Abstract
Studies of molecular, cellular, and pathophysiological parameters in endometriosis are primarily hampered by a lack of in vitro model systems, such as endometriotic cell lines. To overcome this we successfully established cell lines from peritoneal endometriotic biopsies and characterized them at the molecular and cellular level. Two types of cells could be transformed: one exhibiting stromal cell features (cytokeratin/E-cadherin-negative), the other epithelial-like (cytokeratin-positive/E-cadherin-negative, invasive in vitro). Using a Matrigel assay the epithelial-like cell lines proved as invasive as metastatic carcinoma cells, possibly through the influence of N-cadherin implicated as a path-finding cadherin allowing cellular invasion and migration in both normal and pathophysiological processes. Our results support the idea that endometriosis, although not neoplastic, shares features with malignant cells and that metastasis in endometriosis may include mechanisms proposed for micrometastasis in cancer. Thus our cell lines will not only be useful tools for analyzing molecular and cellular events relating to endometriosis, but may also represent a paradigm for invasion and metastasis in general.
Endometriosis is histologically defined by the presence of endometrium-like glands and stroma outside the uterus. This tissue is mostly found in the pelvic cavity, primarily falling into three different categories: ovarian endometriomas; small peritoneal lesions; and deep infiltrating, often rectovaginal or gut lesions. More rarely, endometriosis is also found in organs such as the lung, lymph nodes, or pancreas. Currently, the favored paradigm for the establishment of endometriosis is the implantation theory 1 that postulates that viable endometrial cells are transported to the peritoneal cavity by retrograde menstruation, adhere to the peritoneal wall, proliferate, and form lesions. Ovarian endometriomas and deep infiltrating endometriosis are also thought to arise from retrograde menstruation, whereas lesions at other locations may depend on lymphatic or hematogenic spreading. Furthermore, endometriosis may also arise via metaplasia in some cases. 2,3 Although it is well accepted that endometriotic lesions are composed of stroma and epithelial glandular cells, little is known about the development and characteristics of the cell types contributing to the pathogenesis of endometriosis.
Accompanying the appearance of endometriotic tissue, changes in the peritoneal environment are observed. In the peritoneal fluid in particular, the number of activated macrophages increases 4 and the chemotactic activity of immune system cells is higher compared to controls. 5-8 The levels of cytokines, 9-14 growth factors, 15-17 and prostaglandins 18-20 are elevated whereas angiogenic activity in vivo is increased in patients with endometriosis in comparison to females without the disease. 21-23 It still remains an open question as to what extent the peritoneal environment influences the establishment and/or progression of endometriosis .
Previous studies from our group suggested that the cellular composition of endometriotic lesions might be more heterogeneous than anticipated. This is partly based on the observation that peritoneal lesions contain two types of cytokeratin-expressing epithelial cells. One of them is E-cadherin-positive and not invasive in an in vitro collagen invasion assay. The other, less frequent cell type is E-cadherin-negative and invasive in the same assay. 24 In addition, nonepithelial stroma cells can also be identified. These immunohistological observations are in agreement with analyses showing that the three different cell types identified in vivo are also found in primary endometriotic cell cultures. 24, Thus, it seems reasonable to assume that at least these three types of cells play a role during the establishment and/or progression of the disease. This does not exclude other, as yet unidentified, cell types or developmental stages of the known cells as being also involved.
Our understanding of the etiology and pathogenesis of endometriosis might increase greatly were it possible to study the contributing cell types in more detail. This would require a set of different endometriotic cell lines established from cells of endometriotic lesions that at least partially retain their in vivo phenotype. However, in contrast to tumor cell lines easily established from biopsies without further manipulation, this is impossible with cells isolated from endometriotic lesions that need to be transformed. Constitutive expression of the DNA tumor virus SV40 T-antigen is the most effective means to immortalize primary human cells, or at least to prolong their life span. 25-27 One limitation is that SV40 T-antigen transformation preferentially hits actively proliferating cells, or at least cells that still have the capacity to enter the cell cycle. T antigen can interact with cellular proteins controlling progression through the cell cycle such as the pocket proteins Rb-1, p107, and p130 28-30 as well as p53. 31,32 This subsequently blocks exit from the cell cycle, prevents cell cycle progression-induced apoptosis, and keeps the cell in a proliferative state. Eventually, and irrespective of whether they express SV40 T-antigen or not, human cells reach a point where their proliferative potential is exhausted. In the subsequent period of senescence the cells no longer proliferate but remain viable. This event is called “crisis.” Cells that escape from the crisis are then capable of unlimited proliferation, giving rise to true immortal cell lines. 33,34
In this study we show that it is possible to establish endometriotic cell lines in a reproducible manner using SV40 T-antigen. The cell lines obtained have a prolonged life span before they enter senescence. Some of them escape from the crisis resulting in apparently immortal lines. These cell lines were characterized in terms of immunological markers and functional properties.
Materials and Methods
Endometriotic Tissue and Preparation of Primary Endometriotic Cells
Endometriotic tissue samples were obtained from 64 patients undergoing laparoscopy for unexplained infertility, known endometriosis, or lower abdominal pain. Biopsies were taken during the proliferative phase of the menstrual cycle. Our study was approved by the Local Ethic Committee. In the operation theater, biopsy material was transferred immediately after laparoscopy into phosphate-buffered saline (PBS) containing 0.25% collagenase A and 1.5 U/ml dispase (both Roche, Grenzach-Wyhlen, Germany) and digested at room temperature for 6 hours. Red blood cells and debris were removed by centrifugation on a 45% (v/v) Percoll cushion (Amersham-Pharmacia, Freiburg, Germany). Dissociated cells were plated onto the appropriate tissue culture vessels and maintained in Dulbecco’s modified Eagle’s medium containing antibiotics and 10% fetal calf serum (FCS). For growth of endometriotic cells different batches of FCS were screened: some batches of FCS did not support optimal growth of endometriotic cells.
In Situ Electroporation of Endometriotic Cells
Primary endometriotic cells were prepared as described above and plated onto transwell filter chambers with a diameter of 24 mm and a pore size of 0.4 μm (Corning-Costar, Bodenheim, Germany), allowed to attach, and maintained for 1 to 3 days to allow for adaptation to the culture conditions and some rounds of cell division before electroporation. Cell monolayers were electroporated on the transwell filter using the Equibio in situ electroporation equipment (Peqlab, Erlangen, Germany). Electroporation parameters were set to 70 to 100 V and 150 μF. For expression of SV40 T-antigen a plasmid was used containing the SV40 virus with a deletion of the virus genome late region (bp 1782 to 2533). 35 After electroporation the medium was changed immediately, the electroporated cells were maintained in Dulbecco’s modified Eagle’s medium/10% FCS and the medium was changed at 3- to 4-day intervals. T antigen-expressing cultures started to grow after 3 to 10 days and were expanded. SV40 T antigen-expressing cells were selected by their ability to overgrow the nontransfected cells. As soon as increased growth was observed, cells were passaged to a 15-cm culture flask, followed by passage to a 75-cm culture flask. At this time point, indirect immunofluorescence analysis for expression of T antigen was performed.
Cell Lines and Cell Culture
The human urinary bladder cell lines RT112 and EJ28 were described previously. 24 MCF-7 cells were obtained from ECACC (Salisbury, UK). Ishikawa cells were a gift of H. Hess-Stumpp (Schering AG, Berlin, Germany). All cell lines were cultured in Dulbecco’s modified Eagle’s medium containing antibiotics and 10% FCS. All culture reagents were purchased from Life Technologies (Karlsruhe, Germany).
Antibodies
A hybridoma cell line producing antibodies against a C-terminal epitope of SV40 T-antigen (pAb 101) was obtained from ECACC (Salisbury, UK). Antibodies were purified from cell culture supernatants of cells grown in a bio-perm bioreactor (In Vitro Systems, Göttingen, Germany) using the MAPS antibody purification system (Amersham-Pharmacia, Freiburg, Germany). The following antibodies were purchased: clone pAb 419, a monoclonal antibody against an N-terminal epitope of SV40 T antigen (Dianova, Hamburg, Germany); polyclonal rabbit antibodies against keratins of human epidermis or human factor VIIIa or human CD3 antigen and monoclonal antibodies against human desmin, human CD31, CD45, CD68 antigen (DAKO, Hamburg, Germany); a monoclonal antibody against human vimentin (Progen, Heidelberg, Germany); clone 5H9, a monoclonal antibody against human E-cadherin (Sanbio, Beutelsbach, Germany); polyclonal rabbit antibodies against human calretinin and clone 3B9 a monoclonal antibody against a cytoplasmic epitope of N-cadherin (Zymed, Berlin, Germany); clone TE7, a monoclonal antibody against mesoderm-derived tissue (Harlan Sera-Lab, Loughborough, UK); polyclonal rabbit antibodies against the C-terminal part of most cadherins (pan-cadherin) and monoclonal antibody (clone GC-4) against an N-terminal epitope of N-cadherin (Sigma, Deisenhofen, Germany); antibodies against β-catenin (clone 14) and p120ctn (clone 98) (Signal Transduction Laboratories, Lexington, KY); clone 7.1 and 13.1 both monoclonal antibodies against the green fluorescent protein (Roche); Anti-Xpress antibody (Groningen, The Netherlands). Fluorochrome-conjugated (Alexa 499 or Alexa 564) species-specific secondary antibodies were obtained from Molecular Probes (Leiden, The Netherlands).
