Abstract
Although osteoarthritis is characterized by a progressive loss of the extracellular cartilage matrix, very little is known about the fate of articular chondrocytes during the progression of the disease. In this study we examined the expression of syndecan-3, a marker of early chondrocyte differentiation, and annexin VI, a marker of late chondrocyte differentiation, in mammalian embryonic growth plate cartilage and normal and osteoarthritic human articular cartilage. Whereas syndecan-3 was expressed in the proliferative and hypertrophic zones of growth platecartilage, immunostaining for annexin VI waspredominately found in the hypertrophic and mineralizing zones of fetal bovine growth plate cartilage. Approximately 20% of chondrocytes were immunopositive for syndecan-3 in normal human articular cartilage, the number of syndecan-3-expressing chondrocytes significantly increased during the progression of osteoarthritis with more than 80% syndecan-3-positive cells in the upper zone of severely affected osteoarthritic cartilage. Similarly, the number of annexin VI-expressing cells significantly increased in the upper cartilage zones during the progression of osteoarthritis. Furthermore, immunostaining for proliferating cell nuclear antigen, a marker for cell proliferation, was detected in chondrocytes in the upper zone of osteoarthritic cartilage. Double-labeling experiments with antibodies against syndecan-3 and annexin VI revealed chondrocytes that expressed only syndecan-3, and cells that expressed both syndecan-3 and annexin VI. These results suggest that the expression of early (proliferating cell nuclear antigen, syndecan-3) and late differentiation markers (annexin VI, alkaline phosphatase) is activated in chondrocytes of osteoarthritic cartilage.
Osteoarthritis (OA) is characterized by a progressive damage of articular cartilage, secondary inflammatory processes of the synovialis, the formation of osteophytes, and an increase in subchondral bone mass. 1 In healthy human articular cartilage chondrocytes are responsible for maintaining a balance of anabolic and catabolic pathways to stabilize cartilage integrity. In OA, this balance is disturbed and pathohistological signs of cartilage damage become visible. In particular, fibrillations of the superficial layer, a progressive loss of proteoglycans, and the appearance of both mitotic cell division and chondrocytic cell death are apparent. 2 The coexistence of cell death and mitotic cell division contributes to the typical image of severe OA with extensive chondrocyte clusters and hypocellular areas combined with a total loss of cartilage organization.
During early stages of OA articular chondrocytes try to repair the cartilage matrix by increasing the synthesis of type II collagen and aggrecan in response to the ongoing enzymatic and biomechanical degradation. 3,4 Eventually these reparative processes fail leading to the destruction of cartilage. Although much effort has been devoted to characterize the synthesis patterns of OA chondrocytes, little is known about possible phenotypic changes and the resulting loss of maintenance of cartilage integrity. Several studies have demonstrated the expression of proteins, such as type X collagen, annexins II and V, and alkaline phosphatase. 5-8 These proteins are predominantly expressed by hypertrophic and terminally differentiated, mineralizing chondrocytes during endochondral ossification, and thus are considered as markers for chondrocyte hypertrophy. 8-10 In contrast, other studies have provided evidence that OA chondrocytes express proteins, such as type I and III collagen, which would be indicative for a dedifferentiation process of these cells. 11-13 An exact understanding of the ultimate fate of chondrocytes during the progression of OA might be of great importance, because it could provide novel therapeutic strategies to stop the progression of the disease.
During endochondral ossification, chondrocytes in growth plate cartilage undergo a series of differentiation events, including proliferation, hypertrophy, and terminal differentiation. 9 Each zone of differentiation is characterized by the expression of specific genes. Previous studies have provided evidence that syndecan-3 is restricted to the zone of proliferative chondrocytes in embryonic chicken growth plate cartilage, whereas alkaline phosphatase; annexins II, V, and VI; and type X collagen are highly expressed in the zones of hypertrophic and terminally differentiated chondrocytes. 8-10,14
Syndecan-3 is a member of a family of heparan sulfate proteoglycans that are associated with the cell surface. These macromolecules contain a hydrophobic membrane-spanning domain, a short cytosolic domain, and an extracellular domain. Syndecans are known to interact with several matrix molecules, and thus are thought to serve as structural and functional links between the cell surface and the surrounding extracellular matrix. Furthermore, syndecans interact with growth factors, such as fibroblast growth factor, insulin-like growth factor, and epidermal growth factor. These interactions are required for biological activity, because these factors must first interact with the heparan sulfate chains of the syndecans before they can interact with their high-affinity signaling receptors. Thus, syndecans seem to play important roles in modulating cellular activities, including cell proliferation and differentiation. 15
Annexin VI belongs to the annexin protein family. These proteins have in common that they bind to acidic phospholipids in the presence of calcium. 16 Annexins II, V, and VI, which are expressed in growth plate cartilage, have been shown to form Ca2+ channels, suggesting possible roles in controlling or altering Ca2+ homeostasis in cartilage. 17,18 In addition, annexins II, V, and VI are major components of matrix vesicles that initiate the mineralization process in growth plate cartilage. The annexins enable influx of Ca2+ into the vesicles and the formation of the first crystal phase inside the vesicles. 19,20
Chondrocytes in normal articular cartilage have a stable phenotype in contrast to growth plate chondrocytes, and maintain a functional articular extracellular matrix. However, the possible expression of proteins, which are normally produced by chondrocytes during various stages of differentiation in growth plate cartilage, by chondrocytes in OA cartilage could lead to altered functional activities and the loss of their ability to maintain a functional articular cartilage matrix. Thus, the goal of this study was to examine whether articular chondrocytes express proliferating cell nuclear antigen (PCNA) and syndecan-3, early differentiation markers, which are proposed to play roles in proliferation and differentiation of growth plate chondrocytes, 14,21 and alkaline phosphatase and annexin VI, late differentiation markers, which are involved in Ca2+ homeostasis and mineralization of terminally differentiated chondrocytes. 18,19
Materials and Methods
Tissue Preparation
Seventeen OA human cartilage samples (five samples of mild OA, five samples of moderate OA, and seven samples of severe OA) were obtained from patients undergoing total knee replacement. Clinical data were carefully reviewed to exclude any forms of secondary OA and inflammatory joint diseases. Five normal human cartilage samples were obtained from patients who underwent amputation or during autopsies within 24 hours after dead. In addition, fetal bovine growth plate cartilage was used in this study. Samples were fixed in 4% paraformaldehyde, decalcified in 0.2 mol/L ethylenediaminetetraacetic acid, embedded in paraffin, and 6-μm-thick sections were cut perpendicular to the cartilage surface.
