Abstract
There is considerable evidence that osteoclasts are involved in the pathogenesis of focal bone erosion in rheumatoid arthritis. Tumor necrosis factor-related activation-induced cytokine, also known as receptor activator of nuclear factor-κB ligand (TRANCE/RANKL) is an essential factor for osteoclast differentiation. In addition to its role in osteoclast differentiation and activation, TRANCE/RANKL also functions to augment T-cell dendritic cell cooperative interactions. To further evaluate the role of osteoclasts in focal bone erosion in arthritis, we generated inflammatory arthritis in the TRANCE/RANKL knockout mouse using a serum transfer model that bypasses the requirement for T-cell activation. These animals exhibit an osteopetrotic phenotype characterized by the absence of osteoclasts. Inflammation, measured by clinical signs of arthritis and histopathological scoring, was comparable in wild-type and TRANCE/RANKL knockout mice. Microcomputed tomography and histopathological analysis demonstrated that the degree of bone erosion in TRANCE/RANKL knockout mice was dramatically reduced compared to that seen in control littermate mice. In contrast, cartilage erosion was present in both control littermate and TRANCE/RANKL knockout mice. These results confirm the central role of osteoclasts in the pathogenesis of bone erosion in arthritis and demonstrate distinct mechanisms of cartilage destruction and bone erosion in this animal model of arthritis.
Rheumatoid arthritis (RA) is an immune-mediated inflammatory arthritis that exhibits the capacity to invade and destroy the extracellular matrices of cartilage and bone. Several lines of evidence support a role for osteoclasts in focal bone erosion in RA. Scanning electron microscopic analysis of metacarpal heads taken from areas of pannus invasion have demonstrated resorption bays typical of osteoclastic activity in areas of calcified cartilage and subchondral bone. 1 Analysis of tissue samples from the bone-cartilage interface from patients with RA demonstrated acid phosphatase-positive multinucleated cells in subchondral bone associated with erosions. 2 In situ hybridization studies in similar tissues demonstrated that multinucleated cells in sites of focal bone erosion expressed messenger RNA (mRNA) for tartrate resistant acid phosphatase (TRAP) and cathepsin K, 3,4 both typical but not unique markers of osteoclasts. 5-8 Multinucleated cells in these locations also expressed calcitonin receptor mRNA 9 consistent with their identity as fully functional osteoclasts. 10,11 Other indirect evidence for the role of osteoclasts in bone erosion in arthritis comes from animal models of arthritis in which similar multinucleated cells expressing osteoclast markers have been observed. 12-14
The presence of osteoclasts at sites of focal bone erosion in RA and in animal models of arthritis suggests that factors leading to osteoclast differentiation and activation may play an important role in the pathogenesis of these erosions. An essential factor for osteoclast differentiation, designated osteoprotegerin (OPG) ligand, 15 and osteoclast differentiation factor, 16 has recently been identified. This protein was originally identified as a T-cell product, tumor necrosis factor-related activation-induced cytokine (TRANCE), that can regulate dendritic cell function and survival, 17 and as receptor activator of nuclear factor-κB ligand (RANKL). 18 In this study we refer to this factor as TRANCE/RANKL. In vitro studies using bone marrow co-culture models have demonstrated that many of the factors that enhance osteoclast formation or activity mediate their effects at least in part by up-regulating the expression of TRANCE/RANKL on cells of the osteoblast lineage. 19-22 OPG, 23 also known as osteoclastogenesis inhibitory factor 24 is a soluble decoy receptor for TRANCE/RANKL. OPG binds TRANCE/RANKL with high affinity and thus prevents the interaction of TRANCE/RANKL with its cognate receptor, receptor activator of nuclear factor-κB (RANK).
The essential role of TRANCE/RANKL in osteoclast differentiation is demonstrated by the phenotype of mice in which this gene has been deleted. TRANCE/RANKL knockout (KO) mice exhibit severe osteopetrosis and defective tooth eruption associated with a complete absence of osteoclasts, 25 irregular bone surfaces because of the absence of remodeling, abnormal growth plates with club-shaped long bones, and growth retardation at several skeletal sites. 26 In addition, these mice demonstrate defects in B-cell and T-cell maturation, as well as an absence of lymph nodes, supporting a role for this factor in immune cell differentiation. 25,27 Furthermore, TRANCE/RANKL has been demonstrated to enhance the viability and adjuvant properties of dendritic cells resulting in the activation and proliferation of T-cells. Its expression is also up-regulated in T-cells after T-cell receptor stimulation. 17,18,28
A role for TRANCE/RANKL in bone resorption in RA is suggested by the identification of TRANCE/RANKL mRNA 3,29 and protein 29 in cultured synovial fibroblasts from patients with RA and in CD4+ and CD8+ T lymphocytes in RA synovial tissues. 3,30,31 Additional evidence that TRANCE/RANKL plays a critical role in the pathogenesis of bone erosion in inflammatory arthritis comes from studies in the rat adjuvant arthritis model, a T-cell-driven experimental arthritis. 30 Arthritic rats treated with OPG early in the course of arthritis demonstrated only minimal erosion of cortical and trabecular bone, as compared with severe bone erosion in untreated control animals. Treatment with OPG also prevented osteoclast accumulation, whereas destruction of bone in untreated arthritic animals was accompanied by the accumulation of large numbers of TRAP+ osteoclast-like cells. 30 OPG treatment in an animal model of arthritis that is dependent on T-cell activation, however, has the potential for blocking not only the effects of TRANCE/RANKL on osteoclast differentiation and activation, but also the influence of TRANCE/RANKL on T-cell-dendritic cell interactions.
