at Therapeutically Equivalent Doses
The specific activity of thrombin in Thrombogen and Thrombin-JMI was determined to be 95.7 and 898.0 U/mg, respectively. Consistent with this finding, comparison of Thrombin-JMI and Thrombogen separated by SDS-PAGE under reducing conditions followed by staining with Coomassie blue, demonstrated that Thrombin-JMI is a relatively more pure bovine thrombin preparation than is Thrombogen (Figure 4) ▶ . Nucleic acid was not detected in either preparation (not shown).
Development of Autoantibodies after Exposure to Thrombogen or Thrombin-JMI
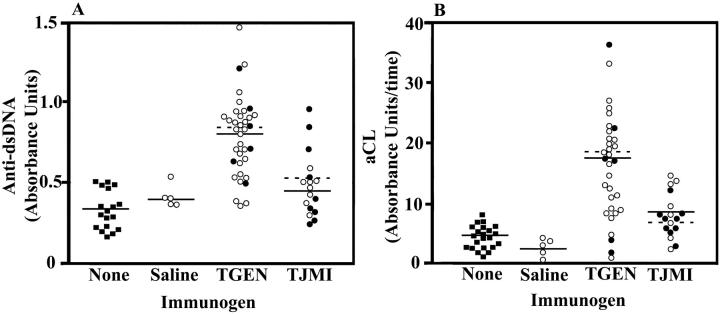
The results of autoimmune studies from mice exposed to Thrombogen or to Thrombin-JMI are presented in Table 2 ▶ and Figures 5 and 6 ▶ ▶ . Consistent with the results observed in mouse study I, mice exposed to Thrombogen, both during surgery and by intraperitoneal injection, developed significant levels of autoantibodies, including antibodies against nuclear antigens, nDNA, dsDNA, and cardiolipin. Further, mice exposed to Thrombin-JMI® also tended to develop autoantibodies. However, as shown in Figures 5 and 6 ▶ ▶ , mice exposed to Thrombogen generally developed higher levels of autoreactive antibodies than mice exposed to a therapeutically equal amount of Thrombin-JMI. These differences were significant for the development of anti-dsDNA antibodies (P = 0.0004) and aCL antibodies (P = 0.0005). Even if the variables of gender and route of exposure, which might potentially confound the results, are eliminated, exposure to Thrombogen still elicited a greater autoimmune response than did exposure to Thrombin-JMI. For example, male mice exposed to Thrombogen by injection developed significantly (P = 0.0099) greater levels of anti-dsDNA antibodies and tended to develop greater levels of antibodies against nuclear antigens, cardiolipin, and native DNA than did male mice exposed to Thrombin-JMI by injection.
Figure 5.
Increased titers of serum ANA and antibodies against nDNA 4 weeks after exposure to Thrombogen or Thrombin JMI in study II. Titers of ANA (A) and anti-nDNA antibodies (B) in serum of mice not exposed to an immunogen (filled square) and mice exposed to saline, Thrombogen, or Thrombin-JMI (TJMI). Mice were exposed to their respective immunogen either through surgery (filled circle) or through intraperitoneal injection (open circle). Dashed lines indicate the mean of serum antibody levels in groups exposed to immunogen through surgery and solid lines indicate the mean of serum antibody levels in groups exposed to immunogen through intraperitoneal injection. Serum titers >1:40 and serum titers >1:10 were considered positive for ANA and anti-nDNA antibodies, respectively.
Figure 6.
Increased levels of serum aCL and anti-dsDNA antibodies 4 weeks after exposure to Thrombogen or Thrombin JMI in study II. Levels of anti-dsDNA antibodies (A) and aCL antibodies (B) in serum of mice not exposed to an immunogen (filled square) and mice exposed to saline, Thrombogen, or Thrombin-JMI (TJMI). Mice were exposed to their respective immunogen either through surgery (filled circle) or through intraperitoneal injection (open circle). Dashed lines indicate the mean of serum antibody levels in groups exposed to immunogen through surgery and solid lines indicate the mean of serum antibody levels in groups exposed to immunogen through intraperitoneal injection. The binding of anti-dsDNA antibodies from the serum of a 14-week-old MRL/MpJ-Tnfrsf6lpr mouse was 0.703 absorbance units.