Immunofluorescence Analysis
For immunofluorescence staining cells were plated onto glass coverslips or were stained directly on the microporous membranes (Becton-Dickinson, Heidelberg, Germany) used for the invasion assay. Briefly cells were fixed in 4% paraformaldehyde and permeabilized by treatment with 0.2% Triton X-100 (both in PBS). Unspecific antibody binding was blocked with PBS/10% FCS. The first antibody was diluted in PBS/10% FCS and incubated with the cells for 1.5 hours at room temperature or overnight at 4°C. Binding of the primary antibody was detected by species-specific fluorochrome-conjugated antibodies. Control stainings in the absence of primary antibody confirmed the specificity of the immunolabeling. Nuclear staining was visualized using Hoechst dye no. 33258 (Sigma). Fluorescence was detected using a Carl Zeiss (Göttingen, Germany) Axiophot microscope or a Leica Microsystems (Heidelberg, Germany) TCS NT confocal laser scanner system. Pictures were taken with ×40 or ×100 objectives and were further processed using Adobe Photoshop (Adobe Systems, Unterschleissheim, Germany).
Western Blot Analysis
Monolayers of cells were washed with PBS and total protein was extracted with RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, 0.25% sodium deoxycholate, 0.1% Nonidet P-40, 0.1% sodium dodecyl sulfate) plus the proteinase inhibitor cocktail Complete (Roche) for 10 minutes of incubation at 4°C. Lysates were cleared by centrifugation for 10 minutes in a microcentrifuge. Twenty μg of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes using HM-transfer buffer (150 mmol/L glycine, 25 mmol/L Tris-HCl, pH 8.8, and 10% methanol) in a semidry blotting chamber (Biometra, Göttingen, Germany). As a molecular size standard prestained proteins were used (SDS7B protein ladder, Sigma; rainbow marker, Amersham-Pharmacia, Freiburg, Germany). Membranes were blocked with 4% nonfat milk powder in TBST (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl; 0.2% Tween 20) for 45 minutes, washed in TBST and incubated with the primary antibody for 90 minutes at room temperature. After intensive washing with TBST the primary antibody was detected with species-specific alkaline phosphatase-conjugated secondary antibodies (Dianova, Hamburg, Germany) using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche) as substrates.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from cultured cells at 80% confluency using the RNAeasy kit (Qiagen, Hilden, Germany). For RT-PCR, cDNA was synthesized in a 30-μl reaction, containing 20 μg of total RNA, 400 U MMTV reverse transcriptase (Life Technologies), the buffer supplied with the enzyme, 10 mmol/L dNTPs, and 10 pmol oligo(dT)22 primer. The cDNA obtained was amplified by PCR, using Taq polymerase according to the manufacturer’s protocol (Life Technologies). The sequences of the sense/antisense primers to amplify a 987-bp cDNA fragment of aromatase cytochrome P-450 were 5′CGG CCT TGT TCG TAT GGT CA 3′/5′GTC TCA TCT GGG TGC AAG GA 3′. 36 The primers to amplify cDNA fragments of estrogen receptor-α and -β and progesterone receptor were provided by Ulrich Gottwald and Holger Hess-Stumpp, Schering AG, Berlin, Germany (estrogen receptor α: 5′GCA GAC AGG GAG CTG GTT CA3′/5′GCC TTT GTT ACT CAT GTG CC3′; estrogen receptor β: 5′GGC AAC TAC TTC AAG GTT TCG AG3′/5′ACT GAG ACT GTG GGT TCT GGG AG3′; progesterone receptor: 5′TTA CCA TGT GGC AGA TCC CAC AG3′/5′ACC ATC CCT GCC AAT ATC TTG GG3′). The housekeeping gene for BiP protein was used as a positive control for successful reverse transcription and to exclude genomic contamination by using primers in two different exons (5′TAC ACT TGG TAT TGA AAC TG/3′GGT GGC TTT CCA GCC ATT C).
Matrigel-Invasion Assay
The ability of cells to migrate or invade through a Matrigel barrier was measured in Falcon BioCoat Matrigel invasion chambers (Becton Dickinson, Heidelberg, Germany) with 6.4-mm diameter and 8-μm pore size. Invasion chambers coated with Matrigel (for invasion measurement) or uncoated filters (for measurement of migration) were used according to standard protocols 37 and the manufacturer’s advice. Cells on the lower surface of the filter were stained with Hoechst dye no. 33258 (Sigma) and microscopically photographed with a ×20 objective. Values for migration were taken as the average number of migrated cells per photographic field over three independent fields per experiment and expressed as averages of triplicate experiments. Values for invasion were similarly recorded, but invasion values were expressed as the percentage of cells penetrating one field of a Matrigel-coated filter, compared to the numbers of the same cell type migrating in one field of a uncoated filter in a parallel control experiment.
Results
In Vitro Growth and Invasive Properties of Primary Endometriotic Cells
Endometriotic peritoneal biopsies were collected from 64 women during laparoscopy and primary cultures prepared as described in Materials and Methods and previously. 24 It was reproducibly found that only cells obtained from biopsies of light red lesions, but not dark red lesions, were able to grow as primary cultures. Furthermore, cells isolated from peritoneal areas without visible lesions also failed to grow in culture. Therefore, the endometriotic cells described in this paper were always isolated from biopsies of light red peritoneal lesions.
Based on morphology and immunocytochemical staining, primary cultures of endometriotic cells appear to comprise three different cell types: epithelial cells with a honeycomb-like morphology, expressing cytokeratins, vimentin, and E-cadherin (Ecad+/CK+ cells); cells of fibroblastoid spindle-shape morphology, containing neither cytokeratins nor E-cadherin but only vimentin (CK− cells); and finally, cells of a flat morphology expressing cytokeratins and vimentin but not E-cadherin (CK+ cells) (this report). 24,38,39
One main characteristic of primary endometriotic cell cultures established from peritoneal biopsies is that they contain invasive cells, as previously shown in an in vitro collagen invasion assay. 24,40 To confirm this and to more easily characterize the invasive cells by immunostaining, we introduced the Matrigel invasion assay (Figure 1) ▶ .
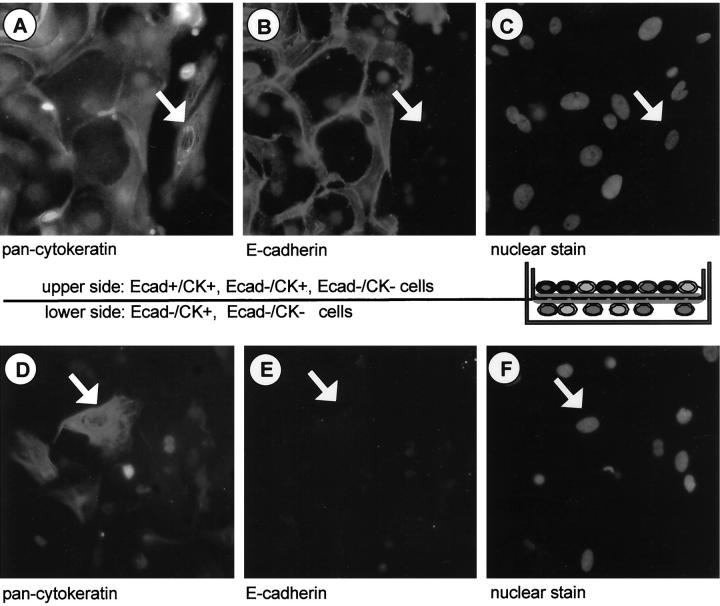
Figure 1.
Matrigel invasion assay of primary endometriotic cell types. Single cells and small cell patches from endometriotic biopsies were isolated and plated onto Matrigel invasion chambers. After 48 hours the cells were fixed and invasive cells migrating to the lower side of the membrane, as well as noninvasive cells found on the upper side of the membrane, were subjected to simultaneous immunocytochemical staining for cytokeratin (A and D) and E-cadherin (B and E). Nuclear counterstain with Hoechst dye was performed to facilitate localization of individual cells (C and F). On the upper side of the membrane all three cell types (Ecad+/CK+, Ecad−/CK+, Ecad−/CK−) were detected, on the lower side only invasive cell types (Ecad−/CK+, Ecad−/CK−) were found. Arrowheads point to the invasive cytokeratin-positive epithelial cell type. Original magnifications, ×40.
After the endometriotic cells were allowed to invade the Matrigel-containing pores for 48 hours, cells from one side of the filter were removed with a cotton swab. Cells on the upper side or lower side of the filters were double stained with antibodies against cytokeratins and E-cadherin on the filters. Three different staining patterns were detected in the upper population, namely CK+/Ecad+, CK+/Ecad−, and no staining (CK−/Ecad−) (Figure 1 ▶ ; A, B, and C). Invasion, meaning cells that appear on the lower side of the membrane (Figure 1 ▶ ; D, E, and F) was only observed for stromal or fibroblast-like CK− cells and the epithelial-like CK+ cells, but not for the CK+/Ecad+ cells (Figure 1B) ▶ . These data confirmed that primary endometriotic cells from peritoneal lesions contain epithelial-like cells with invasive properties. This was simply demonstrated using the Matrigel assay, which was used in all subsequent investigations described here.
Reproducible Establishment of Endometriotic Cell Lines from Peritoneal Lesions
As shown earlier, it is in principle possible to extend the life span of endometriotic cells by stable expression of SV40 T-antigen-encoding DNA. 35 In comparison, primary endometriotic cells as described above become senescent after approximately four passages in culture (unpublished observations). We aimed to establish a reproducible and easy to use protocol for the generation of endometriotic cell lines from primary cultures.
