Abstract
Human immunodeficiency virus (HIV)-infected patients often develop malabsorption and increased intestinal permeability with diarrhea, called HIV enteropathy, even without enteric opportunistic infections. HIV gp120-induced calcium signaling, microtubule loss, and physiological changes resembling HIV enteropathy were previously found in the HT-29 intestinal cell line. How gp120 caused these changes was unclear. We show that the HIV co-receptor Bob/GPR15, unlike CCR5 and CXCR4, is abundant at the basal surface of small intestinal epithelium. The gp120-induced effects on HT-29 cells were inhibited by anti-Bob neutralizing antibodies, the selective G protein inhibitor pertussis toxin, and the phospholipase inhibitor U73122, but not neutralizing antibodies to CXCR4. Gp120 strains that induced signaling in HT-29 cells also induced calcium fluxes in Bob-transfected Ghost (3) cells, whereas gp120 strains not activating HT-29 cells also did not activate Bob-transfected cells. Bob is the first HIV co-receptor shown to be abundantly expressed on the basolateral surface of intestinal epithelium. Although Bob is an inefficient infection-inducing co-receptor, it mediates viral strain-specific gp120-induced calcium signaling at low, physiologically reasonable gp120 concentrations, up to 10,000-fold lower gp120 concentrations than the principal co-receptors. Gp120-induced Bob activation is a plausible cause of HIV enteropathy.
Diarrhea, weight loss, and life-threatening cachexia are frequent problems in advanced human immunodeficiency virus (HIV) infection. Many HIV-infected patients develop increased small intestinal permeability 1 and malabsorption of lipids 2 and sugars 3 with minimal histological findings. 4 Because these occur without identifiable enteric infections, and improve with initial retroviral treatment, 5 these problems were thought to be directly related to HIV and were thus termed “HIV enteropathy.” Gp120 induces calcium signaling and microtubule loss in the HT-29 intestinal cell line, resulting in malabsorption and increases in paracellular permeability resembling HIV enteropathy. 6,7 Antibodies to the glycosphingolipid HIV receptor galactosyl ceramide caused similar changes. Evidence of reduced microtubule stability was also recently shown in the intestinal epithelium of HIV-infected patients. 8 Microtubule disrupting agents such as colchicine also cause malabsorption, 9 increased intestinal permeability, 10 and diarrhea, 11 similar to HIV enteropathy. How gp120 caused calcium signaling and microtubule loss in intestinal epithelium was unknown.
The major HIV co-receptors CCR5 and CXCR4 are both present in enteric epithelium as well as HT-29 cells, but in vivo they are present mainly at and near the luminal surface. 12 However, most productively HIV-infected cells in intestinal mucosa are superficial lamina propria macrophages. 13 A co-receptor present on the basolateral surface of the enteric epithelium would be a more plausible mediator of this effect, but, to our knowledge, none have previously been found.
The orphan G-protein coupled receptor GPR15/Bob (hereafter termed “Bob”) is a previously little studied co-receptor for HIV and simian immunodeficiency virus. 14,15 Although Bob is a frequently used simian immunodeficiency virus co-receptor (ie, promoting viral fusion and infection), HIV-1 co-receptor study results have varied with the sensitivity of the assay used, generally showing either very rare or no Bob usage or common but extremely inefficient Bob usage of uncertain clinical significance. 16-19 Although gp120 is the HIV-1 envelope surface protein, most of it is shed from infected cells rather than incorporated into virions. 20 It is thus a plausible mediator of toxic effects in uninfected cells. The principal HIV co-receptors (CXCR4 and CCR5) mediate gp120-induced calcium signaling, but previous studies demonstrated calcium signaling using these receptors only at relatively high gp120 concentrations (≥200 pmol/L). 21-23 Almost all previous studies, however, focused on the main co-receptors; the possibility that inefficient minor co-receptors could mediate significant HIV-induced activation has, to our knowledge, not previously been explored. Although the gp120 content probably varies widely in different tissue compartments, blood gp120 content in HIV-infected patients is very low (0.075 to 0.80 pmol/L), and is mostly in immune complexes. 24 The goal of this study was to find a HIV co-receptor meeting the following criteria for a putative cause of HIV enteropathy: 1) the receptor is expressed on the basal surface of small intestinal epithelium, where the main exposure to HIV proteins would likely be; 2) it mediates the gp120-induced effects seen in HT-29 cells and either interacts with galactosyl ceramide and/or cross-reacts with anti-galactosyl ceramide antibodies; and 3) it induces calcium signaling at the low gp120 concentrations anticipated in vivo.
Materials and Methods
Reagents
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health: gp120IIIB and gp120MN from DAIDS, NAIAD, produced by ImmunoDiagnostics; gp120CM235 from Protein Sciences Corporation; gp12093TH975 from Steve Showalter, Maria Garcia-Moll, and the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAAD); gp120SF2 from Ms. Margarita Quiroga; anti-CXCR4 and anti-CCR5 antibodies from DAIDS, NIAID, produced by R&D Systems, Minneapolis, MN; Ghost (3) cells and pBABE-Bob plasmid from Dr. Vineet KewalRamani and Dr. Dan Littman; RANTES, and MIP-1β from DAIDS, NIAAD, produced by Piprotech Inc.; MIP-1α from R&D Systems; anti-CXCR4 from Dr. James Hoxie; and anti-CCR5 from DAIDS, NIAID, produced by PharMingen, La Jolla, CA.