There was typically no significant difference between the autoimmune response elicited by exposure to a bovine thrombin preparation by intraperitoneal injection compared to exposure during surgery. The notable exception to this was the significantly greater (P = 0.0142) level of ANA elicited by exposure to Thrombin-JMI during surgery compared to exposure by intraperitoneal injection (Figure 5) ▶ . However, this difference might be because of the fact that the group of mice exposed to Thrombin-JMI by intraperitoneal injection and evaluated for ANA consisted of all males whereas the group exposed during surgery consisted of males and females. Females tended to produce higher levels of autoantibodies in some cases than did males. For example, female mice exposed to Thrombogen by injection developed significantly (P = 0.0228) greater levels of aCL antibodies and tended to develop greater levels of antibodies against nuclear antigens, dsDNA, and native DNA than did the males (Table 3) ▶ . Although additional studies are needed to determine the potential roles that route of exposure and gender play in the induction of autoimmunity by bovine thrombin preparations, it is evident that exposure to these reagents does elicit a strong autoimmune response in nonautoimmune-prone mice.
Table 3.
Autoimmune Induction by Exposure to Thrombogen–Females versus Males
| Immunogen Route of exposure | Surgery | TGEN* IP† | Either | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||||
| n | 3 | 3 | 19 | 11 | 22 | 14 | |||
| ANA | |||||||||
| n‡ | 1 | 2 | 17 | 7 | 18 | 9 | |||
| Percent positive§ | 100 | 100 | 24 | 57 | 28 | 67 | |||
| Mean titer | 160 | 120 | 38 | 74 | 44 | 84 | |||
| P | nd¶ | 0.0729∥ | 0.0601 | ||||||
| Anti-nDNA | |||||||||
| n | 1 | 2 | 17 | 7 | 18 | 9 | |||
| Percent positive | 100 | 100 | 53 | 100 | 56 | 100 | |||
| Mean titer | 80 | 30 | 19 | 31 | 22 | 31 | |||
| P | nd | 0.1897 | 0.3524 | ||||||
| Anti-dsDNA | |||||||||
| n | 3 | 3 | 18 | 10 | 21 | 13 | |||
| Percent positive | 33 | 100 | 61 | 80 | 57 | 85 | |||
| Mean AU∥ | 0.601 | 1.007 | 0.699 | 0.892 | 0.685 | 0.918 | |||
| P | 0.0203 | 0.0614 | 0.0080 | ||||||
| aCL | |||||||||
| n | 3 | 3 | 18 | 10 | 21 | 13 | |||
| Percent positive | 33 | 100 | 72 | 90 | 67 | 92 | |||
| Mean AU/time | 7 | 26 | 14 | 21 | 13 | 22 | |||
| P | 0.0557 | 0.0228 | 0.0019 | ||||||
*Thrombogen.
†Intraperitoneal injection.
‡All tests were not run on some samples because of limited amounts of those samples.
§Values at least double the averages of normal values established from GalT−/− mice not exposed to an immunogen were considered to be positive. Serum titer >1:40 and serum titer >1:10 were considered positive for ANA and anti-nDNA antibodies, respectively.
¶Not determined.
∥Arbitrary Unit.
Development of Antibodies against Thrombogen, Thrombin-JMI, and αGal
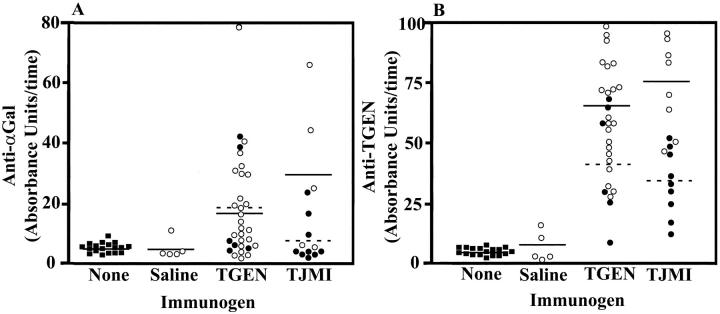
The results of immune studies from these mice are presented in Table 2 ▶ and Figure 7 ▶ . Consistent with the results observed in mouse study I, mice exposed to Thrombogen both during surgery and by intraperitoneal injection developed significant levels of antibodies against both Thrombogen and the xenogeneic carbohydrate αGal. Further, mice exposed to Thrombin-JMI also developed significant levels of antibodies against Thrombin-JMI, and αGal.
