Abstract
Serine proteinases modulate the interaction of tumor cells with extracellular matrix components during extravasation and metastasis. The serine proteinase tissue kallikrein has been previously demonstrated in several human adenocarcinomas, and we presently report the localization of immunoreactive kallikrein and its mRNA in pancreatic adenocarcinoma. In addition, a synthetic peptide-based inhibitor specific for tissue kallikrein (FE999024) was used in our studies to explore a possible role for kallikrein in cancer cell invasiveness. Matrigel invasion assays were performed with a human breast-cancer cell line, MDA-MB-231, which expresses tissue kallikrein in culture. In the presence of FE999024 invasion through Matrigel was inhibited in a dose-dependent manner to a maximum of 39%. We also developed a novel ex vivo assay in which breast cancer cells are infused into the pulmonary circulation of artificially ventilated explanted rat lungs. At intervals up to 6 hours after infusion pulmonary invasion was quantified by bronchial alveolar lavage to recover human cancer cells from the airspace. Invading cells in the lung interstitium were also quantified after immunohistochemistry with a monoclonal antibody specific for human cytokeratin 18. The synthetic kallikrein inhibitor attenuates breast cancer cell invasion into the airspace by 33% when quantified by lavage recovery and up to 34% as quantified in the lung interstitium by cytokeratin 18 immunostaining. Our results indicate tissue kallikrein may participate in the invasion and metastasis of human adenocarcinomas. The newly developed explanted rodent lung assay should be useful for the study of cancer cells, neutrophils, or other extravasating cells.
Cancer cells exploit serine proteinases to influence the local blood supply, extravasate into and out of vessels, and to migrate through tissue matrix during metastasis. The serine proteinase tissue kallikrein has been localized in human adenocarcinomas and related cell lines from a number of organs including prostate, breast, pituitary, colon, ovary, endometrium, kidney, and esophagus. 1-6 Our results demonstrate its expression in pancreatic adenocarcinoma as well. The best known function of kallikrein is the cleavage of low-molecular weight kininogen to release the kinin peptides bradykinin and lys-bradykinin. Kinins are locally active hormones that mediate classical inflammatory responses such as increased vascular permeability, vasodilation, and increased local blood flow. 7 By binding to endothelial bradykinin B2 receptors, kinin stimulates release of potent vasodilators such as nitric oxide, prostacydin, and endothelium-derived hyperpolarizing factor. 8 Kinin’s ability to vasodilate and increase permeability could enhance a tumor’s supply of nutrients and growth factors. Recently, enhanced vascular permeability in a murine sarcoma tumor model was investigated with icatibant (HOE140), a bradykinin B2 receptor antagonist. Icatibant not only decreased tumor vascular permeability, it also reduced primary tumor growth by 32%. 9
Another important new finding indicated that the kallikrein-kinin system stimulated angiogenesis in an in vivo model. 10 In this study the femoral artery was removed in mice to induce hindlimb ischemia, and kallikrein gene delivery significantly increased capillary density and blood flow to the affected limb. This effect was reversed by bradykinin B2 and B1 receptor antagonists, demonstrating a role for both receptors in the angiogenic response. An increased expression of tissue kallikrein and bradykinin B2 receptors, as well as kinin’s promotion of growth, have previously been shown in cultured microvascular endothelial cells. 11,12 In vivo, the kininogen substrate is abundant in plasma and tissues, 7 so the expression and availability of kallikrein are most likely the rate-limiting factors in kinin production.
Our goal was to investigate whether tissue kallikrein possibly facilitates cancer cell invasiveness. Tissue kallikrein has been localized to neutrophils 13 and has been postulated to be involved in neutrophil extravasation through the vascular endothelium and into tissues. Kinin activation of endothelial B2 receptors has been shown to cause increased intracellular calcium and subsequent endothelial retraction, 14 a critical step in diapedesis. In addition to the enzymatic formation of vasoactive kinins, tissue kallikrein in vitro efficiently activates two matrix-degrading metalloproteases that are important for cancer cell mobility: progelatinase A (72-kd gelatinase, MMP-2) and progelatinase B (92-kd gelatinase, MMP-9). 15,16 Our present results demonstrate that a tissue kallikrein inhibitor suppresses cancer cell invasiveness in in vitro assays and provide evidence indicating tissue kallikrein may enhance cancer cell metastasis. Tissue kallikrein inhibition may be of therapeutic value in the treatment of metastatic disease.
Materials and Methods
Tissue Samples for Immunohistochemistry and in Situ Hybridization
Four-μm sections were cut from formalin-fixed, paraffin-imbedded archival specimens from surgical resections for pancreatic adenocarcinoma. Samples of pancreatic adenocarcinoma were taken from 18 patients after Whipple procedures. Sections had previously been histologically examined and categorized by pathologists as moderately (n = 14) to poorly (n = 4) differentiated adenocarcinoma of ductal origin. Before immunohistochemistry for kallikrein, sections were cleared, rehydrated, and antigen retrieval done by steaming sections for 20 minutes while submerged in 0.1 mol/L of citrate buffer, pH 5.0.