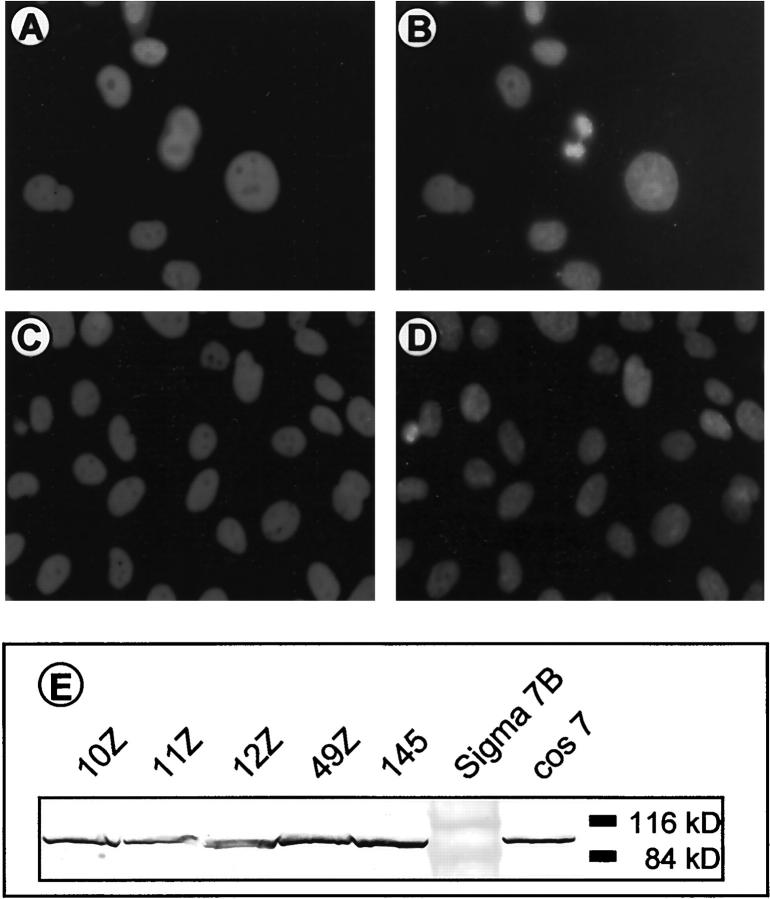
Neither liposome-based transfection of a plasmid encoding SV40 T-antigen nor transfections using conventional electroporation of primary endometriotic cells in suspension were successful. In both cases a substantial portion of the cells died and the remaining cells showed very low transfection efficiency. To overcome this, we decided to establish in situ electroporation of attached primary endometriotic cell monolayers, as described in Materials and Methods. After selection of T-antigen-expressing cells, indirect immunofluorescence analysis revealed that almost all cultures contained 90 to 100% SV40 T-antigen-positive cells (Figure 2 ▶ ; A to D, exemplified for cell lines 11Z and 12Z; Table 1 ▶ ). Immunoblot analysis of the cell lines confirmed the expression of large T antigen (Figure 2E) ▶ .
Figure 2.
Expression of SV40 T antigen in endometriotic cell lines. Immunocytochemistry of T antigen expression in passage 2 of the cell lines 11Z (A and B) and 12Z (C and D). Staining specific for large T antigen (A and C) and nuclear counterstain with Hoechst dye (B and D) show equal and uniform expression of T antigen in every cell of the population. Original magnifications, ×40. E: Western blot analysis of large T-antigen expression in endometriotic cell lines (lanes 1 to 5). A lysate of T-antigen-expressing cos7 cells (lane 7) was included as a control.
Table 1.
Endometriotic Cell Lines Established from Light Red Peritoneal Lesions by Transfection with SV40 T Antigen
| Cell line | T antigen* | Cyto keratin† | Passages 1:8‡ | Growth after crisis (P)§ | ||||
|---|---|---|---|---|---|---|---|---|
| Epithelial-like cell lines | ||||||||
| 10B | + | +/− | 12 | |||||
| 10Z | + | + | 19 | 46 | ||||
| 11Z | + | + | 20 | |||||
| 11E | + | + | 25 | |||||
| 12Z | + | + | 27 | 165 | ||||
| 33Z | + | + | 18 | |||||
| 39Z | + | + | 13 | 34 | ||||
| 42B | + | + | 10 | |||||
| 45Z | + | + | 17 | |||||
| 49Z | + | + | 25 | |||||
| 50Z | + | + | 16 | 20 | ||||
| Stromal/fibroblast-like cell lines | ||||||||
| 3 | + | − | 9 | |||||
| 4 | + | − | 12 | |||||
| 9–4Z | + | − | 17 | |||||
| 9–8Z | + | − | 13 | |||||
| 17B | + | − | 25 | |||||
| 18B | + | − | 22 | |||||
| 20B | + | − | 15 | |||||
| 22B | + | − | 27 | |||||
| 25Z | + | − | 18 | |||||
| 40Z | + | − | n.d. | |||||
| 55Z | + | − | n.d. | |||||
| 57Z–T1 | + | − | n.d. | |||||
| 57Z–T2 | + | − | n.d. | |||||
*Immunocytochemical detection of SV40 T antigen. +, 100% of the cell population stained positive for T antigen after overgrowth of nonexpressing cells. This was reached between passage 3 and 6 depending on the individual cell population.
†Immunocytochemical detection of cytokeratins. +, all T-antigen-positive cells were cytokeratin-positive; −, all T-antigen-positive cells were cytokeratin-negative; +/−, a mixed population of cytokeratin-positive and cytokeratin-negative T-antigen-expressing cells was observed.
‡After passage 3 cell lines were split in a 1:8 ratio, until reaching senescence and entering crisis (see Materials and Methods).
§In some cases cells escaped crisis and became immortal cell lines. All of these are still growing in culture. The present passage is given.
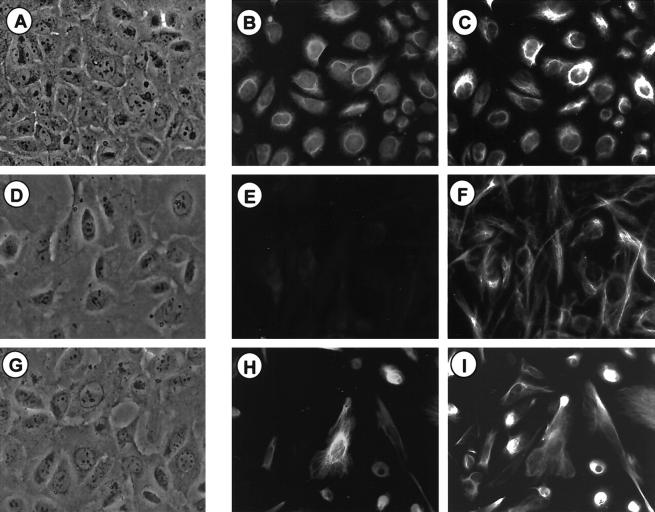
One important feature of peritoneal endometriotic lesions in vivo and in cell cultures derived from such lesions is the presence of E-cadherin-negative epithelial cells within a population of E-cadherin-positive epithelial cells (Figure 1) ▶ . 24 Therefore, expression of the epithelial markers cytokeratin and E-cadherin was investigated in the cell lines by indirect immunofluorescence. None of the cell lines generated were E-cadherin-positive (not shown). Expression of cytokeratins was analyzed using a polyclonal antibody recognizing an epitope common to all cytokeratin isoforms. This revealed that 11 of the T antigen-positive cultures were cytokeratin-positive (Table 1 ▶ ; Figure 3, A and B ▶ ) whereas 13 cell lines were found to be cytokeratin-negative (Table 1 ▶ ; Figure 3, D and E ▶ ). Of the cell lines established, only one contained a mixture of cytokeratin-positive and cytokeratin-negative cells, all of which expressed SV40 T-antigen (Table 1 ▶ , cell line 10B). In addition, all cell lines were positive for vimentin, expressing it either together with cytokeratin (CK+/Vim+) (Figure 3 ▶ ; A, B, and C) or as the only intermediate filament in cytokeratin-negative cell lines (CK−/Vim+) (Figure 3, D, E, and F) ▶ .
Figure 3.
Expression of intermediate filaments in primary endometriotic cells compared to endometriotic cell lines. Immunocytochemical staining for cytokeratins (pan-cytokeratin) and vimentin is shown here in endometriotic cell lines 11Z (A, B, and C) and 22B (D, E, and F), and primary endometriotic cells (passage 1) (G, H, and I). Phase contrast microscopy is shown (A, D, and G) in addition to double-immunofluorescence staining against cytokeratin (B, E, and H) and vimentin (C, F, and I). Original magnifications, ×40. Primary cells and all cell lines express vimentin. Cytokeratin is co-expressed in certain primary cells (H) and in 11 cell lines (represented by 11Z; B). The rest of the primary cells (I) and 13 cell lines (represented by 22B; F) express vimentin as the only intermediate filament.
Both cell types (CK+/Vim+; CK−/Vim+) were also found in the primary endometriotic cell cultures (Figure 3 ▶ ; G, H, and I). This indicates that it is possible to reproducibly establish cell lines from two of the primary cell types found in endometriotic biopsy preparations. A third endometriotic cell sample type, the E-cadherin-positive epithelial cells, was only detectable in freshly plated cells (passage 0, Figure 1B ▶ ) and disappears in the first passage, never giving rise to SV40 T-antigen-transformed cell lines.