Figure 7.
Increased levels of serum anti-αGal and anti-Thrombogen antibodies 4 weeks after exposure to Thrombogen or Thrombin JMI in study II. Levels of anti-αGal antibodies (A) and anti-Thrombogen (TGEN) antibodies (B) in serum of mice not exposed to an immunogen (filled square) and mice exposed to saline, Thrombogen, or Thrombin-JMI (TJMI). Mice were exposed to their respective immunogen either through surgery (filled circle) or through intraperitoneal injection (open circle). Dashed lines indicate the mean of serum antibody levels in groups exposed to immunogen through surgery and solid lines indicate the mean of serum antibody levels in groups exposed to immunogen through intraperitoneal injection.
There was typically no significant difference between the immune response elicited by exposure to a bovine thrombin preparation in females versus males. The notable exception to this was that females developed significantly greater (P = 0.0064) levels of anti-Thrombogen antibodies when exposed to Thrombogen during surgery as compared to levels in males. Although this observation is interesting, additional studies will be required to determine whether gender plays a role in the immune response to bovine thrombin.
Mice exposed to a bovine thrombin preparation by intraperitoneal injection tended to produce higher levels of antibodies than did mice exposed during surgery (Figure 7) ▶ . For example, mice exposed to either Thrombogen or Thrombin-JMI by intraperitoneal injection had significantly greater (P = 0.0447 and 0.003, respectively) levels of anti-Thrombogen than mice exposed during surgery.
Duration of Autoreactive Antibodies Induced by Thrombogen
To determine whether the autoimmune response against a bovine thrombin preparation could be a long-lived response, we evaluated the autoimmune response to thrombin several months after repeated exposure to the therapeutic. Thus, three mice that were initially exposed through intraperitoneal injection to Thrombogen were re-exposed to the same dose of Thrombogen by intraperitoneal injection 12 weeks after the initial exposure. Similarly, three mice that were initially given saline by intraperitoneal injection were re-exposed to saline by intraperitoneal injection 12 weeks after the initial exposure. All six mice were sacrificed 15 weeks after the second injection and autoimmune markers were measured. Analysis of serum obtained from all three mice re-exposed to Thrombogen resulted in positive levels of antibodies against nuclear antigens (titer ranging from 1:80 to 1:160 with peripheral staining), nDNA (titers ranging from 1:20 to 1:80), and cardiolipin. Serum obtained from one of three mice re-exposed to Thrombogen was positive for anti-dsDNA antibodies. Analysis of serum obtained from one mouse re-exposed to saline resulted in a positive titer for ANA (titer = 1:80 with peripheral staining) and anti-nDNA antibodies (titer = 1:20) and negative levels of anti-dsDNA and aCL antibodies. Neither of the serum samples obtained from the two other mice re-exposed to saline resulted in a positive autoimmune test. Further, the mouse immunized with Thrombogen, whose serum was positive for all autoimmune tests, had a larger amount of glomerular IgG deposition as compared to the mouse exposed to saline whose serum was positive for ANA and anti-nDNA antibodies. Thus, although further study is required to determine what fraction of mice have a long-lived autoimmune response after exposure to a bovine thrombin preparation, it is evident that a long-lived response is possible.
Discussion
Our results indicate that exposure to bovine thrombin can induce pathological autoimmunity. No evidence of autoimmune disease was observed in mice exposed to saline indicating that the surgical procedure, intraperitoneal injection, or other factors associated with the experimental procedure (eg, stress) do not account for the autoimmune response that was observed in mice exposed to bovine thrombin. Therefore, this report provides the first unequivocal proof that exposure to bovine thrombin can cause the development of pathological autoreactive antibodies.
Our observation that autoantibodies and pathology consistent with a SLE-like autoimmunity arise after exposure to bovine thrombin has never been reported in mice or in humans. The peripheral pattern of staining observed on HEp-2 slides is considered to be highly suggestive of a clinical diagnosis of SLE. 48 Further, anti-dsDNA autoantibodies are characteristically associated with SLE. 46 It is thought that these autoantibodies are pathogenic, because they can directly attack tissues or form immune complexes that elicit inflammation and damage organs such as the kidney. 49 In this regard, mice exposed to bovine thrombin that developed these autoantibodies were the same mice that developed glomerulonephritis. Thus, the development of these autoantibodies after exposure to bovine thrombin would suggest that the complications because of the use of this therapeutic might also include the induction of serology associated with systemic autoimmunity.
