Abstract
Hodgkin’s disease (HD) is a lymphoproliferative disease of predominantly B-cell origin. However, the reasons for the incomplete development of the B-cell phenotype and lack of immunoglobulin expression in classical HD (cHD) have not been fully explained. We examined the expression of PU.1 in HD, an Ets-family transcription factor, which regulates the expression of immunoglobulin and other genes that are important for B-cell development. Immunohistochemistry for PU.1 was performed on 35 cases of cHD and 15 cases of lymphocyte predominance HD as well as 67 non-Hodgkin’s lymphomas (NHL). Expression of PU.1 was studied by Western blotting in four cHD-derived cell lines and in five NHL cell lines. We also studied the expression of two additional B-cell transcription factors, B-cell-specific activator protein and Oct-2. Our results show a striking lack of PU.1 expression by neoplastic cells in cHD but not in lymphocyte predominance HD. Our study also confirmed that B-cell-specific activator protein but not Oct-2 is not expressed by cHD. Western blotting showed no PU.1 protein expression in the cHD-derived cell lines, with the exception of one cell line of putative monocyte/histiocyte origin. The lack of PU.1 protein expression in cHD likely contributes to the lack of immunoglobulin expression and incomplete B-cell phenotype characteristic of the Reed-Sternberg cells in cHD.
PU.1 (PU.1) belongs to the Ets-family of transcription factors. It is expressed in the myeloid lineage and in immature as well as mature B lymphocytes with the exception of plasma cells. PU.1 is essential during early B-cell differentiation. The absence of PU.1 results in a total block of B-cell development at the pre-pro stage. 1 Very little is known about PU.1 function in later stages of B-cell development. PU.1 does not seem to play a role in the end-stage of B-cell development and is not expressed in plasma cells. Accordingly, PU.1 DNA binding activity, PU.1 mRNA expression and PU.1-dependent transactivation were found to be absent or detectable only at a very low level in a number of multiple myeloma cell lines. 2 PU.1 exerts an important role in the regulation of the expression of crucial B-cell proteins such as immunoglobulin (Ig) genes, CD79, CD20, and CD72. 3 PU.1 binds to the 3′ enhancer region of both the Ig kappa and lambda light chain genes whereas it also regulates the immunoglobulin heavy chain genes through the intron enhancer region. 4-7 In addition, several promotor regions of the immunoglobulin variable genes as well as J chain gene have also been shown to bind PU.1. 8 PU.1 exerts its function in a number of enhancer (eg, the Ig gene) or promotors (eg, the CD20 gene) in cooperation with a second transcription factor Pip. 9 PU.1 has also been shown to act by cooperating with several other transcription factors such as c-jun and c-fos. 10,11
In addition to a number of other genes such as major histocompatibility complex class II, interleukin (IL)-5 receptor α and PU.1 itself, PU.1 targets promoters/enhancers of at least 24 myelomonocyte/granulocyte genes (M-CSF receptor, G-CSF receptor, GM-CSF receptor, CD11b, CD11c, CD18, myeloperoxidase, and others), four megakaryocyte/erythrocyte genes (GPIIb, PBP, β-globin intervening sequence 2, and glutathione peroxidase), and three viral genes (EB virus EBNA2, SV40, and equine infectious anemia virus). 3 The role of the transcription factor PU.1 in hematopoiesis has been extensively reviewed elsewhere. 12-15 It has been recently shown that different levels of expression of PU.1 transcription factor determines cell fate in the hematopoietic system. A high level of expression promotes macrophage differentiation and blocks B-cell development whereas a low level of expression favors B-cell development. 16 Interactions between PU.1 and GATA proteins play a critical role in the decision of stem cells to commit to erythroid versus myeloid lineage. 17 PU.1 also plays a role in dendritic cell development and is required for myeloid-derived but not lymphoid-derived dendritic cells. 18
Oct-2 is an additional important transcription factor in B cells that targets the immunoglobulin promotors. However, it is not necessary for the maintenance of Ig gene expression in a differentiated B cell. 19 The study of Oct-2−/− mice has shown that the Oct-2 gene is essential for survival, but normal numbers of surface Ig-positive B cells develop in the fetal liver. Thus, Oct-2 seems not to be essential for B-cell development nor for regulating the expression of the Ig genes. However, Oct-2 does seem to play a role in germinal center cell formation and further differentiation of B cells into IgG-producing cells and plasma cells and therefore is important for the maintenance of the mature B-cell pool. 20,21
HD has been demonstrated to predominantly represent a clonal disease of B-cell origin by virtue of the frequent rearrangement of immunoglobulin genes by Reed-Sternberg cells (RS), the neoplastic cells of HD. 22-26 Interestingly, Reed-Sternberg cells mostly show a partial B-cell phenotype. Indeed, the Reed-Sternberg cells of classical Hodgkin’s disease (cHD) infrequently express B-cell surface antigens or immunoglobulins in contrast with the neoplastic cells of most non-Hodgkin’s B-cell lymphomas (B-NHL) and lymphocytic predominance Hodgkin’s disease (LPHD). 27-30 Because normal B-cell development is not possible without the transcription factor PU.1 and the B-lymphocyte phenotype is tightly regulated by PU.1, we wanted to investigate in the present study whether the HD phenotype might be related to aberrant PU.1 protein expression.
