Abstract
Alteration in cell adhesion and extracellular matrix deposition is a hallmark of diabetic glomerulosclerosis. Integrin-linked kinase (ILK) is a recently identified integrin cytoplasmic-binding protein that has been implicated in the regulation of cell adhesion and extracellular matrix deposition. To begin to investigate whether ILK is involved in the pathogenesis of diabetic glomerulosclerosis, we have analyzed the distribution and regulation of ILK in normal and diabetic kidneys as well as in isolated mesangial cells. We have found that ILK is normally expressed at high concentration in visceral epithelial cells. In diabetic glomeruli, ILK expression in the mesangium is dramatically increased. The increase in ILK level is associated with diffuse mesangial expansion. In glomeruli where advanced nodular sclerosis and global sclerosis were dominant, ILK level was reduced, suggesting that the increase in ILK expression likely associates with relatively early glomerulosclerosis. Additionally, we have found that exposure of mesangial cells to high concentrations of glucose significantly increased the ILK level. Finally, we show that ILK localizes to regions of cell membranes that are in close contact with mesangial fibronectin matrix. These results suggest that ILK is likely involved in mesangial matrix expansion in response to hyperglycemia in the pathogenesis of diabetic glomerulosclerosis.
Renal failure is a common complication found in patients with diabetes mellitus. 1-4 The pathogenesis of diabetic nephropathy is closely associated with accumulation of extracellular matrix proteins including fibronectin in the glomerular mesangium, 5-8 which contributes to the loss of renal function. Clinical studies have clearly shown that hyperglycemia is an important factor in the progression of diabetic nephropathy. 9-14 Furthermore, extensive experimental studies have demonstrated that exposure of glomerular mesangial cells to high glucose results in increased deposition of extracellular matrix proteins including fibronectin. 8,15-19 Thus, understanding how cells regulate extracellular matrix deposition and how high glucose up-regulates extracellular matrix deposition will help us better understand the molecular mechanism underlying the pathogenesis of diabetic nephropathy.
Integrins are αβ dimeric transmembrane receptors that play important roles in cell-matrix interactions. 20-25 In addition to serving as extracellular matrix receptors mediating cell adhesion and transducing signals from the extracellular matrix, integrins are crucially involved in the cellular control of extracellular matrix deposition. 22,26-28 Using genetic reconstitution and functional blocking antibodies, studies by a number of laboratories including ours have demonstrated that multiple integrins including α5β1, 29-33 α3β1, 34 and αvβ3 integrins 35-37 are involved in the regulation of fibronectin deposition into extracellular matrix. The ability of integrins to promote fibronectin matrix deposition is controlled by both the integrin activation state and the cytoskeletal interaction. 38 Because integrin activation and cytoskeletal interaction are regulated by integrin cytoplasmic domains, 22 these studies suggest that intracellular proteins associated with the integrin cytoplasmic domains likely play important roles in the cellular regulation of fibronectin matrix assembly.
Integrin-linked kinase (ILK) is an intracellular serine/threonine kinase that interacts with multiple integrins (eg, β1 and β3 integrins) 39 and cytoskeleton-associated proteins including PINCH, 40 CH-ILKBP, 41 and paxillin. 42 It becomes increasingly clear now that ILK is crucially involved in the regulation of a number of integrin-mediated processes including cell adhesion, cell shape changes, and gene expression. 41,43,44 We have recently found overexpression of ILK in cultured cells dramatically stimulates the deposition of fibronectin into extracellular matrix, 45 indicating that ILK is involved in the cellular regulation of fibronectin matrix deposition. However, despite our rapidly increasing knowledge on the molecular activities of ILK, nothing was known about the distribution and regulation of ILK in the kidney, either under normal physiological conditions or under pathological conditions. In this study, we have examined the distribution of ILK protein in normal human kidneys. Furthermore, we have analyzed the expression of ILK in diabetic nephropathy. Finally, we have analyzed the regulation of the cellular level of ILK in response to hyperglycemia, a causal factor of diabetic glomerulosclerosis, and the subcellular localization of ILK in mesangial cells that assemble a fibronectin matrix.
Materials and Methods
Antibodies and Other Reagents
Mouse monoclonal anti-ILK antibody 65.1 and rabbit polyclonal anti-fibronectin antibody were generated as previously described. 33,46 Mouse monoclonal anti-actin antibody mAb 1501 was from Chemicon (Temecula, CA). Fluorescein isothiocyanate (FITC)-conjugated AffiniPure goat anti-mouse IgG antibody (minimal cross-reaction with human, bovine, rabbit, and swine serum proteins), Rhodamine Red-conjugated AffiniPure goat anti-rabbit IgG antibody (minimal cross-reaction with human, mouse, and rat serum proteins), and horseradish peroxidase-conjugated goat anti-mouse IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). Rat kidney glomerular mesangial cells were isolated as described. 47 Media for cell culture were from Life Technologies (Grand Island, NY) or Mediatech/CellgroR (Herndon, VA).