To more definitively determine the role of TRANCE/RANKL on bone erosion in arthritis independent of its role in inflammation, we induced arthritis in TRANCE/RANKL KO mice using a serum transfer model. This model is a variant of a well-described animal model in which T-cell transgenic K/B×N mice develop spontaneous autoimmune arthritis. This spontaneous arthritis is dependent on T-cell B-cell interactions resulting in the production of pathogenic anti-glucose-6-phosphate isomerase antibody. 32-34 The arthritis displays many of the characteristic features of RA including leukocyte invasion in affected joints, pannus formation, and cartilage and bone destruction. Transfer of serum containing anti-glucose-6-phosphate isomerase antibody results in the development of arthritis in recipient mice. 33 Serum transfer arthritis is rapid in onset and histologically resembles the spontaneous arthritis. However T-cells and B-cells are not required for this phase of the arthritis and, once produced, the anti-glucose-6-phosphate isomerase antibodies are sufficient to confer disease. 33 The serum transfer model provides an opportunity to investigate the role of TRANCE/RANKL on osteoclastogenesis and bone erosion in an inflammatory arthritis that resembles RA but is independent of cooperative T cell-dendritic cell interactions.
Materials and Methods
Mice
As previously described KRN T-cell transgenic mice maintained on the C57BL/6 background were crossed with nonobese diabetic mice to generate K/B×N mice that develop spontaneous arthritis. 33,34 The generation of the TRANCE/RANKL-deficient mice has been described. 26 The mice used in the present experiments were (C57BL/6 × 129)F2 background. Control mice (Ctl) were matched littermates (+/− or +/+ genotypes). As the TRANCE/RANKL KO mouse phenotype includes severe osteopetrosis with failure of tooth eruption, these mice were maintained with powdered food in the cage. These experiments were reviewed by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC), protocol no. 3224, and the University of Pennsylvania IACUC, approval no. 702636.
Generation of Arthritis by K/B×N Serum Transfer
K/B×N serum pools were prepared from arthritic mice at 60 days of age. Arthritis was induced in recipient mice by intraperitoneal injection (10 to 12.5 μl serum/g weight) in 200 to 250 μl total volume at days 0 and 2, and monitored throughout the next 12 days. For follow-up of longer duration, mice were reinjected with 200 to 250 μl of serum (10 to 12.5 μl serum/g weight) on days 7 and 12 or 14. Five arthritic TRANCE/RANKL KO mice and their matched control littermates were analyzed in five different experiments. Animals were sacrificed 12 to 13 days (experiments A and B) and 21 days (experiments C, D, and E) after initial serum injection to assess both the acute and chronic phases of the arthritis. 33 Phosphate-buffered saline (PBS)-injected controls were sacrificed 21 days after the initial injection (experiments C and D). A clinical index was determined throughout time −1 point for each affected limb; 0.5 for a limb with only mild swelling/redness or only a few digits affected. Ankle thickness was measured by a caliper, 33 and ankle thickening was defined as the difference in ankle thickness vis-a-vis the day 0 measure. Numerical values derived from these measurements were MaxAT (maximum ankle thickness) and AUC(0-12)AT (the integral of ankle thickening throughout the initial 12 day observation period) (Ji et al, submitted). To monitor new bone formation, a feature of this model of arthritis, mice in experiment D were also injected intraperitoneally with 15 mg/kg body weight of tetracycline hydrochloride (Sigma, St. Louis, MO) 2 days before and 18 days after the initial serum injection.
