Abstract
KAI1 is a metastasis suppressor gene located on human chromosome 11p11.2. It belongs to a structurally distinct family of cell surface glycoproteins. Decreased KAI1 expression has been observed in several common solid epithelial tumors, including prostatic, pancreatic, lung, hepatic, colorectal, ovarian, and esophageal cancers. A recent study also observed frequent loss of KAI1 expression in a number of squamous cell carcinomas of the cervix by immunohistochemical technique. To further confirm whether this gene is altered in this malignancy, we analyzed KAI1 expression in various stages of cervical carcinoma by a molecular method. Total cellular RNA was extracted from 84 primary invasive cervical carcinomas and 6 metastatic or recurrent lesions. cDNA was synthesized and was used for real-time quantitative polymerase chain reaction analysis. The level of KAI1 expression was obtained as the value of threshold cycle (Ct) and was quantitated with a comparative Ct method. In addition, paraffin blocks of the tumors were selected and prepared for immunohistochemical study with an anti-KAI1 polyclonal antibody, C-16. Both the real-time quantitative polymerase chain reaction method and immunohistochemical study revealed a frequent decrease in KAI1 expression in invasive cervical cancers and metastatic or recurrent lesions. However, the reduction in KAI1 was not related to progression of the disease. When tumor cell differentiation was analyzed, poorly differentiated tumors showed a greater decrease in KAI1 expression than well or moderately differentiated tumors (P < 0.001). Histologically, KAI1 loss was observed equally in both squamous cell carcinoma and adeno-/adenosquamous carcinoma. Since down-regulation of KAI1 occurs in both early and late stages of cervical cancer, we suggest that its involvement in the progression of this malignancy is an early event.
With the prevalent use of the Pap smear for cervical cancer screening, the incidence of this malignancy has declined in Western countries. However, carcinoma of the uterine cervix continues to be the leading cause of death from cancer among women in most developing countries. 1 Distant metastasis, especially through lymphatic spreading, is a major cause of death in patients with cervical cancer. For example, the 5-year survival rate after a radical hysterectomy can reach 90% in patients with stage IB and IIA cervical cancer if no lymph node metastasis is present, but the rate falls to around 60% if lymph node metastasis occurs. 2 Therefore the status of lymph node metastasis is one of the most important prognostic factors for patients with cervical cancer.
A major cause of this disease is the infection of cervical epithelium with specific strains of human papilloma virus (HPV), whose E6 and E7 proteins inactivate tumor suppressors p53 and pRb, respectively. 3,4 Pathologically, the HPV-infected cervical epithelial cells present morphological changes and cause dysplastic lesions or intraepithelial neoplasia, the precursors of invasive carcinoma. Therefore HPV infection is thought to function as an initiator in cervical carcinogenesis. 5 Additional genetic changes, however, are required to maintain the malignant phenotype. 6 For example, amplification of c-myc and HER2/neu were reported to play a role in the pathogenesis of cervical cancer. 7,8 Another tumor suppressor gene, fragile histidine triad (FHIT), could also be involved in cervical carcinogenesis. 9,10 The genetic and molecular factors contributing to the progression and metastasis of cervical cancer, however, are poorly understood. Recently a metastasis suppressor gene, KAI1, was identified from the p11.2 region of human chromosome 11 in prostatic cancer. KAI1 was able to suppress metastasis when introduced into metastatic rat prostate cancer cells and its expression was reduced in cell lines derived from metastatic human prostate tumors. 11 In addition, KAI1 protein expression was down-regulated during the progression of human prostate cancers. 12 A similar role of the KAI1 gene has also been suggested for lung and pancreatic cancers, as it has been demonstrated that the down-regulation of the KAI1 at the RNA level is correlated with poor survival in patients with lung cancer 13,14 and is correlated with lymph node and distant metastases in patients with advanced stages of pancreatic cancer. 15,16
Recently, down-regulation of KAI1 has also been found in other common types of human malignancies, including bladder, breast, gastric, hepatocellular, colorectal, ovarian, and esophageal cancers. 17-23 These observations suggest that the presence of KAI1 might influence the ability of cancer cells to metastasize.
