Abstract
Mononuclear phagocytes (monocytes, macrophages, and microglia) are considered central to multiple sclerosis (MS) pathogenesis. Molecular cues that mediate mononuclear phagocyte accumulation and activation in the central nervous system (CNS) of MS patients may include chemokines RANTES/CCL5 and macrophage inflammatory protein-1α/CCL3. We analyzed expression of CCR1 and CCR5, the monocyte receptors for these chemokines, on circulating and cerebrospinal fluid CD14+ cells, and in MS brain lesions. Approximately 70% of cerebrospinal fluid monocytes were CCR1+/CCR5+, regardless of the presence of CNS pathology, compared to less than 20% of circulating monocytes. In active MS lesions CCR1+/CCR5+ monocytes were found in perivascular cell cuffs and at the demyelinating edges of evolving lesions. Mononuclear phagocytes in early demyelinating stages comprised CCR1+/CCR5+ hematogenous monocytes and CCR1−/CCR5− resident microglial cells. In later stages, phagocytic macrophages were uniformly CCR1−/CCR5+. Cultured in vitro, adherent monocytes/macrophages up-regulated CCR5 and down-regulated CCR1 expression, compared to freshly-isolated monocytes. Taken together, these findings suggest that monocytes competent to enter the CNS compartment derive from a minority CCR1+/CCR5+ population in the circulating pool. In the presence of ligand, these cells will be retained in the CNS. During further activation in lesions, infiltrating monocytes down-regulate CCR1 but not CCR5, whereas microglia up-regulate CCR5.
Accumulation and activation of mononuclear phagocytes in the human central nervous system (CNS) is thought to be a crucial step in the pathological cascade of multiple sclerosis (MS), which frequently culminates in irreversible injury to myelin and axons. 1 Although MS pathology is heterogeneous among patients, 2 it still remains clear that destruction of myelin and axons as well as oligodendrocyte cell-death are directly related to the numbers of activated inflammatory cells. 3-6 Therefore, the determinants of monocyte recruitment to the CNS in MS and similar pathologies have been examined. Chemokines and their receptors have been implicated in monocyte trafficking under pathological as well as physiological conditions and have emerged as salient targets for investigation. 7,8
The possible role of CC chemokine receptor 1 (CCR1), CCR5, and their ligands in the pathogenesis of MS was first suggested by observations in experimental autoimmune encephalomyelitis (EAE), an animal model for MS. Inhibition/blockade of macrophage inflammatory protein (MIP)-1α (CCL3), a ligand for CCR1 and CCR5, prevented the development of both acute and relapsing paralytic symptoms and infiltration of mononuclear cells into the CNS. Importantly, anti-MIP-1α did not affect the activation of encephalitogenic T cells, suggesting specificity of MIP-1α for chemoattraction of mononuclear inflammatory cells into the CNS in EAE mice. 9-11 Mice lacking CCR1 (CCR1−/−) developed significantly reduced incidence and severity of EAE when compared with wild-type littermates. CCR1−/− spinal cords exhibited less dense cellular infiltrates than cords from symptomatic wild-type mice. 12,13
In contrast, CCR5 seems dispensable for the development of EAE, because CCR5-deficient mice are susceptible to EAE. Further, individuals homozygous for a nonfunctional Δ32 CCR5 develop MS. 14 CCR5 may, however, have a role in determining MS severity, as distinguished from MS susceptibility: genetic studies showed that individuals heterozygous for the Δ32 nonfunctional CCR5 allele experienced prolonged disease-free intervals, compared to individuals with a fully functional CCR5 receptor. 15,16 Consequently, both CCR1 and CCR5 may be implicated in MS pathogenesis, but the relationship between each receptor and disease susceptibility and/or severity may be complex.
The hematogenous inflammatory component in MS can be examined and characterized in tissue sections as perivascular and parenchymal inflammatory cells. CNS-infiltrating leukocytes can also be identified in the lumbar cerebrospinal fluid (CSF). Therefore, we approached our investigation of CCR1 and CCR5 in MS in two ways: CCR1 and CCR5 expression on circulating and CSF CD14+ monocytes was examined by flow cytometry. Quantitative immunohistochemistry was applied to characterize chemokine receptor-positive cells in MS tissue sections during lesion evolution.
The results of these studies implicated CCR1+/CCR5+ cells as infiltrating and activated mononuclear phagocytes in MS.
Materials and Methods
Flow Cytometry
Flow cytometry studies evaluating CCR1 and CCR5 expression on circulating and CSF monocytes, or co-expression of CCR1 with CCR5, were performed in 24 patients with monosymptomatic optic neuritis and 26 patients with MS. In addition, 24 patients with other noninflammatory neurological diseases who underwent diagnostic lumbar puncture were included as controls. Optic neuritis patients had no history of neurological symptoms and were diagnosed using established clinical criteria. 17 MS diagnosis was based on published criteria for clinical research. 18 The patients underwent lumbar puncture and phlebotomy at the Glostrup Hospital, Glostrup, Denmark, or the Department of Neurology, Cleveland Clinic Foundation, Cleveland, Ohio. Patient characteristics are summarized in Table 1 ▶ . The Scientific Ethics Committee of the Government of Denmark approved this study and informed consent was obtained from all participants.
Table 1.
Flow Cytometry Studies: Patient Demographics
| Diagnosis | Gender | Age, years | CSF pleocytosis | CSF oligoclonal bands |
|---|---|---|---|---|
| ON (24) | 14F /10M | 36 (22–54) | 16 (67%) | 17 (71%) |
| MS (26) | 20F /6M | 41 (18–61) | 18 (69%) | 24 (92%) |
| CON (24) | 16F /8M | 54 (23–79) | 3 (13%) | 0 (0%)* |
ON, optic neuritis; MS, multiple sclerosis; CON, neurological controls; F, female; M, male.