Further analysis revealed that all cell lines clearly exhibit a prolonged life span (Table 1) ▶ compared to nontransformed endometriotic cells. After reaching the passages listed in Table 1 ▶ (column 4), cell proliferation ceased. The cells went through a crisis and most of them died. In four samples, however, cells escaped from the crisis and finally appeared as true immortalized cell lines (see Table 1 ▶ , column 5 for current passages). The four cell lines that emerged are still T antigen-positive and retain the cytokeratin expression profile (data not shown).
In summary, 11 cell lines of the cytokeratin-positive epithelial-like and 13 of the stromal/fibroblastoid-like cytokeratin-negative phenotype were obtained. Of these, cell lines 10Z, 11Z, 12Z, 49Z, and the previously established cell line EEC145 35 were selected for further investigation.
Doubling Time of the Established Cell Lines
The endometriotic cell lines analyzed show doubling times between 27 to 50 hours, depending on the individual cell line (Table 2) ▶ . One of the cell lines, 12Z, went through a crisis, postcrisis doubling time subsequently decreasing by approximately one third compared to precrisis passages. Compared to the 4- to 6-day doubling time observed for primary endometriotic cells in culture 41 and unpublished observations, the doubling times observed here are distinctly shorter. Nevertheless, these doubling times are typical for established human cell lines and comparable to those observed for an endometriotic cell line transformed by infection with the SV40 virus. 42
Table 2.
Doubling Time of Endometriotic Cell Lines
| Cell line | 10Z | 11Z | 12Zearly‡ | 12Zlate§ | 49Z | EEC145 |
|---|---|---|---|---|---|---|
| Doubling time* | 27 | 39 | 50 | 31 | 34 | 41 |
| ς n† | 3 | 3 | 13 | 8 | 7 | 13 |
*Average doubling time in hours. Cells were seeded at 1 × 106 cells per 75-cm2 culture flask. After 24, 48, and 72 hours the cells were trypsinized and counted. Each value represents three independent experiments.
†Standard deviation in hours. Each value represents three independent experiments.
‡Cell line 12Z before entering crisis.
§Cell line 12Z after going through crisis.
Exclusion of Contaminating Cells Types in Established Cell Lines
One complication in characterizing endometriotic cells is the lack of positive endometriotic cell marker molecules, such as macrophage-specific CD68. Furthermore, biopsy material collected during laparoscopy may contain, in addition to endometriotic cells, those of mesothelial, smooth muscle, or endothelial origin or cells of the immune system. These cell types have to be excluded as contaminant(s) or indeed the immortalized phenotypes. We therefore conducted immunocytochemical analysis of marker proteins specific for particular cell types with the cell lines 10Z, 11Z, 12Z, and 49Z (Table 3) ▶ . None of the tested markers were found on any of these cell lines. Possibly also present in endometriotic biopsies are epithelial cells of the peritoneum, which cannot be distinguished by immunocytochemical markers. However, considering our numerous failed attempts to cultivate material from lesion-free peritoneum, it is very unlikely that the cell lines originated from the peritoneal layer. Taken together, our data clearly imply that the cell lines described here are of endometriotic origin.
Table 3.
Cell Types Possibly Present in Endometriotic Biopsies: Immunocytochemical Screening of Endometriotic Cell Lines for Contamination by These Cell Types
| Cell type* | Marker on this cell type† | Endometriotic cell lines‡ |
|---|---|---|
| Mesothelial cells | Calretinin | — |
| Mesothelial cells | Clone TE7 | — |
| Muscle cells | Desmin | — |
| Endothelial cells | Factor VIIIa | — |
| Endothelial cells | CD 31 | — |
| Lymphocytes | CD 3 | — |
| Lymphocytes | CD 45 | — |
| Macrophages | CD 68 | — |
*Cell type that may be contaminating or being the origin of cell lines derived from peritoneal endometriotic biopsies.
†Marker protein on this cell type used for immunocytochemistry (for antibodies see Materials and Methods).
‡Expression of the marker protein on the endometriotic cell lines 10Z, 11Z, 12Z, and 49Z between passages 4 and 7.
Expression of Steroid Hormone Receptors and Aromatase Cytochrome P-450
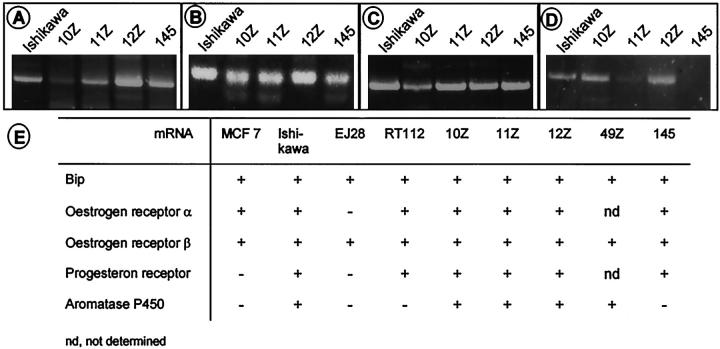
It is postulated that in many cases endometriotic cells are derived from eutopic endometrium. Thus, they may express proteins typically found in endometrial tissue such as receptors for the steroid hormones estrogen and progesterone. In many endometriotic lesions (although not in all), the expression of estrogen and progesterone receptors has been shown and the tissue exhibits biological activities in response to their ligands. 43 To analyze mRNA expression of estrogen receptors-α and -β and progesterone receptor, we performed RT-PCR analysis as described in Materials and Methods. As shown in Figure 4 ▶ , mRNA for estrogen receptors-α and -β and the progesterone receptor was detected in all four cell lines tested. Like estrogen-dependent endometrial cancers, 44 endometriotic lesions often contain aromatase cytochrome P-450, 45,46 which catalyzes conversion of androgens to estrogens. RT-PCR analysis revealed that three out of the four cell lines express mRNA for aromatase cytochrome P-450 (Figure 4) ▶ .
Figure 4.
Expression of steroid hormone receptors and aromatase cytochrome P-450 in endometriotic cell lines, as compared to MCF7, EJ28, RT112, and Ishikawa cancer cell lines used as controls. A to D: Agarose gel electrophoresis of RT-PCR products obtained in the cell lines as indicated with primers for estrogen receptor α—its expression generates a product of 516 bp (A); estrogen receptor β—its expression generates a product of 455 bp (B); progesterone receptor—its expression results in a 379-bp product (C); aromatase cytochrome P-450—its expression generates a 987-bp product (D). E: Tabulated synopsis of all results. BiP was used as a positive control for successful reverse transcription and to exclude genomic contamination. nd, not determined.
Invasion of Endometriotic Cell Lines in the Matrigel Invasion Assay
One important feature of CK+Ecad− endometriotic cells with pathophysiological potential is their invasive behavior. 24 These results correspond well with clinical observations that endometriosis is an invasive and metastasizing disease. Therefore, it was important to determine whether the epithelial endometriotic cell lines EEC145, 10Z, 11Z, and 12Z were also invasive in an in vitro assay. To investigate this question in more detail we examined the invasive capacity of the cell lines relative to their motility. The motility of endometriotic cells was assayed by migration through porous filters in a transwell migration assay (see Materials and Methods) and compared to the migration of the metastatic (EJ28) and nonmetastatic (RT112) bladder cancer cell lines (Figure 5A) ▶ . All four endometriotic cell lines exhibited higher motility than RT112 cells but ∼50% of EJ28 cell motility throughout 48 hours. The invasive capacity of the cell lines in the Matrigel assay was then calculated in relation to their motility, taking into account that invasion also depends on motility (Figure 5B) ▶ .
Figure 5.
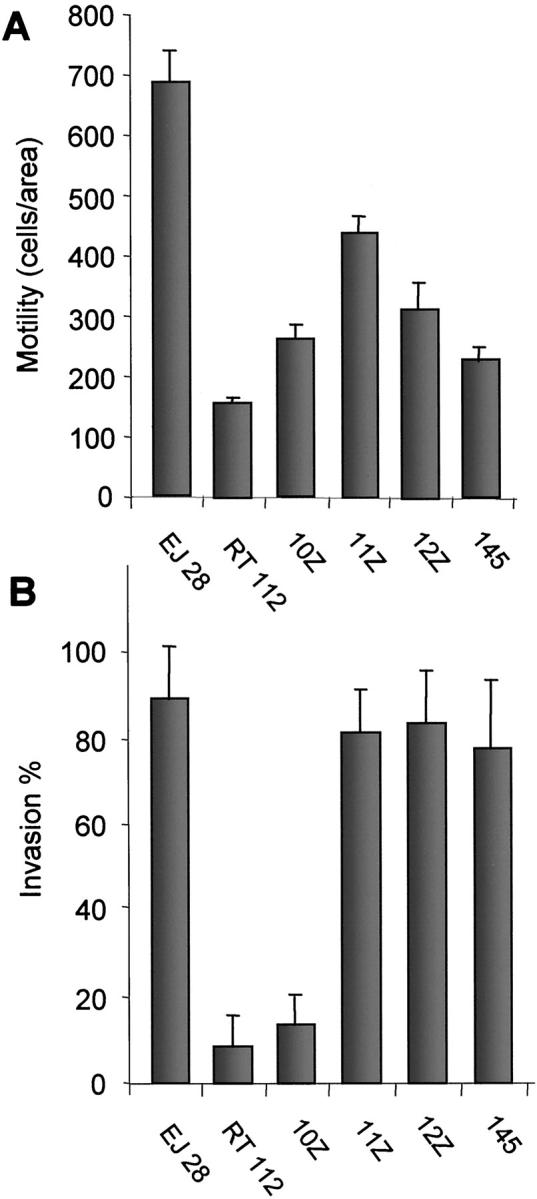
Motility and invasion of endometriotic cell lines in the Matrigel invasion assay. Cell lines were plated on untreated or Matrigel-coated filters and incubated for 48 hours. A: Motility was measured as the movement of cells from a defined area of the untreated micropore filter through the pores. B: Invasion was measured as invasive cells/area on a Matrigel-coated micropore filter and calculated as percent of motile cells/area measured with the same cell line on a noncoated micropore filter in a parallel experiment. Each bar represents two independent experiments; each experiment included counting in three independent areas. The error bars indicate the SD. The bladder carcinoma cell lines EJ28 (invasive) and RT112 (noninvasive) were used as standards to compare motility and invasiveness of the endometriotic cell lines. All four endometriotic cell lines showed higher motility than RT112 cells. Three cell lines (11Z, 12Z, 145) exhibited invasiveness comparable to that of EJ28 cells, one cell line (10Z) showed no invasiveness although it had higher motility than RT112 cells.