Using the immunohistochemical method, a recent study demonstrated that extensive loss of KAI1 staining occurred frequently in squamous cell neoplasms, including cervical carcinoma. 24 To further confirm this finding and investigate the role of KAI1 in the progression of cervical cancer, we analyzed KAI1 expression with a real-time quantitative polymerase chain reaction (PCR) method in various stages of primary cervical carcinomas and metastatic or recurrent lesions. Immunohistochemical staining of KAI1 in these paraffin-embedded tumors was also performed.
Materials and Methods
Tumor Specimens
To avoid contamination with normal tissue, only patients with bulky cervical cancer were selected for tissue sampling. Tumor tissue from 84 primary cervical cancers of various stages (IB, 35 cases; IIA, 3 cases; IIB, 18 cases; III, 22 cases; and IV, 6 cases) was obtained through biopsy or surgical resection. In addition, three metastatic lesions from lymph nodes and three recurrent vaginal tumors were also obtained for study. All of the specimens were cut into two parts. One was taken for pathological examination and the other was stored in RNAlater (Ambion, Austin, TX) at −20°C for subsequent RNA extraction. Microscopically each tissue specimen contained at least 80% tumor cells. Cervical epithelium taken from 22 patients who underwent hysterectomy due to leiomyoma was used as the normal control. All of them had no history of cervical dysplasia and no abnormal finding was noted on their Pap smear. A KAI1-highly expressed colon cancer cell line (SW480) and a KAI1-negatively expressed prostate cancer cell line (PC-3) were used as positive and negative control, respectively. A cervical cancer cell line (HeLa) was also evaluated for KAI1 expression.
RNA Extraction and cDNA Synthesis
Total cellular RNA was isolated from the cervical specimens using the SV Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer’s protocol. RNA was solubilized in RNase free water/ethanol (1:2) and stored at −70°C. First strand cDNA was synthesized from 1 μg of total RNA. Reverse transcription was performed in a volume of 30 μl containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, 2 μmol/L dNTPs, 300 ng of random hexamers (Promega), 600 units of MMLV reverse transcriptase (Life Technologies, Gaithersburg, MD), and 40 units of RNasin (Promega). After denaturation at 95°C for 5 minutes, the reaction was incubated at 42°C for 60 minutes and boiled for 5 minutes.
Real-Time Quantitative PCR
Amplification of KAI1 cDNA was performed using a real-time PCR method and the relative amounts of indicated transcripts were calculated as described previously. 25 Primers and probes used in the PCR were chosen with the assistance of the computer program Primer Expression 1.0 (Perkin-Elmer Applied Biosystems, Foster City, CA). The nucleotide sequences of the primer were as follows: 5′CCGACAAGAGCAGTTTCATCTCT3′ (forward) and 5′AAGACATAGGCCCCCATCCT3′ (reverse). The nucleotide sequence of the probe was 6FAM-CCTGCAAACCTCCTCCAGCTCGCT-TAMRA. The PCR amplification was performed in 25-μl reactions containing 1 μl of first-strand cDNA, 2X Master Mix buffer (TaqMan Universal PCR Master Mix, Perkin-Elmer), 5 μmol/L probe, and 10 μmol/L of forward and reverse primers. The reaction mixture underwent an initial denaturation at 95°C for 10 minutes, then was subjected to 40 PCR cycles with 94°C for 15 seconds, and 60°C for 1 minute. A housekeeping gene 18S rRNA (Perkin-Elmer Applied Biosystems) was used as the internal control. All reactions were performed in the ABI Prism 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems), which detects the signal from the fluorogenic probe during PCR. Samples with high starting copy number of target gene show an increase in fluorescence early in the PCR process, resulting in a low threshold cycle (Ct) number, whereas lower starting copy number results in higher Ct numbers. A comparative Ct method was used to quantitate KAI1 cDNA levels. 26
Immunohistochemistry
The procedure of immunohistochemical staining was described elsewhere. 22 Briefly, paraffin sections of 4 μm were dewaxed, rehydrated, and then heated with citrate buffer (pH 6.0). After being rinsed in phosphate-buffered saline (pH 7.6) and preincubated in serum blocking solution (10% goat serum), slides were reacted with the anti-KAI1 rabbit polyclonal antibody, C-16 (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at a dilution of 1:30. Following incubation with the biotinylated secondary antibody (Histostain-Plus Kit, Zymed, South San Francisco, CA) and peroxidase-labeled streptavidin (Zymed LAB-SA), slides were rinsed again and were developed with the enzyme substrate diaminobenzidine. In each specimen, lymphocytes and endothelial cells in tumor stroma were used as internal positive controls and normal mouse IgG was used as a negative control.