Primary Antibodies
Two sets of three peptide antigens from the human GPR15/Bob sequence were synthesized, namely HAEDFARRRKRSVSL, DKEASLGLWRTGSFLCK, and MDPEETSVYLDYYYATS; and SGLRQEHYLPSAILQ, RELTLIDDKPYCAEKKAT, and KNYDFGSSTETSDSHLTK. Rabbits were injected with either the first three or second three peptides, and immune sera were affinity purified with the corresponding antigen peptides. These antibodies were named anti-Bob37 and anti-Bob39, respectively. This work was performed at Research Genetics, Huntsville, AL. Mouse monoclonal anti-acetylated tubulin (5 μg/ml; Sigma Chemical Co., St. Louis, MO), monoclonal anti-CXCR4 and anti-CCR5 (5 μg/ml; AIDS Research and Reference Reagent Program), polyclonal anti-galactosyl ceramide (Sigma), and monoclonal anti-galactosyl ceramide (Chemicon, Temecula, CA) were also used.
Western Blotting
Western blots were made from tissue or cultured cells; the soluble fractions of homogenates were prepared in Triton X-100-containing lysis buffer 25 at 4°C. Electrophoresis in 10% polyacrylamide gels was done with 50 μg protein per lane, and the protein was electroblotted onto nitrocellulose. The blots were blocked with 20 mmol/L Tris (pH 7.6), 140 mmol/L NaCl, 0.1% Tween 20, and 5 g/100 ml nonfat dry milk for 1 hour, then treated with 0.5 μg/ml of primary antibody in the same buffer for 1 hour. Blots were visualized either by chemiluminescence using ECL reagents (Amersham/Pharmacia, Arlington Heights, IL) or by alkaline phosphatase-conjugated secondary antibodies with NBT/BCIP staining. Negative controls included omitting the primary antibody and overnight preabsorption of the primary antibody with 500-fold excess of the three appropriate peptides.
The HT-29 lysates were immunoprecipitated with 2 μg/ml of Bob37 or polyclonal anti-galactosyl ceramide (1:100 dilution), then incubated with protein A/G Sepharose (1.5% v/v) for 2 hours (both steps on a rocker at 4°C). The Sepharose beads were washed, then boiled and used in Western blotting. These Western blots were stained with anti-Bob37, anti-Bob39, or monoclonal anti-galactosyl ceramide antibodies at 0.5 μg/ml.
RNA in Situ Hybridization
RNA in situ hybridization was done with a digoxygenin-labeled cRNA antisense riboprobe prepared as follows: polymerase chain reaction amplification was made of the pBABE-Bob plasmid (courtesy of Dr. Dan Littman and the AIDS Research and Reference Reagent Program) using primers described by Deng and colleagues 14 to which a T7 RNA polymerase site had been added at the 5′ end of the downstream primer. This yielded a product of expected size (562 bp) and sequence. This was used with T7 polymerase in the presence of digoxygenin-labeled UTP (Dig-RNA labeling kit; Boehringer Mannheim/Roche, Indianapolis, IN) to make the antisense probe. Probe specificity was confirmed by Southern blot analysis of pBABE-BOB and comparing our Northern blot analysis of a human MTCII panel (Clontech, Palo Alto, CA), with results similar to those of Deng and colleagues. 14
In situ hybridization was performed as previously described, 26 except that formalin-fixed, paraffin-embedded colon tissue was used, and protease digestion was performed with pepsin (2 mg/ml in 0.1 N HCl for 1 hour at room temperature). After inhibition of endogenous alkaline phosphatase by 20% acetic acid for 15 seconds at 4°C, immunodetection was performed with alkaline phosphatase-conjugated anti-digoxygenin and overnight NBT/BCIP staining. Slides treated with RNase before in situ hybridization, but otherwise treated identically, were used as negative controls.
Immunostaining
Except for the microtubule staining mentioned below and the CXCR4 and CCR5 staining (for which air-dried, unfixed sections were used), all indirect immunofluorescent staining was done on acetone-fixed frozen tissue sections. Cultured cells for microtubule staining were fixed in paraformaldehyde phosphate-buffered saline (PBS) as previously described. 7 The Bob37 and Bob39 antibodies were preincubated with 100-fold excess of the peptides DKEAS… or KNYDF… , respectively, which was found to increase staining specificity. These were incubated in 10% normal goat serum, then the primary antibody at 5 μg/ml for 1 hour, then washed and incubated with Cy-3-conjugated goat anti-rabbit or anti-mouse IgG (1:100 dilution; Jackson ImmunoResearch, West Grove, PA) as appropriate, all at room temperature. Similar preparations omitting the primary antibody were made as negative controls.
Cell Culture Studies—Calcium Measurements and Microtubules
HT-29 cells (from ATCC, Rockville, MD) were grown in Dulbecco’s modified Eagle’s medium (DMEM)-F12 with 10% bovine calf serum, and were used 24 to 48 hours after plating. The Ghost (3) cells were obtained from the AIDS Research and Reference Reagent Program, and were grown in DMEM with 10% bovine calf serum supplemented with 500 μg/ml G418, 100 μg/ml hygromycin, and 1 μg/ml puromycin. 27 These cells were used 24 to 96 hours after plating.