Materials and Methods
Tissues and Cell Lines
A total of 125 cases were collected from the files of the Department of Pathology, The Norwegian Radium Hospital. Included were the following diagnoses: cHD (35 cases), lymphocyte predominance Hodgkin’s lymphoma (LPHD, 15 cases), various B-cell non-Hodgkin’s lymphomas (B-NHL, 43 cases) and T-cell non-Hodgkin’s lymphomas (T-NHL, 24 cases). Eight reactive lymph nodes were also studied. Included were lymph nodes showing follicular hyperplasia (four cases), dermatopathic lymphadenopathy (one case), sinus histiocytosis (one case), and sarcoidosis (two cases). Formalin-fixed or B5-fixed paraffin-embedded tissues were available for all cases.
The following cell-lines were used: three HD-derived cell lines (KM-H2, L-428, HDLM-2), one putative HD cell line with histiocytic differentiation (HD-MY-Z), two human anaplastic large-cell lymphoma cell lines (SR-786 and SU-DHL-1), one Burkitt’s lymphoma cell line (Namalwa), one T-lymphoblastic leukemia cell line (Jurkat), and one follicular lymphoma cell line (ROS-50). 31-39
All cell lines were obtained from DSMZ (Braunschweig, Germany) with the exception of ROS-50 that was a kind gift from Dr. R. Slater, University Hospitals of Rotterdam, The Netherlands.
Antibodies
The monoclonal anti-human PU.1 antibody (clone G148-74) was purchased from Pharmingen (San Diego, CA), mouse anti-B-cell-specific activator protein (BSAP) (anti-Pax-5, clone 24) from Transduction Laboratories (Lexington, KY), and Oct-2 (AB-1) from Oncogene Research Products (Boston, MA). The monoclonal PU.1, BSAP, and Oct-2 antibodies were used for immunohistochemistry and immunocytochemistry. The polyclonal anti-PU.1 antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and used for Western blot analysis and immunocytochemistry.
Immunohistochemistry and Immunocytochemistry
Formalin-fixed and paraffin-embedded or B5-fixed and paraffin-embedded tissues were cut at 4 μm. The sections were pretreated in a microwave oven (Electrolux microwave, 850 W) by cooking in ethylenediaminetetraacetic acid antigen retrieval solution at pH 8. Subsequently, the sections were incubated with the primary antibody (dilution, 1:10) for 30 minutes and stained using the EnVison kit (DAKO, Glostrup, Denmark). Cytospins prepared from cell cultures were air-dried and stored frozen until use. Before use, cytospins were fixed in acetone for 2 minutes, air-dried again, incubated with primary antibody (dilution, 1:10) for 30 minutes and stained by the EnVision method. Formalin-fixed tissue was available for all of the cases. B5-fixed tissue was available in 40% of the cases and was studied in parallel with formalin-fixed tissue to evaluate differences because of different tissue fixation. The staining pattern was designated as “diffuse” if the staining was found in all malignant cells and as “focal” if present only in a fraction of malignant cells.
Western Blotting
Cells (106) were lysed in Laemmli buffer and heated at 95°C for 5 minutes and subsequently loaded onto the gel. Alternatively, 25 μg of protein as measured by the Bradford assay were used. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% polyacrylamide gels and transfer of proteins to nitrocellulose filters were performed as described previously. 40,41 The transfer of proteins as well as amount of proteins was checked with Ponceaus S solution. After inactivation with 5% milk solution in phosphate-buffered saline with 1% Tween 20, the blots were incubated for 2 hours with the anti-PU.1 polyclonal antibody at a final concentration of 1 μg/ml. After washing, the blots were incubated for 45 minutes with peroxidase-labeled swine anti-rabbit IgG at a final concentration of 0.2 μg/ml. Subsequently, the blots were washed again and incubated with enhanced chemiluminescence detection reagents (Amersham, Buckinghamshire, England) after which they were exposed to autoradiographic film first for 2 minutes, and subsequently for 15 minutes.
Results
Immunohistochemistry and Immunocytochemistry
Reactive Lymph Nodes
The results were very uniform and reproducible with the anti-human PU.1, anti-Pax-5, and anti-Oct-2 antibodies. The anti-human PU.1 antibody labeled lymphocytes in B-cell compartments of the lymph node and histiocytes. Lymphocytes in B-cell compartments, but no other cells of the lymph node showed strong and uniform nuclear positivity with anti-Pax-5. The intensity of staining with anti-human PU.1 varied in various cell types. The staining intensity was the strongest in histiocytes, moderate and uniform in mantle B cells, whereas the majority of germinal center B cells showed a moderate intensity of staining. No expression of either PU.1 or BSAP was detected in plasma cells. Plasmacytoid monocytes expressed PU.1, but not BSAP. Oct-2 was variably expressed in germinal center cells, weak or negative in mantle zone, and weak to moderate in plasma cells. A subpopulation of germinal center cells showed the strongest expression. No expression of Oct-2 was found in any other cell type.