Staining intensity on cell membrane was estimated to be positive when it appeared to be similar to that of lymphocytes and endothelial cells. The staining pattern of KAI1 expression was classified as positive when more then 50% of tumor cells within the tumor tissue were positively stained, decreased if 5 to 50% of tumor cells were positively stained, and negative if less than 5% of tumor cells were positively stained.
Statistical Analysis
One-way analysis of variance test and Scheffé method were used to analyze KAI1 expression in each group of cervical lesions. The relation of KAI1 expression to cell differentiation and histological type were evaluated by t-test and Mann-Whitney U test, respectively. The difference was considered significant when the P value was <0.05.
Results
All these samples were suitable for the real-time quantitative PCR analysis because the Ct values of 18S rRNA in each of the samples were in the range of 18 to 22 cycles, which is requested by the manufacturer’s protocol. The expression of KAI1 was presented as ΔCt (KAI1 Ct −18S rRNA Ct). Real-time quantitative PCR analysis confirmed a high KAI1 expression in the positive control (SW480) with the value of ΔCt = 3.78. In contrast, very low KAI1 expression level was observed in the negative control (PC-3) with the value of ΔCt = 10.36. The cervical cancer cell line HeLa also showed a marked reduction in KAI1, ΔCt = 13.02.
The expression of KAI1 in normal cervical squamous epithelium and various stages of cervical cancer were compared. For statistical analysis, ΔCt was compared in 4 groups of specimens, ie, normal cervical squamous epithelium (n = 22), stages IB and IIA (n = 38), stage IIB (n = 18), and stages III and IV (n = 28). The ΔCt for each group is shown in Table 1 ▶ . Statistically, expression of KAI1 in each cervical cancer group was decreased in comparison with the normal squamous epithelium of the cervix (P < 0.05). However, there was no significant difference in KAI1 expression among the three cervical cancer groups. The sensitivity and specificity for reduced KAI1 expression was 82.1% and 72.7%, respectively, when the cut-off point of ΔCt value was 7, and it was 65.5% and 90.9% when the cut-off point of ΔCt value was 8. Using ΔCt of 8 as the cut-off, frequent reduction of KAI1 expression was seen in each cervical cancer groups with 22 of 38 (60%) stages IB and IIA, 13 of 18 (72%) stage IIB, and 20 of 28 (71%) stages III and IV cases had ΔCt ≧8.
Table 1.
Expression of KAI1 (ΔCt) in Normal Cervical Squamous Epithelium and Various Stages of Cervical Cancer
| Group | N | ΔCt | ||
|---|---|---|---|---|
| Mean | SD | 95% confidence interval | ||
| Normal cervix | 22 | 5.86 | 1.83 | (5.05, 6.67) |
| Stage IB and IIA | 38 | 8.26 | 1.57 | (7.74, 8.78) |
| Stage IIB | 18 | 8.77 | 2.00 | (7.77, 9.76) |
| Stage III/IV | 28 | 9.18 | 2.01 | (8.40, 9.96) |
| Total | 106 | 8.09 | 2.16 | (7.67, 8.51) |
Expression of KAI1 in each cervical cancer group is decreased in comparison with the normal cervix. There is no significant difference in KAI1 expression among the three cervical cancer groups (one-way analysis of variance: F = 15.27, P < 0.001 ***, Scheffé method: P < 0.05*).