Histological/Histochemical Grading
Safranin O-stained sections of normal and OA cartilage were graded by two different observers according to Mankin and colleagues. 22 Furthermore, cartilage samples were classified in normal cartilage with no signs of OA (Mankin 0 to 1), mild OA (no signs of fibrillation, loss of proteoglycans in surface areas; five samples; Mankin 2 to 5), moderate OA (no signs of fibrillation, some loss of superficial zone, some clustering of cells evident; five samples; Mankin 6 to 9), and severe OA (extensive fissuring and fibrillation, clustering of chondrocytes, loss of cartilage; seven samples; Mankin ≥10) as described previously. 23
Immunohistochemistry
After deparaffinization, sections were incubated with sheep testicular hyaluronidase (2 mg/ml; Sigma, St. Louis, MO) in phosphate-buffered saline (PBS), pH 7.5, for 30 minutes at 37°C. Immunostaining was performed using the Histostain SP Kit (Zymed Laboratories Inc., San Francisco, CA) following the manufacturer’s instructions. Briefly, after incubation with a blocking solution for 10 minutes at room temperature, sections were incubated overnight at 4°C with primary antibodies followed by biotinylated secondary antibodies for 10 minutes at room temperature. After washing, sections were incubated with a streptavidin-peroxidase conjugate for 10 minutes at room temperature followed by a solution containing diaminobenzidine (chromogen) and 0.03% hydrogen peroxide for 5 minutes at room temperature. Control sections were incubated with nonimmune rabbit serum. Specimens were viewed and analyzed under a Zeiss-Axiophot microscope (Zeiss, Goettingen, Germany). To determine the percentage of immunostained cells, 100 cells were counted in each of two different areas of each cartilage zone of separately stained sections from different donors. The average of the two counts was used for statistical analysis. Statistical analysis was performed on all data points with regard to immunopositive cells in normal articular cartilage in each zone by an unpaired Student’s t-test. Each data point represented the mean of five or seven samples from different donors with the corresponding SD. P values <0.05 were considered significant.
To stain sections with antibodies specific for PCNA, deparaffinized sections were treated with 0.1% Triton X-100 in PBS (pH 7.4) for 2 minutes at room temperature. After blocking with 5% bovine serum albumin the sections were incubated with primary anti-PCNA antibodies (DAKO, Hamburg, Germany) for 30 minutes at 37°C followed by incubation overnight at 4°C. After washing, sections were incubated with biotinylated secondary antibodies followed by avidin-biotin-alkaline phosphatase conjugate and staining with Fast Red (DAKO). Sections were counterstained with hematoxylin.
Antibodies
Rabbit polyclonal antisera were generated through immunization with recombinant full-length human annexin VI protein or a recombinant fusion protein containing GST and a syndecan-3 fragment encoding amino acids 215 to 313 of chicken syndecan-3 as described previously. 14,18,24 This fragment is located in the extracellular domain of syndecan-3 and shows no homology to other syndecans. Preimmune sera were collected from the same rabbits before immunization. The antisera were produced by Cocalico (Reams, PA). IgG fractions were purified by affinity chromatography using Protein A-Sepharose columns (Pharmacia, Piscataway, NJ). The amino-acid sequence homology between the fragment of chicken syndecan-3 used to prepare the antibodies and the human syndecan-3 is 60%, whereas the amino acid sequence homology between human annexin VI and bovine annexin VI is 98%. Monoclonal antibodies specific for bone and liver human alkaline phosphatase were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and were described by Lawson and colleagues. 25 Monoclonal antibodies specific for PCNA were obtained from DAKO.
Double Immunostaining and Confocal Microscopy
Double immunolabeling with antibodies against annexin VI and syndecan-3 was performed on paraffin-embedded specimens of normal and OA cartilage. Sections were deparaffinized in xylene and a graded series of ethanol.