Hodgkin’s Lymphoma
cHD was classified as follows: nodular sclerosis (20 cases), mixed cellularity (9 cases), lymphocyte depletion (3 cases), and lymphocyte-rich (3 cases). There were 15 cases of LPHD. The immunophenotypes of the neoplastic cells in cHD are summarized as follows: 100% CD30, 70% CD15, 28% CD20 (occasional cells with variable intensity), 23% CD45 (occasional cells), and 7% CD3 (rare cells). The expression of the CD20, CD45, and CD3 was only seen in a small subpopulation of Hodgkin’s cells in these respective cases. The neoplastic cells in all cases of LPHD expressed CD45 and CD20 (diffuse expression, variable intensity), but no positivity was found for either CD30 or CD15.
The results of immunohistological evaluation of PU.1, BSAP, and Oct-2 protein expression are summarized in Tables 1 and 2 ▶ ▶ . Figures 1 and 2 ▶ ▶ show the results of immunohistochemical study of PU.1 and BSAP expression. A striking difference in the expression of the PU.1 protein was found between cHD and LPHD (Figure 1) ▶ . In all cases of LPHD, the neoplastic cells (popcorn cells, L&H cells) showed nuclear positivity for PU.1, whereas only in 1 of the 35 cases of cHD neoplastic cells unequivocally expressed PU.1. In all cases of cHD included in the study, histiocytes as well as small B lymphocytes were invariably positive such as observed in the reactive lymph nodes. Variable cytoplasmic positivity in malignant cells was noted in 62% of cHD and 34% of LPHD cases.
Table 1.
PU.1, BSAP, and Oct-2 Expression in Hodgkin’s Lymphoma
| Diagnosis | PU.1 (%) | BSAP (%) | Oct-2 (%) |
|---|---|---|---|
| Classical HD | 2 /35 (5.7) | 27 /29 (93) | 0 /29 (0) |
| LPHD | 15 /15 (100) | 15 /15 (100) | 15 /15 (0) |
Table 2.
PU.1, BSAP, and Oct-2 Expression in Non-Hodgkin’s Lymphoma
| Diagnosis | PU.1 (%) | BSAP (%) | Oct-2 (%) |
|---|---|---|---|
| B-NHL | 29/43 (67) | 21/22 (95) | 32/36 (89) |
| CLL/SLL* | 6/6 (100) | 2/2 (100) | 3/3 (100) |
| FL | 7/7 (100) | 2/2 (100) | 4/4 (100) |
| MCL | 2/3 (67) | 2/2 (100) | 4/6 (67) |
| MZL | 3/3 (100) | 2/2 (100) | 2/2 (100) |
| DLBCL† | 11/18 (61) | 9/10 (90) | 15/17 (88) |
| TCRBCL | 0/6 (0) | 4/4 (100) | 4/4 (100) |
| PTCL | 0/17 (0) | 0/17 (0) | |
| ALCL | 0/7 (0) | 0/7 (0) |
B-NHL, B-cell non-Hodgkin’s lymphoma; CLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, extranodal marginal zone lymphoma of MALT type; DLBCL, diffuse large B-cell lymphoma; TCRBCL, T-cell-rich B-cell lymphoma; PTCL, peripheral T-cell lymphoma; ALCL, anaplastic large cell lymphoma.
*Including one case of lymphoplasmacytic lymphoma.
†Excluding TCRBCL.
Figure 1.
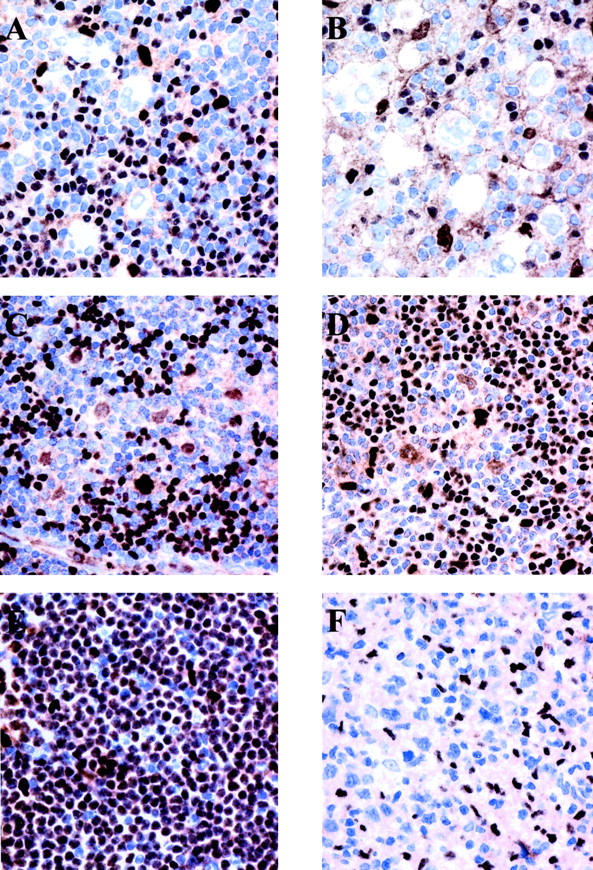
PU.1 is differentially expressed in HD and B-NHL. Immunohistochemical analysis on paraffin-embedded tissues using monoclonal anti-PU.1 antibody (immunostaining by the EnVision method using diaminobenzidine as a chromogen). A and B: Reed-Sternberg cells in nodular sclerosis cHD and mixed cellularity cHD, respectively, show no expression of PU.1. Small B lymphocytes and occasional histiocytes, but not T lymphocytes in the background express the protein in the nuclei. C and D: Neoplastic cells in two cases of LPHD expressing PU.1 in the nucleus. T cells rosetting the neoplastic cells do not express PU.1, whereas small B cells and histiocytes in the background do express the protein. E: PU.1 is strongly and diffusely expressed in small lymphocytic lymphoma/chronic lymphocytic leukemia. F: Absence of PU.1 expression by the neoplastic cells of DLBCL.