Calcium studies were done on HT-29 cells and Ghost (3) cells loaded with Fluo 4. The cells were placed in Locke’s buffer containing 5 μg/ml Fluo 4 a.m. ester (Molecular Probes) for 20 to 30 minutes, then in Locke’s buffer for an additional 30 minutes before testing. Various concentrations of gp120IIIB (X4 trophic), gp120CM235 (R5 trophic), gp120MN (X4 trophic), gp12093TH975 (nonsyncytium-inducing primary isolate from an asymptomatic subject, trophism undetermined but likely R5), and gp120SF2 (X4R5 trophic), ranging from 10 nmol/L to less than 1 pmol/L, were added in Locke’s buffer. 6 The gp120 proteins were obtained from the AIDS Research Reagent Referral Program. Some HT-29 cells were given one of the following pretreatments: 50 μg/ml anti-Bob37 overnight, 1 μg/ml pertussis toxin overnight, or 4.6 μg/ml U73122 for 1 hour. With the HT-29 cells only, similar tests were also performed with SDF-1, MIP-1α, MIP-1β, and RANTES rather than gp120. The cells were visualized every 4 seconds in a confocal microscope, using 488-nm argon laser excitation and detection filters appropriate for fluorescein-like dyes.
To examine the microtubules, HT-29 cells were treated with 10 nmol/L of gp120CM235 or 50 pmol/L of gp120IIIB, then fixed 1 hour later in 4 g/100 ml paraformaldehyde PBS, then stained with mouse anti-acetylated tubulin (5 μg/ml, Sigma) by a method similar to that given above. Some cells were pretreated with 50 μg/ml of anti-Bob37 overnight, 1 μg/ml of pertussis toxin overnight, or 4.6 μg/ml of U73122 for 1 hour before the gp120 was added. Untreated cells were also stained.
Results
Western Blots
In Western blots, homogenates of small bowel and colonic mucosa, lymph node, prostate, testis, and liver, both antibodies stained bands at 36 kd (Figure 1A) ▶ , slightly less than the 40.8-kd molecular weight of Bob estimated from the mRNA sequence. No similar bands were observed in homogenates of brain, placenta, lung, uterus, heart, pancreas, or skeletal muscle. The 36-kd band was absent in Western blots omitting the anti-Bob primary antibody, and the bands were greatly reduced if the anti-Bob was pretreated with excess of the appropriate peptides (not shown).
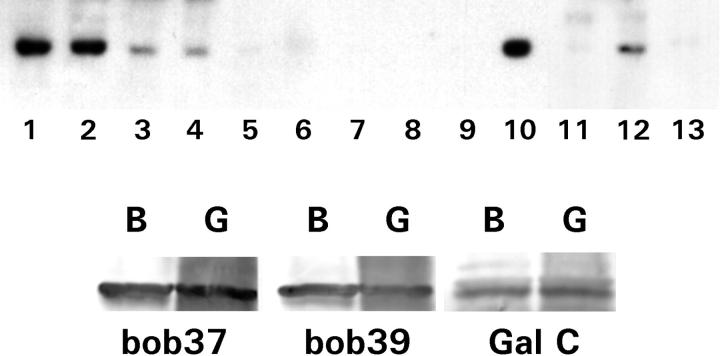
Figure 1.
Tissue and cell line of Bob expression in Western blots. A: Protein homogenates of colonic mucosa (lane 1), small bowel mucosa (lane 2), lymph node (lane 3), testis (lane 4), lung (lane 5), brain (lane 6), placenta (lane 7), skeletal muscle (lane 8), ovary (lane 9), liver (lane 10), heart (lane 11), prostate (lane 12), and pancreas (lane 13). B: HT-29 anti-Bob immunoprecipitation. HT-29 eluates were immunoprecipitated with either Bob37 antibody (indicated by “B”) or polyclonal anti-galactosyl ceramide antibody (indicated by “G”). Western blots were stained with either the Bob37 or Bob39 antibodies or monoclonal anti-galactosyl ceramide as indicated below the band. Each lane had a strong band at 36 kd.
HT-29 homogenates immunoprecipitated with either anti-Bob37 or polyclonal anti-galactosyl ceramide were stained similarly with either anti-Bob37, anti-Bob39, or monoclonal anti-galactosyl ceramide, resulting in similar 36-kd bands (Figure 1B) ▶ . This suggests that both Bob antibodies, formed to different epitopes, recognize the same protein and that both polyclonal and monoclonal anti-galactosyl ceramide antibodies also bind this protein.