Immunohistochemical Staining of Normal and Diabetic Human Kidneys
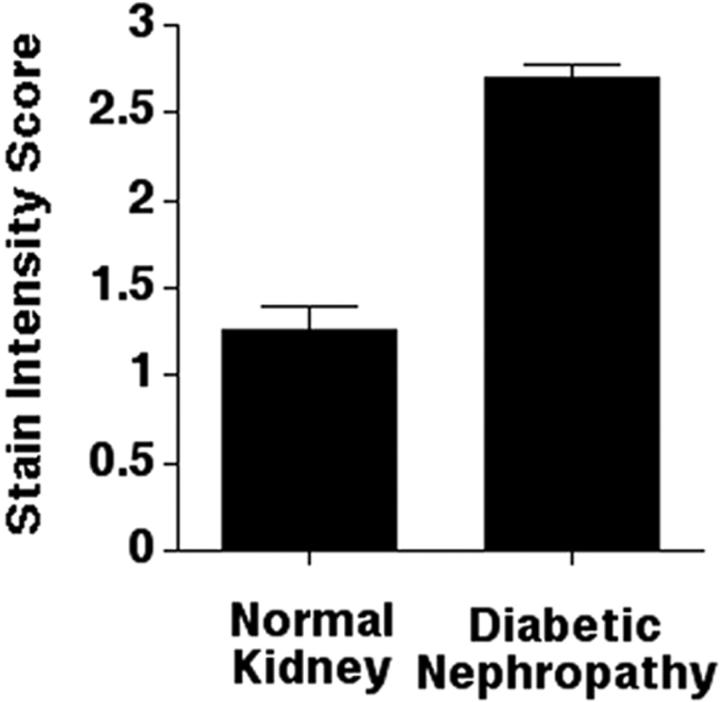
Studies were approved by the Institutional Review Board at the University of Alabama at Birmingham. Kidney tissues from four patients with diabetic nephropathy and five control patients were obtained through the Tissue Procurement Facility at the University of Alabama at Birmingham. Tissues from the control patients showed no morphological abnormalities. Sections (3 μm) of formalin-fixed paraffin-embedded kidney tissues were stained with anti-ILK antibody 65.1. The formalin-fixed kidney sections were peroxidase-blocked with 0.03% hydrogen peroxide for 10 minutes at room temperature and then with 10% normal goat serum in phosphate-buffered saline (PBS) for 1 hour at room temperature. The sections were incubated with mouse anti-ILK monoclonal antibody (0.5 μg IgG/ml) or with an irrelevant mouse IgG (0.5 μg/ml) as a control. The primary antibody was detected with a DAKO EnVision+System (DAKO, Carpinteria, CA) peroxidase (diaminobenzidine) kit and counterstained with hematoxylin. A semiquantitative staining score was created, based on staining of individual glomeruli observed at ×20 magnification. The scoring system ranged from 0 to 3+, with 0 representing no visible staining of the glomerulus; 1+, faint segmental glomerular staining; 2+, segmental increase in staining of the glomerulus; and 3+, globally increased staining of the glomerulus. Twenty-five glomeruli from each kidney were scored and averaged to produce the semiquantitative stain intensity score for each kidney. Glomeruli with morphological evidence of advanced sclerosis were omitted from analysis. Data were presented as means ± SE. Significant differences were determined using the unpaired t-test. A P value <0.05 assigned statistical significance.
We also analyzed frozen sections of normal human kidney (frozen sections of diabetic human kidney were not available) by immunofluorescent staining with monoclonal anti-ILK antibody based on a previously described method. 48 The primary antibody was detected with FITC-conjugated goat anti-mouse IgG (35 μg/ml) and the images were observed under a fluorescence microscope and photographed at ×20 magnification with a digital camera (model C5810, Hamamatsu Photonics K.K.).
Immunofluorescent Staining of Mesangial Cells
Rat kidney glomerular mesangial cells were plated in wells of Lab-Tek 8-chamber culture slides (Nunc, Inc.) in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1× insulin-transferrin-selenium-A solution supplement (Life Technologies, Inc.). The cells were fixed with 3.7% paraformaldehyde in PBS, permeabilized with 0.1% Triton X-100 in PBS containing 1 mg/ml bovine serum albumin, and then stained with mouse anti-ILK antibody (0.5 μg/ml) and rabbit anti-fibronectin antibody (8 μg/ml). After rinsing, the bound mouse IgG and rabbit IgG were detected with a FITC-conjugated AffiniPure goat anti-mouse IgG antibody and a Rhodamine Red-conjugated AffiniPure goat anti-rabbit IgG antibody, respectively. Stained cells were observed under a fluorescence microscope equipped with rhodamine and FITC filters. In control experiments, no cross-reactivity between the mouse monoclonal antibodies and the Rhodamine Red-conjugated AffiniPure goat anti-rabbit IgG antibody or that between the rabbit polyclonal antibodies and the FITC-conjugated AffiniPure goat anti-mouse IgG antibody was observed.