One mg of the polyclonal rabbit anti-annexin VI IgG fraction (1 mg/ml) was labeled with Alexa Fluor 568 dye, whereas 1 mg of the polyclonal rabbit anti-syndecan-3 IgG fraction was labeled with Alexa Fluor 488 dye using the Alexa Fluor protein-labeling kits (Molecular Probes, Eugene, OR) following the manufacturer’s instructions. Labeling of the anti-annexin VI IgG fraction resulted in 3.8 mol of Alexa 568 dye per mol of antibody, whereas labeling of the anti-syndecan-3 IgG fraction resulted in 2.4 mol Alexa 568 dye per mol of antibody.
After pretreatment with hyaluronidase for 30 minutes at 37°C, sections were blocked with 5% bovine serum albumin in PBS for 10 minutes. The two fluorescence dye-labeled antibodies against annexin VI and syndecan-3 were mixed and incubated on the sections for 2 hours at room temperature. After washing three times with PBS, sections were mounted and viewed under an Olympus Fluoview laser-scanning confocal microscope.
Results
Embryonic Bovine Growth Plate Cartilage
In the first set of experiments we analyzed the immunolocalization of syndecan-3 and annexin VI in the embryonic bovine growth plate. The antibodies specific for syndecan-3 showed staining in the zones of proliferative, hypertrophic, and terminally differentiated chondrocytes, whereas the zone of reserve chondrocytes showed no staining (Figure 1A) ▶ . In contrast, most intensive staining for annexin VI was obtained in the zones of hypertrophic and terminally differentiated chondrocytes, whereas little or no staining was observed in the zones of proliferative and resting chondrocytes (Figure 1B) ▶ . In 18-day chicken embryonic tibia immunostaining for syndecan-3 was restricted to the zone of BrdU-positive, proliferating chondrocytes, 14 suggesting possible species differences between the mammalian and the chicken growth plate.
Figure 1.
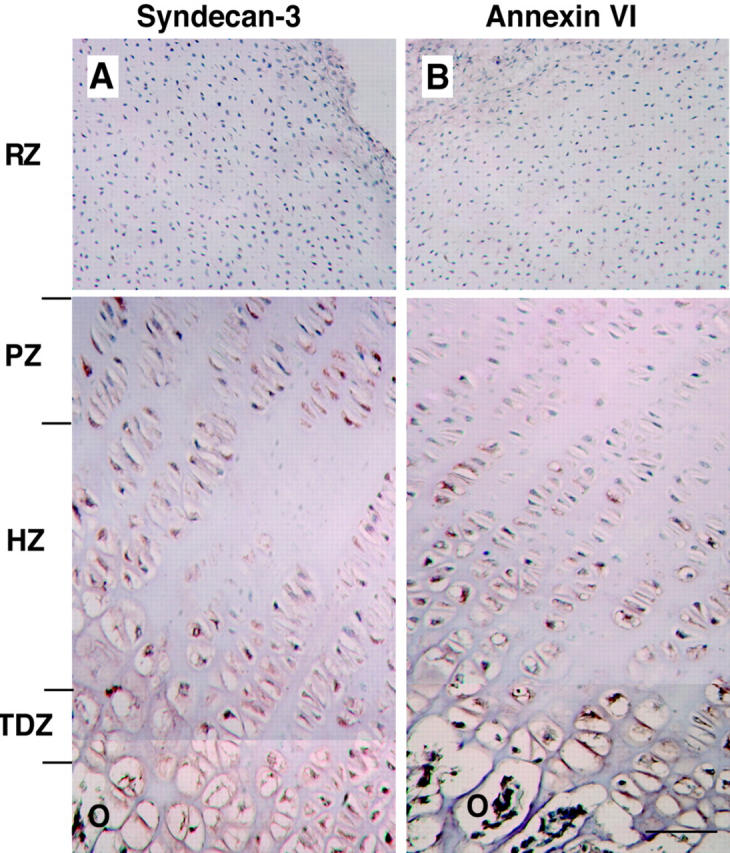
Immunohistochemical analysis of syndecan-3 (A) and annexin VI (B) in sections of embryonic bovine growth-plate cartilage. Sections were stained with anti-syndecan-3 IgG fraction (A) or anti-annexin VI IgG fraction (B) as described in Material and Methods. Staining for syndecan-3 was obtained in the zone of proliferative (PZ), hypertrophic (HZ), and terminally differentiated (TDZ) chondrocytes, whereas no staining was obtained in the zone of resting chondrocytes (RZ). In contrast, staining for annexin VI was obtained in the zones of hypertrophic and terminally differentiated chondrocytes, whereas little or no staining was detected in the zones of proliferative and resting chondrocytes. O, osteoid. Scale bar, 100 μm.