Figure 2.

BSAP is expressed in all subtypes of HD and B-NHL. Immunohistochemical analysis on paraffin-embedded tissues using monoclonal anti-Pax-5 antibody (immunostaining by the EnVision method using diaminobenzidine as a chromogen). A, B, and C: Reed-Sternberg cells in nodular sclerosis cHD, mixed cellularity cHD, and neoplastic cells of LPHD, respectively, show nuclear BSAP expression. The level of BSAP expression by neoplastic cells varies from case to case as illustrated by the low level of expression of the case shown in A. Small B lymphocytes, but not T lymphocytes or histocytes in the background also express the protein. D: BSAP is expressed in neoplastic cells of DLBCL.
In all cases of LPHD and all, but two cases of cHD, neoplastic cells were positive for BSAP (Figure 2) ▶ . The intensity of the staining varied from weak to strong in the neoplastic cells, whereas it was invariably strong in the small benign B lymphocytes. Oct-2 was not expressed in any of the cases of cHD in our study. Also, all cases of LPHD expressed Oct-2.
NHL
The results are shown in Table 2 ▶ . B-NHLs were positive for BSAP except for a single case of plasmablastic lymphoma that was negative. Interestingly, 33% of B-NHL were negative for PU.1 (Figure 1) ▶ and 11% for Oct-2.
PU.1−/Oct-2− Phenotype
Double-negative phenotype, PU.1−/Oct-2−, was found characteristically in all but two cases of cHD. In contrast to cHD, all cases of LPHD were positive for PU.1 and Oct-2. Also, 1 of 17 cases of diffuse large B-cell lymphoma (DLBCL) (5.8%) was double-negative for PU.1 and Oct-2. Forty-seven percent of DLBCL had double-positive phenotype such as LPHD.
Cell Lines
The results of PU.1 expression by the cell lines are shown in Table 3 ▶ . The HD-MYZ cell line showed cytoplasmic positivity with polyclonal PU.1 antibody. However, monoclonal PU.1 antibody detected very weak nuclear expression in an occasional cell. The L428, HDLM-2, and KM-H2 HD-derived cell lines were negative using both polyclonal and monoclonal PU.1 antibody. The Namalwa cell line showed occasional cells with weak, but definite positivity. The Jurkat cell line was negative with both antibodies. The SU-DHL-1 anaplastic large-cell lymphoma cell line showed borderline cytoplasmic and nuclear positivity with monoclonal, but not polyclonal PU.1 antibody. However, SR-786, another anaplastic large-cell lymphoma cell line was found to be negative with both PU.1 antibodies. The control cell line ROS.50 showed strong nuclear positivity with both monoclonal and polyclonal anti-PU.1 antibody.
Table 3.
Immunocytochemical Detection of PU.1 in Hodgkin’s and Non-Hodgkin’s Lymphoma-Derived Cell Lines
| Cell line | Monoclonal PU.1 | Polyclonal PU.1 |
|---|---|---|
| HD-MY-Z | +/− | − |
| HDLM-2 | − | − |
| L-428 | − | − |
| KM-H2 | − | − |
| Namalwa | + | + |
| Jurkat | − | − |
| SU-DHL-1 | −/+ | − |
| SR-786 | − | − |
| ROS-50 | ++ | ++ |
++, strong; +, weak; +/−, very weak; −/+, borderline.
Western Blotting
The results are illustrated in Figure 3 ▶ . The ROS-50 follicular lymphoma cell line showed the strongest band of the appropriate molecular weight (∼42 kd) that was also present in the peripheral blood mononuclear cell sample. An identical, but much weaker band was detected after prolonged exposure of the radiographic film in the Namalwa cell line. Three of the HD-derived cell lines did not reveal PU.1 expression. The HD-MYZ, a putative HD-derived cell line with monocytic differentiation displayed an anti-PU.1 reactive protein migrating at ∼50 kd. The Jurkat and the two anaplastic large cell lymphoma (ALCL) cell lines were negative for PU.1 expression.
Figure 3.