RNA in Situ Hybridization and Immunostaining
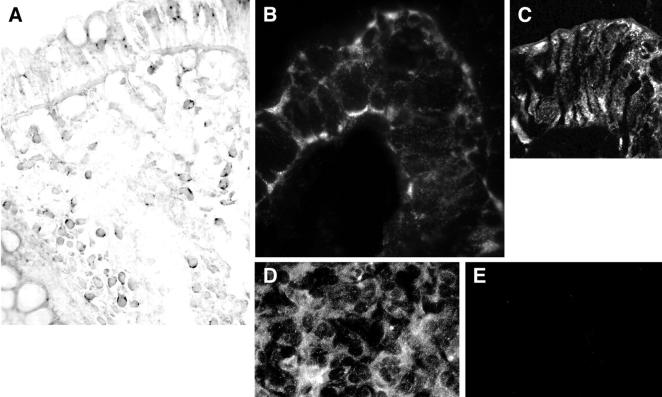
RNA in situ hybridization using a Bob antisense riboprobe showed granular cytoplasmic staining of both surface and crypt epithelial cells and lamina propria mononuclear cells in colonic mucosa (Figure 2A) ▶ . Indirect immunofluorescent staining with anti-Bob antibodies showed extensive staining of intestinal epithelium and lamina propria mononuclear cells. In essentially all small intestinal epithelial cells and in a few colonic crypt cells, there was granular membranous staining, mostly of the basal membrane and just under the brush border of the luminal surface (Figure 2B) ▶ . The staining was similar throughout the villi and crypts. In colonic epithelium, Bob staining was mostly granular and cytoplasmic (Figure 2C) ▶ , although a few crypt cells had membranous staining resembling that of small bowel.
Figure 2.
Cellular localization of Bob. In situ hybridization showed granular staining of rectal epithelium and most lamina propria lymphocytes (A). Immunofluorescent staining (with Bob37) of a small bowel villus tip, with finely granular membranous staining, much of which was at the basal surface and near the apical surface and, to a lesser degree, along the lateral sides (B). By confocal microscopy, most colonic epithelial cells had granular cytoplasmic staining (C). Bob-transfected Ghost (3) cells had finely granular, predominantly cytoplasmic staining when stained with anti-Bob37 (D). CXCR4-transfected Ghost (3) cells, stained and photographed like the Bob-transfected cells, had no staining (E). Original magnifications: ×450 (A); ×550 (B); ×700 (C); ×270 (D and E).
Either anti-Bob antibody stained the Bob-transfected Ghost (3) cells in a finely granular, predominantly cytoplasmic pattern, but did not stain the Ghost (3) parent cell line or cells transfected with CCR5 or CXCR4 (Figure 2, D and E) ▶ . Both the small bowel and the colonic epithelium had weak staining for CXCR4 and CCR5, which was mostly at or near the luminal surface. Small bowel epithelial cell staining was very weak for both CXCR4 and CCR5.
Calcium Flux Studies
Gp120IIIB and gp120CM235 induced an identical, immediate (within 4 seconds) increase in free calcium in HT-29 cells (Figure 3A) ▶ . The signals lasted from ∼12 seconds to several minutes, and varied from cell to cell. The signals were generally longer when more gp120 was added. Serial dilution studies showed that the minimal concentration causing calcium signaling was 1 pmol/L for gp120IIIB and 2.5 nmol/L for gp120CM235. Gp120MN, gp120SF2, and gp12093TH975 induced no calcium signaling, even at 10 nmol/L concentrations.
Figure 3.
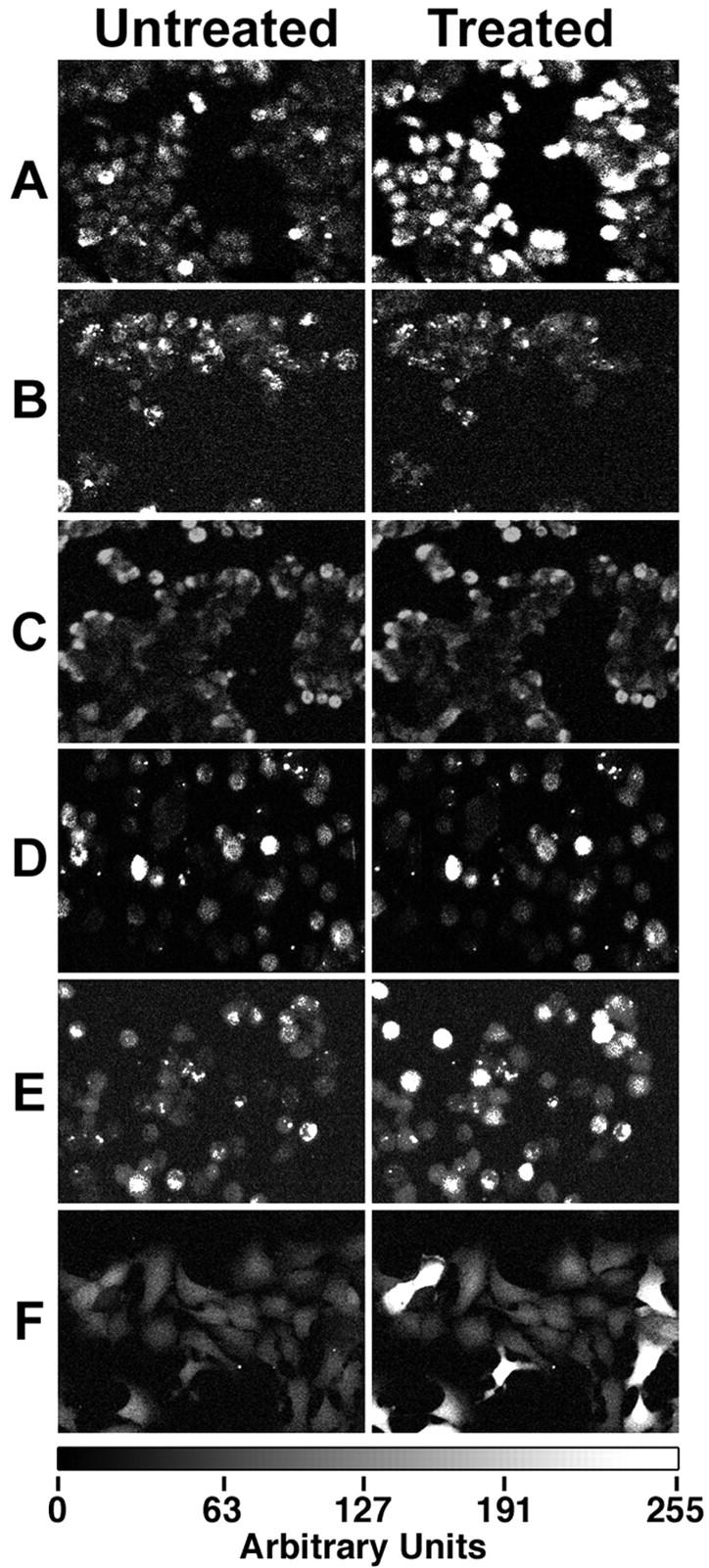