Immunoblotting Analysis
Rat mesangial cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 1× insulin-transferrin-selenium-A supplement and either high (30 mmol/L) or normal (5.6 mmol/L) concentration of glucose for 21 days. The cells were harvested and then lysed with 1% sodium dodecyl sulfate in 50 mmol/L of Tris-HCl buffer (pH 7.5) containing150 mmol/L NaCl, 0.2 mmol/L 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 μg/ml aprotinin, 5 μmol/L leupeptin, and 1 μmol/L pepstatin. Protein concentrations of the cell lysates were determined using bicinchoninic acid protein assay reagents (Pierce Chemical Co., Rockford, IL). Cell lysates were separated on 10% reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and ILK protein was detected by immunoblotting with anti-ILK antibody 65.1. Equal loading of cell lysates was confirmed by probing the membranes with anti-actin mAb1501.
Results
Expression of ILK in Normal Human Kidney
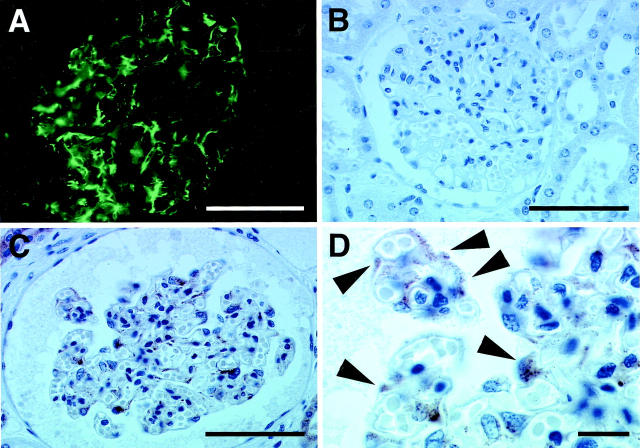
To analyze ILK distribution in normal human kidney, we stained sections derived from normal human kidneys with monoclonal anti-ILK antibody. Immunofluorescent staining of frozen sections of normal human kidneys revealed that ILK was present primarily in the glomerulus in a peripheral pattern (Figure 1A) ▶ . To further analyze this, we stained sections of formalin-fixed paraffin-embedded normal human kidney tissues, in which morphology was better preserved, with the monoclonal anti-ILK antibody. Consistent with the results of immunofluorescent staining, immunohistochemical analyses revealed that ILK is present to a large extent in the periphery of normal glomeruli (Figure 1C) ▶ . Examination of the sections at higher magnifications showed visceral epithelial cell staining (Figure 1D ▶ , arrowheads) by the monoclonal anti-ILK antibody. Faint staining of mesangial cells was also observed in some glomeruli of normal kidneys. Tubular cell staining, primarily in the distal tubule, was also present. Vascular staining was not apparent using the present techniques. In control experiments, no specific staining was detected when the monoclonal anti-ILK antibody was replaced with an irrelevant mouse IgG (Figure 1B) ▶ , confirming the specificity of the staining.
Figure 1.
Immunofluorescent and immunohistochemical staining of ILK in normal human kidney tissue. A: Immunofluorescent staining of frozen sections of normal human kidney tissues with anti-ILK antibody. Note that the monoclonal anti-ILK antibody stains the glomerulus in a peripheral pattern. This staining pattern was absent in specimens when the primary ILK antibody was omitted (not shown in the figure). B–D: Immunoperoxidase staining of formalin-fixed paraffin-embedded sections of normal human kidney tissues. The background staining was minimal in the immunohistochemistry studies (B). The anti-ILK antibody stains primarily the periphery of normal glomeruli (C). Tubular cell staining was localized to the distal tubules (not shown). Higher magnification demonstrates visceral epithelial cell staining (arrowheads) by the anti-ILK antibody, with only faint staining of mesangial cells (D). Scale bars, 50 μm (A–C) and 10 μm (D). Sections from five normal human kidneys were analyzed and similar results were obtained.
Expression of ILK in Diabetic Glomeruli
To determine whether ILK expression in the kidney is altered in patients with diabetic nephropathy, we analyzed sections of kidney tissues from patients with diabetic nephropathy by immunohistochemical staining with the monoclonal anti-ILK antibody. The results showed a striking increase in the ILK protein level in diabetic glomeruli, with strong staining detected in both mesangial cells and epithelial cells (Figure 2 ▶ ; A, B, and C, compare to Figure 1C ▶ ). Tubular cell staining was also increased focally in the proximal tubules in these specimens. We have compared the ILK staining in glomeruli from four patients with diabetic nephropathy with that in glomeruli from five normal control patients (25 glomeruli from each kidney were scored). The results showed that the ILK protein level was significantly increased in diabetic glomeruli (Figure 3) ▶ . The increase in ILK staining seemed to be associated with diffuse mesangial expansion (Figure 2, B and C) ▶ . ILK was not detected in nodular sclerotic lesions (Figure 2A ▶ , arrow). In glomeruli where advanced nodular sclerosis and global sclerosis were dominant, ILK staining was reduced (Figure 2 ▶ ; D, E, and F, arrows). Taken together, these results suggest that the amount of ILK expressed by mesangial cells was significantly increased in response to diabetic milieu and the increase in ILK expression was a relatively early event in the development of glomerulosclerosis.