Microcomputed Tomographic (MicroCT) Imaging and Histology
Inflamed hind limbs were assessed by microCT and histology. Hind limbs were collected and the knee and ankle joints were separated at the mid tibia. Specimens were fixed in 4% paraformaldehyde for a minimum of 12 hours. For microCT imaging, ankle and forefoot joints were analyzed by means of a compact fan-beam-type microCT system (μCT 20; Scanco Medical AG, Bassersdorf, Switzerland 35 ). This system has been used in previous studies in which microCT images have been demonstrated to closely correlate with bone histomorphometric analysis in murine and human samples. 36,37
Lower hind limbs were placed in formalin-filled air-tight cylindrical containers marked with an axial alignment line to allow for consistent positioning of the specimens. Analysis consisted of a scout view, selection of the examination volume, automatic positioning, measurement, off-line reconstruction, and evaluation. For each sample, ∼200 microCT slices 17-μm apart were acquired, covering the entire medial-lateral width of the ankle. Images were reconstructed in 1024 × 1024 pixel matrices providing a nominal resolution of 17 μm. A constrained three-dimensional Gaussian filter was used for partial suppression of the noise in the volumes. 38 All samples were filtered according to the same parameters for filter width (1.0) and filter support. 1 Three-dimensional visualization was performed according to a previously described method. 39 All two-dimensional slices from the three-dimensional stack of microCT images were assessed for bone erosion by two independent observers (D.S. and R.M.). A two-dimensional mid-sagittal slice comprising a detailed view of tibia, talus, and forefoot bones was chosen for careful correlation with histological sections.
For histological analysis specimens were dissected to remove skin and outer muscle and demineralized for ∼2 weeks in 14% ethylenediaminetetraacetic acid followed by paraffin embedding (Citadel 1000; Shandon, Pittsburgh, PA). For each specimen at least 40 5-μm sagittal serial sections were cut. At least every fifth section was stained with hematoxylin and eosin (H&E) (Sigma) for evaluation of inflammation, bone erosion, and cartilage destruction. An adjacent section was stained with toluidine blue (Sigma) for specific evaluation of proteoglycan. TRAP staining was performed by a modification of a previously described method. 40 Briefly, sections were incubated for 15 minutes at 37°C in freshly prepared 0.1 mol/L Tris buffer, pH 5.0, 1.35 mmol/L naphthol AS-MX phosphate (Sigma), 0.362 mol/L N,N-dimethylformamide, 3.88 mmol/L Violet LB salt (Sigma), and 25 mmol/L sodium tartrate. Slides were rinsed for 10 minutes and counterstained with hematoxylin. For assessment of new bone formation, one hind paw from the tetracycline-injected group of mice was fixed as for histology followed by dehydration in ascending grades of ethanol and embedding in OsteoBed resin (methacrylate; Polysciences, Warrington, PA) according to the manufacturer’s instructions. One-μm sections were cut using a Sorvall Porterblum MT-2B ultramicrotome with a glass knife. New bone formation was visualized using fluorescent microscopy (Nikon, Tokyo, Japan).
Histopathological Scoring
Histopathological scoring was performed as previously described 41 with the following minor modifications. The tibio-talar joint and forefoot joints were scored separately and the scoring criteria were altered to allow for independent scoring of these joint areas. Mild edema was included only in the minimal inflammation score because it is an early feature of this animal model of arthritis. 34 Osteoclast quantification was removed from the criteria for bone erosion because of the total absence of osteoclasts in the TRANCE/RANKL KO mice. 25,26 At least six H&E-stained sections were scored by two independent observers (A.R.P. and E.M.G.) at low power for inflammation, and low and high power for bone erosion. Serial toluidine blue-stained sections were scored for cartilage damage and proteoglycan loss in areas remote from inflamed synovium (pannus), and cartilage damage in areas adjacent to pannus was scored on H&E-stained sections as outlined in Table 1 ▶ . The mean score for each histopathological feature was calculated.
Table 1.
Histopathological Scoring Criteria
| Score | Inflammation | Bone erosion | Cartilage damage | |
|---|---|---|---|---|
| Remote from pannus | Adjacent to pannus | |||
| 0 | Normal | Normal | Normal | Normal |
| 1 | Minimal infiltration of inflammatory cells and/or mild edema | Small areas of resorption, not readily apparent on low magnification, in trabecular or cortical bone. | Minimal to mild loss of cartilage with no obvious chondrocyte loss or collagen disruption | Pannus formation with superficial cartilage destruction |
| 2 | Mild infiltration | More numerous areas of resorption, not readily apparent on low magnification, in trabecular or cortical bone. | Mild loss of cartilage with mild (superficial) chondrocyte loss and/or collagen disruption | Pannus formation with moderate cartilage destruction (depth to the middle zone) |
| 3 | Moderate infiltration | Obvious resorption of trabecular and cortical bone, without full thickness defects in the cortex; loss of some trabeculae; lesions apparent on low magnification. | Moderate loss of cartilage with moderate multifocal (depth to middle zone) chondrocyte loss and/or collagen disruption | Pannus formation with marked cartilage destruction (depth to the tidemark) |
| 4 | Marked infiltration | Full thickness defects in the cortical bone and marked trabecular bone loss, without distortion of the profile of the remaining cortical surface. | Marked loss of cartilage with marked multifocal (depth to deep zone) chondrocyte loss and/or collagen disruption | N/A |
| 5 | Severe infiltration | Full thickness defects in the cortical bone and marked trabecular bone loss, with distortion of the profile of the remaining cortical surface. | Severe diffuse loss of cartilage with severe multifocal (depth to tidemark) chondrocyte loss and/or collagen disruption | N/A |
Histopathological criteria was modified from a previously described method. 41
Abbreviation: N/A, not applicable.