Calcium signaling was induced by gp120 and blocked by pertussis toxin, U73122, and anti-Bob antibodies. Treatment with gp120IIIB (50 pmol/L in Locke’s buffer) induced an immediate calcium signal (A). Similar signals (not shown) were seen with gp120CM235 (10 nmol/L). No significant gp120IIIB-induced calcium signaling was seen after pretreatment with pertussis toxin (500 ng/ml for 18 hours) (B) or U73122 (10 μmol/L for 30 minutes) (C). U73343, an inactive analog of U73122, had no inhibitory effect (data not shown). Anti-Bob37 (100 μg/ml for 18 hours) also inhibited the gp120IIIB-induced calcium signaling (D). These cells were then treated with SDF-1, causing a substantial calcium signal (E). Calcium signals were observed in Ghost (3) cell Bob cells with 15 fmol/L gp120IIIB (F). Original magnifications, ×160.
Each of the natural ligands of CXCR4 and CCR5–100 ng/ml SDF-1, 50 ng/ml MIP-1α, or MIP-1β, and 10 ng/ml RANTES, induced similar calcium signaling. Calcium signaling induced by either gp120 or SDF-1 was inhibited by the selective G protein inhibitor pertussis toxin and by the phospholipase inhibitor U73122 (Figure 3, B and C) ▶ . Pretreatment with anti-Bob37 antibodies inhibited the calcium signaling by both gp120CM235 and gp120IIIB (Figure 3D) ▶ . In cells pretreated with anti-Bob, calcium signaling could then be induced by 100 ng/ml of SDF-1, implying that the CXCR4 receptors and the G protein-induced calcium-signaling pathway remained functional (Figure 3E) ▶ . Although HIVIIIB is an X4 or T lymphocyte-trophic virus, gp120IIIB-induced calcium signaling was not inhibited by anti-CXCR4 neutralizing antibodies. Comparable results, demonstrating that the gp120-induced signaling by the gp120s of two different viral strains were inhibited by anti-Bob37 but not by anti-CXCR4 antibodies, were found independently in a second laboratory (that of JF), using confluent, polarized monolayers of the D4 clone of HT-29 (results not shown). Furthermore, the polarized cells could be stimulated by gp120IIIB from either the basal or top surface, and anti-Bob37 neutralized activation from either side (results not shown).
To confirm that Bob is the receptor mediating gp120-induced calcium signals, gp120-induced calcium signals were also examined in Ghost (3) cells transfected to express Bob, CXCR4, or CCR5, as well as the parental cell line. 27 Calcium signaling was induced by as little as 0.015 pmol/L of gp120IIIB (Figure 3F) ▶ or 8 pmol/L of gp120CM235 in Bob-transfected Ghost (3) cells, whereas Ghost (3) CXCR4-transfected cells required at least 150 pmol/L gp120IIIB and did not respond to gp120CM235 at any concentration tested. Both Ghost (3) CCR5-transfected cells and the parental cell line (which expresses CXCR4 at low levels) required ∼1 nmol/L of gp120IIIB and did not respond to gp120CM235 at any concentration tested. Gp120 from each of the three viral strains (MN, SF2, and 93TH975) that did not cause calcium signals in HT-29 cells similarly did not cause calcium signals in the Ghost (3) Bob-transfected cells at any concentration tested.
The calcium signals in the Ghost (3) cells were usually very intense and often lasted for several minutes. At the minimal concentrations, only some of the cells exhibited calcium signals (Figure 3F) ▶ , but at higher concentrations most cells exhibited signaling. However, at very high gp120 concentrations, calcium signaling became weak or negative.