*Oligoclonal bands were not tested in one control patient.
Preparation of Cells, Staining, and Flow Cytometry
CSF was collected directly on ice; centrifuged within 10 minutes after lumbar puncture at 250 × g for 10 minutes at 4°C, washed once in phosphate-buffered saline (PBS) with 1% human serum albumin and 0.1% sodium azide [fluorescence-activated cell sorting (FACS buffer)], and resuspended in ice cold FACS buffer. Phlebotomy was performed simultaneously with lumbar puncture; peripheral blood mononuclear cells were obtained by density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway), washed three times at 4°C in PBS with 1% human serum albumin and resuspended in ice-cold FACS buffer. One hundred μl of CSF cells (minimum 4000 mononuclear cells) or 100 μl of peripheral blood mononuclear cells (100,000 mononuclear cells) were incubated on ice with antibodies for 30 minutes, washed twice in FACS buffer, and fixed with 1% paraformaldehyde. Analysis was performed on a FACSCalibur (Glostrup Hospital) or FACScan (Cleveland Clinic Foundation) flow cytometer (BD Biosciences, San Jose, CA), using CellQuest software (BD Biosciences). Cells were gated according to forward- and side-light-scattering properties and positively or negatively selected for CD14 or CD3 expression, respectively.
The following antibodies were used: phycoerythrin-conjugated anti-CCR1 (clone 53504.111; R&D Systems, Minneapolis, MN), fluorescein isothiocyanate- and phycoerythrin-conjugated anti-CCR5 (clone 2D7; BD PharMingen, San Diego, CA), allophycocyanin and peridinin chlorophyll protein-conjugated anti-CD14 (clone MøP9, BD Biosciences), fluorescein isothiocyanate-conjugated anti-CD3 (clone SK7, BD Biosciences) and phycoerythrin and fluorescein isothiocyanate-conjugated mouse isotype controls (BD Biosciences).
MS Autopsy Material
We focused these studies on active MS lesions, because cellular infiltration is proposed to be an initiating event in acute lesions. These studies, therefore, afforded an opportunity to examine the fate of CCR1+/CCR5+ monocytes that had newly entered the CNS. Paraffin-embedded archival autopsy material of five MS patients was available. The material was collected and neuropathologically examined at the Brain Research Institute, University of Vienna, Vienna, Austria. All five cases showed a prominent deposition of immunoglobulins and complement C9neo antigen at sites of active myelin destruction. Together with abundant T-cell and macrophage infiltrates and active demyelination, this form of tissue injury has been designated pattern II. 2
In a total of 10 tissue sections 23 active lesions were identified and according to previously published criteria 19-21 the following stages of demyelinating activity were defined: early-active (EA) regions were located at borders between demyelinating plaques and periplaque white matter. Macrophages contained myelin-degradation products, which stained with luxol fast blue myelin stain and were immunoreactive for all myelin proteins including minor proteins such as myelin oligodendrocyte glycoprotein. In late-active (LA) areas, myelin degradation was more advanced with macrophages containing myelin degradation products immunoreactive for the major myelin products myelin basic protein and proteolipid protein, but not for myelin oligodendrocyte glycoprotein. Inactive (IA) areas showed complete demyelination. Macrophages contained either empty vacuoles or periodic acid-Schiff reaction-positive degradation products.
In 11 of 23 lesions, all three stages of demyelinating activity could be identified. Four lesions contained EA and LA areas. Six lesions were entirely EA and two lesions IA. Ten representative regions outside lesions, showing neither macroscopic nor histological evidence of demyelination, were identified as internal controls (periplaque white matter) (Table 2) ▶ .
Table 2.
Autopsy Material: Patient Characteristics
| Case number | Gender | Age, years | Disease course | Disease duration, months | Number of tissue sections | Number of lesions | Early-active regions | Late-active regions | Inactive regions | Periplaque WM regions |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 47 | Acute | 3.5 | 2 | 3 | 3 | 2 | 2 | 2 |
| 2 | F | 46 | Acute | 0.4 | 3 | 4 | 4 | 4 | 4 | 3 |
| 3 | F | 28 | SP | 12 | 2 | 7 | 6 | 4 | 3 | 2 |
| 4 | M | 52 | Acute | 1.5 | 2 | 7 | 6 | 3 | 2 | 2 |
| 5 | F | 34 | SP | 144 | 1 | 2 | 2 | 2 | 2 | 1 |
| 5 Patients | 4F/1M | 41 (28–52) | 3 Acute/2 SP | 32.3 (0.4–144) | 10 | 23 | 21 | 15 | 13 | 10 |
F, female; M, male; SP, secondary progressive; WM, white matter.
Immunocytochemical Techniques
Immunocytochemical analysis was performed using an avidin-biotin-horseradish peroxidase complex procedure and 3,3-diaminobenzidine as described previously. 22 Berlex Biosciences provided a rabbit polyclonal anti-CCR1 antibody. 23 Murine monoclonal anti-human CCR5 (clone 45549.111, mouse IgG2B) was obtained from R&D Systems, murine monoclonal anti-human MRP14 from Bachem Bioscience Inc., King of Prussia, PA (clone S 36.48, mouse IgG1), and murine monoclonal anti-CD68 (clone KP1, mouse IgG1) from DAKO Corporation, Carpinteria, CA. Primary antibodies were omitted in controls.
For analysis of co-localizations of CCR1 with CCR5, CCR1 with MRP14, and CCR5 with CD68 sections were simultaneously labeled with primary antibodies and then incubated with Texas Red- and fluorescein isothiocyanate-conjugated secondary antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). In controls, primary antibodies were omitted, and tests for cross-reactivity by secondary antibodies were performed. Sections were analyzed on a Leica TCS-NT confocal scanning laser microscope or a Leica DMR microscope (Leica Wetzlar, Heidelberg, Germany).