Antibodies for Immunohistochemistry
Rabbit anti-human tissue kallikrein antiserum was used at a dilution of 1:1200 to detect kallikrein and an equal dilution of normal rabbit sera served as negative control. The specificity of the rabbit antiserum against human tissue kallikrein has been previously published. 17 To identify human cancer cells a commercially available monoclonal antibody to cytokeratin 18 was used at a dilution of 1:100 (Novocastra Laboratories, Newcastle on Tyne, UK). The manufacturer reports this monoclonal is specific to human cytokeratin, and by immunohistochemistry and Western blot is not cross-reactive with rat proteins. A monoclonal to von Willebrand factor (DAKO, Carpinteria, CA) was used at a dilution of 1:200 to identify the endothelium of lung vessels. Visualization of primary antibodies was performed using the Vectastain Elite Universal avidin-biotin-peroxidase complex kit as directed by the manufacturer (Vector Laboratories, Burlingame, CA).
For the quantification of interstitial breast cancer cells in rat lungs after the explanted lung invasion assay a double-label immunohistochemistry was performed with the anti-cytokeratin 18 monoclonal used as the first primary antibody, followed by color development with diaminobenzidine tetrahydrochloride plus 1% nickel chloride to darken the chromogenic reaction product to brownish black. After incubating sections in 3% hydrogen peroxide to irreversibly inhibit the peroxidase bound to anti-cytokeratin, the antibody to von Willebrand factor was used as a second primary antibody in the same sections and subsequent development was done with 3-amino-9-ethylcarbazole to produce a red stain. A similar double label was performed with the anti-tissue kallikrein sera as the first primary antibody and anti-cytokeratin 18 as the second primary to co-localize the human-specific marker with tissue kallikrein expressed in human breast cancer cells invading rat lung during the ex vivo assay.
In Situ Hybridization Histochemistry in Tissue Sections of Pancreatic Adenocarcinoma
In situ hybridizations specific for human tissue kallikrein were performed as previously reported. 18,19 A 186-bp human tissue kallikrein cDNA fragment was used to generate digoxygenin-UTP labeled antisense and sense riboprobes. Antisense riboprobe specific for tissue kallikrein was used to identify the kallikrein mRNA. Control sections were incubated with a labeled sense riboprobe. Additional controls were pretreated with RNase A before incubating with an antisense riboprobe.
Cell Line and Reagents
The human breast cancer cell line MDA-MB-231 (ATCC, Rockville, MD) was maintained in Dulbecco’s modified Eagle’s medium (DMEM) plus antibiotic (penicillin 100 U/ml, streptomycin 100 μg/ml) and 10% fetal bovine serum. MDA-MB-231 was chosen for assays because this cell line metastasizes aggressively and has demonstrated high constitutive expression of progelatinases A and B (MMP-2 and MMP-9). 20 MDA-MB-231 cells are estrogen receptor-negative and hormone-independent. The synthesis, characterization, and in vivo use of the peptide-based human tissue kallikrein inhibitor FE999024 (Ferring Research, Southampton, UK), formerly designated CH2856, has been previously described. 21 FE999024 has a Ki of 2.2 nmol/L toward tissue kallikrein and displayed high selectivity for tissue kallikrein (potency ratios in parentheses) over plasma kallikrein (454), trypsin (454), thrombin (16,000), and plasmin (4900).
Enzyme-Linked Immunosorbent Assay for Human Tissue Kallikrein
Levels of human tissue kallikrein in MDA-MB-231 culture media and cell lysates were determined by enzyme-linked immunosorbent assay as previously described. 22
Matrigel Invasion Assay
Matrigel matrix (Becton Dickinson Labware, Franklin Lakes, NJ) was applied and polymerized in 24-well 9-mm inserts containing polyethylene terephtphalate (PET) membranes with 8-μm pores to create invasion chambers as directed by the supplier (Becton Dickinson). MDA-MB-231 cells were grown to near confluence, harvested by trypsinization, which was inactivated with media containing bovine calf serum, and cells were subsequently washed twice in DMEM without added serum or proteinase inhibitor. The cells were suspended in DMEM at 1 × 105/ml. DMEM (0.6 ml) containing 5% fetal bovine serum was added to each plate well as a chemoattractant, and 0.2 ml (2 × 10 4 cells) of cell suspension was added to each insert. Assays were performed with triplicate wells for each condition. In experimental groups the tissue kallikrein inhibitor FE999024 was used at concentrations of 0.05, 0.5, and 5 μmol/L. Cells treated with the inhibitor were preincubated 20 minutes before applying to chambers. The plates of inserts were incubated for 6 hours at 37°C. Noncoated membrane inserts were also seeded to serve as controls. After incubation the chambers were processed and stained as directed by the supplier (Becton Dickinson). The cells were enumerated by counting four fields per chamber under ×100 magnification with the aid of a ruled grid. Data were expressed as percent invasion, ie, the ratio of cells invading through the Matrigel-coated inserts relative to the uncoated control inserts.