The results indicated that three endometriotic cell lines had invasive potential equal or similar to that of EJ28 cells (Figure 5B) ▶ . RT112 cells and one endometriotic cell line, 10Z, showed no invasive potential despite the fact that cell line 10Z exhibited higher motility than RT112 control cells (Figure 5, A and B) ▶ . The doubling times of the endometriotic cell lines (Table 2) ▶ , EJ28 cells and RT112 cells, assayed by counting the cells, were comparable. Thus the differences in motility and invasion observed between the cell lines are unlikely to depend on unequal cell-doubling times.
Endometriotic Cell Lines Express N-Cadherin, an Invasion/Migration-Related Cadherin
As shown previously, primary peritoneal endometriotic cells 24,40 and endometriotic cell lines derived from peritoneal lesions (this article and Starzinski-Powitz et al 35 ) share features with carcinoma cells (absence of E-cadherin and invasive capacity) although they are not neoplastic. The question was whether additional parameters typical for invasive/metastatic tumor cells might be found in the endometriotic cell lines.
In normal epithelial cells, the cytoplasmic tail of the metastasis suppressor molecule E-cadherin, a molecule responsible for the tight interaction between epithelial cells, is usually complexed with cytoplasmic proteins of the catenin family such as α-catenin, β-catenin, plakoglobin, and p120ctn. 47-52 In carcinoma cells, the malignant phenotype is related to impaired expression or functionality of E-cadherin 53-58 as well as increased transcriptional activity of β-catenin via its interaction with the transcription factor LEF-1/Tcf. 59,60 More recent experiments implied that p120ctn, another protein found in the E-cadherin/catenin complex, might also contribute to the transformed phenotype. 61,62
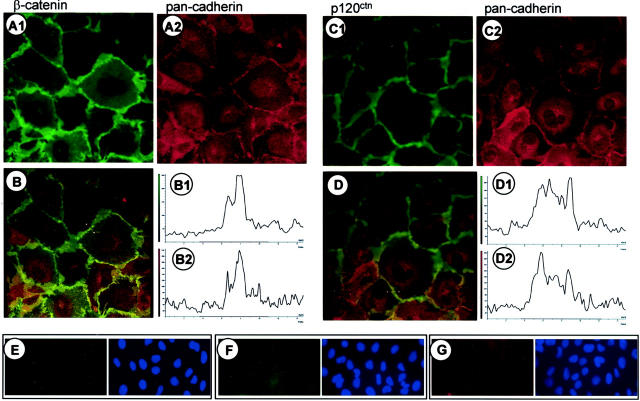
As the endometriotic cell lines do not express E-cadherin, we analyzed the cellular distribution of β-catenin (Figure 6 ▶ A1) and p120ctn (Figure 6 ▶ C1) by immunofluorescence. Surprisingly, both proteins were found almost exclusively at the membrane and no nuclear and barely any cytoplasmic localization was detected (Figure 6 ▶ , A1 and C1). Localization of the catenins at the cell membrane suggested the presence of a catenin-binding protein, presumably a cadherin in the membrane of the endometriotic cell lines. By applying a pan-cadherin antibody that recognizes an epitope highly conserved in most cadherins, immunocytochemistry revealed a cadherin in the endometriotic cell lines tested (Figure 6 ▶ , A2 and C2, shown for 12Z). This cadherin co-localized with β-catenin (Figure 6B) ▶ and with p120ctn (Figure 6D) ▶ .
Figure 6.
Co-localization of β-catenin and p120ctn with cadherin in endometriotic cell lines. Double-immunofluorescence labeling of β-catenin (A1) and a cadherin (A2) (stained with the pan-cadherin antibody). B: Merged images of the two-color channels recorded simultaneously and intensity profile of the different fluorescence labels (B1 for A1 and B2 for A2) recorded at the position of the bar in B. β-catenin co-localized with a cadherin at the sites of cell-cell contact. Co-localization of p120ctn (C1) with a cadherin (C2) (stained with the pan-cadherin antibody) at the site of cell-cell contact. D, D1, D2: Merged images and intensity profile analysis at the position of the bar in D. Original magnification, ×100. E and F: Control stainings with unrelated antibodies (E: anti-green fluorescent protein; F: anti-Xpress) of the same IgG subclass (IgG1) used in A1 and C1, and in Figures 7B ▶ and Figure 8 ▶ (A, C, and E). G: Control staining with normal rabbit serum for the rabbit antisera used in A2 and C2, and in Figure 7A ▶ and Figure 8 ▶ (B, D, and F).
Recent reports 63-65 have implied that expression of N-cadherin is compatible with cell migration, invasion, and metastasis. Thus, N-cadherin was a candidate ligand for the pan-cadherin antibodies in the endometriotic cell lines. To test this, double immunostainings, in addition to immunoblotting, were performed with a N-cadherin and pan-cadherin antibodies (Figure 7) ▶ . The double immunostainings revealed a co-localization of the antigens recognized by both these antibodies (Figure 7 ▶ ; A to D), strongly suggesting that the endometriotic cell lines express N-cadherin. This was further supported by the immunoblots (Figure 7E) ▶ . In cell lysates of the endometriotic cell lines the pan-cadherin antibodies detected a 135-kd protein typical for N-cadherin, 66 which was similar if not identical in size to the protein recognized by the N-cadherin antibodies (Figure 7E) ▶ .
Figure 7.
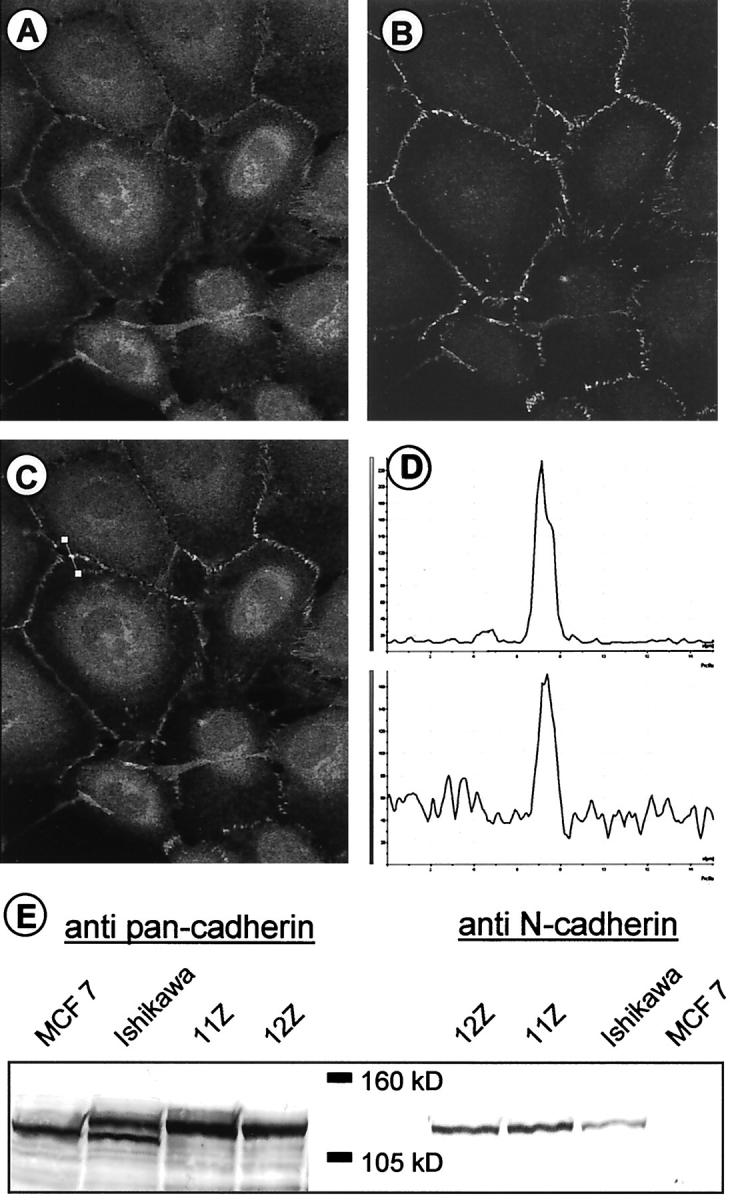
Identification of the cadherin expressed in endometriotic cell lines as N-cadherin. Double-immunofluorescence labeling of the cadherin expressed in endometriotic cell lines using a pan-cadherin antibody (A) and an N-cadherin antibody (B). Merged image of both color channels (C) and intensity profile of the different fluorescence labels [D; A (top) and B (bottom)] recorded at the position of the bar in C. Both fluorescence signals co-localize perfectly with each other. Original magnifications, ×100. E: Western blot analysis of cadherin expression in cell lysates with the pan-cadherin antibody and an antibody specific for N-cadherin. Left: The pan-cadherin antibody recognizes E-cadherin in MCF-7 cells (lane 1), E-cadherin, and a 135-kd signal in Ishikawa cells (lane 2) and a 135-kd signal in cell lysates of the endometriotic cell lines 11Z and 12Z (lanes 3 and 4). The N-cadherin antibody (right) detects only a 135-kd protein in lysates of the endometriotic cell lines 11Z and 12Z (lanes 5 and 6) and Ishikawa cells (lane 7).