Figure 2.
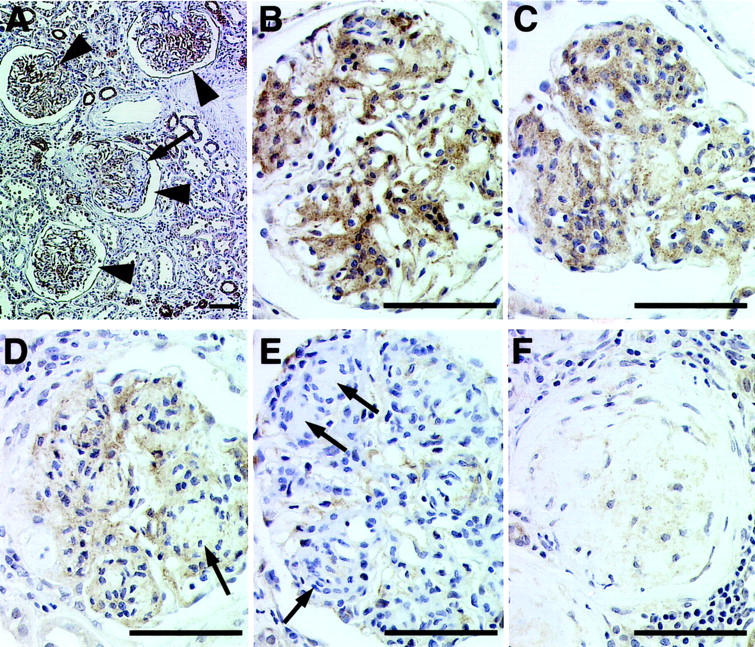
Representative immunohistochemical studies of diabetic glomeruli. Sections of diabetic human kidney tissues were stained with monoclonal anti-ILK antibody 65.1 as described in Materials and Methods. A: A low-power view of diabetic glomeruli, demonstrating segmental and global increases in ILK staining (arrowheads). A nodular sclerotic lesion (arrow) lacks ILK staining. Focal increases in staining of proximal tubular segments were also observed. B and C: Global increases in ILK staining are shown. The antibody appears to stain both mesangial and epithelial cells. As glomeruli undergo progressive sclerosis (D–F), ILK staining decreases. Scale bars, 50 μm.
Figure 3.
Mean stain intensity scores of normal and diabetic kidneys. ILK staining in glomeruli from four patients with diabetic nephropathy and five normal control patients (25 glomeruli from each kidney were scored) were analyzed as described in Materials and Methods. The mean score for diabetic kidneys, 2.7 ± 0.09, was significantly greater (P < 0.05) than the mean score for control kidneys, 1.3 ± 0.1.
High Glucose Concentration Increases the Amount of ILK Protein Expressed by Mesangial Cells
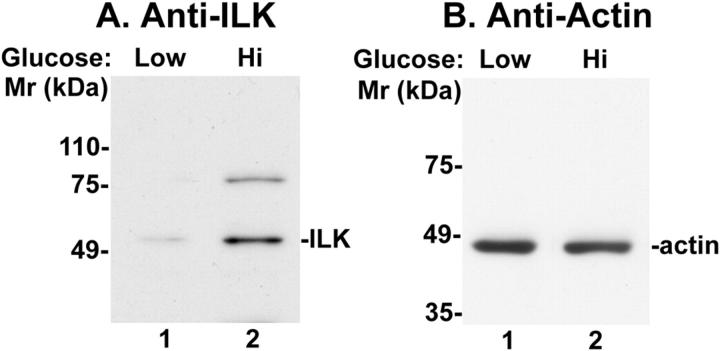
Hyperglycemia is an important factor contributing to mesangial expansion in the progression of diabetic nephropathy. To test whether an elevated concentration of glucose exerts an effect on the ILK protein level in mesangial cells, we cultured rat kidney glomerular mesangial cells in medium containing either normal concentration (5.6 mmol/L) or high concentration (30 mmol/L) of glucose. ILK expressed by mesangial cells that were exposed to normal or high concentration of glucose was detected by immunoblotting with monoclonal anti-ILK antibody. The results showed that exposure of mesangial cells to high glucose concentration significantly increased the amount of ILK protein expressed by the mesangial cells (Figure 4) ▶ , suggesting that hyperglycemia is likely a causal factor for the up-regulation of ILK protein level in mesangium (Figures 2 and 3) ▶ ▶ .
Figure 4.