Results
Clinical and Histological Features of Inflammation Are Similar in TRANCE/RANKL KO and Ctl Mice with Serum Transfer Arthritis
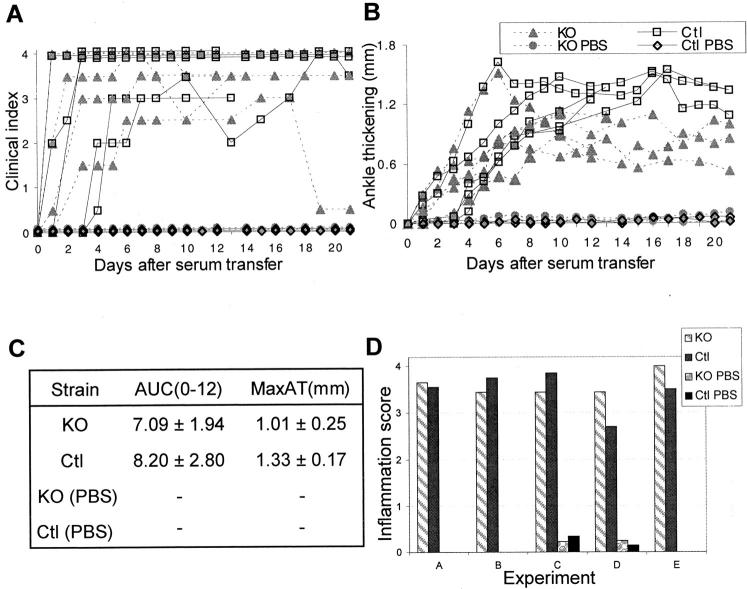
The susceptibility of TRANCE/RANKL-deficient mice to K/B×N arthritis was tested in a serum transfer protocol. Knockout mice and control littermates (Ctl) were injected with serum from 60-day-old K/B×N arthritic mice, and the progression of arthritis was followed throughout the next 3 weeks. The onset of clinical signs of arthritis and ankle thickening occurred between days 1 to 3 in both the KO and Ctl mice (Figure 1A) ▶ . The disease then progressed in a similar manner in both types of mice, with a rate of ankle thickening consistent with previous observations in inbred mouse strains 33,42 The only clinical difference observed was a consistently but modestly lower plateau level of ankle thickening beyond day 7 in the TRANCE/RANKL KO mice (Figure 1B) ▶ . AUC(0-12)AT (the integral of ankle thickening throughout the initial 12-day observation period) and MaxAT (maximum ankle thickness) demonstrated a similar severity and rapidity of disease development in TRANCE/RANKL KO and Ctl mice (Figure 1C) ▶ . Inflammation was specifically assessed using histopathological scoring as outlined in Table 1 ▶ . Both the TRANCE/RANKL KO and Ctl mice demonstrated moderate to marked inflammation (Figure 1D) ▶ at the time of sacrifice. Taken together, these data indicate that there is little difference in the degree of inflammation in TRANCE/RANKL KO and Ctl with serum transfer arthritis. Neither the PBS-injected Ctl or TRANCE/RANKL KO mice showed signs of inflammation (Figure 1) ▶ .
Figure 1.
TRANCE/RANKL KO and Ctl mice with serum transfer arthritis develop inflammation that is clinically and histologically similar. TRANCE/RANKL KO (KO) and Ctl mice were injected with K/B×N serum or PBS (experiments C and D only) and sacrificed at 12 to 13 days (experiments A and B) or 21 days (experiments C to E) after the initial serum injection. Arthritis was monitored by clinical index (A) and measurement of ankle thickness, represented as ankle thickening (B) and AUC(0-12)AT and MaxAT (C). Histopathological scoring of inflammation in H&E-stained sections (D) was performed as outlined in Table 1 ▶ .
Bone Erosion Is Dramatically Reduced in TRANCE/RANKL KO Mice with Serum Transfer Arthritis
MicroCT and histopathological analyses were used to assess the degree of bone erosion in TRANCE/RANKL KO and Ctl mice with serum transfer arthritis. Areas of bone erosion were apparent on microCT images of all arthritic Ctl mice at both time points studied, and appeared as ragged bone surfaces and gaps in the cortical and trabecular bone (Figure 2A) ▶ . The degree of bone erosion in the arthritic Ctl mice was striking even in mice sacrificed on day 12 (data not shown). Erosion of cortical and trabecular bone evident on the microCT images correlated well with focal and full thickness cortical and trabecular bone erosions present in the matched H&E sections as demonstrated in a representative arthritic Ctl mouse sacrificed on day 21 (Figure 2, A and B ▶ , asterisks).