Furthermore, although it was not determined if the aCL antibodies that developed in mice exposed to bovine thrombin induced a procoagulant phenotype, the development of these antibodies suggests that that the complications because of use of this therapeutic are not limited to postoperative hemorrhagic complications, but also may include chronic prothrombotic complications. Antibodies specific for cardiolipin are a subset of antiphospholipid antibodies, which have been recently reported to develop in humans after exposure to bovine thrombin. 32 It has been hypothesized that these antibodies can develop from the immune presentation of xenogeneic coagulation proteins bound to phospholipid. 32 Anti-phospholipid antibodies have been studied extensively and are associated with systemic autoimmune syndromes and prothrombotic complications such as stroke, myocardial infarctions, and deep venous thrombosis. 50 In this regard, a recent report demonstrated that patients who received a polytetrafluoroethylene arterial venous fistula and developed an immune response to bovine thrombin were significantly more likely to develop graft thrombosis than patients who did not develop an immune response to bovine thrombin. 30 Evidence in this study is also consistent with the idea that bovine thrombin may induce autoantibodies associated with a procoagulant phenotype, because most mice exposed to bovine thrombin developed aCL antibodies.
In addition, the results in our study suggest that the exposure of GalT−/− mice to bovine thrombin both during surgery and by intraperitoneal injection can be used as an animal model to study the mechanisms that trigger, and the pathology that results from, the development of autoantibodies associated with SLE-like syndromes. This autoimmune syndrome can be detected in some mice 15 weeks after a secondary exposure to bovine thrombin, indicating that this autoimmune syndrome can persist well after the inducing antigen has been processed and cleared. The levels of anti-dsDNA antibodies in mice exposed to bovine thrombin were comparable or higher than those of a mouse used as a model for SLE-like autoimmune syndromes. Additionally, in humans SLE develops more frequently in females than in males. In this regard, female GalT−/− mice exposed to bovine thrombin developed significantly greater levels of antibodies against dsDNA and cardiolipin and tended to develop greater levels of antibodies against nuclear antigens and native DNA than did the males. Although the characteristics of these autoantibodies are similar to those observed in other models of SLE-like autoimmunity, it remains to be determined whether the mechanisms that drive the development of these antibodies are similar.
GalT−/− mice exposed to bovine thrombin were also found to develop significant levels of antibodies against bovine thrombin and the xenogeneic carbohydrate αGal. These responses are similar to those characteristic of the human immune response after exposure to bovine thrombin during surgery. 1-3 Further, results indicate that some of the mice develop autoantibodies against clotting factors. This would suggest that the exposure of GalT−/− mice to bovine thrombin during surgery could be used as a model for the human immune response to bovine thrombin. Further studies using this model may help determine the mechanisms that trigger, and the pathology that results from, the development of autoreactive anti-clotting factor antibodies after exposure to bovine thrombin during surgery.
Although the mechanisms associated with the development of autoimmunity in this model require additional study, the present findings have immediate clinical implications. The results suggest that exposure to either of the two currently Food and Drug Administration approved preparations of bovine thrombin may pose serious immunological risk to patients. We recommend that use of this therapeutic be limited to life-saving procedures, and that alternative, nonimmunogenic, hemostatic agents be developed. If bovine thrombin must be used in a life-saving procedure we would recommend using Thrombin-JMI in lieu of Thrombogen. Although both preparations elicited significant levels of autoantibodies, exposure to Thrombogen generally resulted in greater levels of these antibodies and significantly greater levels of antibodies against dsDNA and cardiolipin. In these studies mice were exposed to therapeutically equivalent amounts (measured in thrombin units) of Thrombogen and Thrombin-JMI. However, because the specific activities of Thrombogen and Thrombin-JMI are dramatically different, mice exposed to Thrombogen were exposed to ∼10-fold more protein than were mice exposed to Thrombin-JMI. It is unknown whether this difference in total protein or whether some qualitative difference(s) between the proteins present in the two preparations account for the greater induction of autoantibody levels by Thrombogen than by Thrombin-JMI. Regardless of the mechanisms underlying these findings, the results strongly discourage the use of bovine thrombin, particularly Thrombogen, in humans.