The E-cadherin protein of 120 kd, which is the only cadherin in MCF-7 cells (Figure 7E) ▶ , 64 was not found in the endometriotic cell lines. In Ishikawa cells, E-cadherin is co-expressed with N-cadherin. The two cadherins detected by the pan-cadherin antibodies could be distinguished by their different electrophoretic mobilities whereas the specific N-cadherin antibody detected only the slower migrating 135-kd protein in this cell lysate.
N-Cadherin-Positive Cell Populations in Primary Endometriotic Cells
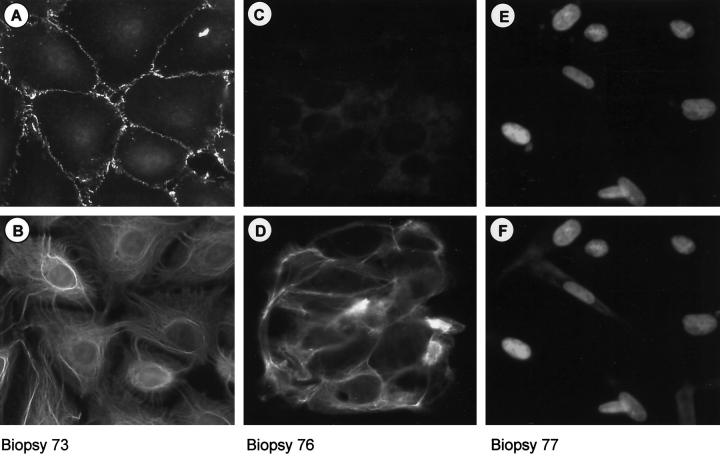
To investigate whether the cytokeratin and N-cadherin-positive profile identified in the cell lines is present in endometriotic lesions we performed double-immunocytochemical staining against cytokeratin and N-cadherin in primary endometriotic cells. Primary cell cultures were prepared from biopsies as described and cells allowed to attach to the coverslip were fixed at 18 hours. Immunocytochemical staining of cells from nine biopsies revealed five lesions containing N-cadherin and cytokeratin-expressing cells (Figure 8, A and B ▶ , shown for biopsy 73). In one lesion (Figure 8, C and D ▶ , biopsy 76) only cytokeratin-positive, N-cadherin-negative cells were detected. In the three remaining biopsies, the cells expressed neither protein (Figure 8, E and F) ▶ .
Figure 8.
N-cadherin-expressing epithelial-like cells are found in endometriotic lesions. Double-immunofluorescence labeling of primary endometriotic cells 18 hours after preparation from biopsy material. Cells are double stained with anti-N-cadherin (A, C, and E) and anti-cytokeratins (B, D, and F) antibodies. Individual biopsies were found to contain either CK+/Ncad+ cells (A and B) or CK+/Ncad− cells (C and D) or only CK−/Ncad− cells (E and F) (see text for details). In the case of CK−/Ncad− cells (E and F) the nuclei of the cells are counterstained with Hoechst dye to highlight the cells. Original magnification, ×40.
Discussion
We demonstrate here that it is possible to reproducibly establish cell lines from peritoneal endometriotic biopsies by transformation with SV40 T-antigen. Two types of cells could be transformed, one of which is of epithelial nature (CK+) and the other not (CK−). Both cell types are E-cadherin-negative. The CK+/Ecad− cell type most likely represents the phenotype we identified previously in the glands of peritoneal endometriotic biopsies. 24 The inability to transform CK+/Ecad+ cells probably reflects the fact that these cells are terminally differentiated and destined to die in culture. This assumption correlates well with what we see in primary endometriotic cell cultures prepared from peritoneal lesions: the CK+/Ecad+ cells disappear rather rapidly (normally during the first passage of the primary culture) whereas the CK+/Ecad− cell type can be detected for a limited number of passages (3 to 4), before all cells exhibit an CK−/Ecad− phenotype. In view of these observations, the CK−/Ecad− cell lines may originate either from stromal or fibroblast cells, or, alternatively, from cells that have lost cytokeratin expression after some doublings in culture. To our knowledge, there are currently no markers available that could help to distinguish between these two possibilities.
Our experiments raise an important question about the developmental stage of the immortalized parental cell. One attractive speculation is that the CK+Ecad− endometriotic cells are in a type of transition state between a rather undifferentiated precursor cell (CK−Ecad−) and the terminally differentiated CK+Ecad+ epithelial cell. Relating to this is the assumption that a mesenchymal-epithelial transformation occurs in the ectopic endometrial cells, perhaps induced and/or controlled by factors in the peritoneal fluid, for example cytokines. These might be the cytokines and their receptors that also play a role in the mesenchymal-epithelial transition of cells in the kidney. 67,68 Secondly, arrest of the endometriotic cells in transition may reflect their ability to exhibit some stem cell characteristics, such as the capacity for self-renewal and generation of differentiated daughter cells. Indeed, the observation that only cell preparations from light red lesions could be cultured in vitro may support this idea. Attempts to cultivate cells from dark red lesions, which represent a later stage in the pathogenesis of endometriosis, and perhaps contain only differentiated or even senescent endometriotic cells, failed. These cells may lack the plasticity or viability necessary to grow in culture. This also implies that a cell type with stem cell characteristics will be found only in fresh endometriotic lesions.
As we have documented previously, primary endometriotic cells are invasive in a collagen invasion assay. 24,40 It is conceivable that the invasion and metastasis of endometriotic cells resembles in some ways early micrometastasis of carcinomas. Here, it is postulated that a few CK+ cells can reach distant locations such as lymph nodes and bone marrow because of early disruption of homotypic cell-cell contacts in the primary tumor. This disruption requires dysregulation of cell adhesion molecules like E-cadherin in the metastasizing cell (epithelial-mesenchymal transition), which finally may become dormant for a long time. 69,70
In parallel with these ideas, the E-cadherin-negative cells in endometriotic lesions may be the result of such an epithelial-mesenchymal transition. In any case, those cells, which must be unable to make intact contacts with their surrounding E-cadherin-positive cells, are not fully differentiated and thus might migrate out easily. Dormancy may also play a role in endometriosis, in particular during the periods when estrogen depletion is used as therapy. This presumption is based on the high relapse rate after estrogen depletion that may be explained, at least in part, by dormant endometriotic cells.
When the CK+/Ecad− epithelial-like endometriotic cell lines were compared to bladder carcinoma cell lines EJ28 (invasive) and RT122 (noninvasive) using the Matrigel assay, the endometriotic cell lines exhibited invasiveness comparable to the cell line EJ28. Significantly, not only is E-cadherin absent in both cell types, but N-cadherin is expressed in endometriotic cell lines as well as EJ28 cells. This may be of relevance for the pathogenesis of endometriosis because several reports have indicated that N-cadherin may act as a path-finding cadherin allowing cells to be invasive and migratory in both normal development and pathophysiological processes. 71-75 In congruence with this idea are studies of carcinoma cells showing that invasion/metastasis requires the absence or inactivation of E-cadherin, which is often substituted by the expression of N-cadherin. 63,76-78 Furthermore, ectopic expression of N-cadherin in differentiated, E-cadherin-positive, MCF-7 breast cancer cells switched their phenotype from noninvasive to invasive. Apparently, this N-cadherin-mediated switch is dominant because MCF-7 cells continue to express E-cadherin. 62
Both E-cadherin and N-cadherin belong to the so-called classical/type-I cadherins that comprise two subgroups: E-cadherin and P-cadherin, or N-cadherin and R-cadherin. Functional comparison of E- and N-cadherin domains showed that a chimeric E-cadherin containing the extracellular domain 4 (EC4) of N-cadherin behaves like N-cadherin and causes epithelial to mesenchymal transition and increased motility when transfected into noninvasive carcinoma cells. 79 Although the structural and molecular basis of different cadherin family member functions is relatively well understood, much less is known about the biological processes caused and/or regulated by changing expression from E- to N-cadherin. Furthermore, it was suggested that N-cadherin can interact with, and activate fibroblast growth factor receptors. Moreover, N-cadherin-mediated cell motility can be decreased by an inhibitor of fibroblast growth factor-mediated signal transduction, 75 whereas fibroblast growth factor itself causes a dramatic increase in motility in N-cadherin-expressing cells. 62 However, the mechanism by which fibroblast growth factor receptor-signaling influences N-cadherin-dependent motility and whether this requires direct interactions between N-cadherin and the fibroblast growth factor receptor is not clear at this time. Other recent experiments using trojan peptides that compete with the interaction between specific effectors and the cytoplasmatic domain of N-cadherin resulted in both inhibition of N-cadherin- and β1-integrin-mediated motility as well as neurite outgrowth. This treatment also results in release of the nonreceptor tyrosine kinase Fer from cadherin and its accumulation in the integrin complex, suggesting cross-talk between these two receptor complexes. 80
In addition to the endometriotic cell lines, we also found N-cadherin-positive cells expressing cytokeratin in endometriotic lesions. These cells can be found in the majority, but not all, of the biopsies investigated so far, possibly reflecting differences in the type or developmental stage of individual biopsies. Clearly, whether N-cadherin expression in primary endometriotic cells is of biological relevance for the disease needs further investigation. Similarly, it remains to be seen whether these cells are stromal/fibroblast-like cells making a mesenchymal-epithelial transition, or epithelial cells undergoing an epithelial-mesenchymal transition.