Morphometric Analysis
The number of immunostained cells was determined in at least four standardized fields (146,200 μm2, defined by a morphometric grid) from each of the distinct lesional areas. Sections were photographed on a Leica DMR microscope and an Optronix Magnafire digital camera system and analyzed using Image Pro Plus (Media Cybernetics, Silver spring, MD).
Isolation and Culture of Human Monocytes
To examine CCR1 and CCR5 expression during monocyte differentiation in vitro, monocytes were obtained from freshly donated human peripheral blood from five healthy donors (three females and two males; mean age, 33 years; range, 21 to 43 years). The blood was immediately diluted 1:1 with PBS containing 1% human albumin (Sigma, St. Louis, MO) and underlayered with Ficoll-Paque (Pharmacia, Piscataway, NJ) for separation of mononuclear cells by density centrifugation. Monocytes were separated by adherence to serum-coated flasks according to the method of Kumagai and colleagues 24 and subsequently detached using 0.5 mmol/L of ethylenediaminetetraacetic acid. This preparation contained >95% monocytes as determined by flow cytometry for CD14 surface expression. Isolated monocytes were cultured in Dulbecco’s modified Eagle Medium (Mediatech Cellgro Inc., Herndon, VA) supplemented with l-glutamine, 4.5 mg/L glucose, and 10% bovine calf serum (Hyclone, Logan, UT) for 7 days at 37°C and 10% CO2. Under these conditions monocytes differentiate into macrophages after 7 days. 25,26 Granulocyte macrophage-colony-stimulating factor was omitted in the cell culture medium because this might alter chemokine surface expression levels. 26 After 1 day or 7 days in culture cells were detached from the flask using 0.5 mmol/L of ethylenediaminetetraacetic acid and gentle scraping and resuspended in PBS. CCR1 and CCR5 expression was determined by flow cytometry.
Statistical Analysis
Nonparametric tests (Mann-Whitney test and Wilcoxon signed rank test) were applied because the data were not normally distributed (Kolmogorovv-Smirnov test). A P value <0.05 was considered statistically significant.
Results
CCR1+/CCR5+ Monocytes Accumulate Preferentially in the CSF Compartment
CCR1 and CCR5 expression on CD14+ monocytes in peripheral blood and CSF was compared in patients with optic neuritis, MS, and neurological controls. Approximately 90% of CSF monocytes expressed CCR1 and ∼80% expressed CCR5, which was significantly (P < 0.001) higher compared to peripheral blood. There were no differences between the three patient groups examined (Figure 1) ▶ .
Figure 1.

CCR1 and CCR5 expression on CD14+ monocytes in peripheral blood and CSF. CCR1 (A) and CCR5 (B) expression on CD14+ monocytes in peripheral blood and CSF was compared in patients with optic neuritis (ON), MS, and in neurological control patients (CON) by flow cytometry. Numbers of examined sample pairs are given in brackets for each group. Values represent percentages of receptor+/CD14+ cells and are given as means ± SEM. A: In all patient groups CCR1+/CD14+ monocytes were enriched in the CSF compartment compared to peripheral blood (P < 0.001). CCR1+/CD14+ cells comprised a mean of ∼90% of all CD14+ monocytes in the CSF. B: In all patient groups CCR5+/CD14+ monocytes were enriched in the CSF compartment compared to peripheral blood (P < 0.001). CCR5+/CD14+ cells comprised a mean of ∼80% of all CD14+ monocytes in the CSF.
To define the distribution of the CCR1+/CCR5+ phenotype on monocytes, we examined co-expression of CCR1 and CCR5 on circulating and CSF monocytes from five patients with MS and five controls. Significantly more CSF monocytes (70%, 18.8 to 88.7%, P < 0.01) had the CCR1+/CCR5+ phenotype, compared to 19% (9.7 to 34.2%) of circulating monocytes. Virtually all CCR1+ CSF monocytes (96.6%) also expressed CCR5, compared to 23.3% of circulating CCR1+ monocytes. By comparison, a majority of CCR5+ monocytes both in the circulation and CSF were also CCR1+ (79.5% and 83.9%, respectively).
Together, these results indicate that CCR1+/CCR5+ monocytes, although a minority of the circulating monocyte pool, are highly enriched in the CSF population, regardless of the presence of CNS inflammatory pathology.
Distribution of Mononuclear Phagocytes in Acute MS Lesions
To follow the fate of CCR1+/CCR5+ hematogenous monocytes in MS lesions, immunohistochemistry for CD68, CCR5, and CCR1 was performed on 10 tissue sections of five patients with MS and distribution of immunoreactive cells in relation to demyelinating activity was established. In the 10 available tissue sections 23 lesions were identified containing 21 areas of EA demyelination, 15 LA regions, and 13 IA areas.
In EA zones two populations of CD68+ cells were observed: perivascular CD68+ cells had the appearance of activated monocytes with prominent granular cytoplasm, whereas parenchymal CD68+ cells exhibited predominantly the morphology of activated process-bearing microglia (Figure 2A) ▶ .
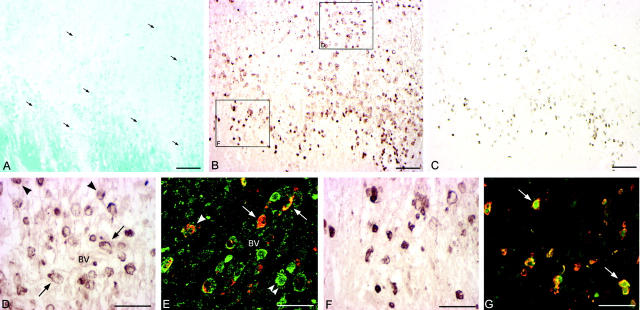
Figure 2.