Explanted Rodent Lung Invasion Assay
Sprague-Dawley rats weighing between 225 and 275 g (Sprague-Dawley Harlan, Indianapolis, IN) were anesthetized by an intraperitoneal injection of ketamine (9 mg/100g body weight)/xylazine (1 mg/100g body weight). The procedure was performed aseptically and all solutions contained penicillin 100 U/ml and streptomycin 100 μg/ml. The trachea was exposed and a 0.05 × 0.09-inch tygone tube inserted and placed above the bifurcation, secured by ligation, and connected to a model 683 rodent respirator (Harvard Instruments, South Nautik, MA) set at a volume of 1.0 ml of room air and a rate of 60/minute. The chest was opened and the rat heparizined by injection of 300 U of heparin into the left ventricle. The heart and lungs were carefully removed as a single block and placed in sterile phosphate-buffered saline (PBS). The pulmonary artery and left atrium were then catheterized with 0.04 × 0.07-inch tygone tubing and the pulmonary circulation perfused with 50 ml of DMEM entering the pulmonary artery by gravity flow and draining from the left atrium. Bronchial alveolar lavage was accomplished by gently instilling 5 ml of DMEM per lavage through the tracheal tube with a needle small enough to allow backflow through the endotracheal tube. The lungs were manipulated gently and inverted to drain. A total of 30 ml of DMEM was used for lavage. At the beginning of the assay 5 ml of DMEM plus 5% fetal bovine serum was instilled as in lavage to wet the airspace with chemoattractant. Five ml of DMEM with 10% bovine albumin containing 1.0 × 10 5 MDA-MB-231 cells, prepared the same as for invasion assays, were slowly perfused through the pulmonary artery, Lungs were then placed in DMEM maintained at 37°C, and incubated 6 hours while respirated.
Cells within the airspace were recovered by lavage performed with a total of 30 ml of sterile PBS. After airspace recovery, cells were recovered from the vasculature by perfusion of 30 ml of PBS through the left atrium and draining from the pulmonary artery and trachea. Cells were centrifuged and resuspended in 2 ml of PBS, an aliquot was diluted 1:1 with 0.2% trypan blue solution and counted in a hemocytometer. Cells were distinguishable as either large or small in size, and only large cells were counted. Microscopic examination after hematoxylin and eosin (H&E) staining confirmed large cells as morphologically anaplastic and cancerous. Small cells, ie, red blood cells or occasional leukocytes, were ignored. An invasion index was expressed as a percentage and calculated as the number of cells recovered from the airspace divided by the total cells recovered from the airspace and vessels. Recovered cancer cells were >95% viable. Five ml of zinc formalin was instilled into lungs to inflate and fix before submerging lungs completely in fixative for subsequent embedding in paraffin.
As a second index of invasion paraffin sections underwent a double-label immunohistochemistry as described above with antibodies to cytokeratin 18 and von Willebrand factor (factor VIII). The darkly stained human breast adenocarcinoma cells were counted with the aid of a counting grid in 20 random ×100 fields per animal and the interstitial localization of these cells was verified at ×400. Endothelial visualization helped to assure cells lodged in capillaries were not counted as invading cells. Invasion index was expressed as cytokeratin 18-positive cells embedded in interstitial matrix per mm2. Cells were also qualitatively evaluated in sections in which cytokeratin 18 immunohistochemistry was followed by picro-sirius red staining to visualize cancer cells embedded in collagen matrix. As an additional control rat hepatocytes were harvested by collagenase digestion and were washed twice in DMEM to remove enzyme and fetal bovine serum. Hepatocytes were resuspended at 1.0 × 10 5 cells/ml, perfused, incubated, recovered, and quantified in the same manner as breast cancer cells. Visualization of hepatocytes in lung sections was accomplished by periodic acid-Schiff (PAS) staining to demonstrate abundant cytoplasmic glycogen.
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons among groups were made by analysis of variance with Fisher’s PLSD (protected least significant difference). Differences were considered significant at P < 0.05.
Results
Cellular Localization and Expression of Tissue Kallikrein in Pancreatic Adenocarcinoma
Figure 1 ▶ illustrates the immunohistochemical reactivity for human tissue kallikrein typical in pancreatic ductal adenocarcinoma both within the pancreas and at sites of metastasis. Adenocarcinoma samples from 14 of 18 patients with pancreatic cancer were positive for kallikrein. All four samples of poorly differentiated adenocarcinoma were positive. Metastatic cancers in lymph nodes were examined from eight patients (16 nodes), and in samples from six patients they were positive for kallikrein (10 nodes). Low-power magnification typically revealed an overall heterogeneous distribution at the sites of cancer with the most frequent staining occurring apically. However, basolateral staining, as indicated by the smaller arrows in Figure 1, A and C ▶ , was also evident. Cells morphologically identified as neutrophils, lymphocytes, and fibroblasts were frequently positive for tissue kallikrein in inflamed tissue adjacent to adenocarcinoma. The larger arrows in Figure 1, A and B ▶ , point to an area of inflammatory infiltrate that was positive for kallikrein. High-power magnification revealed a distinct, finely granular cytoplasmic reactivity. No reactivity was observed when normal rabbit serum was used as a negative control (not shown). H&E staining demonstrates morphological features, such as atypical malignant glands lined with anaplastic cells, consistent with pancreatic adenocarcinoma of ductal origin.