Figure 2.
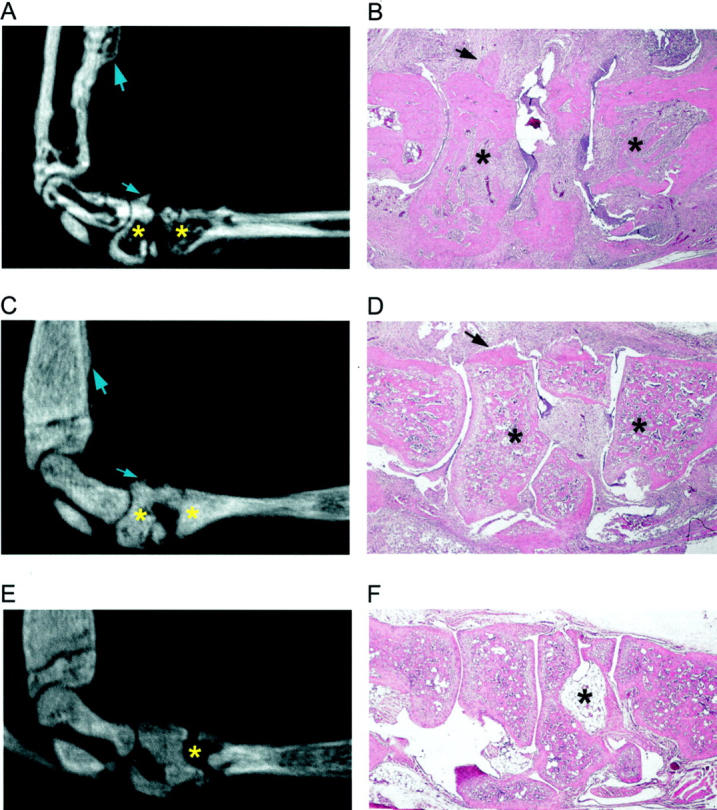
MicroCT and histological analysis of bone erosion in TRANCE/RANKL KO and Ctl mice. To assess bone erosion in TRANCE/RANKL KO and Ctl mice with serum transfer arthritis microCT images and histological sections were analyzed and correlated. Matched sagittal microCT images and H&E-stained sections for each sample were identified and compared: A and B, arthritic Ctl mouse, day 21; C and D, arthritic TRANCE/RANKL KO mouse, day 21; E and F, PBS-injected TRANCE/RANKL KO mouse, day 21. In A to C, asterisks indicate examples of significant erosion in Ctl mice, and the absence of erosion at the same anatomical location in TRANCE/RANKL KO mice in matched microCT images and H&E sections. In D and E, asterisks indicate the same anatomical location in the matched microCT image and H&E section. Arrows indicate sites of new bone formation. Original magnifications: ×4 (B, D, and F).
The osteopetrotic phenotype of the TRANCE/RANKL KO mice was immediately evident on microCT and on histological analysis (Figure 2 ▶ ; C to F). 25,26 PBS-injected TRANCE/RANKL KO mice (Figure 2, E and F) ▶ were used as controls for the assessment of bone erosion in arthritic TRANCE/RANKL KO mice. Bone in the TRANCE/RANKL KO mice is primarily woven bone because of the absence of bone remodeling. 26 Thin cortical bone is present but has an irregular surface (Figure 2F) ▶ and trabecular bone, although mineralized (as detected by microCT analysis; Figure 2, C and E ▶ ), has a high proteoglycan content, demonstrated by toluidine blue staining. In contrast to the findings in arthritic Ctl mice, microCT images of TRANCE/RANKL KO mice with arthritis demonstrated continuous bone surfaces and showed evidence of dramatic reduction in bone erosion at both time points studied (Figure 2C) ▶ . Histological analysis was performed on matched H&E sections to correlate with the microCT observations (Figure 2, C and D) ▶ . H&E sections confirmed that cortical bone surfaces were predominantly intact (Figure 2D) ▶ indicating that the TRANCE/RANKL KO mice are protected from bone erosion in this model of arthritis. TRAP histochemistry was performed to determine whether TRAP+ multinucleated cells were present at sites of bone erosion in this model. Numerous TRAP+ multinucleated cells were present on bone at sites of cortical and trabecular bone erosion in arthritic Ctl mice (Figure 3 ▶ ; A, B, and E). In contrast, TRAP staining confirmed the complete absence of TRAP+ multinucleated cells in TRANCE/RANKL KO mice (Figure 3, C and D) ▶ .