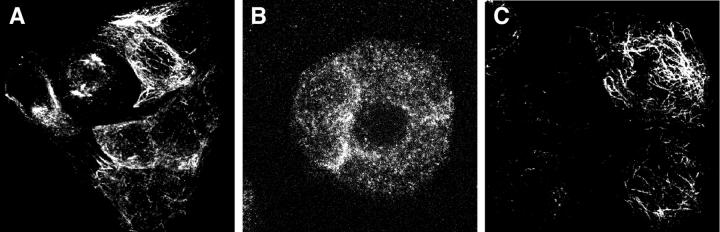
Microtubule Staining
Corresponding experiments were done to demonstrate gp120-induced microtubule alterations in HT-29 cells. Either gp120IIIB or gp120CM235 caused a marked loss of acetylated tubulin-staining microtubules (Figure 4, A and B) ▶ . This effect was most apparent in clusters of several cells, large cell clusters (>20 cells) often retained stainable microtubules. The loss of microtubule staining was inhibited by pretreatment with anti-Bob (Figure 4C) ▶ , pertussis toxin, or U73122 under the same conditions used for calcium signaling, except that the cells were in DMEM-F12 rather than Locke’s buffer. The loss of microtubules induced by gp120IIIB, and that this effect could be inhibited by anti-Bob, was confirmed with differentiated, polarized HT-29 cells in a second laboratory (that of JF, results not shown).
Figure 4.
Gp120 effects on microtubules were blocked by anti-Bob, pertussis toxin, and U73122. Anti-acetylated tubulin antibodies stained microtubules in many HT-29 cells (A). Staining was markedly reduced by a 1-hour treatment with gp120CM235 (B), particularly among clusters of several cells. The gp120-induced microtubule loss was inhibited by anti-Bob37 antibodies, 100 μg/ml for 18 hours before the gp120 treatment (C). Original magnifications, ×460.
Discussion
The Western blot results with both anti-Bob antibodies demonstrate a band at an appropriate molecular weight, suggesting that Bob is present in small bowel and colonic mucosa, lymphoid tissue, prostate, testis, and liver, but not in lung, pancreas, brain, placenta, heart, or skeletal muscle. This is in agreement with previous Northern blot studies, 14 except for the Western blot band suggesting that Bob is in liver. This discrepancy could be because of the increased sensitivity of Western blots. The 36-kd band also appears in Western blots of homogenates immunoprecipitated with one anti-Bob antibody and stained with the other antibody, which was raised and affinity purified to different epitopes. Staining with anti-Bob is present in Bob transfected Ghost (3) cells but not the parent cell line or cells transfected with the major co-receptors, and the distribution of Bob immunostaining matches that of Bob in situ hybridization. Together, these findings strongly suggest that the anti-Bob antibodies do in fact stain Bob. Bob also appears to cross-react with anti-galactosyl ceramide antibodies. Further work is needed to determine the relationship between Bob and galactosyl ceramide; there are proteins that both interact with galactosyl ceramide and cross-react with anti-galactosyl ceramide antibodies. 28
Both in situ hybridization and immunostaining with two different antibodies demonstrate that Bob is present in intestinal epithelium and in lymphocytes. Also, Bob is abundantly expressed at the basal and apical surfaces of small bowel epithelium. In agreement with previous results 12 and in contrast to Bob, CCR5 and CXCR4, although present in intestinal epithelium, are mostly at the luminal surface. In chronic HIV infection, most productively HIV-infected cells in intestinal mucosa are superficial lamina propria macrophages; 13 intestinal epithelium is probably exposed to gp120 on the basal surface. Thus Bob, unlike CCR5 or CXCR4, is present at the correct site to mediate gp120-induced signaling.
The inhibition of the gp120-induced calcium fluxes and microtubule loss in HT-29 cells by pertussis toxin and U73122, as well as induction of similar calcium signals by the natural ligands of CCR5 and CXCR4, strongly implicate a G protein-coupled receptor/pertussis toxin-sensitive G protein/phospholipase mechanism. We have shown, for both of our strains of gp120 that induce these changes, that they are inhibited by affinity-purified anti-Bob antibodies. Also, picomolar or lower concentrations of gp120 of either strain induced calcium fluxes in Bob-transfected Ghost (3) cells but not in the parental cell line or in CCR5 or CXCR4-transfected Ghost (3) cells. All three gp120s of strains not inducing signaling in HT-29 cells also do not induce calcium fluxes in Bob-transfected Ghost (3) cells. Thus we believe that Bob mediates these gp120-induced effects in HT-29 cells, which are very similar to those of HIV enteropathy. 7
Together, these findings suggest that Bob is present at the right site, that it induces physiological changes in HT-29 cells resembling HIV enteropathy, it cross-reacts with anti-galactosyl ceramide antibodies, and that, unlike the principal co-receptors CCR5 and CXCR4, it induces calcium signaling at extremely low gp120 concentrations. Recently, we also showed a decrease in acetylated tubulin staining in the intestinal epithelium of HIV-infected patients, suggesting decreased microtubule stability in vivo. 8 Together, these findings strongly implicate gp120-induced Bob activation as a likely cause of the calcium signaling and microtubule loss resulting in HIV-associated enteropathy. However, further work is needed, particularly to correlate the presence of a HIV strain inducing Bob activation with HIV enteropathy in vivo.