Up-regulation of ILK protein level in response to high concentration of glucose. Rat mesangial cells were cultured in RPMI 1640 medium containing a normal concentration (5.6 mmol/L) (lane 1) or a high concentration (30 mmol/L) (lane 2) of glucose for 21 days. ILK and actin in cell lysates (0.25 mg/ml) were detected by immunoblotting (20 μl lysates/lane) with monoclonal anti-ILK antibody 65.1 (A) or anti-actin antibody mAb1501 (B) as described in Materials and Methods. Note that the amount of ILK (59 kd) was significantly increased in mesangial cells exposed to high glucose concentration. The amount of a higher molecular mass band (80 kd) that was recognized by the monoclonal anti-ILK antibody, which likely represents a sodium dodecyl sulfate-resistant ILK-containing protein complex or an ILK variant, 41 was also increased in response to high glucose.
ILK Co-Localizes with Fibronectin Matrix Deposited by Mesangial Cells
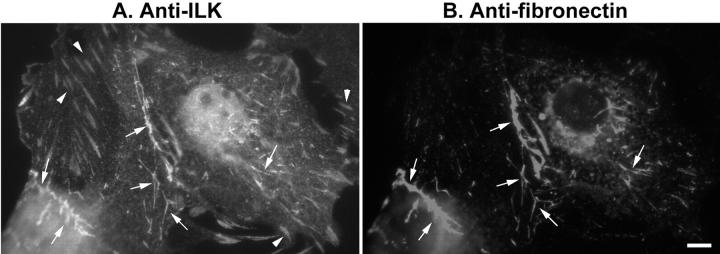
It has been well documented that kidney glomerular mesangial cells deposit fibronectin into extracellular matrix and the amount of fibronectin matrix deposited by mesangial cells increase in response to high concentration of glucose. 16,19 We have previously shown that in cells cultured in plastic dishes, ILK is clustered in focal adhesions, 46 regions of close contacts between the substrate and the plasma membrane on the basal surface of the cells. 49,50 In cells that assemble fibronectin matrix, cell membranes are also in close contact with fibronectin fibrils at sites termed as extracellular matrix contacts that are morphologically and structurally distinct from the focal adhesions. 51-57 Because extracellular matrix contacts are active sites for the deposition of fibronectin into extracellular matrix, 54,58 we have tested whether ILK also localizes in the extracellular matrix contacts. To do this, we stained mesangial cells with both mouse monoclonal anti-ILK antibody and rabbit polyclonal anti-fibronectin antibody. As expected, we have detected abundant ILK clusters in focal adhesions (Figure 5A ▶ , arrowheads). In addition, we have found that ILK was also clustered in sites where fibronectin fibrils were in contact with the cells (Figure 5, A and B ▶ , arrows). These results are highly consistent with our previous studies showing that overexpression of ILK promotes fibronectin matrix assembly 45 and suggest that ILK is likely directly involved in the initiation of fibronectin matrix assembly in mesangial cells.
Figure 5.
Co-localization of ILK with fibronectin fibrils. Rat kidney glomerular mesangial cells were double-stained with mouse monoclonal anti-ILK antibody (A) and rabbit polyclonal anti-fibronectin antibody (B). Arrows in A and B indicate extracellular matrix contacts where ILK and fibronectin fibrils were co-localized. Arrowheads in A indicate ILK clusters in focal adhesions. Scale bar, 2 μm.
Discussion
Diabetic glomerulosclerosis is characterized by the accumulation of extracellular matrix proteins including fibronectin in the mesangium. 5-8 Extensive studies in model cell culture systems including fibroblasts, Chinese hamster ovary cells, and rat intestinal epithelial cells and model animal systems have shed light on the general mechanisms by which cells regulate the deposition of fibronectin into extracellular matrix. Using genetic reconstitution approaches and functional blocking antibodies, studies by a number of laboratories including ours have demonstrated that the deposition of fibronectin into extracellular matrix can be regulated by multiple integrins (eg, α5β1, α3β1, and αvβ3 integrins). 32,34-38 Cell biological and genetic knock-out studies have also implied an important role of integrins in the assembly of basement membranes. 59-62 Using model cell culture systems, we have found that overexpression of ILK promotes fibronectin matrix deposition. 34 These studies suggest that integrins and integrin cytoplasmic domain-binding proteins such as ILK are important elements in the signaling pathway that controls fibronectin matrix assembly. It was not known, however, whether the cellular level of ILK is elevated in pathological situations such as diabetic nephropathy that associate with abnormal fibronectin matrix deposition. In this study, we have obtained evidence showing that ILK expression is markedly increased in glomerular mesangium in patients with diabetic nephropathy. The increase in ILK expression seems to be associated with diffuse mesangial expansion and therefore the up-regulation of ILK is likely a relatively early event in the pathogenesis of diabetic nephropathy. Additionally, we have found that exposure of mesangial cells to high glucose significantly increased the level of ILK in these cells. It is interesting to note that the levels of several integrins, including β1 integrins to which ILK binds, are also increased in the mesangium of human diabetic kidneys. 63 Exposure of cultured mesangial cells to high glucose concentration also increased the cellular level of several integrins. 64 Based on these studies, we propose that ILK, together with integrins and other associated proteins, are coordinately regulated in response to hyperglycemia, which in turn contributes to the expansion of mesangial matrix in patients with diabetic nephropathy. Clearly, future studies are required to further analyze the role of ILK and associated proteins in this pathological process.