Normal and OA Human Articular Cartilage
We next analyzed the expression of syndecan-3 and annexin VI in normal and OA human articular cartilage samples by immunohistochemistry. Five normal articular cartilage samples, five mild, five moderate, and seven severe OA cartilage samples from different donors were investigated in this study. Normal cartilage samples were uniformly stained with safranin O (Figure 2A) ▶ , indicating no loss of proteoglycans, and with antibodies specific for type II collagen (data not shown). However, most of the cells showed no immunostaining for syndecan-3 and annexin VI (Figure 2, B and C) ▶ . To obtain the percentage of syndecan-3 and annexin VI-positive cells in normal articular and OA cartilage, we counted 100 cells in each of two separated areas of the superficial, middle, and deep cartilage zones in normal and mild OA cartilage, and the middle and deep zones of moderate and severe OA cartilage (superficial zone is lost in moderate and severe OA cartilage). Approximately 20% of cells showed immunostaining for syndecan-3 in the superficial and middle zones of normal articular cartilage, whereas ∼13% of immunopositive cells were detected in the deep zone (Table 1) ▶ . Only between 9 and 15% of cells in the superficial and middle zones of normal articular cartilage showed immunostaining for annexin VI, whereas the percentage of positive cells was higher in the deep zone (∼30%; Table 1 ▶ ). There was a slight increase in syndecan-3-positive cells in the superficial, middle, and deep zones of mild OA cartilage, whereas a slight increase of annexin VI-positive cells was only observed in the middle zone of mild OA cartilage (Table 1) ▶ . The percentage of syndecan-3-positive cells increased to ∼57% in the middle zone and ∼40% in the deep zone of moderately affected OA cartilage (Figure 2E ▶ , Table 1 ▶ ). Moderately affected OA cartilage was characterized by a loss of the superficial zone, loss of safranin O staining in the middle zone, indicative for proteoglycans loss, some evidence of cell clustering, and some beginning fissuring (Figure 2D) ▶ . The percentage of annexin VI-positive cells increased to ∼29% in the middle zone of moderately affected OA cartilage (Figure 2F ▶ , Table 1 ▶ ). Severely affected OA cartilage was characterized by extensive chondrocyte clusters, fissuring, fibrillation, and loss of cartilage (Figure 2G) ▶ . Most of cells in clusters showed strong immunostaining for syndecan-3, resulting in ∼87% positive cells in the middle zone and >60% positive cells in the deep zone of severely affected OA cartilage (Figure 2, H and J ▶ ; Table 1 ▶ ). Also intensive staining of many cells in clusters for annexin VI was detected in severely affected OA cartilage (Figure 2, I and K) ▶ , resulting in ∼45% immunopositive cells in the middle zone and ∼34% immunopositive cells in the deep zone of severely affected OA cartilage (Table 1) ▶ . Furthermore, many cells in clusters immunostained with antibodies specific for alkaline phosphatase (Figure 2L) ▶ , which is another marker for hypertrophic chondrocytes. 10,26
Figure 2.
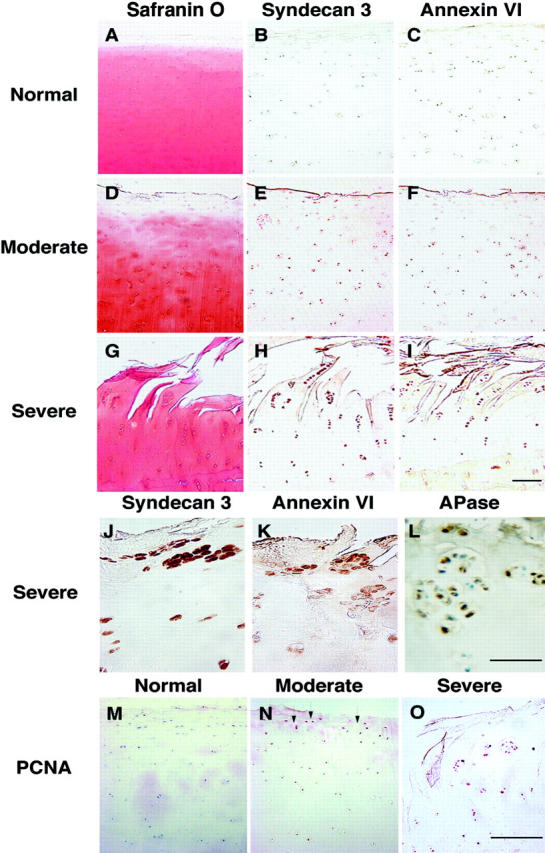
Immunohistochemical analysis of syndecan-3 (B, E, H, and J), annexin VI (C, F, I, and K), alkaline phosphatase (L), and PCNA (M–O) in sections of human normal and osteoarthritic cartilage. A: Safranin O staining of normal articular cartilage. B and C: Note the absence of staining for syndecan-3 (B) and annexin VI (C). D: Safranin O staining of moderately affected OA cartilage. Note the loss of the superficial zone and the loss of safranin O staining in the middle zone. E and F: Cells in the middle and deep zones of moderately affected OA cartilage showed immunostaining for syndecan-3 (E) and annexin VI (F). G: Safranin O staining of severely affected OA cartilage (deep clefts down into the deep zone, extensive chondrocyte clusters, and loss of cartilage). H and I: Note the intense staining for syndecan-3 (H) and annexin VI (I) of chondrocytes in the middle and deep zones of severe OA cartilage. J, K, and L: Higher magnification of cell clusters showing immunostaining for syndecan-3 (J), annexin VI (K), and alkaline phosphatase (APase, L). M: Note the absence of staining for PCNA in normal articular cartilage. N: Chondrocytes close to the joint surface in moderate OA cartilage showed immunostaining for PCNA (arrows). O: Chondrocytes in clusters of severe OA cartilage showed immunostaining for PCNA. B, C, E, F, H, I–L: Brown staining indicates immunopositive cells. M–O: Red staining indicates immunopositive cells. Scale bar, 100 μm.