Acknowledgments
We thank Dr. Garnett Kelsoe and Dr. David Pisetsky for their thoughtful recommendations; Ryan C. Fields, Quinton V. Cancel, Dr. Robert J. Lefkowitz, Russell Boreland, and Susan H. Schoenecker for their assistance; and the Durham Veterans Affairs Hospital electron microscopy service for specimen processing and photography.
Footnotes
Address reprint requests to Dr. Jeffrey H. Lawson, Duke University Medical Center, Room 481 MSRB/Box 2622, Research Dr., Durham, NC 27710. E-mail: lawso717@duke.edu.
Supported by The American Heart Association (to J. H. L. and T. L. O.), the Department of Surgery at Duke University Medical Center, the Fannie E. Rippel Foundation (to W. P.), Baxter Health Care Corporation and the Lupus Foundation of America (to J. G. S.). Dr. Lawson is a Clinician Scientist Awardee from the American Heart Association and Genentech, Inc. (95004380).
References
- 1.Ortel TL, Mercer MC, Thames EH, Moore KD, Lawson JH: The immunologic impact and clinical outcomes following surgical exposure to bovine thrombin. Ann Surg 2001, 233:88-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorion RP, Hamati HF, Landis B, Frey C, Heydt D, Carey D: Risk and clinical significance of developing antibodies induced by topical thrombin preparations. Arch Pathol Lab Med 1998, 122:887-894 [PubMed] [Google Scholar]
- 3.Schoenecker JG, Hauck RK, Mercer MC, Parker W, Lawson JH: Exposure to topical bovine thrombin during surgery elicits a response against the xenogeneic carbohydrate galactose(alpha1-3)galactose. J Clin Immunol 2000, 20:434-444 [DOI] [PubMed] [Google Scholar]
- 4.Jackson MR, MacPhee MJ, Drohan WN, Alving BM: Fibrin sealant: current and potential clinical applications. Blood Coagul Fibrinolysis 1996, 7:737-746 [PubMed] [Google Scholar]
- 5.Spotnitz W: Fibrin sealant in the United States: clinical use at the University of Virginia. Semin Thromb Hemost 1995, 74:482-485 [PubMed] [Google Scholar]
- 6.Prior JJ, Wallace DG, Harner A, Powers N: A sprayable hemostat containing fibrillar collagen, bovine thrombin, and autologous plasma. Ann Thorac Surg 1999, 68:479-485 [DOI] [PubMed] [Google Scholar]
- 7.Lawson JH, Pennell BJ, Olson JD, Mann KG: Isolation and characterization of an acquired antithrombin antibody. Blood 1990, 76:2249-2257 [PubMed] [Google Scholar]
- 8.Berruyer M, Amiral J, French P, Belleville J, Bastien O, Clerc J, Kassir A, Estanove S, Dechavanne M: Immunization by bovine thrombin used with fibrin glue during cardiovascular operations. Development of thrombin and factor V inhibitors. J Throrac Cardiovasc Surg 1993, 105:892-897 [PubMed] [Google Scholar]
- 9.Ortel TL: Clinical and laboratory manifestations of anti-factor V antibodies. J Lab Clin Med 1999, 133:326-334 [DOI] [PubMed] [Google Scholar]
- 10.Ortel TL, Moore KD, Quinn-Allen MA, Okamura T, Sinclair AJ, Lazarchick J, Govindan R, Carmagnol F, Kane WH: Inhibitory anti-factor V antibodies bind to the factor V C2 domain and are associated with hemorrhagic manifestations. Blood 1998, 91:4188-4196 [PubMed] [Google Scholar]
- 11.Ortel TL, Charles LA, Keller FG, Marcom PK, Oldham HN, Jr, Kane WH, Macik BG: Topical thrombin and acquired coagulation factor inhibitors: clinical spectrum and laboratory diagnosis. Am J Hematol 1994, 45:128-135 [DOI] [PubMed] [Google Scholar]
- 12.Spero JA: Bovine thrombin-induced inhibitor of factor V and bleeding risk in postoperative neurosurgical patients. Report of three cases. J Neurosurg 1993, 78:817-820 [DOI] [PubMed] [Google Scholar]