Distribution, morphology, and co-localization of CD68+ and CCR5+ cells in EA and LA zones of demyelination. Immunohistochemistry for CD68 (A and D) and CCR5 (B and E) was performed on serial sections. Additionally, dual-label immunofluorescence histochemistry for CD68 and CCR5 and confocal microscopy were performed (C and F). CCR5 immunoreactivity is shown in green; red indicates CD68 immunoreactivity. Shown are areas of EA (A–C) and LA (D–F) demyelination of a single lesion. Scale bars, 100 μm. BV indicates blood vessel. A: In EA areas two populations of CD68+ cells were found: perivascular cells (arrows) with the appearance of monocytes and parenchymal cells (arrowheads) exhibiting the morphology of activated microglia. B: CCR5+ cells in EA areas were predominantly perivascular with morphological character of monocytes (arrows). C: In EA areas CCR5+/CD68+ cells were predominantly perivascular and exhibited the morphology of activated monocytes (arrows). CCR5−/CD68+ cells in EA regions were parenchymal cells with the morphology of activated microglia or small phagocytic cells (arrowheads). Within EA areas only occasional CCR5+/CD68+ cells were detected in the parenchyma (double arrowheads). D: No distinct subpopulations of CD68+ cells were found in LA zones, which contained a homogeneous population of large, CD68+ phagocytic macrophages (arrowheads). E: CCR5+ cells in LA areas resembled large phagocytic cells (arrowheads) and were predominantly found in the parenchyma. F: Concomitant with the transition from EA to LA areas of demyelination, there was a dramatic change in distribution, morphology, and frequency of CCR5+/CD68+ cells. CCR5+/CD68+ cells in LA regions formed a homogeneous population of parenchymal (double arrowheads) and perivascular (arrow) phagocytic macrophages. CCR5−/CD68+ cells were rarely found (arrowhead).
CCR5 immunoreactivity was almost exclusively found on perivascular cells and on activated monocytes in zones of early active demyelination (Figure 2B) ▶ . Perivascular CCR5+ cells were comprised both of CD68+ monocytes and CD3+ lymphocytes. Dual-label immunofluorescence histochemistry and confocal microscopy for CD68 and CCR5 localized CD68+/CCR5+ double-positive cells to perivascular accumulations of EA regions, with CD68-single-positive microglial cells detected in the parenchyma (Figure 2C) ▶ .
In contrast to EA zones, CD68+ cells in LA areas comprised a homogenous population of large, strongly CD68-immunoreactive phagocytic macrophages (Figure 2D) ▶ .
CCR5+ cells in LA regions resembled large phagocytic cells and were predominantly found in the parenchyma (Figure 2E) ▶ . In serial section analysis and dual-label immunohistochemistry, CD68+ phagocytic parenchymal macrophages in LA regions uniformly expressed CCR5 (Figure 2F) ▶ .
CD68 and CCR5 immunoreactivity in IA zones was essentially identical with that observed in LA regions (data not shown).
CCR1+ cells were predominantly localized at lesion edges and within areas of EA demyelination (Figure 3 ▶ ; A, B, D, and F). In serial section analyses CCR1+ cells co-localized with CD68+ cells, but not with CD3 immunoreactivity (data not shown). CCR1+ cells in EA areas were either perivascular or in parenchymal foci of active demyelination (Figure 3B) ▶ . Both perivascular and parenchymal CCR1+ cells exhibited the morphology of monocytes (Figure 3D) ▶ . Dual-label immunofluorescence histochemistry and confocal microscopy localized CCR1+/CCR5+ cells predominantly to perivascular cell aggregates in EA regions (Figure 3E) ▶ . As in CSF, all CCR1+ cells were CCR5+, whereas CCR5+ cells were not invariably CCR1+. CCR1+ cells at lesion edges were small, round cells (Figure 3F) ▶ that co-localized with macrophage-related protein (MRP)14+ cells (Figure 3, C and G) ▶ .
Figure 3.
Characterization of CCR1 expression in EA region and expanding lesion edge of actively demyelinating MS lesion. Actively demyelinating and expanding lesion stained with luxol fast blue myelin stain (LFB, A) indicating the lesion edge and adjacent EA area. Macrophages contained myelin degradation products indicating ongoing demyelination (A, arrows). Immunohistochemistry for CCR1 (B, D, and F) located CCR1+ cells to the immediate lesion edge and within EA regions. CCR1+ cells within EA areas were either perivascular (D, arrows) or dispersed in the parenchyma (D, arrowheads). Dual-label immunohistochemistry for CCR1 and CCR5 (E, CCR1 immunoreactivity shown in red, green indicates CCR5 immunoreactivity) and confocal microscopy characterized perivascular (E, arrows) as well as parenchymal (E, arrowhead) CCR1+/CCR5+ cells. Interestingly, all CCR1+ cells co-expressed CCR5, whereas CCR5+ cells not necessarily were also CCR1+ (E, double arrowheads). CCR1+ cells at the lesion edge (F) had the morphology of small, round cells. In a serial section analysis these cells co-localized with MRP14 immunoreactivity (C). Dual-label immunohistochemistry confirmed co-expression of CCR1 with MRP14 as shown in G (arrows, CCR1 immunoreactivity shown in red, green indicates MRP14 immunoreactivity). Scale bars: 100 μm (A–C), 50 μm (D–G). BV, blood vessel.
In contrast to EA areas, only occasional cells in LA and IA regions exhibited CCR1 immunoreactivity and were predominantly perivascular (data not shown).
In summary, in EA zones mononuclear phagocytes formed a heterogeneous population of CCR1+/CCR5+ perivascular monocytes and parenchymal CCR1−/CCR5− microglial cells. In LA and IA zones a homogenous population of monocyte- and microglia-derived mononuclear phagocytes were CCR1−/CCR5+.