Table 1.
Percentage of Cells in Normal, Mild, Moderate, and Severe OA Cartilage Showing Immunostaining for Syndecan-3 or Annexin VI
| Cartilage | Superficial zone | Middle zone | Deep zone |
|---|---|---|---|
| Normal (n = 5) | |||
| Syndecan-3 | 23.6 ± 7.4 | 20.8 ± 9.5 | 13.2 ± 8.4 |
| Annexin VI | 9.5 ± 6.1 | 14.7 ± 11.2 | 29.8 ± 9.5 |
| Mild OA (n = 5) | |||
| Syndecan-3 | 32.0 ± 9.8 | 31.3 ± 16.5 | 27.8 ± 11.4† |
| Annexin VI | 11.9 ± 8.4 | 26.2 ± 14.5 | 31.0 ± 8.5 |
| Moderate OA (n = 5) | |||
| Syndecan-3 | 57.4 ± 27.6† | 40.8 ± 21.7† | |
| Annexin VI | 28.9 ± 12.1 | 22.1 ± 17.9 | |
| Severe OA (n = 7) | |||
| Syndecan-3 | 86.7 ± 14.9* | 65.7 ± 16.4* | |
| Annexin VI | 44.9 ± 10.0* | 34.1 ± 9.9 |
One hundred cells were counted in each of two separate areas of the various zones of normal articular cartilage, mild, moderate, and severe OA cartilage using a high-power magnification field (superficial, middle, and deep zones in normal and mild OA cartilage; middle and deep zones in moderate and severe OA cartilage; the superficial zone is lost in moderate and severe OA cartilage). Data are expressed as the mean ± SD of the percentage of total cells which show cell-associated staining.
*P < 0.01.
†P < 0.05 (versus percentage of immunopositive cells in normal articular cartilage).
We next determined the expression of PCNA, a marker of cell proliferation. 27,28 Although no immunostaining for PCNA was detected in normal human articular cartilage (Figure 2M) ▶ , some chondrocytes close to the joint surface showed staining for PCNA in moderately affected OA cartilage (Figure 2N) ▶ . Furthermore, chondrocytes in clusters in severely affected OA cartilage showed immunostaining for PCNA (Figure 2O) ▶ .
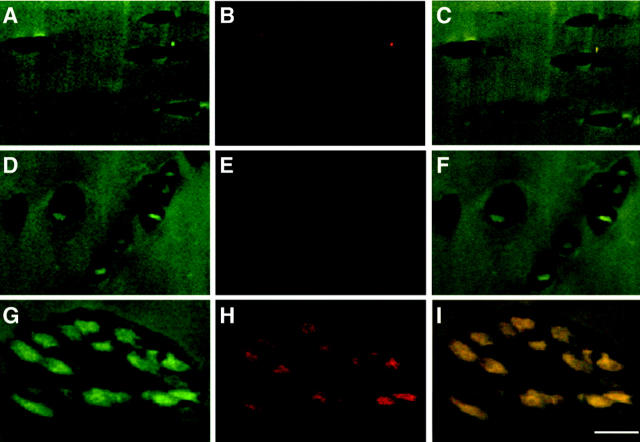
As shown in Table 1 ▶ , more chondrocytes were immunopositive for syndecan-3 than for annexin VI, suggesting a similar sequence of differentiation events in OA cartilage as in growth plate cartilage. To further test this hypothesis, we used double staining with antibodies against syndecan-3 and annexin VI to determine whether chondrocytes in OA cartilage, like growth plate chondrocytes first express syndecan-3 followed by the synthesis of annexin VI. For double staining, the anti-syndecan-3 IgG fraction was labeled with the fluorescence dye Alexa Fluor 488-nm dye, whereas the anti-annexin VI IgG fraction was labeled with Alexa Fluor 568-nm dye; double-stained sections were viewed under a confocal microscope. In normal articular cartilage the majority of cells showed no staining for syndecan-3 and annexin VI (Figure 3 ▶ ; A, B, and C). Normal and OA articular cartilage matrix showed autofluorescent, resulting in a relatively high background in the matrix (Figure 3) ▶ . In moderately affected OA cartilage, some cells were detected that showed staining for both syndcan-3 and annexin VI (data not shown). However, many cells that were immunopositive for syndecan-3 (Figure 3D) ▶ , showed no staining for annexin VI (Figure 3E) ▶ . The overlay of both images revealed only a green staining of the cells (Figure 3F) ▶ . In severely affected OA cartilage, most of the cells in clusters showed immunostaining for both syndecan-3 (Figure 3G) ▶ and annexin VI (Figure 3H) ▶ , resulting in a yellow signal in the overlay of both images (Figure 3I) ▶ . No cells were detected in moderate or severe OA cartilage that showed immunostaining for annexin VI but not for syndecan-3. Thus, the majority of cells in OA cartilage first start to synthesize syndecan-3 followed by synthesis of annexin VI similar to chondrocytes in mammalian growth plate cartilage.
Figure 3.