- 13.Cmolik BL, Spero JA, Magovern GJ, Clark RE: Redo cardiac surgery: late bleeding complications from topical thrombin-induced factor V deficiency. J Throrac Cardiovasc Surg 1993, 105:222-228 [PubMed] [Google Scholar]
- 14.Fastenau DR, Hormuth DA, McIntyre JA: Antiphospholipid antibodies in left-ventricular assist system recipients after exposure to topical bovine thrombin. Transplant Proc 1999, 31:141-142 [DOI] [PubMed] [Google Scholar]
- 15.Chouhan VD, De La Cadena RA, Nagaswami C, Weisel JW, Kajani M, Rao AK: Simultaneous occurrence of human antibodies directed against fibrinogen, thrombin, and factor V following exposure to bovine thrombin: effects on blood coagulation, protein C activation and platelet function. Semin Thromb Hemost 1997, 77:343-349 [PubMed] [Google Scholar]
- 16.Zehnder JL, Leung LL: Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombin. Blood 1990, 76:2011-2016 [PubMed] [Google Scholar]
- 17.Israels SJ, Leaker MT: Acquired inhibitors to factors V and X after exposure to topical thrombin: interference with monitoring of low molecular weight heparin and warfarin. J Pediatr 1997, 131:480-483 [DOI] [PubMed] [Google Scholar]
- 18.McKie JS, Herzenberg JE: Coagulopathy complicating intraoperative blood salvage in a patient who had idiopathic scoliosis. A case report. J Bone Joint Surg Am 1997, 79:1391-1394 [DOI] [PubMed] [Google Scholar]
- 19.Muntean W, Zenz W, Edlinger G, Beitzke A: Severe bleeding due to factor V inhibitor after repeated operations using fibrin sealant containing bovine thrombin. Semin Thromb Hemost 1997, 77:1223. [PubMed] [Google Scholar]
- 20.Christie RJ, Carrington L, Alving B: Postoperative bleeding induced by topical bovine thrombin: report of two cases. Surgery 1997, 121:708-710 [DOI] [PubMed] [Google Scholar]
- 21.Tarantino MD, Ross MP, Daniels TM, Nichols WL: Modulation of an acquired coagulation factor V inhibitor with intravenous immune globulin. J Pediatr Oncol Hematol 1997, 19:226-231 [DOI] [PubMed] [Google Scholar]
- 22.Banninger H, Hardegger T, Tobler A, Barth A, Schupbach P, Reinhart W, Lammle B, Furlan M: Fibrin glue in surgery: frequent development of inhibitors of bovine thrombin and human factor V. Br J Hematol 1993, 85:528-532 [DOI] [PubMed] [Google Scholar]
- 23.Rapaport SI, Zivelin A, Minow RA, Hunter CS, Donnelly K: Clinical significance of antibodies to bovine and human thrombin and factor V after surgical use of bovine thrombin. Am J Clin Pathol 1992, 97:84-91 [DOI] [PubMed] [Google Scholar]
- 24.Carroll JF, Moskowitz KA, Edwards NM, Hickey TJ, Rose EA, Budzynski AZ: Immunologic assessment of patients treated with bovine fibrin as a hemostatic agent. Semin Thromb Hemost 1996, 76:925-931 [PubMed] [Google Scholar]
- 25.Diez-Martin J, Sikkink RA, Gilchrist GS, Bowie EJ, Fass DN: Development of anti-bovine thrombin antibodies following neurosurgical procedures. Br J Hematol 1990, 74:369-370 [DOI] [PubMed] [Google Scholar]
- 26.Knobl P, Lechner K: Acquired factor V inhibitors. Baillieres Clin Hematol 1998, 11:305-318 [DOI] [PubMed] [Google Scholar]
- 27.Spero JA, Triplett DA, Cmolik BL, Reid C, Clark RE: A family of clotting factor inhibitors primarily involving FV and FXI occurring after cardiovascular and neurosurgical procedures. Blood 1991, 78(Suppl):63A1712645 [Google Scholar]
- 28.Burak WJ, Goodman P, Young D, Farrar W: Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. J Surg Oncol 1997, 64:27-31 [DOI] [PubMed] [Google Scholar]
- 29.Flaherty MJ, Henderson R, Wener MH: Iatrogenic immunization with bovine thrombin: a mechanism for prolonged thrombin times after surgery. Ann Intern Med 1989, 111:631-634 [DOI] [PubMed] [Google Scholar]