Quantification of Mononuclear Phagocytes in Relation to Demyelinating Activity
The density of CD68+ cells was significantly (P < 0.001) higher in EA, LA, and IA zones, compared to periplaque white matter, with consistent CD68 counts within the different zones of individual lesions (Table 3 ▶ and Figure 4A ▶ ), indicating that the transition from EA to LA and IA demyelination in these lesions was characterized by monocyte and microglia activation and redistribution, rather than continuous accumulation of newly infiltrating cells from the bloodstream.
Table 3.
Quantitation of CD68 in Early-Active, Late-Active, and Inactive Zones of Demyelination and in the Periplaque White Matter
| Case number | Tissue section | Periplaque white matter | Lesion assignment | Early-active | Late-active | Inactive |
|---|---|---|---|---|---|---|
| 1 | 1A | 315 | Lesion 1 | 1317 | 1231 | 1387 |
| Lesion 2 | 825 | |||||
| 1B | 235 | Lesion 3 | 1603 | 1822 | 1353 | |
| 2 | 2A | 318 | Lesion 1 | 1756 | 1563 | 1554 |
| Lesion 2 | 1856 | 2761 | 2535 | |||
| 2B | 226 | Lesion 3 | 2704 | 1866 | 2708 | |
| 2C | 158 | Lesion 4 | 1827 | 2156 | 2249 | |
| 3 | 3A | 158 | Lesion 1 | 1763 | 1969 | |
| Lesion 2 | 1260 | 1587 | ||||
| Lesion 7 | 1087 | |||||
| 3B | 127 | Lesion 3 | 2181 | 2141 | 2086 | |
| Lesion 4 | 1531 | 1195 | 1665 | |||
| Lesion 5 | 2267 | |||||
| Lesion 6 | 1517 | |||||
| 4 | 4A | 139 | Lesion 1 | 1257 | 1228 | |
| Lesion 2 | 1415 | 1380 | ||||
| Lesion 3 | 1247 | |||||
| 4B | 170 | Lesion 4 | 1952 | 1307 | 1379 | |
| Lesion 5 | 1428 | |||||
| Lesion 6 | 1874 | |||||
| Lesion 7 | 2266 | |||||
| 5 | 5A | 188 | Lesion 1 | 1545 | 1626 | 1133 |
| Lesion 2 | 1870 | 1781 | 1531 | |||
| Mean± SEM | 203.3 ± 21.8 | 23 lesions | 1666.4 ± 90.8 | 1707.5 ± 112.1 | 1763.9 ± 149.7 |
Numbers are given in cells/mm2.
Figure 4.

Quantitation of CD68, CCR1, and CCR5 in EA, LA, and IA zones of demyelination and in the periplaque white matter. CD68, CCR1, and CCR5 expression was quantified on serial sections in 59 regions of 23 lesions with zones of varying demyelinating activity: 21 EA, 15 LA, 13 IA, and 10 regions of periplaque white matter (WM). Values are given as means ± SEM. A: A statistically significant increase (P < 0.001) of the number of CD68+ cells between periplaque WM and EA regions was observed. There was no significant change in the absolute numbers of CD68+ cells in EA, as compared to LA and to completely demyelinated IA regions. B: Significantly more CCR1+ cells were found within the lesion, compared to periplaque white matter (P < 0.0001). There was a highly significant decrease in CCR1+ cells in areas of IA demyelination, compared to EA and LA regions (P < 0.001 for EA versus IA and P < 0.05 for LA versus IA). C: Significantly more CCR5+ cells were found within the lesion, compared to periplaque white matter (P < 0.05 for WM versus EA and P < 0.001 for WM versus LA and IA, respectively). There was a highly significant increase (P < 0.001) in CCR5+ cells in areas of LA demyelination, compared to EA regions.
EA areas contained 417 ± 55.1 CCR1+ cells/mm 2 (mean ± SEM) (Figure 4B) ▶ . This density was significantly (P < 0.001), 100-fold higher than in periplaque white matter (4.9 ± 2.2 cells/mm2) and represented a mean of 25% (5 to 54%) of CD68+ cells in EA areas. In LA areas, 271 ± 54.3 cells/mm 2 (16%, 3 to 44% of CD68+ cells) expressed CCR1. Compared to EA and LA, IA areas had a significant (EA versus IA P < 0.001, LA versus IA P < 0.05) lower density of CCR1+ cells (128.9 ± 25.1 cells/mm2; 7%, 1 to 27%).
Quantitative immunohistochemical analysis of CCR5 expression in EA areas revealed 705 ± 64 cells/mm 2 (mean ± SEM, Figure 4C ▶ ). This number represented significantly (P < 0.05) more CCR5+ cells than found in periplaque white matter (65 ± 25 cells/mm2), and constituted a mean of 42% (range, 13 to 67%) of CD68+ cells within EA areas. The density of CCR5+ cells was significantly (P < 0.001) higher in areas of LA demyelination, compared with EA regions. In LA areas, a mean of 1599 ± 145 cells/mm 2 expressed CCR5. In these regions, CCR5+ cells represented 94% (62 to 205%) of CD68+ cells. In IA areas, CCR5 expression was 1480 ± 181 cells/mm2, representing 84% (18 to 152%) of CD68+ cells.