Figure 1.
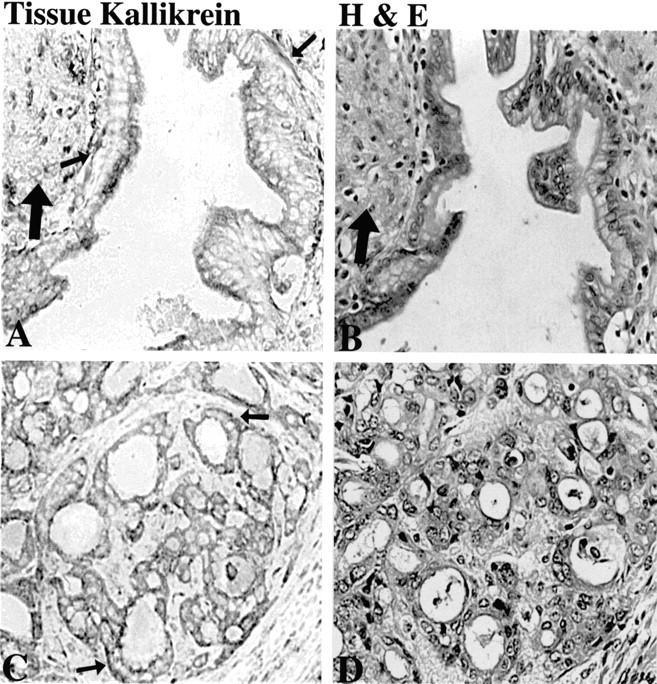
Immunohistochemical localization of tissue kallikrein in human pancreatic adenocarcinoma. Left: Results shown in the column are typical of localization seen using a polyclonal antiserum specific for tissue kallikrein both in the pancreas and at sites of metastasis. Right: The column shows results of H&E staining in adjacent sections. Ductal adenocarcinoma is identified in H&E sections by the presence of irregular glands lined with anaplastic cells. A and B are adjacent sections of pancreatic cancer in the pancreas and C and D are cancer that metastasized to a lymph node. Small arrows point toward basolateral-positive staining for kallikrein in adenocarcinoma cells. Large arrows indicate an area of inflammatory infiltrate in which neutrophils, leukocytes, and fibroblasts were also positively stained. Original magnifications, ×400; sections in A and C are not counterstained.
Figure 2 ▶ shows the in situ localization of tissue kallikrein mRNA using specific digoxygenin-labeled antisense riboprobes. These results indicated that tissue kallikrein mRNA is synthesized by pancreatic adenocarcinoma cells within the pancreas as shown in Figure 2 ▶ . By in situ hybridization, seven samples were positive for kallikrein mRNA, including two samples of adenocarcinoma that had metastasized to lymph nodes. Reactivity was absent when sense riboprobe was hybridized as a negative control. Also, no staining was seen when sections were treated with RNase A before hybridization with the antisense probe (not shown). These results demonstrate the specificity of labeled antisense probe for kallikrein transcript.
Figure 2.
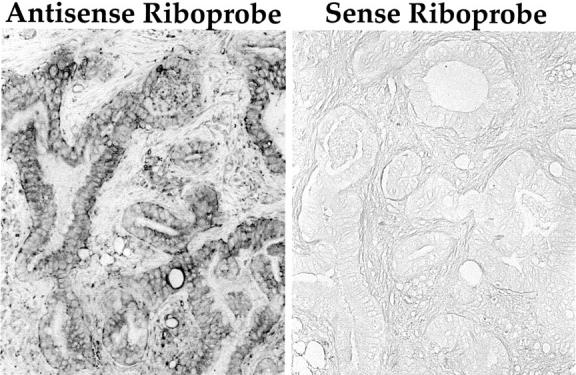
In situ hybridization histochemical localization of tissue kallikrein mRNA in pancreatic adenocarcinoma. Positive reactivity is seen in adenocarcinoma cells when an antisense probe specific for tissue kallikrein was used for hybridization. Also shown is the absence of reactivity when tissue kallikrein sense riboprobe was used as a negative control. Original magnifications, ×400; sections are not counterstained.
Matrigel Invasion Assays
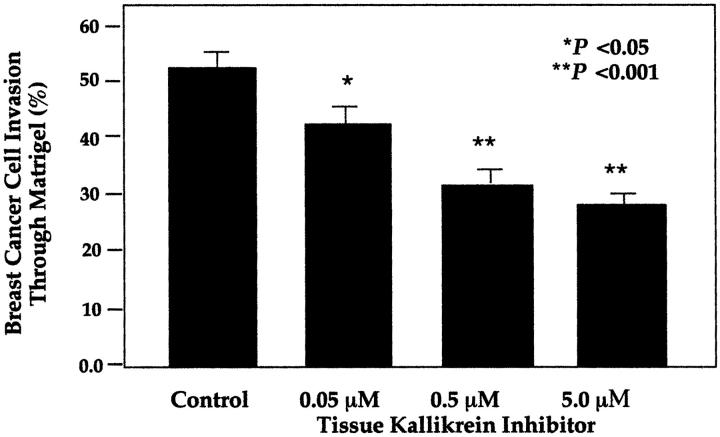
Figure 3 ▶ demonstrates the inhibition of breast cancer cell invasion of Matrigel matrix when the human tissue kallikrein inhibitor FE999024 was used at doses of 0.05, 0.5, and 5 μmol/L. Compared to untreated breast cancer cells, the kallikrein inhibitor maximally suppressed invasion by 39% (P < 0.001 as compared to untreated control) in a dose-dependent manner. MDA-MB-231 breast cancer cells in culture secreted 8 ng of human tissue kallikrein per ml media and had 17 ng of kallikrein per mg of cellular protein as determined by a specific enzyme-linked immunosorbent assay.