Previous studies have shown that sites between cell membrane and fibronectin fibrils can serve as fibronectin matrix assembly sites initiating the deposition of fibronectin into extracellular matrix. 54,58 In this study, we have demonstrated that ILK is localized to fibronectin matrix contact sites. These observations, together with our previous results showing that overexpression of ILK promotes fibronectin matrix assembly, 45 raises an interesting possibility that ILK is directly involved in the organization of fibronectin matrix assembly sites and thereby play a direct role promoting fibronectin matrix assembly. We have previously demonstrated that integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. 38 ILK associates with multiple integrins including members of both β1 and β3 integrin families. 39,43 Recently, we have found that ILK can be physically connected to actin cytoskeleton through a novel ILK- and actin-binding protein that we termed as CH-ILKBP. 41 Thus, ILK could participate in the deposition of fibronectin into extracellular matrix by influencing the activation of integrins and/or by providing a connection between integrins that mediate fibronectin matrix assembly and the actin cytoskeleton. Regardless of the specific mechanism involved, the data shown in this and other studies strongly suggest that ILK is one important element in the cellular control of deposition of fibronectin into extracellular matrix, including that occurring in the mesangium in diabetic nephropathy. One important goal of future studies is to develop tools that down-regulate ILK expression, which is stimulated by hyperglycemia, as well as to develop tools that disrupt ILK function in mesangial cells. These tools will allow us to further test the notion that ILK promotes mesangial matrix accumulation found in patients with diabetic nephropathy and to develop new approaches to intervene in the progressive renal failure in diabetes patients.
In addition to regulating fibronectin matrix assembly, ILK is also involved in other integrin-mediated cellular process including cell adhesion, cell shape changes, and anchoring the actin cytoskeleton to the extracellular matrix. 39,43,44,65 In normal human kidneys, ILK is expressed at relatively high concentration in visceral epithelial cells or podocytes (Figure 1, C and D) ▶ . Visceral epithelial cells are involved in production of the outer portion of the glomerular basement membranes and maintenance of the filtration slit structure that prevents protein escape from the glomerular capillaries. 8,66-69 Recent studies have shown that visceral epithelial cells, such as mesangial cells, are also involved in progressive glomerular damage in diabetic patients. 67,68,70,71 Although alteration of ILK level in diabetic visceral epithelial cells was difficult to assess (in comparing to the readily detectable changes in mesangium), it is quite likely that ILK may play an important physiological role in visceral epithelial cell functions given its relatively high level of expression in normal visceral epithelial cells. Although the specific functions of ILK in visceral epithelial cell remain to be determined, data from studies in other systems suggest that ILK could be potentially involved in anchoring the actin cytoskeleton to the glomerular basement membranes, organization of the glomerular basement membranes, or maintenance of the cell shape of visceral epithelial cells. 39,41,45,72
Diseases are frequently caused by disorders of basic cell biological mechanisms that govern the functions of individual tissues and organs 73 and detailed knowledge of the molecular pathogenesis is a prerequisite for the design of specific therapies. 66 The progression of diabetic nephropathy is a complex process involving a number of factors including extracellular matrix, growth factors and other cytokines, and intracellular signaling proteins. The findings described in this report provide novel in vivo and in vitro evidence suggesting that ILK is one of the factors involved in the pathogenesis of diabetic glomerulosclerosis. Future studies aimed at defining the molecular mechanism by which ILK functions in kidney mesangial and visceral epithelial cells will likely help us to better understand the pathogenesis of diabetic nephropathy as well as other renal diseases associated with glomerulosclerosis.
Footnotes
Address reprint requests to Chuanyue Wu, 707B Scaife Hall, Department of Pathology, University of Pittsburgh, 3550 Terrace St., Pittsburgh, PA 15261. E-mail: carywu@imap.pitt.edu.
Supported by the National Institutes of Health (grants DK54639 to C. W. and DK46199 to P. W. S.), and the Research Service of the Department of Veterans Affairs (to P. W. S).
Current address of L. G. and C. W.: Department of Pathology, University of Pittsburgh, 3550 Terrace St., Pittsburgh, PA 15261.