Figure 3.
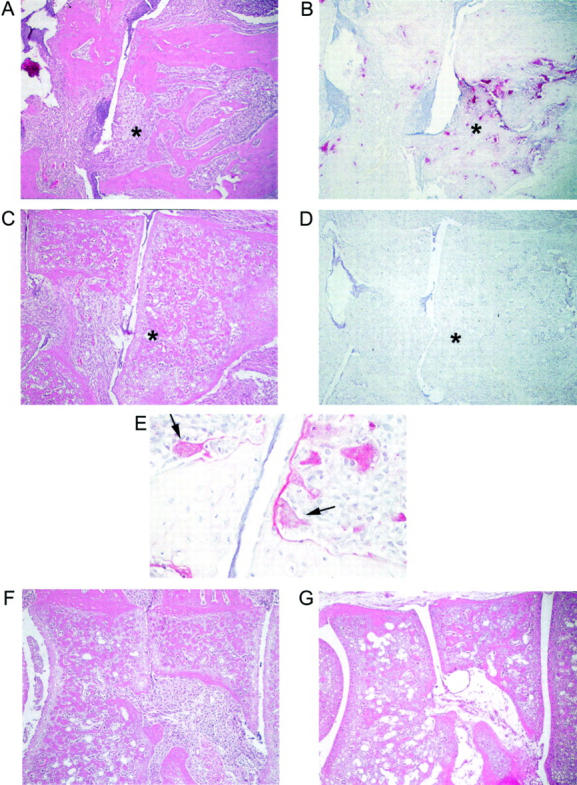
TRAP+ multinucleated cells are present in focal bone erosion in arthritic Ctl mice. H&E and TRAP histochemistry were performed in serial sections of arthritic Ctl and TRANCE/RANKL KO mice. A: H&E section illustrating focal bone erosion in an arthritic Ctl mouse sacrificed on day 21. B: Serial section stained for TRAP. Numerous multinucleated TRAP+ cells are present on the surface of the cortical and trabecular bone in areas of erosion. C: H&E section of an arthritic TRANCE/RANKL KO mouse sacrificed at day 21. D: Serial section stained for TRAP. Asterisks indicate the same anatomical site in A to D. E: High-power view of arthritic Ctl mouse demonstrating multinucleated (arrows) and occasional mononuclear TRAP+ cells in subchondral bone erosions. F and G: H&E-stained sections illustrating areas of irregular osteopetrotic bone present in both the arthritic (F) and PBS-injected (G) TRANCE/RANKL KO mice. Note that the inflamed tissue fills spaces to which it has direct access, including nutrient artery entry sites into bone. Original magnifications: ×10 (A–D, F, and G); ×40 (E).
To confirm the protection of bone from focal erosion in TRANCE/RANKL KO mice, histopathological scoring of bone erosion was performed in Ctl and TRANCE/RANKL KO mice using the criteria outlined in Table 1 ▶ . Bone erosion was more prominent in the forefoot in arthritic Ctl mice. Therefore, forefoot and tibio-talar joints were scored separately to more accurately reflect the disease process. Figure 4A ▶ demonstrates bone erosion scores for paired Ctl and TRANCE/RANKL KO mice with arthritis (and PBS-injected controls in some experiments) and confirms that bone erosion is dramatically reduced in TRANCE/RANKL KO mice with serum transfer arthritis. Careful histological analysis of ankle and forefoot joints was performed in arthritic TRANCE/RANKL KO mice and comparisons were made to PBS-injected TRANCE/RANKL KO mice. The abnormal osteopetrotic bone phenotype in the TRANCE/RANKL KO mice with irregular bone shapes and prominent nutrient artery entry points made definitive exclusion of bone erosion impossible (Figure 3F and G) ▶ . Any irregular bone feature in an area of synovitis that could not be definitively identified as a manifestation of the osteopetrotic bone phenotype was scored as erosion. The degree of focal bone erosion that was present in the TRANCE/RANKL KO mice with arthritis was small.
Figure 4.