Figure 3.
Inhibition of MDA-MB-231 breast cancer cells in the Matrigel invasion assay with the human tissue kallikrein inhibitor FE999024. Assays were performed at least three times, and in triplicate wells. Data represents percentage of cells migrating through the Matrigel-coated filters compared to uncoated filters seeded with the same number of cells, and is expressed as mean ± SEM.
Explanted Rodent Lung Invasion Assay
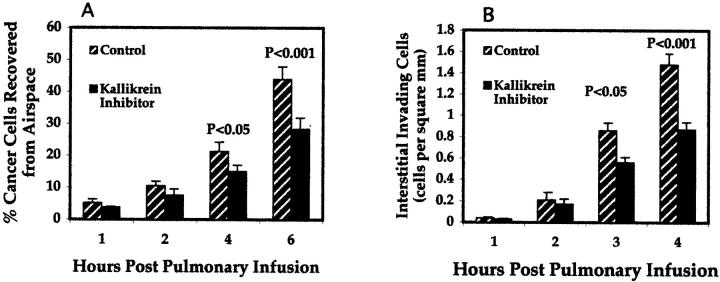
Figure 4 ▶ shows the inhibition of invasion of MDA-MB-231 breast cancer cells in the explanted lung invasion assay as quantified after recovery of breast cancer cells by lavage (Figure 4A) ▶ or identification of breast cancer cells in the interstitium by immunohistochemistry for human cytokeratin 18 (Figure 4B) ▶ . The results are obtained during a time course of 1, 2, 4, and 6 hours after infusion of MDA-MB-231 cells into the pulmonary circulation. The presence of 5 μmol/L of tissue kallikrein inhibitor resulted in a 33% reduction in breast cancer cell invasion into the airspace as quantified by lavage (graph A, 6 hours, P = 0.002), and a 34% reduction of invading cells in the lung interstitium as quantified by cytokeratin immunohistochemistry (graph B, 6 hours, P = 0.02).
Figure 4.
Inhibition of lung invasion by breast cancer cells in explanted rodent lung invasion assay. A: During a time course of 1, 2, 4, and 6 hours after infusion into pulmonary circulation breast cancer cells that had extravasated from the pulmonary vasculature were recovered from the lung airspace by bronchial alveolar lavage. Data are represented as percent lavage-recovered cells compared to total cells recovered by both lavage and from the vasculature. B: Breast cancer cells invading lung interstitium and identified by immunohistochemistry with an anti-cytokeratin 18 monoclonal antibody. Cells were counted with the aid of a counting grid and quantified per square mm. Data are expressed as mean ± SEM (n = 8).
Figure 5 ▶ shows results in sections of the explanted rodent lung invasion assay after four histochemical procedures. Figure 5A ▶ shows an invading breast cancer cell (arrow) identified by anti-cytokeratin 18 immunostaining (black). The red staining for factor VIII localizes vascular endothelium. The double-label procedure was used so that during quantification, cancer cells entrapped in capillaries are not counted as interstitial. Breast cancer cells invading the interstitium were usually seen in close proximity to capillaries, postcapillary venules, or occasionally small arterioles. Only breast cancer cells embedded in interstitial matrix, such as the one shown in Figure 5A ▶ , were counted for quantification (Figure 4B) ▶ .
Figure 5.
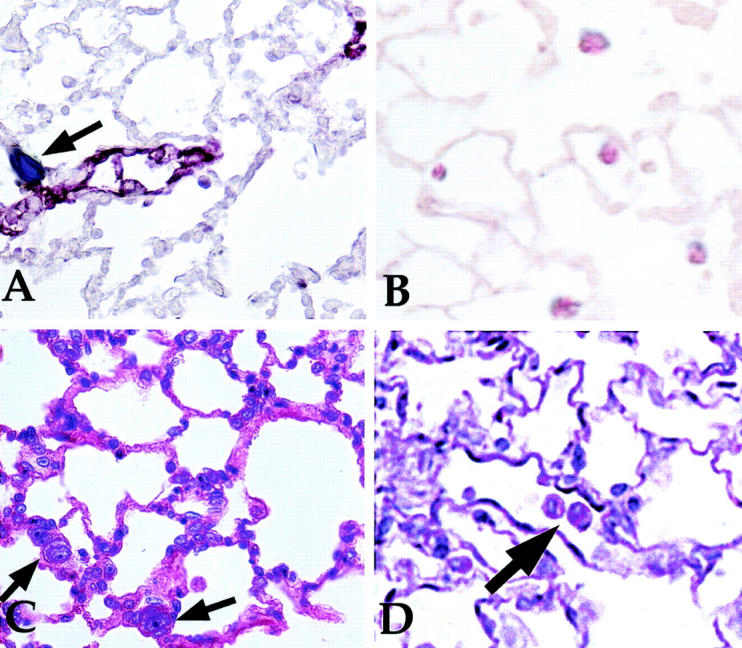
Histological examination of paraffin sections after infusion of human breast cancer cells into pulmonary circulation of explanted rodent lungs. A: A section that underwent double immunostain to demonstrate a human cytokeratin 18-positive breast cancer cell embedded in lung extracellular matrix (arrow, dark stain). Factor VII reactivity appears red and delineates vascular endothelium. B: Tissue kallikrein reactivity (dark stain) co-localized with reactivity for human cytokeratin 18 that was used as a breast cancer cell marker (red stain). C: The relatively large size and irregular nuclei associated with breast cancer cells (arrows) that was evident by H&E staining. PAS staining of lung sections like that in D identified hepatocytes that were used in explanted lung invasion assay as noninvasive control cells. Glycogen in hepatocytes (arrow) appears intensely PAS-positive. Original magnifications, ×600.