In nontumor cells β-catenin and p120ctn are mostly complexed with E-cadherin or other cadherins at the membrane. Under genetic or epigenetic influence, for example in tumor cells, the cytoplasmic pool of β-catenin can be stabilized and therefore increased. This may lead to the formation of β-catenin complexes with Tcf/Lef in the nucleus and finally to changes in gene expression, for example, regarding cell cycle proteins such as myc and cyclin D1. 59,60,81,82 Nuclear localization and interaction with the transcription factor, Kaiso, is also possible for p120ctn. 83 In this context, it was interesting to note that the catenins β-catenin and p120ctn are exclusively membrane-bound although endometriotic cells exhibit some features of tumor cells.
The most important concern about using a cell culture model for molecular and cellular studies of endometriosis is whether and to what extent these cell lines maintain their in vivo characteristics. So far, this question can only be answered concerning molecules also found in endometriotic lesions in vivo. Such marker molecules included cytokeratin, 84,85 aromatase P-450, 45,46 estrogen receptor-α and -β, and progesterone receptor. 43 Not only were all three steroid hormone receptors detected by RT-PCR in most of the endometriotic cell lines, but as in endometriotic lesions, they are expressed in low amounts. Attempts to confirm expression and protein function using estrogen- and progesterone-responsive promoter elements and a luciferase reporter gene, failed (unpublished observations). All but one of the cell lines tested express aromatase cytochrome P-450. Whether aromatase protein or mRNA is expressed in the eutopic endometria of normal menstruating women is still controversial (Kitawaki et al 86 and references therein) but both are found in endometriotic lesions. Thus, the presence of aromatase cytochrome P-450 suggests a local estrogen production that may influence the disease. 45,46 Furthermore, endometriotic cell lines also express gp130, the common chain of the interleukin-6 receptor family that is typically found in sections of peritoneal endometriotic lesions (A. Mayer and A. Schreiner, unpublished observation).
In summary, it seems that a number of proteins that are typical and possibly also functionally important for endometriosis are expressed in the cell lines we established. This, combined with the fact that the endometriotic cell lines are invasive will make them a useful tool for identifying and characterizing endometriosis-related genes or immunological markers.
Acknowledgments
We thank Avril Arthur-Goettig for critical reading of the manuscript, Anette Mayer and Alexander Schreiner for valuable conceptual contributions, Günter Herrmann for the gift of antibodies, Holger Hess-Stumpp for Ishikawa cells and oligonucleotide primers, and Beata Krebs for technical assistance.
Footnotes
Address reprint requests to Anna Starzinski-Powitz, Institut der Anthropologie und Humangenetik fuer Biologen, Johann-Wolfgang-Goethe-Universitaet Frankfurt, Siesmayerstrasse 70, D-60054 Frankfurt/Main, Germany. E-mail: starzinski-powitz@em.uni-frankfurt.de.
Supported by grant Sta 187/13-1 from the Deutsche Forschungsgemeinschaft (to A. S.-P.), and by a fellowship from the FAZIT-Stiftung (to A. Z.).
References
- 1.Sampson JA: Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Obstet Gynecol 1927, 14:422-469 [Google Scholar]
- 2.Suginami H: A reappraisal of the coelomic metaplasia theory by reviewing endometriosis occurring in unusual sites and instances. Am J Obstet Gynecol 1991, 165:214-218 [DOI] [PubMed] [Google Scholar]
- 3.Matsuura K, Ohtake H, Katabuchi H, Okamura H: Coelomic metaplasia theory of endometriosis: evidence from in vivo studies and an in vitro experimental model. Gynecol Obstet Invest 1999, 47(Suppl 1):18-22 [DOI] [PubMed] [Google Scholar]
- 4.Halme J, Becker S, Hammond MG, Raj MGH, Raj S: Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol 1983, 145:333-337 [DOI] [PubMed] [Google Scholar]
- 5.Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV: Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol 1993, 169:1545-1549 [DOI] [PubMed] [Google Scholar]
- 6.Akoum A, Lemay A, Brunet C, Hebert J: Cytokine-induced secretion of monocyte chemotactic protein-1 by human endometriotic cells in culture. Am J Obstet Gynecol 1995, 172:594-600 [DOI] [PubMed] [Google Scholar]
- 7.Weil SJ, Wang S, Perez MC, Lyttle CR: Chemotaxis of macrophages by a peritoneal fluid protein in women with endometriosis. Fertil Steril 1997, 67:865-869 [DOI] [PubMed] [Google Scholar]
- 8.Murphy AA, Santanam N, Morales AJ, Parthasarathy S: Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab 1998, 83:2110-2113 [DOI] [PubMed] [Google Scholar]
- 9.Taketani Y, Kuo TM, Mizuno M: Comparison of cytokine levels and embryo toxicity in peritoneal fluid in infertile women with untreated or treated endometriosis. Am J Obstet Gynecol 1992, 167:265-270 [DOI] [PubMed] [Google Scholar]
- 10.Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN: Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril 1995, 63:929-932 [PubMed] [Google Scholar]
- 11.Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP: Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril 1996, 65:925-930 [PubMed] [Google Scholar]
- 12.Schroder W, Gaetje R, Baumann R: Interleukin-6 and soluble interleukin-6 receptor in peritoneal fluid and serum of patients with endometriosis. Clin Exp Obstet Gynecol 1996, 23:10-14 [PubMed] [Google Scholar]
- 13.Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, Terakawa N: Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol 1997, 176:593-597 [DOI] [PubMed] [Google Scholar]
- 14.Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR: Peritoneal fluid concentrations of interleukin-8 in women with endometriosis: relationship to stage of disease. Hum Reprod 1998, 13:1957-1961 [DOI] [PubMed] [Google Scholar]
- 15.Surrey ES, Halme J: Effect of peritoneal fluid from endometriosis patients on endometrial stromal cell proliferation in vitro. Obstet Gynecol 1990, 76:792-797 [DOI] [PubMed] [Google Scholar]
- 16.Koutsilieris M, Niklinski W, Frenette G, Lemay A: Heparin-Sepharose binding growth factors in peritoneal fluid of women with endometriosis. Fertil Steril 1993, 59:93-97 [PubMed] [Google Scholar]
- 17.Giudice LC, Dsupin BA, Gargosky SE, Rosenfeld RG, Irwin JC: The insulin-like growth factor system in human peritoneal fluid: its effects on endometrial stromal cells and its potential relevance to endometriosis. J Clin Endocrinol Metab 1994, 79:1284-1293 [DOI] [PubMed] [Google Scholar]
- 18.Koskimies AI, Tenhunen A, Ylikorkala O: Peritoneal fluid 6-keto-prostaglandin F1 alpha, thromboxane B2 in endometriosis and unexplained infertility. Acta Obstet Gynecol Scand 1984, 123(Suppl):S19-S21 [DOI] [PubMed] [Google Scholar]
- 19.Dawood MY, Khan-Dawood FS, Wilson L, Jr: Peritoneal fluid prostaglandins and prostanoids in women with endometriosis, chronic pelvic inflammatory disease, and pelvic pain. Am J Obstet Gynecol 1984, 148:391-395 [DOI] [PubMed] [Google Scholar]
- 20.Mudge TJ, James MJ, Jones WR, Walsh JA: Peritoneal fluid 6-keto prostaglandin F1 alpha levels in women with endometriosis. Am J Obstet Gynecol 1985, 152:901-904 [DOI] [PubMed] [Google Scholar]
- 21.Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR: Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril 1993, 59:778-782 [DOI] [PubMed] [Google Scholar]
- 22.Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR: Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol 1994, 83:287-292 [PubMed] [Google Scholar]
- 23.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK: Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest 1996, 98:482-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaetje R, Kotzian S, Herrmann G, Baumann R, Starzinski-Powitz A: Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am J Pathol 1997, 150:461-467 [PMC free article] [PubMed] [Google Scholar]
- 25.Sack GH, Jr: Human cell transformation by simian virus 40—a review. In Vitro 1981, 17:1-19 [DOI] [PubMed] [Google Scholar]
- 26.Pantel K, Dickmanns A, Zippelius A, Klein C, Shi J, Hoechtlen-Vollmar W, Schlimok G, Weckermann D, Oberneder R, Fanning E, Riethmüller G: Establishment of micrometastatic carcinoma cell lines: a novel source of tumor cell vaccines. J Natl Cancer Inst 1995, 87:1162-1168 [DOI] [PubMed] [Google Scholar]
- 27.Brosens JJ, Takeda S, Acevedo CH, Lewis MP, Kirby PL, Symes EK, Krausz T, Purohit A, Gellersen B, White JO: Human endometrial fibroblasts immortalized by simian virus 40 large T antigen differentiate in response to a decidualization stimulus. Endocrinology 1996, 137:2225-2231 [DOI] [PubMed] [Google Scholar]
- 28.DeCaprio JA, Ludlow JW, Figge J, Shew J, Huang C, Lee W, Marsilio E, Paucha E, Livingstone DM: SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 1988, 54:275-283 [DOI] [PubMed] [Google Scholar]
- 29.Ewen ME, Ludlow JW, Marsilio E, DeCaprio JA, Millikan RC, Cheng SH, Paucha E, Livingston DM: An N-terminal transformation-governing sequence of SV40 large T-antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell 1989, 58:257-267 [DOI] [PubMed] [Google Scholar]