We suspect that gp120 causes HIV enteropathy by the following mechanism. Gp120 activates intestinal epithelial cell Bob, inducing G protein and phospholipase activation, and thus inositol triphosphate and calcium signaling. Increased cytosolic calcium depolymerizes microtubules. 29 Microtubule loss alters RhoA and Rac1 activation, increasing actin-myosin contraction 30 near the tight junctions, causing increased paracellular permeability and diarrhea. 31 Microtubule loss also reduces enterocyte lipid transport, 9 causing lipid malabsorption.
Previous studies of co-receptor activation by gp120 focused almost exclusively on the major co-receptors CCR5 and CXCR4, with the implicit assumption that the activation was an incidental phenomenon with receptors for which viral fusion was the main goal. Finding extremely sensitive gp120-induced activation of Bob, a very inefficient co-receptor, by both an X4 trophic strain (HIVIIIB) and an R5 trophic strain (HIVCM235) indicates that different receptors can be activated than those used as main co-receptors for viral fusion. However, like co-receptor activity, activation with calcium signaling seems to be a viral strain-specific phenomenon. Correspondingly, Baik and colleagues 32 showed that efficient co-receptor activity/fusion can occur without avid gp120 to co-receptor binding. Activation and viral fusion are distinct phenomena; the search for receptors activated by HIV should not be limited to the major co-receptors and might even include those with no co-receptor activity at all.
The extraordinary potency of gp120IIIB for Bob activation seems unlikely to be random and presumably was selected for. We speculate that this activation could give an HIV strain some selective advantage, although it seems implausible that it is because of the enteropathy. Possibly, gp120-induced Bob activation induces similar calcium signaling in other Bob-expressing cells such as lymphocytes, explaining the long known gp120-induced alterations in lymphocyte inositol phosphate metabolism. 33 Potentially, Bob activation could inhibit the anti-HIV immune response or induce the gp120IIIB-induced, actin-mediated co-localization of CD4 and the major co-receptors advantageous for HIV infection. 34 Further work is needed to explore this and the significance of Bob expression in other cell types.
In summary, both the effects of co-receptor ligands and inhibition studies suggest that HIV co-receptor activation causes calcium signaling and microtubule loss in HT-29 cells. These calcium and microtubule changes were previously shown to induce HIV enteropathy-like malabsorption and increased paracellular permeability. Unlike the major HIV co-receptors, the inefficient, little studied co-receptor Bob is abundant at the basal surface of small intestinal epithelium. Both neutralizing antibody studies and co-receptor-transfected cell studies demonstrate that Bob mediates the gp120-induced calcium signaling and microtubule loss. HIV gp120-induced Bob activation is a very plausible cause of HIV enteropathy. Further study is needed to explore the possibility that gp120-induced activation of Bob or similar receptors could hasten immunodeficiency or induce other, as yet poorly understood side effects of HIV infection.
Footnotes
Address reprint requests to Dr. Frederic Clayton, Pathology Department, Salt Lake VA, 500 Foothill Dr., Salt Lake City, UT 84148. E-mail: drfclayton@aol.com.
Supported by the Veteran’s Administration Merit Review, Associated Regional and University Pathologists, and Western Institute for Biomedical Research (to F. C.), the National Institute of Diabetes and Digestive and Kidney Disease (grant K08-DK-02531 to S. K. K.), and the National Institutes of Health (grant AI211414 to D. P. K.).
References
- 1.Stockmann M, Fromm M, Schmitz H, Schmidt W, Riecken EO, Schulzke JD: Duodenal biopsies of HIV-infected patients with diarrhoea exhibit epithelial barrier defects but no active secretion. AIDS 1998, 12:43-51 [DOI] [PubMed] [Google Scholar]
- 2.Benhamou Y, Hilmarsdottir I, Desportes Livage I, Hoang C, Datry A, Danis M, Gentilini M, Opolon P: Association of lipid accumulation in small intestinal mucosa with decreased serum triglyceride and cholesterol levels in AIDS. Dig Dis Sci 1994, 39:2163-2169 [DOI] [PubMed] [Google Scholar]
- 3.Ehrenpreis ED, Gulino SP, Patterson BK, Craig RM, Yokoo H, Atkinson AJ, Jr: Kinetics of D-xylose absorption in patients with human immunodeficiency virus enteropathy. Clin Pharmacol Ther 1991, 49:632-640 [DOI] [PubMed] [Google Scholar]
- 4.Ullrich R, Zeitz M, Heise W, L’Age M, Hoffken G, Riecken EO: Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med 1989, 111:15-21 [DOI] [PubMed] [Google Scholar]
- 5.Kotler DP, Shimada T, Snow G, Winson G, Chen W, Zhao M, Inada Y, Clayton F: Effect of combination antiretroviral therapy upon rectal mucosal HIV RNA burden and mononuclear cell apoptosis. AIDS 1998, 12:597-604 [DOI] [PubMed] [Google Scholar]
- 6.Dayanithi G, Yahi N, Baghdiguian S, Fantini J: Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: a putative mechanism for HIV-1 enteropathy. Cell Calcium 1995, 18:9-18 [DOI] [PubMed] [Google Scholar]