To evaluate the association between KAI1 expression and cancer cell differentiation, the 84 primary invasive tumors were classified into two groups according to their cellular differentiation: well and moderately differentiated (WD and MD) versus poorly differentiated (PD). It was found that KAI1 expression was significantly decreased in poorly differentiated tumors compared to that of well and moderately differentiated tumors (P < 0.001) (Table 2) ▶ . When histological type was analyzed, there was no significant difference in KAI1 expression between the squamous cell carcinoma (SCC) group and the adenocarcinoma and adenosquamous carcinoma (AC and AS) group (Table 3) ▶ .
Table 2.
Expression of KAI1 (ΔCt) in Relation to Cellular Differentiation
| Group | N | ΔCt | ||
|---|---|---|---|---|
| Mean | SD | 95% CI | ||
| Well differentiated and moderately differentiated | 41 | 7.96 | 1.59 | (7.46, 8.46) |
| Poorly differentiated | 43 | 9.35 | 1.83 | (8.79, 9.92) |
t-test; P < 0.001***.
Table 3.
Expression of KAI1 (ΔCt) in Relation to Histological Type
| Group | N | ΔCt | |
|---|---|---|---|
| Mean | SD | ||
| Squamous cell carcinoma | 69 | 8.74 | 1.78 |
| Adenocarcinoma, and adenosquamous carcinoma | 15 | 8.29 | 1.66 |
Mann-Whitney U test: P = 0.268.
Of the 6 metastatic/recurrent lesions, decreased KAI1 expression was noted in 5 cases with ΔCt from 8.7 to 10.48. No apparent decrease in KAI1 (ΔCt = 6.33) was noted in one lesion taken from a vaginal recurrence. Immunohistochemical stain of the recurrent tumor was also positive (Figure 1D) ▶ .
Figure 1.
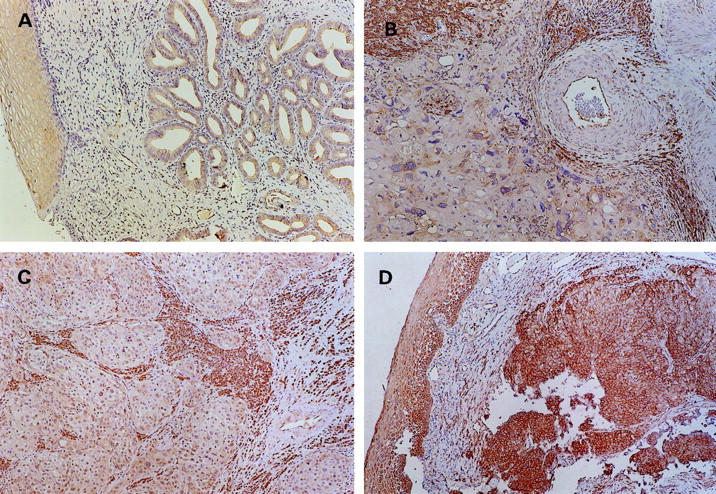
Immunohistochemical staining of KAI1 in cervical carcinomas (×100). A: A well differentiated adenocarcinoma showing positive KAI1 expression over the cell membrane in more than 50% tumor cells (ΔCt = 6.6). B: Negative KAI1 expression (<5% tumor cells were positively stained) in a poorly differentiated squamous cell carcinoma (ΔCt = 10.08); note the positively stained stromal cells, lymphocytes, and vascular endothelium. C: Lymph node metastasis from a squamous cell carcinoma with negative KAI1 expression (ΔCt = 10.37). D: Positive KAI1 expression in a recurrent squamous cell carcinoma (ΔCt = 6.33); left, normal vaginal squamous epithelium.