- 30.Zalvide J, DeCaprio JA: Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol 1995, 15:5800-5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JY, Simmons DT: The ability of large T antigen to complex with p53 is necessary for the increased lifespan and partial transformation of human cells by SV40. J Virol 1991, 65:6447-6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kierstead TD, Tevethia MJ: Association of p53 binding and immortalization of primary C57B/6 mouse embryo fibroblasts using SV40 T antigen mutants bearing internal overlapping deletion mutations. J Virol 1993, 65:1817-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang SE: In vitro transformation of human epithelial cells. Biochim Biophys Acta 1986, 823:161-194 [DOI] [PubMed] [Google Scholar]
- 34.Shay JW, Wright WE, Werbin H: Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta 1991, 1072:1-7 [DOI] [PubMed] [Google Scholar]
- 35.Starzinski-Powitz A, Gaetje R, Zeitvogel A, Kotzian S, Handrow-Metzmacher H, Herrmann G, Fanning E, Baumann R: Tracing cellular and molecular mechanisms involved in endometriosis. Hum Reprod Update 1998, 4:724-729 [DOI] [PubMed] [Google Scholar]
- 36.Mu YM, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, Takayanagi R, Nawata H: Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun 2000, 271:710-713 [DOI] [PubMed] [Google Scholar]
- 37.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987, 47:3239-3245 [PubMed] [Google Scholar]
- 38.Osteen KG, Hill GA, Hargrove JT, Gorstein F: Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil Steril 1989, 52:965-972 [DOI] [PubMed] [Google Scholar]
- 39.Matthews CJ, Redfern CP, Hirst BH, Thomas EJ: Characterization of human purified epithelial and stromal cells from endometrium and endometriosis in tissue culture. Fertil Steril 1992, 57:990-997 [DOI] [PubMed] [Google Scholar]
- 40.Gaetje R, Kotzian S, Herrman G, Baumann R, Starzinski-Powitz A: Invasiveness of endometriotic cells in vitro. Lancet 1995, 346:1463-1464 [DOI] [PubMed] [Google Scholar]
- 41.Ryan IP, Schriock ED, Taylor RN: Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 1994, 78:642-649 [DOI] [PubMed] [Google Scholar]
- 42.Akoum A, Lavoie J, Drouin R, Jolicoeur C, Lemay A, Maheux R, Khandjian EW: Physiological and cytogenetic characterization of immortalized human endometriotic cells containing episomal simian virus 40 DNA. Am J Pathol 1999, 154:1245-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segars JH: Endometriosis and nuclear hormone receptors. Diamond MP Osteen KG eds. Endometrium and Endometriosis. 1997, :pp 321-332 Blackwell Science, Malden [Google Scholar]
- 44.Bulun SE, Economos K, Miller D, Simpson ER: CYP19 (aromatase cytochrome P450) gene expression in human malignant endometrial tumors. J Clin Endocrinol Metab 1994, 79:1831-1834 [DOI] [PubMed] [Google Scholar]
- 45.Noble LS, Simpson ER, Johns A, Bulun SE: Aromatase expression in endometriosis. J Clin Endocrinol Metab 1996, 81:174-179 [DOI] [PubMed] [Google Scholar]
- 46.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H: Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 1997, 57:514-519 [DOI] [PubMed] [Google Scholar]
- 47.Ozawa M, Baribault H, Kemler R: The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989, 8:1711-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H: Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 1994, 107:3655-3663 [DOI] [PubMed] [Google Scholar]
- 49.Butz S, Kemler R: Distinct cadherin-catenin complexes in Ca(2+)-dependent cell-cell adhesion. FEBS Lett 1994, 355:195-200 [DOI] [PubMed] [Google Scholar]
- 50.Hinck L, Nathke IS, Papkoff J, Nelson WJ: Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol 1994, 125:1327-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ: Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol 1994, 125:1341-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniel JM, Reynolds AB: The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol 1995, 15:4819-4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W: E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umbas R, Schalken JA, Aalders TW, Carte BS, Karthaus HF, Schaffsma HE, Debryne FM, Isaacs BS: Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res 1992, 52:5104-5109 [PubMed] [Google Scholar]
- 55.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H: E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994, 54:3845-3852 [PubMed] [Google Scholar]
- 56.Risinger JI, Berchuck A, Kohle MF, Boyd J: Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet 1994, 7:98-102 [DOI] [PubMed] [Google Scholar]
- 57.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T: Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995, 92:7416-7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G: A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392:190-193 [DOI] [PubMed] [Google Scholar]
- 59.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 60.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R: Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 1996, 59:3-10 [DOI] [PubMed] [Google Scholar]
- 61.van Hengel J, Vanhoenacker P, Staes K, van Roy F: Nuclear localization of the p120(ctn) armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc Natl Acad Sci USA 1999, 96:7980-7985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aono S, Nakagawa S, Reynolds AB, Takeichi M: p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol 1999, 145:551-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran NL, Nagle RB, Cress AE, Heimark RL: N-cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am J Pathol 1999, 155:787-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA: Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000, 148:779-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA: Cadherin switching in human prostate cancer progression. Cancer Res 2000, 60:3650-3654 [PubMed] [Google Scholar]
- 66.Volk T, Geiger B: A-CAM: a 135-kD receptor of intercellular adherens junctions. II. Antibody-mediated modulation of junction formation. J Cell Biol 1986, 103:1451-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kispert A, Vainio S, McMahon AP: Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998, 125:4225-4234 [DOI] [PubMed] [Google Scholar]
- 68.Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA: Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 1999, 99:377-386 [DOI] [PubMed] [Google Scholar]
- 69.Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmuller G, Schlimok G: Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000, 342:525-533 [DOI] [PubMed] [Google Scholar]
- 70.Zippelius A, Pantel K: RT-PCR-based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors. An overview. Ann NY Acad Sci 2000, 906:110-123 [DOI] [PubMed] [Google Scholar]
- 71.Hatta K, Takagi S, Fujisawa H, Takeichi M: Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol 1987, 120:215-227 [DOI] [PubMed] [Google Scholar]
- 72.Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE: Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron 1996, 17:837-848 [DOI] [PubMed] [Google Scholar]
- 73.Brand-Saberi B, Gamel AJ, Krenn V, Mueller TS, Wilting J, Christ B: N-Cadherin is involved in myoblast migration and muscle differentiation in the avian limb bud. Dev Biol 1996, 178:160-173 [DOI] [PubMed] [Google Scholar]
- 74.Hazan RB, Kang L, Whooley BP, Borgen PI: N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun 1997, 4:399-411 [DOI] [PubMed] [Google Scholar]
- 75.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ: N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 1999, 147:631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR: Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 1996, 135:1643-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Husmark J, Heldin NE, Nilsson M: N-cadherin-mediated adhesion and aberrant catenin expression in anaplastic thyroid-carcinoma cell lines. Int J Cancer 1999, 83:692-699 [DOI] [PubMed] [Google Scholar]
- 78.Giroldi LA, Bringuier PP, Shimazui T, Jansen K, Schalken JA: Changes in cadherin-catenin complexes in the progression of human bladder carcinoma. Int J Cancer 1999, 82:70-76 [DOI] [PubMed] [Google Scholar]
- 79.Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR: N-cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 2000, 151:1193-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arregui C, Pathre P, Lilien J, Balsamo J: The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol 2000, 149:1263-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H: Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 1997, 88:789-799 [DOI] [PubMed] [Google Scholar]
- 82.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H: XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86:391-399 [DOI] [PubMed] [Google Scholar]
- 83.Daniel JM, Reynolds AB: The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol 1999, 19:3614-3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nisolle M, Casanas-Roux F, Donnez J: Coexpression of cytokeratin and vimentin in eutopic endometrium and endometriosis throughout the menstrual cycle: evaluation by a computerized method. Fertil Steril 1995, 64:69-75 [PubMed] [Google Scholar]
- 85.Kruitwagen RF, Poels LG, Willemsen WN, Jap PH, de Ronde IJ, Hanselaar TG, Rolland R: Immunocytochemical marker profile of endometriotic epithelial, endometrial epithelial, and mesothelial cells: a comparative study. Eur J Obstet Gynecol Reprod Biol 1991, 41:215-223 [DOI] [PubMed] [Google Scholar]
- 86.Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H: Expression of aromatase cytochrome P450 in eutopic endometrium and its application as a diagnostic test for endometriosis. Gynecol Obstet Invest 1999, 48(Suppl 1):S21-S28 [DOI] [PubMed] [Google Scholar]