- 7.Delezay O, Yahi N, Tamalet C, Baghdiguian S, Boudier JA, Fantini J: Direct effect of type 1 human immunodeficiency virus (HIV-1) on intestinal epithelial cell differentiation: relationship to HIV-1 enteropathy. Virology 1997, 238:231-242 [DOI] [PubMed] [Google Scholar]
- 8.Clayton F, Kapetanovic K, Kotler DP: Enteric microtubule depolymerization in HIV infection: a possible cause of HIV-associated enteropathy. AIDS 2001, 15:123-124 [DOI] [PubMed] [Google Scholar]
- 9.Glickman RM, Perrotto JL, Kirsch K: Intestinal lipoprotein formation: effect of cholchicine. Gastroenterology 1976, 70:347-352 [PubMed] [Google Scholar]
- 10.Fradkin A, Yahav J, Diver Haber A, Zemer D, Jonas A: Colchicine enhances intestinal permeability in patients with familial Mediterranean fever. Eur J Clin Pharmacol 1996, 51:241-245 [DOI] [PubMed] [Google Scholar]
- 11.Dukes MNG (Ed): Meyler’s Side Effects of Drugs, ed 13. Amsterdam, Elsevier, 1996
- 12.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF: Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 1999, 117:359-367 [DOI] [PubMed] [Google Scholar]
- 13.Fox CH, Kotler D, Tierney A, Wilson CS, Fauci AS: Detection of HIV-1 RNA in the lamina propria of patients with AIDS and gastrointestinal disease. J Infect Dis 1989, 159:467-471 [DOI] [PubMed] [Google Scholar]
- 14.Deng HK, Unutmaz D, KewalRamani VN, Littman DR: Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997, 388:296-300 [DOI] [PubMed] [Google Scholar]
- 15.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J: Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med 1997, 186:405-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, He T, Huang Y, Chen Z, Guo Y, Wu S, Kunstman KJ, Brown RC, Phair JP, Neumann AU, Ho DD, Wolinsky SM: Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J Virol 1998, 72:9307-9312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger AL, Hoffman TL, Sharron M, Lee B, O’Dowd B, Doms RW: Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology 1998, 249:367-378 [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Rudolph DL, Owen SM, Spira TJ, Lal RB: Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 1998, 12:F137-F143 [DOI] [PubMed] [Google Scholar]
- 19.Pohlmann S, Krumbiegel M, Kirchhoff F: Coreceptor usage of BOB/GPR15 and Bonzo/STRL33 by primary isolates of human immunodeficiency virus type 1. J Gen Virol 1999, 80:1241-1251 [DOI] [PubMed] [Google Scholar]
- 20.Schneider J, Kaaden O, Copeland TD, Oroszlan S, Hunsmann G: Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol 1986, 67:2533-2538 [DOI] [PubMed] [Google Scholar]
- 21.Weissman D, Rabin RL, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber JM, Fauci AS: Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature 1997, 389:981-985 [DOI] [PubMed] [Google Scholar]
- 22.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien WA, Verdin E: Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 1998, 395:189-194 [DOI] [PubMed] [Google Scholar]
- 23.Arthos J, Rubbert A, Rabin RL, Cicala C, Machado E, Wildt K, Hanbach M, Steenbeke TD, Swofford R, Farber JM, Fauci AS: CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J Virol 2000, 74:6418-6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, Kornfeld H: Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr 1992, 5:251-256 [PubMed] [Google Scholar]
- 25.Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, Wiley HS: Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am J Physiol 1998, 275:C1419-C1428 [DOI] [PubMed] [Google Scholar]
- 26.Morgan T, Craven C, Ward K: Human spiral artery renin-angiotensin system. Hypertension 1998, 32:683-687 [DOI] [PubMed] [Google Scholar]
- 27.Morner A, Bjorndal A, Albert J, Kewalramani VN, Littman DR, Inoue R, Thorstensson R, Fenyo EM, Bjorling E: Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol 1999, 73:2343-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaurin J, Moscarello MA: Reactivity of two anti-galactosyl ceramide antibodies towards myelin basic protein. J Neurol Sci 1992, 108:73-79 [DOI] [PubMed] [Google Scholar]
- 29.O’Brien ET, Salmon ED, Erickson HP: How calcium causes microtubule depolymerization. Cell Motil Cytoskeleton 1997, 36:125-135 [DOI] [PubMed] [Google Scholar]
- 30.Waterman Storer CM, Salmon E: Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol 1999, 11:61-67 [DOI] [PubMed] [Google Scholar]
- 31.Madara JL, Pappenheimer JR: Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol 1987, 100:149-164 [DOI] [PubMed] [Google Scholar]
- 32.Baik SS, Doms RW, Doranz BJ: HIV and SIV gp120 binding does not predict coreceptor function. Virology 1999, 259:267-273 [DOI] [PubMed] [Google Scholar]
- 33.Nye KE, Knox KA, Pinching AJ: Lymphocytes from HIV-infected individuals show aberrant inositol polyphosphate metabolism which reverses after zidovudine therapy. AIDS 1991, 5:413-417 [DOI] [PubMed] [Google Scholar]
- 34.Iyengar S, Hildreth JE, Schwartz DH: Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol 1998, 72:5251-5255 [DOI] [PMC free article] [PubMed] [Google Scholar]