References
- 1.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T: Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983, 25:496-501 [DOI] [PubMed] [Google Scholar]
- 2.: Renal failure in diabetics in the UK: Deficient provision of care in 1985. Joint Working Party on Diabetic Renal Failure of the British Diabetic Association, the Renal Association, and the Research Unit of the Royal College of Physicians. Diabet Med 1988, 5:79-84 [PubMed] [Google Scholar]
- 3.: System USRD: USRDS 1990 Annual Data Report, The National Institutes of Health, The National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 1990. August
- 4.Stephens GW, Gillaspy JA, Clyne D, Mejia A, Pollak VE: Racial differences in the incidence of end-stage renal disease in types I and II diabetes mellitus. Am J Kidney Dis 1990, 15:562-567 [DOI] [PubMed] [Google Scholar]
- 5.Abrass CK, Peterson CV, Raugi GJ: Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes 1988, 37:1695-1702 [DOI] [PubMed] [Google Scholar]
- 6.Abrass CK: Diabetic nephropathy. Mechanisms of mesangial matrix expansion. West J Med 1995, 162:318-321 [PMC free article] [PubMed] [Google Scholar]
- 7.Adler S: Structure-function relationships in diabetic nephropathy: lessons and limitations. Kidney Int 1997, 60(Suppl):S42-S45 [PubMed] [Google Scholar]
- 8.Phillips A, Janssen U, Floege J: Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press Res 1999, 22:81-97 [DOI] [PubMed] [Google Scholar]
- 9.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR: The changing natural history of nephropathy in type I diabetes. Am J Med 1985, 78:785-794 [DOI] [PubMed] [Google Scholar]
- 10.Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D: Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 1989, 261:1155-1160 [PubMed] [Google Scholar]
- 11.Kunzelman CL, Knowler WC, Pettitt DJ, Bennett PH: Incidence of proteinuria in type 2 diabetes mellitus in the Pima Indians. Kidney Int 1989, 35:681-687 [DOI] [PubMed] [Google Scholar]
- 12.Kobrin SM: Diabetic nephropathy. Dis Mon 1998, 44:214-234 [DOI] [PubMed] [Google Scholar]
- 13.Marcantoni C, Ortalda V, Lupo A, Maschio G: Progression of renal failure in diabetic nephropathy. Nephrol Dial Transplant 1998, 13:16-19 [DOI] [PubMed] [Google Scholar]
- 14.Rachmani R, Ravid M: Risk factors for nephropathy in type 2 diabetes mellitus. Compr Ther 1999, 25:366-369 [DOI] [PubMed] [Google Scholar]
- 15.Roy S, Sala R, Cagliero E, Lorenzi M: Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA 1990, 87:404-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayo SH, Radnik RA, Garoni JA, Glass WF, Kreisberg JI: High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol 1990, 136:1339-1348 [PMC free article] [PubMed] [Google Scholar]
- 17.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI: High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol 1991, 261:F571-F577 [DOI] [PubMed] [Google Scholar]
- 18.Nahman NS, Leonhart KL, Cosio FG, Hebert CL: Effects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cells. Kidney Int 1992, 41:396-402 [DOI] [PubMed] [Google Scholar]
- 19.Kreisberg JI, Ayo SH: The glomerular mesangium in diabetes mellitus. Kidney Int 1993, 43:109-113 [DOI] [PubMed] [Google Scholar]
- 20.Hynes RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 21.Clark EA, Brugge JS: Integrins and signal transduction pathways: the road taken. Science 1995, 268:233-239 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz MA, Schaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995, 11:549-599 [DOI] [PubMed] [Google Scholar]
- 23.Yamada KM, Miyamoto S: Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol 1995, 7:681-689 [DOI] [PubMed] [Google Scholar]
- 24.Dedhar S: Integrins and signal transduction. Curr Opin Hematol 1999, 6:37-43 [DOI] [PubMed] [Google Scholar]
- 25.Giancotti FG, Ruoslahti E: Integrin signaling. Science 1999, 285:1028-1032 [DOI] [PubMed] [Google Scholar]
- 26.Mosher DF, Sottile J, Wu C, McDonald JA: Assembly of extracellular matrix. Curr Opin Cell Biol 1992, 4:810-818 [DOI] [PubMed] [Google Scholar]
- 27.Wu C: Roles of integrins in fibronectin matrix assembly. Histol Histopathol 1997, 12:233-240 [PubMed] [Google Scholar]
- 28.Schwarzbauer JE, Sechler JL: Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol 1999, 11:622-627 [DOI] [PubMed] [Google Scholar]
- 29.Akiyama SK, Yamada SS, Chen WT, Yamada KM: Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol 1989, 109:863-875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darribere T, Guida K, Larjava H, Johnson KE, Yamada KM, Thiery JP, Boucaut JC: In vivo analyses of integrin beta 1 subunit function in fibronectin matrix assembly. J Cell Biol 1990, 110:1813-1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF: Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol 1990, 111:699-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giancotti FG, Ruoslahti E: Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 1990, 60:849-859 [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Bauer JS, Juliano RL, McDonald JA: The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem 1993, 268:21883-21888 [PubMed] [Google Scholar]
- 34.Wu C, Chung AE, McDonald JA: A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci 1995, 108:2511-2523 [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Hughes PE, Ginsberg MH, McDonald JA: Identification of a new biological function for the integrin alpha v beta 3: initiation of fibronectin matrix assembly. Cell Adhesion Commun 1996, 4:149-158 [DOI] [PubMed] [Google Scholar]
- 36.Yang JT, Hynes RO: Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell 1996, 7:1737-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R: Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol 1996, 132:227-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Keivens VM, Te OT, McDonald JA, Ginsberg MH: Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995, 83:715-724 [DOI] [PubMed] [Google Scholar]