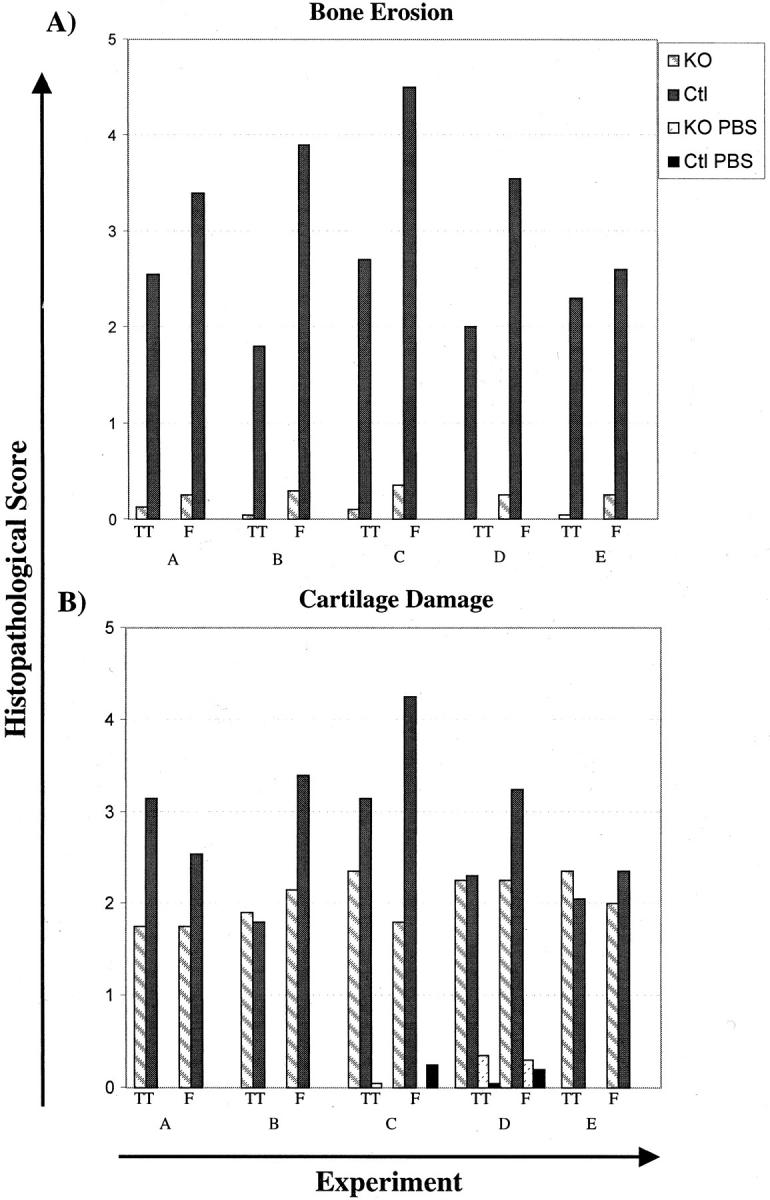
TRANCE/RANKL KO mice with serum transfer arthritis are protected from bone erosion. Histopathological scoring of bone erosion (A) and cartilage damage (B) were performed using the criteria outlined in Table 1 ▶ . The tibio-talar (TT) and forefoot (F) joints were scored separately and the average score for each site was compared with its matched pair. TRANCE/RANKL KO and Ctl mice were injected with K/B×N serum and sacrificed at days 12 to 13 (experiments A and B) or day 21 (experiments C to E) after the initial serum injection. In experiments C and D control TRANCE/RANKL KO (KO PBS) and Ctl (Ctl PBS) mice were injected with PBS. PBS injected control mice are not pictured in A because of the absence of bone erosion in these mice.
MicroCT and histopathological analyses indicated that new bone deposition is a feature of serum transfer arthritis. Sites of new bone deposition were similar in arthritic Ctl and arthritic TRANCE/RANKL KO mice and included the anterior tibia, the dorsal surfaces of forefoot bones (Figure 2 ▶ ; A to D, arrows), and the volar surface of the calcaneus (not shown). These bone deposits were confirmed as sites of new bone formation by tetracycline labeling (data not shown). The new bone was remodeled by TRAP+ multinucleated cells in Ctl but not in TRANCE/RANKL KO mice. This is reflected by the higher density of areas of bone deposition in the TRANCE/RANKL KO mice demonstrated on microCT (Figure 2C) ▶ .
Cartilage Destruction Occurs in TRANCE/RANKL KO Mice
Cartilage destruction was assessed in the tibio-talar joint and in joints within the forefoot in Ctl and TRANCE/RANKL KO mice by scoring of sections stained with toluidine blue, which stains cartilage proteoglycan, and by examination of loss of cartilage in direct contact with pannus tissue on H&E-stained sections. Toluidine blue-stained sections revealed loss of cartilage proteoglycan in arthritic Ctl and TRANCE/RANKL KO mice as compared to PBS-injected control mice (Figure 5 ▶ ; A to C). Scoring of toluidine blue-stained sections revealed loss of articular cartilage in all mice with arthritis. The degree of proteoglycan and chondrocyte loss showed a trend toward higher scores in the arthritic Ctl mice (Figure 4B) ▶ . However in these mice, complete loss of articular cartilage was noted in areas of subchondral bone erosion where the scaffolding subchondral bony plate deep to articular cartilage was completely lost (Figure 5D) ▶ . Similar areas of subchondral bone erosion and associated loss of articular cartilage were absent in KO mice (Figure 5E) ▶ . Cartilage loss in areas of articular cartilage adjacent to inflamed synovium was present in both Ctl and TRANCE/RANKL KO mice (Figure 5F) ▶ , with a similar trend toward higher scores in Ctl mice (data not shown). The results indicate that cartilage damage occurs in both the TRANCE/RANKL KO and Ctl mice with serum transfer arthritis although the degree of damage seems to be milder in the TRANCE/RANKL KO mice.