The result of immunohistochemical study showed a good correlation with that of real-time quantitative PCR analysis (Table 4) ▶ . A decreased pattern of KAI1 staining presented in the majority of cervical cancers (57/90) and 12 of the 16 tumors showing markedly decreased KAI1 in real-time PCR (ΔCt ≧10) had a negative staining pattern. Examples of the staining are shown in Figure 1 ▶ .
Table 4.
KAI1 Expression of Cervical Cancer by Immunohistochemical Staining and Real-Time Quantitative PCR (ΔCt)
| ΔCt | Immunohistochemical staining | ||
|---|---|---|---|
| Negative | Decreased | Positive | |
| <8 | 0 | 12 | 18 |
| 8–10 | 2 | 41 | 1 |
| ≧10 | 12 | 4 | 0 |
Negative: <5%, decreased: 5–50%, positive: >50% tumor cells were positively stained.
Discussion
It is known that in the oncogenic process some genetic changes would result in an imbalance of cellular growth regulation, and this will lead to uncontrolled proliferation. However, unrestrained growth does not, by itself, result in invasion and metastasis. It is clear now that metastasis requires additional genetic changes to occur. 27 Loss of function of the metastasis suppressor gene(s) is an important event during the progression of a tumor cell from a nonmetastatic to a metastatic phenotype. 28 KAI1 is a recently identified metastasis suppressor gene for prostatic cancer. The expression of KAI1 was reduced in human cell lines derived from metastatic prostate tumors as compared with its expression in normal prostate tissue. 11 It was noted later that KAI1 protein expression was down-regulated during the progression of human prostate cancers. 12
KAI1 specifies a protein of 267 amino acids. It belongs to a structurally distinct family of cell surface glycoproteins, the transmembrane 4 superfamily (TM4SF), which all have four hydrophobic transmembrane domains and one large extracellular N-glycosylated domain. Because of their membrane localization and extensive glycosylation, it is suggested that these proteins may function via cell-cell and cell-extracellular matrix interactions, thereby potentially influencing the ability of cancer cells to invade and to metastasize. 11 Transcript of KAI1 was detected in all of the human tissues tested, including various surface epithelia and activated lymphocytes. 11,24,29 In addition, the KAI1 coding sequence is conserved in various animals. These findings suggest that KAI1 has an essential biological function in evolution. 11
Using an immunohistochemical study, Geradts et al 24 noted a loss of KAI1 expression in 12 of 14 cervical squamous cell carcinomas and suggested an important role of the gene in the suppression of invasion in this malignancy.
In the present study, we further confirmed that KAI1 is down-regulated in cervical carcinoma by a real-time quantitative PCR method. Because the high and low KAI1 expression levels were seen in positive and negative control cell lines (SW480 and PC-3), respectively, this recently developed methodology is confirmed to be a reliable and practical technique for determining KAI1 mRNA expression. To exclude contamination of non-neoplastic cells that may express KAI1 at high levels, only bulky cervical tumors were used in this study and the tumor content in each specimen was more than 80% as confirmed by pathological examination. The data presented in this study should reflect properly the level of KAI1 expression in cervical cancers.