Double immunostaining for syndecan-3 (A, D, G) and annexin-VI (B, E, H) of sections from normal (A–C), moderate (D–F), and severe OA specimens (G–I). Sections of normal, moderate, and severe OA cartilage were double immunostained with antibodies specific for syndecan-3 and annexin VI and viewed under a confocal microscope as described in Material and Methods. In normal articular cartilage most of the cells showed no immunostaining for syndecan-3 (A) and annexin VI (B). Because of the autofluorescence of the cartilage matrix, the background in the matrix was relatively high. C: Overlay image of A and B. In moderate OA cartilage cells were detected that showed staining for syndecan-3 (D), but no staining for annexin VI (E). F: Overlay image of D and E. In severe OA cartilage-clustered chondrocytes showed immunostaining for both syndecan-3 (G) and annexin VI (H). I: Overlay image of G and H. Scale bar, 50 μm.
Discussion
In this study, we provide evidence that the percentage of chondrocytes expressing syndecan-3 and annexin VI increases significantly in OA cartilage compared to normal healthy human articular cartilage. The percentage of immunopositive chondrocytes gradually increases in the various stages of OA to up to 87% cells positive for syndecan-3 and 45% positive for annexin VI in the middle zone, and 66% cells positive for syndecan-3 and 34% for annexin VI in the deep zone of severe OA cartilage. Furthermore, chondrocytes in OA cartilage also show immunostaining for PCNA and alkaline phosphatase, clearly establishing that these cells activate the expression of proteins, which are produced during early (PCNA, syndecan-3) and late stages (alkaline phosphatase, annexin VI) of maturation of growth plate chondrocytes.
Our double-staining experiments with antibodies specific for syndecan-3 and annexin VI reveal cells that are positive for syndecan-3 but not for annexin VI, and cells that are positive for both syndecan-3 and annexin VI. However, no cell was detected that shows only staining for annexin VI but not for syndecan-3. These findings suggest that the temporal expression pattern of syndecan-3 and annexin VI is similar to the pattern found in embryonic mammalian growth plate cartilage, where syndecan-3 immunostaining is found in the proliferative and hypertrophic zones, whereas annexin VI staining is restricted to the hypertrophic zone. The immunostaining pattern for syndecan-3 in mammalian growth plate cartilage is different from the pattern obtained in embryonic chicken growth-plate cartilage, where syndecan-3 staining is restricted to the proliferative zone. 14 Based on the restricted localization of syndecan-3 to the proliferative zone in embryonic chicken growth-plate cartilage and the fact that it binds mitogenic factors, such as fibroblast growth factor-2 or insulin-like growth factor-1, it has been suggested that syndecan-3 might be involved in regulating chondrocyte proliferation. 14 However, based on its localization in the proliferative and hypertrophic zones of the embryonic mammalian growth plate, the protein might have other additional functions than regulating cell proliferation. Interestingly, syndecans have been shown to bind to extracellular matrix proteins, and it has been suggested that these molecules might be involved in cell-matrix interactions. 15 Thus, the functions of syndecan-3 during normal chondrocyte differentiation and in OA cartilage remain to be established.
Previous studies from our and other laboratories have demonstrated that OA chondrocytes express annexins II and V. 7,8 Here we show that OA chondrocytes also activate the expression of annexin VI. Thus, OA chondrocytes, like terminally differentiated growth plate chondrocytes, express annexins II, V, and VI. These three annexins are major components of matrix vesicles. 18,20 These vesicles are budded off from the plasma membrane of terminally differentiated chondrocytes, and after being released initiate the mineralization process in growth plate cartilage. 19,20 By forming Ca2+ channels in these vesicles, annexins II, V, and VI play crucial roles in regulating matrix vesicle-mediated mineralization. 18,20 Interestingly, mineral deposits and matrix vesicles are also present in OA cartilage. 8,29-31 Thus, OA chondrocytes activate the expression of major components (alkaline phosphatase; and annexins II, V, and VI) of mineralization-competent matrix vesicles.
Interestingly, chondrocytes in the upper zones of moderate and severe OA cartilage are immunopositive for PCNA. PCNA, also known as cyclin, is a 36-kd intranuclear protein that is synthesized in the late G1 and S phase, and is localized to sites of DNA synthesis. 32,33 Monoclonal antibodies against PCNA have been used to detect proliferative cells in a variety of different tissues. 27,28 Studies on rat growth plate cartilage have demonstrated that PCNA staining is restricted to chondrocytes in the proliferative zone. 21 In addition, PCNA-positive, proliferating chondrocytes were detected in the fracture callus. 34 We detected staining for PCNA of articular chondrocytes close to the joint surface in moderate and severe OA cartilage, especially in cell clusters, further confirming our hypothesis that articular chondrocytes in OA cartilage undergo similar differentiation events as growth plate chondrocytes, including cell proliferation and hypertrophy. In addition, these findings are in agreement with previous studies suggesting that cell clustering in OA cartilage results from proliferative events. 35,36
The final fate of chondrocytes in OA cartilage is not clear yet. Several studies have provided evidence that chondrocytes in the upper zones of OA cartilage undergo programmed cell death (apoptosis). 8,29,37 However, it is still controversial whether OA chondrocytes before undergoing cell death, maintain their articular phenotype, or undergo similar differentiation events as seen in growth plate cartilage. Several studies have demonstrated the expression of alkaline phosphatase, annexin II, annexin V, osteopontin, osteocalcin, and type X collagen that are all considered as markers for hypertrophic and terminally differentiated chondrocytes in OA cartilage. 5-10,26,38-40 In this study we extend these findings by showing that the expression of another marker for hypertrophic chondrocytes, namely annexin VI, is activated in OA chondrocytes. Furthermore, we provide evidence for the first time, that OA chondrocytes not only produce markers of hypertrophic chondrocytes, but also activate expression of the proliferation and early differentiation markers, PCNA and syndecan-3. Taken together, these findings suggest that chondrocytes in OA cartilage indeed undergo a differentiation process similar to growth plate chondrocytes, possibly resulting in terminal differentiation and ultimately in cell death. However, it is possible that OA chondrocytes besides undergoing these terminal differentiation events activate the expression of proteins that are normally not found in growth plate cartilage, including type III collagen. 13 Nonetheless, our and other findings suggest that instead of repairing and producing a functional articular cartilage matrix, differentiated chondrocytes in late stages of OA produce a matrix, which probably closely assembles the matrix produced by hypertrophic and terminally differentiated growth plate chondrocytes.