Expression of CCR1 and CCR5 Was Differentially Regulated during Monocyte Differentiation in Vitro
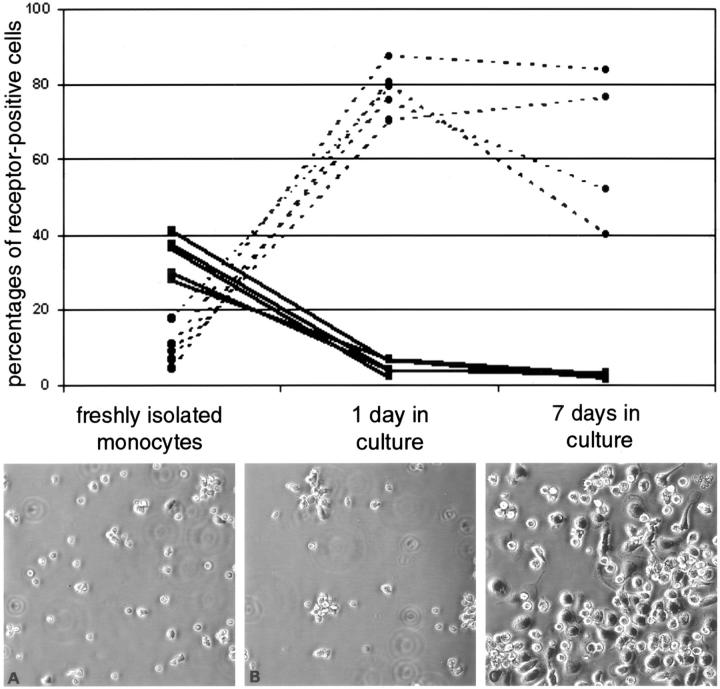
To investigate regulation of CCR1 and CCR5 expression during monocyte maturation in vitro, chemokine receptor expression on freshly isolated monocytes and at two different time points during monocyte differentiation in culture was examined by flow cytometry. Thirty-four percent (27.9 to 41%) of freshly-isolated monocytes expressed CCR1, whereas 9.7% (4.5 to 17.6%) expressed CCR5 (Figure 5 ▶ , top). After 7 days in culture the cells had become enlarged or spindle-shaped with extended processes (Figure 5 ▶ ; A to C, bottom). After 1 and 7 days in culture CCR1 was expressed by 4.7% (2.1 to 6.7%, P < 0.01) and 2.5% (1.5 to 3.4%), respectively.
Figure 5.
CCR1 and CCR5 expression is differentially regulated on human monocytes during differentiation in vitro. Freshly isolated monocytes were obtained from five healthy donors by density centrifugation and adherence to serum-coated flasks. Monocytes were allowed to differentiate in culture to mature macrophages. Shown are freshly isolated monocytes (A) and monocytes in culture at two different time points (B, 1 day; C, 7 days). CCR1 and CCR5 surface receptor expression was determined by flow cytometry on freshly isolated monocytes after 1 day and 7 days in culture. CCR1 (solid line) was expressed on 34.5% (28 to 41%) on freshly isolated monocytes. After 1 day in culture CCR1 expression decreased dramatically to 4.7% (2 to 7%) and after 7 days to 2.5% (2 to 3%). In contrast, CCR5 (dotted line) was expressed on 9.7% (5 to 18%) on freshly isolated monocytes and increased during culture to 78.7% (70 to 88%) after 1 day and 63.1% (40 to 84%) after 7 days in culture.
After 1 day in culture, 78.7% (70.3 to 87.5%) of cells expressed CCR5, as compared to 9.7% (4.5 to 17.6%, P < 0.01) of freshly isolated monocytes. After 7 days in culture 63.1% (40 to 83.8%) of macrophages expressed CCR5.
These in vitro results indicate that mature macrophages express very low levels of CCR1 and high levels of CCR5 expression, compared to freshly isolated monocytes.
Discussion
This report described CCR1 and CCR5 expression on mononuclear phagocytes in CSF and CNS of MS patients. This investigation was initiated to characterize chemokine receptor involvement in recruitment and activation of mononuclear phagocytes in the human CNS during early demyelination. The study was motivated by evidence from human material and animal models, implicating two major ligands for these receptors, RANTES/CCL5 and MIP-1α/CCL3, in the pathogenesis of inflammatory demyelination. Both chemokine receptors were highly enriched on monocytes in the CSF compartment and in perivascular cell accumulations in acute MS lesions. Furthermore, differential expression patterns for CCR1 and CCR5 were observed during lesion evolution and during culture of human monocytes in vitro. These results provide insight into pathogenetic mechanisms during early demyelinating events in MS and identify targets for future study and potential therapeutic intervention.
Hematogenous Monocytes that Enter the CNS Are CCR1+/CCR5+
In this report, we provide evidence that hematogenous monocytes that infiltrate the CNS are CCR1+/CCR5+. This evidence is primarily based on findings of our flow cytometry studies showing that a majority of monocytes in the CSF compartment were CCR1+/CCR5+ (Figure 1) ▶ . This CCR1+/CCR5+ population constituted a minority of the circulating pool of monocytes. Consistent with the hypothesis that the lumbar CSF is in equilibrium with the perivascular space of the CNS white matter, dual-label immunohistochemistry revealed CCR1+/CCR5+ perivascular cell accumulations in early demyelinating regions (Figure 3E) ▶ .
Although enrichment of CCR1+/CCR5+ monocytes in the CSF compartment was observed independent of CNS pathology, CCR1+/CCR5+ perivascular cell accumulations have not been detected in noninflamed brain sections (unpublished observations). We therefore propose that CCR1+/CCR5+ monocytes will only be retained in the CNS perivascular space in the presence of appropriate ligands, such as RANTES, which is present in CSF of MS patients and is abundantly expressed by the perivascular elements of MS lesions. 22
CCR1+ cells at MS lesion edges co-expressed the macrophage-related protein (MRP) 14 (Figure 3G) ▶ , a 14-kd calcium-binding protein expressed primarily by circulating human neutrophils and monocytes. 27 In vitro, this antigen shows a decline in expression during monocyte differentiation. 28 In MS lesions, MRP-14 expression is associated with the earliest stage of macrophage-mediated demyelinating activity. 19 It is uncertain whether MRP14 can be expressed by microglia under certain pathological conditions. In MS lesions, we and others detected MRP-14 expression only on small round, nonprocess-bearing cells, morphologically consistent with monocytes (Figure 3C) ▶ . 19 Co-expression of CCR1 and MRP-14 was therefore interpreted as identifying a population of newly recruited hematogenous monocytes.