The reduction of KAI1 expression was observed in all stages of cervical cancer with no significant difference between different stages (Table 1) ▶ . This finding indicates that down-regulation of KAI1 in cervical cancer, unlike in prostate, lung, and pancreatic cancers, is not only limited to late stage or metastatic/recurrent tumors. A similar finding was also noted in colorectal and ovarian carcinomas. 21,22 Moreover, loss of KAI1 protein expression in a subset of high-grade squamous intraepithelial lesions of the cervix has been reported. 24 Therefore, our finding is consistent with the hypothesis that down-regulation of KAI1 is an important event in the progression of squamous cell carcinoma from the in situ to the invasive stage. In fact, it has been suggested that loss of CD82 (KAI1) is crucial for stromal invasion in squamous cell carcinoma of the skin. 30
In this study we did not perform mutational analysis of KAI1 in the cervical tumors. It is generally believed that mutation of the gene is rare in human tumors that revealed expression reduction. Although methylation of the 5′ promoter region has been speculated as a possible mechanism for the down-regulation of KAI1, 24 another study found no evidence for hypermethylation of the CpG island within the promoter region. 31 In addition, it was found that the tumor suppressor gene p53 can directly activate the KAI1 gene by interacting with its 5′ promoter region. 32 Whether HPV induced inactivation of p53 in cervical cancer plays a role in the down-regulation of KAI1 is recently investigated by Schindl et al. 33 In their study, KAI1 down-regulation was found by immunohistochemistry in 68% of 67 cervical cancer patients. However, the HPV infection rate was 91%. They concluded that KAI1 down-regulation in cervical cancer seems independent of HPV-E6 induced p53 inactivation. The mechanism of KAI1 down-regulation is thus still to be determined.
We also observed that poorly differentiated tumors showed further reduced expression than did well and moderately differentiated tumors. This finding is in agreement with the clinical observation that poorly differentiated cervical cancers are more apt to metastasize than well or moderately differentiated tumors. In addition, we noted that loss of KAI1 expression occurred in both the squamous cell carcinoma and adeno-/adenosquamous carcinoma with no difference in extent of expression loss.
Although five of the six metastatic/recurrent tumors displayed decreased expression of KAI1, one tumor from a vaginal recurrence did not show apparent KAI1 reduction. In addition, four of the 28 cases of stages III and IV also revealed a preserved KAI1 level with the value of ΔCt ≦ 7. The higher KAI1 expression in these tumors needs further investigation. Adachi et al suggested that an aberrant glycosylation instead of down-regulation may occur at the N-linked glycosylation sites of KAI1 protein, resulting in the loss of the protein’s function. 13 One study also noted that KAI1 can be re-expressed by addition of nerve growth factor in some prostate cancer cell lines which are originally KAI1 negative. 34 However, whether nerve growth factor is expressed in cervical cancer remains to be investigated. Finally, other metastasis suppressor gene(s) than KAI1 may also be involved in the metastasis of cervical cancer. For example, some studies have shown that reduced expression of nm23 was associated with lymph node metastasis in cervical cancer. 35,36 It is more likely that more than one metastasis suppressor gene are involved in cervical cancer metastasis.
Since all of the tumor specimens analyzed in this study were taken within the last 2 years, the follow-up time is too short to provide survival information. However, KAI1 expression may not have prognostic significance in cervical cancer, because down-regulation in both early and late stages of tumor may preclude it as an effective prognostic factor.
In conclusion, using a real-time quantitative PCR method, we confirmed that KAI1 was frequently down-regulated in invasive, metastatic, and recurrent cervical carcinomas. Because decreased KAI1 expression is seen in early stages of cervical cancer and it has been shown in a subset of high-grade cervical dysplasias, 24 we speculate that down-regulation of this gene may occur as an early event in the development of cervical cancer.
Footnotes
Address reprint requests to Fu-Shing Liu, M.D., Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Taichung Veterans General Hospital, Taichung, Taiwan 40705, Republic of China. E-mail: fsliu@vghtc.vghtc.gov.tw.
Supported by grant TCVGH-896401C from the Taichung Veterans General Hospital (to F. S. L.).