- 39.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S: Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 1996, 379:91-96 [DOI] [PubMed] [Google Scholar]
- 40.Tu Y, Li F, Goicoechea S, Wu C: The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 1999, 19:2425-2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu Y, Huang Y, Zhang Z, Hua Y, Wu C: A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 2001, 153:585-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolopoulos SN, Turner CE: Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem 2001, 276:23499-23505 [DOI] [PubMed] [Google Scholar]
- 43.Dedhar S, Williams B, Hannigan G: Integrin linked kinase (ILK): a regulator of integrin and growth-factor signaling. Trends Cell Biol 1999, 9:319-323 [DOI] [PubMed] [Google Scholar]
- 44.Wu C: Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci 1999, 112:4485-4489 [DOI] [PubMed] [Google Scholar]
- 45.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S: Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem 1998, 273:528-536 [DOI] [PubMed] [Google Scholar]
- 46.Li F, Zhang Y, Wu C: Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci 1999, 112:4589-4599 [DOI] [PubMed] [Google Scholar]
- 47.Thomas GJ, Shewring L, McCarthy KJ, Couchman JR, Mason RM, Davies M: Rat mesangial cells in vitro synthesize a spectrum of proteoglycan species including those of the basement membrane and interstitium. Kidney Int 1995, 48:1278-1289 [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Kleppel MM, Butkowski R, Mauer SM, Wieslander J, Michael AF: Differential expression of basement membrane collagen chains in diabetic nephropathy. Am J Pathol 1991, 138:413-420 [PMC free article] [PubMed] [Google Scholar]
- 49.Burridge K, Chrzanowska-Wodnicka M: Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 1996, 12:463-518 [DOI] [PubMed] [Google Scholar]
- 50.Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J: The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol 1995, 11:379-416 [DOI] [PubMed] [Google Scholar]
- 51.Chen WT, Singer SJ: Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol 1982, 95:205-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada KM, Geiger B: Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol 1997, 9:76-85 [DOI] [PubMed] [Google Scholar]
- 53.Singer II, Scott S, Kawka DW, Kazazis DM, Gailit J, Ruoslahti E: Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol 1988, 106:2171-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM: Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol 2000, 148:1075-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z: Molecular diversity of cell-matrix adhesions. J Cell Sci 1999, 112:1655-1669 [DOI] [PubMed] [Google Scholar]
- 56.Katz BZ, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B: Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell 2000, 11:1047-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B: Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol 2000, 2:191-196 [DOI] [PubMed] [Google Scholar]
- 58.Christopher RA, Kowalczyk AP, McKeown-Longo PJ: Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci 1997, 110:569-581 [DOI] [PubMed] [Google Scholar]
- 59.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO: Alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol 1997, 137:729-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki T, Forsberg E, Bloch W, Addicks K, Fassler R, Timpl R: Deficiency of beta 1 integrins in teratoma interferes with basement membrane assembly and laminin-1 expression. Exp Cell Res 1998, 238:70-81 [DOI] [PubMed] [Google Scholar]
- 61.Schwarzbauer J: Basement membranes: putting up the barriers. Curr Biol 1999, 9:R242-R244 [DOI] [PubMed] [Google Scholar]
- 62.Colognato H, Winkelmann DA, Yurchenco PD: Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol 1999, 145:619-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, Kim Y: Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol 1996, 7:2636-2645 [DOI] [PubMed] [Google Scholar]
- 64.Setty S, Anderson SS, Wayner EA, Kim Y, Clegg DO, Tsilibary EC: Glucose-induced alteration of integrin expression and function in cultured human mesangial cells. Cell Adhes Commun 1995, 3:187-200 [DOI] [PubMed] [Google Scholar]
- 65.Huang Y, Li J, Zhang Z, Wu C: The roles of integrin-linked kinase in the regulation of myogenic differentiation. J Cell Biol 2000, 150:861-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerjaschki D: Dysfunctions of cell biological mechanisms of visceral epithelial cell (podocytes) in glomerular diseases. Kidney Int 1994, 45:300-313 [DOI] [PubMed] [Google Scholar]
- 67.Rennke HG: How does glomerular epithelial cell injury contribute to progressive glomerular damage? Kidney Int 1994, 45(Suppl):S58-S63 [PubMed] [Google Scholar]
- 68.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997, 99:342-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kriz W, Lemley KV: The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens 1999, 8:489-497 [DOI] [PubMed] [Google Scholar]
- 70.Regoli M, Bendayan M: Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 1997, 40:15-22 [DOI] [PubMed] [Google Scholar]
- 71.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 2000, 15:1379-1383 [DOI] [PubMed] [Google Scholar]
- 72.Zervas CG, Gregory SL, Brown NH: Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol 2001, 152:1007-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virchow R: Cellular pathology. Diseases, Life and Man. 1958, :pp 71-101 Stanford University Press, Stanford [Google Scholar]