Hematogenous Monocytes and Resident Microglia Show Different Chemokine Receptor Expression Patterns during Early Demyelination
In EA zones, a mean of 25% of CD68+ mononuclear phagocytes expressed CCR1 whereas a mean of 42% of CD68+ cells expressed CCR5. If the majority of hematogenous monocytes in the CNS are CCR1+/CCR5+, the remaining 58 to 75% of CCR1−/CCR5−/CD68+ mononuclear phagocytes most plausibly represent resident microglial cells. This interpretation was also supported by the distribution and morphology of CD68+ cells in EA areas, where we identified two distinct populations. One CD68+ population was identical to CCR1+/CCR5+ perivascular monocytes. The second population of CD68+ cells exhibited the morphology of parenchymal microglia (Figure 2A) ▶ . CD68+ cells with microglial morphology did not co-localize with CCR1 or CCR5 immunoreactivity and were concentrated in and around regions of EA demyelination (Figure 2C) ▶ . Based on these data, we propose that initial effectors of demyelination in MS lesions include CCR1+/CCR5+ hematogenous monocytes as well as CCR1−/CCR5− resident parenchymal microglial cells.
Lesion Evolution Is Characterized by Activation, rather than Accumulation of Monocytes from the Blood Stream
We and others found that numbers of CD68+ cells in EA, LA, and IA regions of individual active MS lesions remained virtually unchanged (Figure 4A ▶ and Table 3 ▶ ). 19,29 These observations led to the interpretation that changes in the CD68+ population occurred via activation and redistribution in the absence of meaningful continuous invasion of cells from the bloodstream. Another possibility is that large numbers of CD68+ cells undergo apoptosis in EA regions of MS lesions and are quantitatively replaced by hematogenous cells. Arguing against this interpretation, analyses of apoptotic cells in MS lesions have not reported dying macrophages or microglial cells in numbers sufficient to offer an alternative explanation of our results. 30,31
Expression of CCR1 and CCR5 Are Differentially Regulated during Lesion Evolution
The number of CCR5+ cells was significantly higher in LA and IA regions compared to EA regions (Figure 4C) ▶ , suggesting that CCR5 expression is not only maintained by the infiltrating hematogenous monocyte population throughout time but was also up-regulated by resident, initially CCR5-negative, microglial cells during activation and transformation into mature macrophages. In contrast, we observed a significant lower number of CCR1-expressing monocytes in IA regions compared to EA and LA areas (Figure 4B) ▶ . How can differential regulation of CCR1 and CCR5 expression during MS lesion evolution be explained? Expression of chemokine receptors on the cell surface is a result of a complex interplay of regulatory mechanisms including cytokine stimulation, ligand-induced internalization, as well as differentiation and activation of the receptor-bearing cell. Here, we report that the expression of CCR1 and CCR5 on human monocytes is differentially regulated during monocyte maturation in vitro (Figure 5) ▶ . As reported previously, we found higher CCR5 surface expression on macrophages when compared to freshly isolated monocytes. 26,32 Our data constitute the first report that CCR1 surface expression is down-regulated during monocyte differentiation in vitro. The loss of CCR1 and the gain of CCR5 expression during MS lesion evolution might therefore be explained by an increased activation and maturation of the infiltrating hematogenous monocyte population.
In considering the implications of our findings for MS pathogenesis, it is pertinent that chemokine receptors mediate effects beyond chemotaxis, some of which could be important for MS pathogenesis. 33 These effects include direct induction of IL-12 34 and nitric oxide production as well as modulation of cytokine release. 35-37
Our results provide insight into potential mechanisms of trafficking and activation of hematogenous monocytes in the CNS of MS patients. The challenge for the future is to define the roles of these components in disease pathogenesis.
Acknowledgments
We thank Barbara Tucky and Anne Petersen for excellent technical assistance, Grahame Kidd for help with confocal microscopy and image processing, Carl Bjartmar for helpful comments, and the Nancy Davis Center Without Walls for providing a morphometric image analysis station.
Footnotes
Address reprint requests to Richard M. Ransohoff, M.D., Department of Neurosciences, Mail Code NC30, The Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195. E-mail: ransohr@ccf.org.
Supported by the National Institutes of Health (1PO1 NS38667 to R. M. R.), the Williams Fund for MS Research (to R. M. R.), the Deutsche Forschungsgemeinschaft, Germany (TR463/1-1 to C. T.), the P. Carl Petersen Foundation, Denmark (to T. L. S.), the Niels Ydes Foundation, Denmark (to T. L. S.), and Bundesministerium für Bildung, Wissenschaft und Kultur, Austria (GZ 70.056/2-Pr/4/99 to H. L.).