Figure 5B ▶ shows the co-localization of immunoreactive tissue kallikrein with cytokeratin 18 as demonstrated by a double-immunostain procedure. Tissue kallikrein reactivity in breast cancer cells appears black and immunostaining for human cytokeratin 18 appears red. The results show tissue kallikrein localizes to human breast cancer cells during the explanted lung invasion assay.
In the H&E-stained section of rat lung at 6 hours after infusion (Figure 5C) ▶ , the cancer cells in the lung interstitium (arrows) are morphologically identified by their large size relative to lung epithelial cells and their large, irregular nuclei. Six hours after infusion, the respiratory epithelium and vascular endothelium appeared intact and healthy morphologically, except in large vessels and airways where some sloughing of cells was seen. Small areas of the lung also appeared to have cells in the lung parenchyma with condensed nuclei, however, the lung extracellular matrix appeared intact. These areas were not used for quantification of interstitial invading cells. These observations indicated that 6 hours represent approaches the maximal time during which the assay can be conducted.
PAS staining was used to identify hepatocytes (large arrow, Figure 5D ▶ ) that were used as noninvasive control cells. Hepatocytes stained by PAS are identifiable by an intensely positive cytoplasm because of their abundant intracellular glycogen. Hepatocytes were not seen in the lung interstitium, although occasional hepatocytes were seen in alveoli, indicating that some leakage was occurring from vessels. By lavage recovery at 4 hours after infusion, 4.2% of hepatocytes were in the airspace and this number increased to 5.8% by 6 hours after infusion. Therefore, the invasion assay must be performed within 6 hours after infusion.
Discussion
Kallikrein is usually associated with kinin production, and kinin has been known for some time to be a major mediator of the vascular inflammatory responses such as vasodilation and increased vascular permeability. The potential roles tissue kallikrein may play in the growth and mobility of cancer cells, however, remain primarily unknown. The recent discovery linking the kallikrein-kinin system with angiogenesis 4 certainly underscores the importance of kallikrein’s potential involvement with cancer. In the present study we explored a potential role for tissue kallikrein in the degradation of the extracellular matrix, which is an important step in preparation for angiogenesis and for cancer cell metastasis. The use of a synthetic tissue kallikrein inhibitor resulted in the significant inhibition of cancer cell invasion in in vitro assays. The suppression of cancer cell invasiveness in Matrigel assays was corroborated by results from a novel assay of cancer cell invasion in explanted rat lungs. These data advance earlier observations that tissue kallikrein in vitro activates metalloproteinases and might therefore be associated with cancer cell mobility. 14,15 In addition, kinin generation may contribute to this effect. Kinin has been previously shown to be a potent stimulus for the release of tissue plasminogen activator in the vasculature, which by a proteolytic cascade can lead to plasmin activation of metalloproteinases and subsequent matrix degradation. 23,24 Our results prompt further investigations into the use of tissue kallikrein inhibitors, such as FE999024, either alone or in combination with other agents, to inhibit cancer growth and metastasis in in vivo models.
The explanted lung assay differs qualitatively from Matrigel-coated chambers in that there is extravasation through the vascular endothelium followed by invasion into a natural extracellular matrix. This assay is more closely related to in vivo metastasis. However, problems did arise in this assay that indicated that a significant level of vascular leakage unrelated to invasion occurs in a time-dependent manner. An increasing number of hepatocytes used as noninvasive control cells were recovered from the airspace and by 6 hours after infusion, swelling of the lungs became apparent and fluid escaped through trachea during vascular recovery. Also at 6 hours after infusion, histological changes, such as sloughing of the endothelium or epithelium, were seen in the large vessels and airways. These observations indicate 6 hours is the maximum time in which the explanted lung invasion assay can be performed as detailed. The smaller vessels, which are the sites of extravasation, appeared morphologically healthy and intact at 6 hours. This is probably because of adequate aeration of the smaller vessels and hypoxia in larger vessels. However, the walls of larger vessels and airways remained intact and for our purposes of examining invasion, the 6-hour assay as performed proved adequate. The explanted lung invasion assay should be generally useful for assaying extravasation and invasion by not only cancer cells, but for neutrophils and other inflammatory cells as well. Neutrophils and other cells found in inflammatory infiltrates can be easily recovered from the pulmonary airspace and vasculature. Additionally, the short time period required for assaying neutrophils and leukocytes should facilitate interaction with a fully functional endothelium.