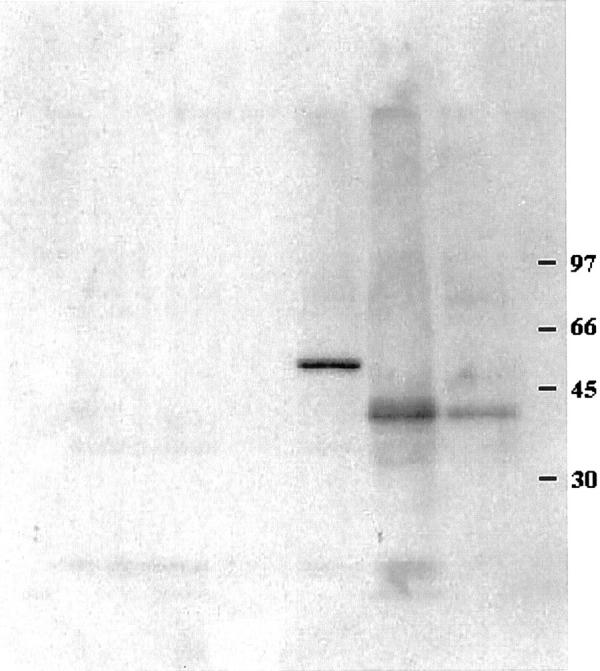
Normal PU.1 is not expressed in HD-derived cell lines by Western blotting. HD-derived cell lines L428, KM-H2, and HDLM-2 do not show PU.1 expression. In contrast, the peripheral blood cells as well as the follicular lymphoma cell line ROS-50 reveal expression of 42-kd PU.1 protein. HD-derived cell line HD-MYZ with monocytic differentiation shows an aberrant anti-PU.1 reactive 50-kd protein. Molecular weight markers are depicted at the right.
Discussion
Our study shows a striking absence of PU.1 expression by Hodgkin cells in cHD as compared to LPHD. In addition, normal PU.1 protein was not found to be expressed by three cHD-derived cell lines and the HD-MY-Z, a putative HD cell line. PU.1 is a transcription factor necessary for B-cell development and regulates a diversity of genes important for B-cell differentiation. Among the genes regulated by PU.1 are the Ig genes including heavy chain, J-chain, and both light chain genes, as well as the CD20 gene and the mb-1 gene. 4-8,11,42 Thus, it is likely that the lack of PU.1 expression is at least partly responsible for an abnormal and/or incomplete B-cell phenotype of the Hodgkin cells in cHD. The absence of immunoglobulin expression by Hodgkin cells in cHD was previously in part explained by crippling somatic hypermutations of the rearranged Ig genes such as the introduction of stop codons. 43 However, in one study, the Ig-coding capacity was found to be preserved in 18 of 24 cases (75%), but the expression of Ig mRNA was not detectable in the RS cells with the exception of Ig kappa light chain expression in some tumor cells implying other mechanisms leading to the lack of Ig expression by Hodgkin cells. 44 Two recent studies suggested that down-regulation of BOB.1/OBF.1 and Oct-2 in cHD but not in LPHD may be responsible for the lack of Ig gene expression. 45,46 In addition, the study of Stein and colleagues 45 demonstrated that the activity of co-transduced Ig promoter constructs in cultured HRS cells can be restored by transfection with BOB.1 and Oct-2. This study dismissed the concept that the absence of immunoglobulin expression in cHD, but not in LPHD is because of disrupting mutations of Ig V genes in cHD. The latter data strongly suggest that Hodgkin cells have lost their Ig gene transcription ability, because of functional defects in the Ig gene regulatory elements. However, Oct-2 does not seem to be essential for Ig gene expression nor for normal development of B cell before terminal differentiation into plasma cells as was shown previously. 19-21 However, it is likely that the transcription factor PU.1 plays a more direct role in the regulation of Ig gene expression. 47 The study of Nelsen and colleagues 47 showed that the co-expression of both PU.1 and Ets-1 transcription factors in nonlymphoid cells trans-activated reporter plasmids that contained the minimal Igμ enhancer. In addition, absence of PU.1 expression leads to the absence of B-cell development. Because PU.1 nuclear protein expression was not found in all but 2 of the 35 studied cases, it is likely that the lack of PU.1 protein expression is responsible for the lack of normal B-cell development in cHD including immunoglobulin expression. Because 49% of DLBCL were also found to lack PU.1 expression, it is likely that other mechanisms also contribute to the development of a characteristic phenotype of cHD. It is of particular interest in that regard that cHD seems to be characterized by the absence of both PU.1 and Oct-2, whereas absence of both transcription factors is notably rare in DLBCL (1 case in the current study, and 8 of 127 cases we have studied so far, unpublished results).
The mRNA expression of PU.1 was previously studied in multiple myeloma and lymphoblastic cell lines and acute lymphoblastic leukemia (ALL). 12,48 Hromas and co-authors 49 studied several cell lines derived from malignant lymphomas by Northern expression analysis and found that PU.1 mRNA was expressed in all of the four B-cell leukemia lines assayed. There are no previous studies of PU.1 protein expression in malignant lymphoma by immunohistochemistry even though the protein activity and subcellular localization of some transcription factors, including PU.1 might be more representative of their activity/function than mRNA expression. 50 Only two studies investigated PU.1 protein expression using immunohistochemistry; however, no lymphomas were analyzed. 49,51 As opposed to BSAP, we have found that PU.1 protein is differentially expressed in B-NHLs. Lack of PU.1 expression in 7 of 18 cases (39%) of DLBCL, as well as 1 of 3 mantle cell lymphomas. An extended study of PU.1 expression in DLBCL is currently being performed in our laboratory. Small lymphocytic lymphoma and marginal zone lymphomas uniformly and strongly expressed PU.1. A single case of lymphoplasmacytic lymphoma expressed PU.1 only in a subpopulation of malignant cells, which is in concordance with its partial plasmacytic differentiation. Although PU.1 was expressed in LPHD, it was not in four cases of T-cell-rich B-cell lymphoma after LPHD and in an additional two de novo cases of T-cell-rich B-cell lymphoma. These preliminary findings suggest that PU.1 may be used as a useful diagnostic marker distinguishing LPHD from T-cell-rich B-cell lymphoma.