C. T. and T. L. S. contributed equally to this work.
References
- 1.Sørensen TL, Ransohoff RM: Etiology and pathogenesis of multiple sclerosis. Semin Neurol 1998, 18:287-294 [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H: Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000, 47:707-717 [DOI] [PubMed] [Google Scholar]
- 3.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Brück W: Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 2000, 123:1174-1183 [DOI] [PubMed] [Google Scholar]
- 4.Bitsch A, Wegener C, Da Costa C, Bunkowski S, Reimers CD, Prange HW, Bruck W: Lesion development in Marburg’s type of acute multiple sclerosis: from inflammation to demyelination. Multiple Sclerosis 1999, 5:138-146 [DOI] [PubMed] [Google Scholar]
- 5.Ferguson B, Matyszak MK, Esiri MM, Perry VH: Axonal damage in acute multiple sclerosis lesions. Brain 1997, 120:393-399 [DOI] [PubMed] [Google Scholar]
- 6.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L: Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998, 338:278-285 [DOI] [PubMed] [Google Scholar]
- 7.Luster AD: Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 8.Zlotnik A, Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity 2000, 12:121-127 [DOI] [PubMed] [Google Scholar]
- 9.Karpus WJ, Kennedy KJ: MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol 1997, 62:681-687 [PubMed] [Google Scholar]
- 10.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD: An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 1995, 155:5003-5010 [PubMed] [Google Scholar]
- 11.Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ: Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol 1998, 92:98-108 [DOI] [PubMed] [Google Scholar]
- 12.Rottman JB, Slavin AJ, Silva R, Weiner HL, Gerard CG, Hancock WW: Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur J Immunol 2000, 30:2372-2377 [DOI] [PubMed] [Google Scholar]
- 13.Rottman JB, Silva R, Slavin A, Weiner HL, Gerard CG, Hancock WW: Central role of CCR1+ cells in the immunopathogenesis of experimental allergic encephalomyelitis (EAE). FASEB J 1999, 13:666(abstract) [Google Scholar]
- 14.Bennetts BH, Teutsch SM, Buhler MM, Heard RN, Stewart GJ: The CCR5 deletion mutation fails to protect against multiple sclerosis. Hum Immunol 1997, 58:52-59 [DOI] [PubMed] [Google Scholar]
- 15.Barcellos LF, Schito AM, Rimmler JB, Vittinghoff E, Shih A, Lincoln R, Callier S, Elkins MK, Goodkin DE, Haines JL, Pericak-Vance MA, Hauser SL, Oksenberg JR: CC-chemokine receptor 5 polymorphism and age of onset in familial multiple sclerosis. Multiple Sclerosis Genetics Group. Immunogenetics 2000, 51:281-288 [DOI] [PubMed] [Google Scholar]
- 16.Sellebjerg F, Madsen HO, Jensen CV, Jensen J, Garred P: CCR5 delta32, matrix metalloproteinase-9 and disease activity in multiple sclerosis. J Neuroimmunol 2000, 102:98-106 [DOI] [PubMed] [Google Scholar]
- 17.Francis DA: Demyelinating optic neuritis: clinical features and differential diagnosis. Br J Hosp Med 1991, 45:376-379 [PubMed] [Google Scholar]
- 18.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW: New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983, 13:227-231 [DOI] [PubMed] [Google Scholar]
- 19.Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H: Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995, 38:788-796 [DOI] [PubMed] [Google Scholar]
- 20.Brück W, Schmied M, Suchanek G, Brück Y, Breitschopf H, Poser S, Piddlesden S, Lassmann H: Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol 1994, 35:65-73 [DOI] [PubMed] [Google Scholar]
- 21.Lucchinetti CF, Brück W, Rodriguez M, Lassmann H: Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol 1996, 6:259-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sørensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM: Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999, 103:807-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R: CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol 1997, 7:112-121 [DOI] [PubMed] [Google Scholar]
- 24.Kumagai K, Itoh K, Hinuma S, Tada M: Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods 1979, 29:17-25 [DOI] [PubMed] [Google Scholar]
- 25.Hariharan D, Douglas SD, Lee B, Lai JP, Campbell DE, Ho WZ: Interferon-gamma upregulates CCR5 expression in cord and adult blood mononuclear phagocytes. Blood 1999, 93:1137-1144 [PubMed] [Google Scholar]
- 26.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM: Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol 1998, 72:4962-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C: Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330:80-82 [DOI] [PubMed] [Google Scholar]
- 28.Goebeler M, Roth J, Henseleit U, Sunderkotter C, Sorg C: Expression and complex assembly of calcium-binding proteins MRP8 and MRP14 during differentiation of murine myelomonocytic cells. J Leukoc Biol 1993, 53:11-18 [DOI] [PubMed] [Google Scholar]
- 29.Brück W, Sommermeier N, Bergmann M, Zettl U, Goebel HH, Kretzschmar HA, Lassmann H: Macrophages in multiple sclerosis. Immunobiology 1996, 195:588-600 [DOI] [PubMed] [Google Scholar]
- 30.Bonetti B, Raine CS: Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol 1997, 42:74-84 [DOI] [PubMed] [Google Scholar]
- 31.Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W: Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med 1996, 184:1513-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Marzio P, Tse J, Landau NR: Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses 1998, 14:129-138 [DOI] [PubMed] [Google Scholar]
- 33.Bacon KB, Premack BA, Gardner P, Schall TJ: Activation of dual T cell signaling pathways by the chemokine RANTES. Science 1995, 269:1727-1730 [DOI] [PubMed] [Google Scholar]
- 34.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A: CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat Immunol 2000, 1:83-87 [DOI] [PubMed] [Google Scholar]
- 35.Fahey TJ, 3rd, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, Cerami A, Sherry B: Macrophage inflammatory protein 1 modulates macrophage function. J Immunol 1992, 148:2764-2769 [PubMed] [Google Scholar]
- 36.Aliberti JC, Machado FS, Souto JT, Campanelli AP, Teixeira MM, Gazzinelli RT, Silva JS: Beta-chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect Immun 1999, 67:4819-4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villalta F, Zhang Y, Bibb KE, Kappes JC, Lima MF: The cysteine-cysteine family of chemokines RANTES, MIP-1alpha, and MIP-1beta induce trypanocidal activity in human macrophages via nitric oxide. Infect Immun 1998, 66:4690-4695 [DOI] [PMC free article] [PubMed] [Google Scholar]