It is becoming apparent that a significant number of adenocarcinomas express kallikrein. These tumors likely take advantage of kallikrein’s enzymatic properties to influence growth and metastasis. Our results demonstrate the expression and localization of kallikrein in pancreatic adenocarcinoma of ductal origin. A direct comparison with normal pancreatic tissue in our samples proved difficult because pancreatitis was evident in most of the uninvolved pancreas adjacent to adenocarcinoma in our samples. However, the reactivity of morphologically normal pancreas that was present (results not shown) were consistent with earlier studies establishing the expression and localization of tissue kallikrein in acinar cells. 22,26,27 Immunoreactive kallikrein and kallikrein mRNA are not normally present in the ductal epithelium. Our results suggest a possible differential expression in adenocarcinoma derived from ductal epithelium. Not all samples of adenocarcinoma examined were positive for immunoreactive kallikrein, and detection of kallikrein mRNA proved more problematic than the protein. This is probably because of the low level of expression usually seen for proteinases such as kallikrein, coupled with the variations in fixation and processing used on these archival specimens.
Our study supports the hypothesis that tissue kallikrein may contribute to extracellular matrix degradation related not only to metastasis, but potentially during tissue remodeling and repair as well. The observation that kallikrein localizes to inflammatory cells and fibroblasts is consistent with a possible contribution to matrix degradation during inflammation and repair. Potential roles for kallikrein in cancer may also include the processing of novel substrates such as growth factors. For example, kallikrein has been shown to process somatostatin in vitro. 28 Recent studies, mostly related to cancer, have also revealed that novel members of the human kallikrein family exist, 29,30 although their functions remain to be elucidated. The accumulating body of evidence implicating roles for tissue kallikrein and novel members of the kallikrein family in tumor biology certainly warrants confirmation and further investigation. Because the growth and mobility of many soft tissue cancers, including adenocarcinoma, use common proteinases, inhibition of these proteinases may be beneficial for the treatment of these cancers. Combination therapies incorporating proteinase inhibitors may be particularly useful, and may even prove to be effective alternatives to the less specific and much more toxic conventional chemotherapies.
Footnotes
Address reprint requests to Dr. Julie Chao, Department of Biochemistry and Molecular Biology, Medical University of South Carolina, 171 Ashley Ave., Charleston, South Carolina 29425. E-mail: chaoj@musc.edu.
Supported by National Institutes of Health grants HL 44083 and HL 29397.
References
- 1.Chen LM, Richards GP, Chao L, Chao J: Molecular cloning, purification and in situ localization of human colon kallikrein. Biochem J 1995, 307:481-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements J, Mukhtar A: Tissue kallikrein and the bradykinin B2 receptor are expressed in endometrial and prostate cancers. Immunopharmacology 1997, 36:217-220 [DOI] [PubMed] [Google Scholar]
- 3.Jones TH, Figueroa CD, Smith C, Cullen DR, Bhoola KD: Characterization of a tissue kallikrein in human prolactin-secreting adenomas. J Endocrinol 1990, 124:327-331 [DOI] [PubMed] [Google Scholar]
- 4.Rae F, Bulmer B, Nicol D, Clements J: The human tissue kallikreins (KLKs 1–3) and a novel KLK1 mRNA transcript are expressed in a renal cell carcinoma cDNA library. Immunopharmacology 1999, 45:83-88 [DOI] [PubMed] [Google Scholar]
- 5.Dlamini Z, Raidoo D, Bhoola K: Visualisation of tissue kallikrein and kinin receptors in oesophageal carcinoma. Immunopharmacology 1999, 43:303-310 [DOI] [PubMed] [Google Scholar]
- 6.Rehbock J, Buchinger P, Hermann A, Figueroa C: Identification of immunoreactive tissue kallikrein in human ductal breast carcinomas. J Cancer Res Clin Oncol 1995, 121:64-68 [DOI] [PubMed] [Google Scholar]
- 7.Bhoola KD, Figueroa CD, Worthy K: Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmocol Rev 1992, 44:1-80 [PubMed] [Google Scholar]
- 8.Hornig D, Drexler H: Endothelial function and bradykinin in humans. Drugs 1997, 54(Suppl 5):S42-S47 [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Akaike T, Maeda H: Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res 1998, 58:159-165 [PubMed] [Google Scholar]