References
- 1.Morris M, Tortolero-Luna G, Malpica A, Baker VV, Cook E, Johnson E, Follen-Mitchell M: Cervical intraepithelial neoplasia and cervical cancer. Obstet Gynecol Clin North Am 1996, 23:347-410 [PubMed] [Google Scholar]
- 2.Hacker NF: Cervical cancer. Berek JS Hacker NF eds. Practical Gynecologic Oncology. 2000, :pp 345-405 Lippincott Williams and Wilkins, Philadelphia [Google Scholar]
- 3.Scheffner M, Werness BA, Huibregste JM, Levine AJ, Howley PM: The E6 oncoprotein encoded by human papilloma virus types 16 and 18 promotes the degradation of p53. Cell 1990, 63:1129-1136 [DOI] [PubMed] [Google Scholar]
- 4.Dyson N, Howley P, Munger K, Harlow E: The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243:934-937 [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H: HPV in the pathogenesis of anogenital cancer. Virology 1991, 184:9-13 [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H: Are human papillomavirus infections not necessary or sufficient causal factors for invasive cancer of the cervix? Int J Cancer 1995, 63:315-316 [DOI] [PubMed] [Google Scholar]
- 7.Baker VV, Hatch KD, Shingleton HM: Amplification of the c-myc proto-oncogene in cervical carcinoma. J Surg Oncol 1988, 39:225-228 [DOI] [PubMed] [Google Scholar]
- 8.Mitra AB, Murty VV, Pratap M, Sodhani P, Chaganti RS: ERBB2 (HER2/neu) oncogene is frequently amplified in squamous cell carcinoma of the uterine cervix. Cancer Res 1994, 54:637-639 [PubMed] [Google Scholar]
- 9.Birrer MJ, Hendricks D, Farley J, Sundborg MJ, Bonome T, Walts MJ, Geradts J: Abnormal fhit expression in malignant and premalignant lesions of the cervix. Cancer Res 1999, 59:5270-5274 [PubMed] [Google Scholar]
- 10.Yoshino K, Enomoto T, Nakamura T, Sun H, Ozaki K, Nakashima R, Wada H, Saitoh J, Watanabe Y, Noda K, Murata Y: FHIT alterations in cancerous and non-cancerous cervical epithelium. Int J Cancer 2000, 85:6-13 [DOI] [PubMed] [Google Scholar]
- 11.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC: KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995, 268:884-886 [DOI] [PubMed] [Google Scholar]
- 12.Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT: Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res 1996, 56:4387-4390 [PubMed] [Google Scholar]
- 13.Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M: Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res 1996, 56:1751-1755 [PubMed] [Google Scholar]
- 14.Adachi M, Taki T, Konishi T, Huang CL, Higashiyama M, Miyake M: Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J Clin Oncol 1998, 16:1397-1406 [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M: KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res 1996, 56:4876-4880 [PubMed] [Google Scholar]
- 16.Friess H, Guo XZ, Berberat P, Graber HU, Zimmermann A, Korc M: Reduced KAI1 expression in pancreatic cancer is associated with lymph node and distant metastases. Int J Cancer 1998, 79:349-355 [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Yang JL, Markovic B, Jackson P, Yardley G, Barrett J, Russell PJ: Loss of KAI1 messenger RNA expression in both high-grade and invasive human bladder cancers. Clin Cancer Res 1997, 3:1045-1049 [PubMed] [Google Scholar]
- 18.Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M: Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol 1998, 153:973-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinoda Y, Adachi Y, Takaoka A, Mitsuuchi H, Satoh Y, Itoh F, Kondoh Y, Imai K: Decreased expression of the metastasis suppressor gene KAI1 in gastric cancer. Cancer Lett 1998, 129:229-234 [DOI] [PubMed] [Google Scholar]
- 20.Guo XZ, Friess H, Di Mola FF, Heinicke JM, Abou-Shady M, Graber HU, Baer HU, Zimmermann A, Korc M, Buchler MW: KAI1, A new metastasis suppressor gene, is reduced in metastatic hepatocellular carcinoma. Hepatology 1998, 28:1481-1488 [DOI] [PubMed] [Google Scholar]