Our study demonstrated that the B-cell-specific transcription factor (BSAP, the product of PAX-5) is expressed by all except two cases of cHD studied and all cases of LPHD. That BSAP is expressed in most B-cell lymphomas was also confirmed in our study. 52 Because we used a monoclonal anti-Pax-5 antibody, our findings are very similar to that of Foss and co-authors 52 who showed universal expression of this transcription factor in both Hodgkin’s lymphoma and non-Hodgkin B-cell lymphoma. Using two polyclonal antibodies, the relative number of B-NHLs and Hodgkin’s lymphomas expressing BSAP in the study of Krenacs and colleagues 53 was smaller. This is likely because of the lack of sensitivity of the antibodies or the immunohistochemical technique used by the authors. The only B-cell lymphoma negative for this transcription factor in our study was a plasmablastic lymphoma in a HIV-positive patient. This finding is expected because BSAP is not expressed at the plasma cell level of differentiation. 54,55
We agree with Foss and co-authors 52 that BSAP may be clinically used to distinguish between cHL and ALCL. Three of our cases of cHD included in this study were previously classified as HD-like ALCL. The expression of BSAP by the Reed-Sternberg cells in these cases illustrates that BSAP expression is a good marker to distinguish HD from ALCL, especially in those cases of HD with numerous neoplastic cells. Also, our finding that two cHD cases are negative for BSAP is in accordance with previous findings by Foss and co-authors. 52 These cases may be of non-B-cell origin.
In summary, PU.1 transcription factor is not expressed in cHD in contrast to LPHD. The lack of PU.1 expression in addition to the previously described lack of Oct-2 expression are likely causes of the incomplete B-cell phenotype characteristic of the Reed-Sternberg cells in cHD. Especially the absence of PU.1 expression is likely to contribute to the absence of immunoglobulin expression in cHD. The lack of both PU.1 and Oct-2 in cHD but not in B-NHL suggests different mechanisms of PU.1 deregulation in cHD versus B-NHL. These mechanisms need yet to be elucidated. Transfection studies are also needed to further evaluate the functional consequences of the loss of PU.1 expression in cHD.
Footnotes
Address reprint requests to Emina Torlakovic, M.D., Department of Pathology, The Norwegian Radium Hospital, and Institute for Cancer Research, Montebello, 0310 Oslo, Norway. E-mail: emina.torlakovic@labmed.uio.no.
Supported in part by the Norwegian Cancer Society (grant B00001/004).
References
- 1.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA: Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J 1996, 15:5647-5658 [PMC free article] [PubMed] [Google Scholar]
- 2.Pettersson M, Sundstrom C, Nilsson K, Larsson LG: The hematopoietic transcription factor PU.1 is downregulated in human multiple myeloma cell lines. Blood 1995, 86:2747-2753 [PubMed] [Google Scholar]
- 3.Oikawa T, Yamada T, Kihara-Negishi F, Yamamoto H, Kondoh N, Hitomi Y, Hashimoto Y: The role of Ets family transcription factor PU.1 in hematopoietic cell differentiation, proliferation and apoptosis. Cell Death Differ 1999, 6:599-608 [DOI] [PubMed] [Google Scholar]
- 4.Pongubala JM, Nagulapalli S, Klemsz MJ, McKercher SR, Maki RA, Atchison ML: PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3′ enhancer activity. Mol Cell Biol 1992, 12:368-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenbeis CF, Singh H, Storb U: PU.1 is a component of a multiprotein complex which binds an essential site in the murine immunoglobulin lambda 2-4 enhancer. Mol Cell Biol 1993, 13:6452-6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelsen B, Tian G, Erman B, Gregoire J, Maki R, Graves B, Sen R: Regulation of lymphoid specific immunoglobulin mu heavy chain gene enhancer by ETS-domain proteins. Science 1993, 261:82-86 [DOI] [PubMed] [Google Scholar]
- 7.Rivera RR, Stuiver MH, Steenbergen R, Murre C: Ets proteins: new factors that regulate immunoglobulin heavy-chain gene expression. Mol Cell Biol 1993, 13:7163-7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin MK, Koshland ME: Ets-related protein PU.1 regulates expression of the immunoglobulin J-chain gene through a novel Ets-binding element. Genes Dev 1993, 7:2006-2015 [DOI] [PubMed] [Google Scholar]
- 9.Himmelmann A, Riva A, Wilson GL, Lucas BP, Thevenin C, Kehrl JH: PU.1/Pip and a basic helix loop helix zipper transcription factors interact with binding sites in the CD20 promoter to help confer lineage- and stage-specific expression of CD20 in B lymphocytes. Blood 1997, 90:3984-3995 [PubMed] [Google Scholar]
- 10.Eisenbeis CF, Singh H, Storb U: Pip, a novel IRF family member, is a lymphoid-specific PU.1-dependent transcriptional activator. Genes Dev 1995, 9:1377-1387 [DOI] [PubMed] [Google Scholar]