Acknowledgments
We thank Drs. Bob Kosher, Stephen Moss, and Maurizio Pacifici for providing us with the full-length annexin VI cDNA and syndecan-3 cDNA; and Jerry A. Katzmann for developing the monoclonal antibodies against human alkaline phosphatase that were obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, under contract N01-HD-7-3263 from the National Institute of Child Health and Human Development.
Footnotes
Address reprint requests to Thorsten Kirsch, Ph.D., Penn State College of Medicine, Hershey Medical Center, Department of Orthopaedics and Rehabilitation, H089, 500 University Dr., Hershey, PA 17033-2390. E-mail: tkirsch@psu.edu.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR-43732 and AR-46245 to T. K.).
References
- 1.Kuettner KE, Goldberg V: Osteoarthritic Disorders. 1995:pp 27-45 V Goldberg. Rosemont, American Academy of Orthopaedic Surgeons Symposium Series, Edited by KE Kuettner
- 2.Reginster JY, Pelletier JP, Martel-Pelletier J, Henrotin Y: Osteoarthritis. Clinical and Experimental Aspects. 1999, JP Pelletier, J Martel-Pelletier, Y Henrotin. Berlin, Springer, Edited by JY Reginster
- 3.Matyas JR, Adams ME, Huang D, Sandell LJ: Discoordinate gene expression of aggrecan and type II collagen in experimental osteoarthritis. Arthritis Rheum 1995, 38:420-425 [DOI] [PubMed] [Google Scholar]
- 4.Cs-Szabo G, Melching LI, Roughley PJ, Glant TT: Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum 1997, 40:1037-1045 [DOI] [PubMed] [Google Scholar]
- 5.von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K: Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum 1992, 35:806-811 [DOI] [PubMed] [Google Scholar]
- 6.Hoyland JA, Thomas JT, Donn R, Marriott A, Ayad S, Boot-Handford RP, Grant ME, Freemont AJ: Distribution of type X collagen mRNA in normal and osteoarthritic human cartilage. Bone Miner 1991, 15:151-163 [DOI] [PubMed] [Google Scholar]
- 7.Mollenhauer J, Mok MT, King KB, Gupta M, Chubinskaya S, Koepp H, Cole A: Expression of anchorin CII (cartilage annexin V) in human young, normal adult, and osteoarthritic cartilage. J Histochem Cytochem 1999, 47:209-220 [DOI] [PubMed] [Google Scholar]
- 8.Kirsch T, Swoboda B, Nah H-D: Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage 2000, 8:294-302 [DOI] [PubMed] [Google Scholar]
- 9.von der Mark K: Differentiation, modulation and dedifferentiation of chondrocytes. Rheumatology 1986, 10:272-315 [Google Scholar]
- 10.Matsuzawa T, Anderson HC: Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem 1971, 19:801-808 [DOI] [PubMed] [Google Scholar]
- 11.Nimni M, Deshmukh K: Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science 1973, 181:751-752 [DOI] [PubMed] [Google Scholar]
- 12.Adam M, Deyl Z: Altered expression of collagen phenotype in osteoarthrosis. Clin Chim Acta 1983, 133:25-32 [DOI] [PubMed] [Google Scholar]
- 13.Aigner T, Bertling W, Stoess H, Weseloh G, von der Mark K: Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest 1993, 91:829-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazu A, Nah H-D, Kirsch T, Koyama E, Leatherman JL, Golden EB, Kosher RA, Pacifici M: Syndecan-3 and the control of chondrocyte proliferation during endochondral ossification. Exp Cell Res 1996, 229:126-136 [DOI] [PubMed] [Google Scholar]
- 15.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ: Biology of syndecans: a family of heparan sulfate proteoglycans. Annu Rev Cell Biol 1992, 8:365-393 [DOI] [PubMed] [Google Scholar]
- 16.Geisow MJ, Walker JH, Boustead C, Taylor W: Annexins—a new family of Ca2+ regulated phospholipid-binding proteins. Thorn NA Traiman M Peterson OH eds. Molecular Mechanisms in Secretion. 1988, :pp 598-608 Munskgard Copenhagen [Google Scholar]
- 17.Arispe N, Rojas E, Genge BR, Wu LNY, Wuthier RE: Similarity in calcium channel activity of annexin v and matrix vesicles in planar lipid bilayers. Biophys J 1996, 71:1764-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsch T, Harrison G, Golub EE, Nah H-D: The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem 2000, 275:35577-35583 [DOI] [PubMed] [Google Scholar]
- 19.Anderson HC: Molecular biology of matrix vesicles. Clin Orthop Rel Res 1995, 314:266-280 [PubMed] [Google Scholar]