- 21.Lombardi DP, Geradts J, Foley JF, Chiao C, Lamb PW, Barrett JC: Loss of KAI1 expression in the progression of colorectal cancer. Cancer Res 1999, 59:5724-5731 [PubMed] [Google Scholar]
- 22.Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ESC, Hung MJ: Frequent down-regulation and lack of mutation of the KAI1 metastasis suppressor gene in epithelial ovarian carcinoma. Gynecol Oncol 2000, 78:10-15 [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Kato H, Shitara Y, Yoshikawa M, Tajima K, Masuda N, Shouji H, Tsukada K, Nakarama T, Kuwano H: Mutation and expression of the metastasis suppressor gene KAI1 in esophageal squamous cell carcinoma. Cancer 2000, 89:955-962 [DOI] [PubMed] [Google Scholar]
- 24.Geradts J, Maynard R, Birrer MJ, Hendricks D, Abbondanzo SL, Fong KM, Barrett JC, Lombardi DP: Frequent loss of KAI1 expression in squamous and lymphoid neoplasms. Am J Pathol 1999, 154:1665-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heid CA, Stevens J, Livak KJ, Williams PM: Real time quantitative PCR. Genome Res 1996, 6:986-994 [DOI] [PubMed] [Google Scholar]
- 26.Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB: Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem 2000, 278:175-184 [DOI] [PubMed] [Google Scholar]
- 27.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa T, Ichikawa Y, Isaacs JT: Genetic factors and metastatic potential of prostatic cancer. Cancer Surv 1991, 11:35-42 [PubMed] [Google Scholar]
- 29.Huang CL, Taki T, Adachi M, Yagita M, Sawada S, Takabayashi A, Inufusa H, Yoshie O, Miyake M: MRP-1/CD9 and KAI1/CD82 expression in normal and various cancer tissues. Int J Oncol 1997, 11:1045-1051 [DOI] [PubMed] [Google Scholar]
- 30.Okochi H, Kato M, Nashiro K, Yoshie O, Miyazono K, Furue M: Expression of tetra-spans transmembrane family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and neoplastic human keratinocytes: an association of CD9 with α3β1 integrin. Br J Dermatol 1997, 137:856-863 [PubMed] [Google Scholar]
- 31.Jackson P, Millar D, Kingsley E, Yardley G, Ow K, Clark S, Russell PJ: Methylation of a CpG island within the promoter region of the KAI1 metastasis suppressor gene is not responsible for down-regulation of KAI1 expression in invasive cancers or cancer cell lines. Cancer Lett 2000, 157:169-176 [DOI] [PubMed] [Google Scholar]
- 32.Mashimo T, Watabe M, Hirota S, Hosobe S, Miura K, Tegtmeyer PJ, Rinker-Shaeffer CW, Watabe K: The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc Natl Acad Sci USA 1998, 95:11307-11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindl M, Bachtiary B, Dreier B, Birner P, Latinovic L, Karner B, Breitenecker G, Oberhuber G: Impact of human papillomavirus infection on the expression of the KAI1 metastasis suppressor protein in invasive cervical cancer. Cancer Lett 2001, 162:261-266 [DOI] [PubMed] [Google Scholar]
- 34.Sigala S, Faraoni I, Botticini D, Paez-Pereda M, Missale C, Bonmassar E, Spano P: Suppression of telomerase, reexpression of KAI1, and abrogation of tumorigenicity by nerve growth factor in prostate cancer cell lines. Clin Cancer Res 1999, 5:1211-1218 [PubMed] [Google Scholar]
- 35.Marone M, Scambia G, Ferrandina G, Giannitelli C, Benedetti-Panici P, Iacovella S, Leone A, Mancuso S: Nm23 expression in endometrial and cervical cancer: inverse correlation with lymph node involvement and myometrial invasion. Br J Cancer 1996, 74:1063-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarac E, Ayhan A, Ertoy D, Tuncer ZS, Yasui W, Tahara E, Ayhan A: nm23 expression in carcinoma of the uterine cervix. Eur J Gynaecol Oncol 1998, 19:312-315 [PubMed] [Google Scholar]