- 11.Pongubala JMR, Atchison ML: PU.1 can participate in an active enhancer complex without its transcriptional activation domain. Biochemistry 1997, 94:127-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenen DG, Hromas R, Licht JD, Zhang DE: Transcription factors, normal myeloid development, and leukemia. Blood 1997, 90:489-519 [PubMed] [Google Scholar]
- 13.McKercher SR, Henkel GW, Maki RA: The transcription factor PU.1 does not regulate lineage commitment but has lineage-specific effects. J Leukoc Biol 1999, 66:727-732 [DOI] [PubMed] [Google Scholar]
- 14.Simon MC: PU.1 and hematopoiesis: lessons learned from gene targeting experiments. Semin Immunol 1998, 10:111-118 [DOI] [PubMed] [Google Scholar]
- 15.Fisher RC, Scott EW: Role of PU.1 in hematopoiesis. Stem Cells 1998, 16:25-37 [DOI] [PubMed] [Google Scholar]
- 16.DeKoter RP, Singh H: Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 2000, 288:1439-1441 [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Radomska HS, Auron PE, Tenen DG, Sun Z: Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc Natl Acad Sci USA 1999, 96:8705-8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerriero A, Langumuir PB, Spain LM, Scott EW: PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 2000, 95:879-885 [PubMed] [Google Scholar]
- 19.Feldhaus AL, Klug CA, Arvin KL, Singh H: Targeted disruption of the Oct-2 locus in a B-cell provides genetic evidence for two distinct cell-type specific pathways of octamer element-mediated gene activation. EMBO J 1993, 12:2763-2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corcorean LM, Karvelas M, Nossal GJV, Ye Z-S, Jacks T, Baltimore D: Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev 1993, 7:570-582 [DOI] [PubMed] [Google Scholar]
- 21.Schubart K, Massa S, Schubart D, Corcoran LM, Rolink AG, Matthias P: B cell development and immunoglobulin gene transcription in the absence of Oct-2 and OBF-1. Nature Immunol 2001, 2:69-74 [DOI] [PubMed] [Google Scholar]
- 22.Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, Hansmann ML: Hodgkin’s disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B-cells at various stages of development. Proc Natl Acad Sci USA 1994, 91:10962-10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamaru J, Hummel M, Zemlin M, Kalvelage B, Stein H: Hodgkin’s disease with a B-cell phenotype often shows a VDJ rearrangements and somatic mutations in the VH genes. Blood 1994, 84:708-715 [PubMed] [Google Scholar]
- 24.Ohno T, Stribley JA, Wu G, Hinrichs SH, Weisenburger DD, Chan WC: Clonality in nodular lymphocyte-predominant Hodgkin’s disease. N Engl J Med 1997, 337:459-465 [DOI] [PubMed] [Google Scholar]
- 25.Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Falini B, Delsol G, Isaacson PG, Pileri S, Stein H: Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B-cells. N Engl J Med 1997, 337:453-458 [DOI] [PubMed] [Google Scholar]
- 26.Braeuninger A, Küppers R, Strickler JG, Wacker HH, Rajewsky K, Hansman ML: Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin’s disease represent clonal populations of germinal center-derived tumor B-cells. Proc Natl Acad Sci USA 1997, 94:9337-9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason DY, Banks PM, Chan J, Cleary ML, Delsol G, de Wolf Peeters C, Falini B, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES, Knowles DM, Müller-Hermelink HK, Pileri S, Ralfkiaer E, Stein H, Warnke R: Nodular lymphocyte predominance Hodgkin’s disease. A clinicopathological entity. Am J Surg Pathol 1994, 18:526-530 [DOI] [PubMed] [Google Scholar]
- 28.Schmid C, Pan L, Diss T, Isaacson PG: Expression of B-cell antigens by Hodgkin’s and Reed-Sternberg cells. Am J Pathol 1991, 139:701-707 [PMC free article] [PubMed] [Google Scholar]
- 29.Carbone A, Gloghini A, Gaidano G, Franceschi S, Capello D, Drexler HG, Falini B, Dalla-Favera R: Expression status of BCL-6 and syndecan-1 identifies distinct histogenetic subtypes of Hodgkin’s disease. Blood 1998, 92:2220-2228 [PubMed] [Google Scholar]
- 30.Watanabe K, Yamashita Y, Nakayama A, Hasegawa Y, Kojima H, Nagasawa T, Mori N: Varied B-cell immunophenotypes of Hodgkin/Reed-Sternberg cells in classic Hodgkin’s disease. Histopathology 2000, 36:353-361 [DOI] [PubMed] [Google Scholar]
- 31.Kamesaki H, Fukuhara S, Tatsumi E, Uchino H, Yamabe H, Miwa H, Shirakawa S, Hatanaka M, Honjo T: Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin’s disease. Blood 1986, 68:285-292 [PubMed] [Google Scholar]
- 32.Schaadt M, Fonatsch C, Kirchner H, Diehl V: Establishment of a malignant, Epstein-Barr-virus (EBV)-negative cell-line from the pleura effusion of a patient with Hodgkin’s disease. Blut 1979, 38:185-190 [DOI] [PubMed] [Google Scholar]
- 33.Drexler HG, Gaedicke G, Lok MS, Diehl V, Minowada J: Hodgkin’s disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res 1986, 10:487-500 [DOI] [PubMed] [Google Scholar]