- 30.Sands JJ, Nudo SA, Ashford RG, Moore KD, Ortel TL: Antibodies to topical bovine thrombin correlate with access thrombosis. Am J Kidney Dis 2000, 35:796-801 [DOI] [PubMed] [Google Scholar]
- 31.Stricker RB, Lane PK, Leffert JD, Rodgers GM, Shuman MA, Corash L: Development of antithrombin antibodies following surgery in patients with prosthetic cardiac valves. Blood 1988, 72:1375-1380 [PubMed] [Google Scholar]
- 32.Fastenau DR, McIntyre JA: Immunochemical analysis of polyspecific antibodies in patients exposed to bovine fibrin sealant. Ann Thorac Surg 2000, 69:1867-1872 [DOI] [PubMed] [Google Scholar]
- 33.Kovanen P, Manttari M, Palosuo T, Manninen V, Aho K: Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med 1998, 159:2364-2365 [DOI] [PubMed] [Google Scholar]
- 34.Manzi S: Systemic lupus erythematosus: a model for atherogenesis? Rheumatology 2000, 39:353-359 [DOI] [PubMed] [Google Scholar]
- 35.Gallucci S, Lolkema M, Matzinger P: Natural adjuvants: endogenous activators of dendritic cells. Nat Med 1999, 5:1249-1255 [DOI] [PubMed] [Google Scholar]
- 36.Thall AD, Maly P, Lowe JB: Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem 1995, 270:21437-21440 [DOI] [PubMed] [Google Scholar]
- 37.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ: Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 1978, 148:1198-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love S, Lee W, Nakamura Y, Platt J, Bollinger R, Parker W: Unexpected anti-αGalNAc antibodies in alpha-galactosyltransferase deficient mice: complex relationship between genotype and the natural antibody repertoire. Immunobiology 2001, 203:650-658 [DOI] [PubMed] [Google Scholar]
- 39.Collins BH, Cotterell AH, McCurry KR, Alvarado CG, Magee JC, Parker W, Platt JL: Cardiac xenografts between primate species provide evidence for the importance of the alpha-galactosyl determinant in hyperacute rejection. J Immunol 1995, 154:5500-5510 [PubMed] [Google Scholar]
- 40.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM: Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 1988, 56:1730-1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love S, Lee W, Nakamura Y, Platt J, Bollinger R, Parker W: Natural anti-carbohydrate IgM in mice: dependence on age and strain. J Immunol Methods 2000, 246:61-68 [DOI] [PubMed] [Google Scholar]
- 42.Holland PC: Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process 1977, 3:77-104 [DOI] [PubMed] [Google Scholar]
- 43.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA: Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988, 263:17755-17762 [PubMed] [Google Scholar]
- 44.Pyun EH, Pisetsky DS, Gilkeson GS: The fine specificity of monoclonal anti-DNA antibodies induced in normal mice by immunization with bacterial DNA. J Autoimmun 1993, 6:11-26 [DOI] [PubMed] [Google Scholar]
- 45.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 46.Ruddy S, Harris E, Sledge C, Kelley, W. Kelley’s Textbook of Rheumatology. Philadelphia, W.B. Saunders Company, 2001, pp 161–174
- 47.Koopman WJ (Ed): Arthritis and Allied Conditions. Philadelphia, Lippincott, Williams and Wilkins, 2001, pp 1480–1502
- 48.Tan EM: Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol 1989, 44:93-151 [DOI] [PubMed] [Google Scholar]
- 49.Pisetsky DS, Grudier JP, Gilkeson GS: A role for immunogenic DNA in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum 1990, 33:153-159 [DOI] [PubMed] [Google Scholar]
- 50.Roubey RA: Antigenic specificities of antiphospholipid autoantibodies: implications for clinical laboratory testing and diagnosis of the antiphospholipid syndrome. Lupus 1996, 5:425-430 [DOI] [PubMed] [Google Scholar]