- 10.Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Bianchini G, Tozzi G, Capogrossi C, Madeddu P: Local delivery of human kallikrein gene accelerates spontaneous angiogenesis in a mouse model of hindlimb ischemia. Circulation 2001, 103:125-132 [DOI] [PubMed] [Google Scholar]
- 11.Plendl J, Snyman C, Naidoo S, Sawant S, Mahabeer R, Bhoola KD: Expression of tissue kallikrein and kinin receptors in angiogenic microvascular endothelial cells. Biol Chem 2000, 381:1103-1115 [DOI] [PubMed] [Google Scholar]
- 12.Morbidelli L, Parenti A, Giovannelli L, Granger HJ, Ledda F, Ziche M: B1 receptor involvement in the effect of bradykinin on venular endothelial cell proliferation and potentiation of FGF-2 effects. Br J Pharmacol 1998, 124:1286-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa CD, MacIver AG, Bhoola KD: Identification of a tissue kallikrein in human polymorphonuclear leucocytes. Br J Haematol 1989, 72:321-328 [DOI] [PubMed] [Google Scholar]
- 14.Oyvin IA, Gaponyuk PY, Volodin VM, Oyvin VI, Tokaryev OY: Mechanism of blood vessel permeability derangement under the influence of permeability factors (histamine, serotonin, kinins) and inflammatory agents. Biochem Pharmacol 1972, 21:89-95 [DOI] [PubMed] [Google Scholar]
- 15.Tschesche H, Michaelis J, Kohnert U, Fedrowitz J, Oberhoff R: Tissue kallikrein effectively activates latent matrix degrading metalloenzymes. Adv Ex Med Biol 1989, 247A:545-548 [DOI] [PubMed] [Google Scholar]
- 16.Desrivieres S, Lu H, Peyri N, Soria C, Legrand Y, Menashi S: Activation of the 92 kDa type IV collagenase by tissue kallikrein. J Cell Physiol 1993, 157:587-593 [DOI] [PubMed] [Google Scholar]
- 17.Proud D, Vio CP: Localization of immunoreactive tissue kallikrein in human trachea. Am J Respir Cell Mol Biol 1993, 8:16-19 [DOI] [PubMed] [Google Scholar]
- 18.Wang DZ, Song Q, Chen LM, Chao L, Chao J: Expression and cellular localization of tissue kallikrein-kinin system in human adrenal gland. Am J Physiol 1996, 271:F709-F716 [DOI] [PubMed] [Google Scholar]
- 19.Chen LM, Song Q, Chao L, Chao J: Cellular localization of tissue kallikrein and kallistatin mRNAs in human kidney. Kidney Int 1995, 48:690-697 [DOI] [PubMed] [Google Scholar]
- 20.Rose DP, Connolly JM, Liu XH: Effects of linoleic acid on the growth and metastasis of two human breast cancer cell lines in nude mice and the invasive capacity of these cell lines in vitro. Cancer Res 1994, 54:6557-6562 [PubMed] [Google Scholar]
- 21.Evans DM, Jones DM, Pitt GR, Ashworth D, De Clerck F, Verheyen F, Szelke M: Synthetic inhibitors of human tissue kallikrein. Immunopharmacology 1996, 32:117-118 [DOI] [PubMed] [Google Scholar]
- 22.Wolf WC, Harley RA, Sluce D, Chao L, Chao J: Cellular localization of kallistatin and tissue kallikrein in human pancreas and salivary glands. Histochem Cell Biol 1998, 110:477-484 [DOI] [PubMed] [Google Scholar]
- 23.Smith D, Gilbert M, Owen WG: Tissue plasminogen activator release in vivo in response to vasoactive agents. Blood 1985, 66:835-839 [PubMed] [Google Scholar]
- 24.Brown NJ, Gainer JV, Stein CM, Vaughan DE: Bradykinin stimulates tissue plasminogen activator release in human vasculature. Hypertension 1999, 33:1431-1435 [DOI] [PubMed] [Google Scholar]
- 25.Figueroa CD, Gonzalez CB, Muller-Esterl W, Bhoola KD: Cellular localization of human kininogens. Agents Actions Supple 1992, 38:617-626 [DOI] [PubMed] [Google Scholar]
- 26.Orstavik TB, Brandtzaeg P, Nustad K, Pierce JV: Immunohistochemical localization of kallikrein in human pancreas and salivary glands. J Histochem Cytochem 1980, 28:557-562 [DOI] [PubMed] [Google Scholar]
- 27.Pinkus GS, Maier M, Seldin DC, ole-MoiYoi O, Austen KF, Spragg J: Immunohistochemical localization of glandular kallikrein in the endocrine and exocrine human pancreas. J Histochem Cytochem 1983, 31:1279-1288 [DOI] [PubMed] [Google Scholar]
- 28.Pimenta DC, Chao J, Chao L, Juliano MA, Juliano L: Specificity of human tissue kallikrein towards substrates containing Phe-Phe pair of amino acids. Biochem J 1999, 339:473-479 [PMC free article] [PubMed] [Google Scholar]
- 29.Yousef GM, Luo LY, Diamandis EP: Identification of novel human kallikrein-like genes on chromosome 19q13.3-q13.4. Anticancer Res 1999, 19:2843-2852 [PubMed] [Google Scholar]
- 30.Yousef GM, Diamandis EP: The expanded human kallikrein gene family: locus characterization and molecular cloning of a new member, KLK-L3 (KLK9). Genomics 2000, 65:184-194 [DOI] [PubMed] [Google Scholar]