- 20.Kirsch T, Harrison G, Golub EE: Regulatory roles of zinc in matrix vesicle-mediated mineralization of growth plate cartilage. J Bone Miner Res 2000, 15:261-270 [DOI] [PubMed] [Google Scholar]
- 21.Tajima Y, Kato K, Maruyama S, Hosoi K: In vivo modulation of proliferating cell nuclear antigen in growth plate chondrocytes from normal, hypophysectomized, and growth hormone-treated hypophysectomized rats: a comparative immunohistochemical study with image analysis. J Histochem Cytochem 1996, 44:713-720 [DOI] [PubMed] [Google Scholar]
- 22.Mankin H, Dorfman H, Lippiello L, Zarins H: Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology and metabolic data. J Bone Joint Surg Am 1971, 53:523-537 [PubMed] [Google Scholar]
- 23.Pfander D, Cramer T, Deuerling D, Weseloh G, Swoboda B: Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann Rheum Dis 2000, 59:448-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould SE, Upholt WB, Kosher RA: Characterization of chicken syndecan-3 as a heparan sulfate proteoglycan and its expression during embryogenesis. Dev Biol 1995, 168:438-451 [DOI] [PubMed] [Google Scholar]
- 25.Lawson GM, Katzmann JA, Kimlinger TK, O’Brien JF: Isolation and preliminary characterization of a monoclonal antibody that interacts preferentially with the liver isoenzyme of human alkaline phosphatase. Clin Chem 1985, 31:381-385 [PubMed] [Google Scholar]
- 26.Roach HI: Association of matrix acid and alkaline phosphatases with mineralization of cartilage and endochondral bone. Histochem J 1999, 31:53-61 [DOI] [PubMed] [Google Scholar]
- 27.Garcia RL, Coltrera MD, Gown AM: Analysis of proliferative grade using anti-PCNA cyclin monoclonal antibodies in fixed, embedded tissues. Am J Pathol 1989, 134:733-739 [PMC free article] [PubMed] [Google Scholar]
- 28.Hall PA, Levison DA, Woods AL, Yu CCW, Kellock DB, Watkins JA: Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 1990, 162:285-294 [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto S, Ochs RL, Rosen F, Quach J, Mccabe G, Solan J, Seegmiller JE, Terkeltaub R, Lotz M: Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Nat Acad Sci USA 1998, 95:3094-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einhorn TA, Gordon SL, Siegel SA, Hummel CF, Avitable MJ, Carty RP: Matrix vesicle enzymes in human osteoarthritis. J Orthop Res 1985, 3:160-169 [DOI] [PubMed] [Google Scholar]
- 31.Derfus B, Kranendonk S, Camacho N, Mandel N, Kushnaryov V, Lynch K, Ryan L: Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif Tissue Int 1998, 63:258-262 [DOI] [PubMed] [Google Scholar]
- 32.Matthews MB, Bernstein RM, Franza BR, Garrels JI: Identity of the proliferating cell nuclear antigen and cyclin. Nature 1984, 309:374-376 [DOI] [PubMed] [Google Scholar]
- 33.Bravo R, Flank R, Blundell PA: Cyclin/PCNA is the auxiliary protein of DNA polymerase delta. Nature 1987, 326:515-517 [DOI] [PubMed] [Google Scholar]
- 34.Iwaki A, Jingushi S, Oda Y, Izumi T, Shida J-I, Tsuneyoshi M, Sugioka Y: Localization and quantification of proliferating cells during rat fracture repair: detection of proliferating cell nuclear antigen by immunohistochemistry. J Bone Miner Res 1997, 12:96-102 [DOI] [PubMed] [Google Scholar]
- 35.Kouri JB, Jimenez SA, Quintero M, Chico A: Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage 1996, 106:111-125 [DOI] [PubMed] [Google Scholar]
- 36.Hulth A, Lindberg L, Telhag H: Mitosis in human articular osteoarthritic cartilage. Clin Orthop 1972, 84:197-199 [DOI] [PubMed] [Google Scholar]
- 37.Blanco FJ, Guitian R, Vazquez-Martul E, De Toro FJ, Galdo F: Osteoarthritis chondrocytes die by apoptosis—a possible pathway for osteoarthritis pathology. Arthritis Rheum 1998, 41:284-289 [DOI] [PubMed] [Google Scholar]
- 38.Rees JA, Ali SY: Ultrastructural localisation of alkaline phosphatase activity in osteoarthritic human articular cartilage. Ann Rheum Dis 1988, 47:747-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pullig O, Weseloh G, Ronneberger D, Kakonen S, Swoboda B: Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif Tissue Int 2000, 67:230-240 [DOI] [PubMed] [Google Scholar]
- 40.Pullig O, Weseloh G, Gauer S, Swoboda B: Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol 2000, 19:245-255 [DOI] [PubMed] [Google Scholar]