- 34.Epstein AL, Kaplan HS: Biology of the human malignant lymphomas. I. Establishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer 1974, 34:1851-1872 [DOI] [PubMed] [Google Scholar]
- 35.Nadkarni JS, Nadkarni JJ, Clifford P, Manolov G, Fenyo EM, Klein E: Characteristics of new cell lines derived from Burkitt lymphomas. Cancer 1969, 23:64-79 [DOI] [PubMed] [Google Scholar]
- 36.Schneider U, Schwenk HU, Bornkamm G: Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer 1977, 19:521-526 [DOI] [PubMed] [Google Scholar]
- 37.Bargou RC, Mapara MY, Zugck C, Daniel PT, Pawlita M, Dohner H, Dorken B: Characterization of a novel Hodgkin cell line, HD-MyZ, with myelomonocytic features mimicking Hodgkin’s disease in severe combined immunodeficient mice. J Exp Med 1993, 177:1257-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckwith M, Longo DL, O’Connell CD, Moratz CM, Urba WJ: Phorbol ester-induced, cell-cycle-specific, growth inhibition of human B-lymphoma cell lines. J Natl Cancer Inst 1990, 82:501-509 [DOI] [PubMed] [Google Scholar]
- 39.van Ooteghem RB, Smit EM, Beishuizen A, Lambrechts AC, vd Blij-Philipsen M, Smilde TJ, Hagemeijer A: A new B-cell line showing a complex translocation (8;14;18) and BCL2 rearrangement. Cancer Genet Cytogenet 1994, 74:87-94 [DOI] [PubMed] [Google Scholar]
- 40.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 277:680-685 [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979, 76:4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagman J, Grosschedl R: An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of Ets family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci USA 1992, 89:8889-8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jox A, Zander T, Kuppers R, Irsch J, Kanzler H, Kornacker M, Bohlen H, Diehl V, Wolf J: Somatic mutations within the untranslated regions of rearranged Ig genes in a case of classical Hodgkin’s disease as a potential cause for the absence of Ig in the lymphoma cells. Blood 1999, 93:3964-3972 [PubMed] [Google Scholar]
- 44.Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, Lammert H, Demel G, Theil J, Wirth T, Stein H: Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000, 95:1443-1450 [PubMed] [Google Scholar]
- 45.Stein H, Marafioti T, Foss HD, Laumen H, Hummel M, Anagnostopoulos I, Wirth T, Demel G, Falini B: Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 2001, 97:496-501 [DOI] [PubMed] [Google Scholar]
- 46.Re D, Müschen M, Ahmadi T, Wickenhauser C, Staratschek-Jox A, Holtick U, Diehl V, Wolf J: Oct-2 and Bob-1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res 2001, 61:2080-2084 [PubMed] [Google Scholar]
- 47.Nelsen B, Tian G, Erman B, Gregoire J, Maki R, Graves B, Sen R: Regulation of lymphoid-specific immunoglobulin μ heavy chain gene enhancer by ETS-domain proteins. Science 1993, 261:82-86 [DOI] [PubMed] [Google Scholar]
- 48.Nishii K, Kita K, Miwa H, Shikami M, Taniguchi M, Usui E, Katayama N, Shiku H: Expression of B-cell-associated transcription factors in B-cell precursor acute lymphoblastic leukemia cells: association with PU.1 expression, phenotype, and immunogenotype. Int J Hematol 2000, 71:372-378 [PubMed] [Google Scholar]
- 49.Hromas R, Orazi A, Neiman RS, Maki R, Van Beveran C, Moore J, Klemsz M: Hematopoietic lineage- and stage-restricted expression of the ETS oncogene family member PU.1. Blood 1993, 82:2998-3004 [PubMed] [Google Scholar]
- 50.Georgopoulos K: Transcription factors required for lymphoid lineage commitment. Curr Opin Immunol 1997, 9:222-227 [DOI] [PubMed] [Google Scholar]
- 51.Walton MR, Gibbons H, MacGibbon GA, Sirimanne E, Saura J, Gluckman PD, Dragunow M: PU.1 expression in microglia. J Neuroimmunol 2000, 104:109-115 [DOI] [PubMed] [Google Scholar]
- 52.Foss HD, Reusch R, Demel G, Lenz G, Anagnostopoulos I, Hummel M, Stein H: Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood 1999, 94:3108-3113 [PubMed] [Google Scholar]
- 53.Krenacs L, Himmelmann AW, Quintanilla-Martinez L, Fest T, Riva A, Wellmann A, Bagdi E, Kehrl JH, Jaffe ES, Raffeld M: Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B-cells and in subsets of B-cell lymphomas. Blood 1998, 92:1308-1316 [PubMed] [Google Scholar]
- 54.Usui T, Wakatsuki Y, Matsunaga Y, Kaneko S, Koseki H, Kita T, Kosek H: Overexpression of B-cell-specific activator protein (BSAP/Pax-5) in a late B-cell is sufficient to suppress differentiation to an Ig high producer cell with plasma cell phenotype. J Immunol 1997, 158:3197-3204 [PubMed] [Google Scholar]
- 55.Morrison AM, Nutt SL, Thevenin C, Rolink A, Busslinger M: Loss- and gain-of-function mutations reveal an important role of BSAP (Pax-5) at the start and end of B-cell differentiation. Semin Immunol 1998, 10:133-142 [DOI] [PubMed] [Google Scholar]