Figure 5.
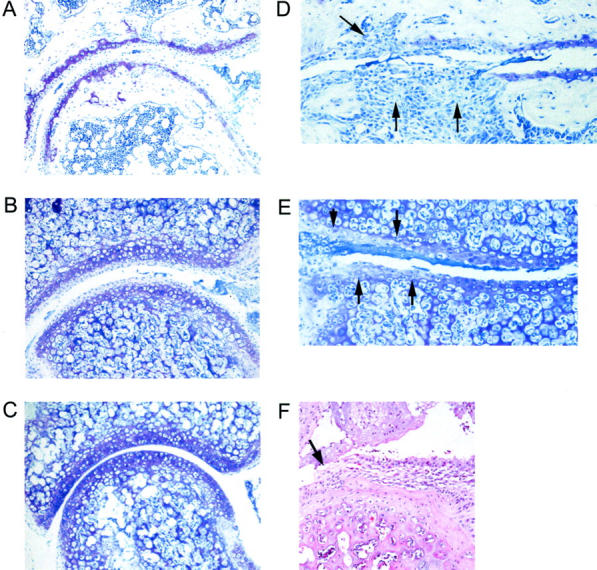
Cartilage damage in TRANCE/RANKL KO mice. Cartilage damage remote from pannus tissue in Ctl and TRANCE/RANKL KO mice was assessed in toluidine blue-stained sections. Mild to marked cartilage proteoglycan, chondrocyte, and articular cartilage loss were observed in both the Ctl (A) and TRANCE/RANKL KO (B) arthritic mice. Toluidine blue-stained proteoglycan is present throughout articular cartilage in PBS-injected TRANCE/RANKL KO (C) and Ctl (data not shown) mice. Full-depth articular cartilage loss was observed in Ctl mice at sites of subchondral bone erosion where the scaffolding subchondral bony plate deep to articular cartilage was completely lost (D, arrows). These areas of subchondral bone erosion and associated full-depth articular cartilage loss were absent in TRANCE/RANKL KO mice (E, same anatomical location as illustrated in D). Areas of proteoglycan, chondrocyte, and cartilage loss ranging from superficial to deep zones were observed in TRANCE/RANKL KO mice (E, arrows). Destruction of articular cartilage directly adjacent to pannus was also observed in TRANCE/RANKL KO (F) and Ctl mice (data not shown). Original magnifications: ×10 (A–C); ×20 (D–F).
Discussion
Blockade of TRANCE/RANKL with OPG has been shown to protect cortical and trabecular bone from erosion in rat adjuvant arthritis, demonstrating the importance of this factor in the pathogenesis of bone erosion in a model of inflammatory arthritis. 30 Our results support these findings in an animal model 26 in which TRANCE/RANKL is completely absent at all stages of arthritis. However, in the adjuvant arthritis model, a model of arthritis that is dependent on T-cell activation, OPG treatment may block not only the effects of TRANCE/RANKL on osteoclast differentiation and activation, but also the influence of TRANCE/RANKL on T-cell-dendritic cell interactions. 17,18,30 The serum transfer model of arthritis used in this study provided an opportunity to investigate the role TRANCE/RANKL on osteoclastogenesis in a model of arthritis that resembles RA but bypasses cooperative T-cell-dendritic cell interactions, thus eliminating the potential effect of blocking TRANCE/RANKL-RANK signaling on the inflammatory response. 33
We demonstrate in this murine model that on transfer of arthritogenic serum, TRANCE/RANKL KO mice develop inflammation similar to that seen in the Ctl mice. On the other hand, bone erosion in the absence of TRANCE/RANKL is dramatically reduced compared to Ctl mice. Large numbers of multinucleated TRAP-positive cells are present in resorption lacunae in areas of bone erosion in arthritic Ctl mice, and are completely absent in the arthritic TRANCE/RANKL KO mice, demonstrating the absolute requirement for this factor in osteoclastogenesis in this model of inflammatory arthritis. Small foci of bone irregularity adjacent to areas of synovitis were present in the arthritic TRANCE/RANKL KO mice that could not be definitively identified as a manifestation of the osteopetrotic bone phenotype. These were scored as erosions. These mice do have synovial fibroblasts, activated macrophages, and other cell types that have been implicated as playing a primary role in bone erosion. However, culture of monocyte/macrophage lineage cells on dentine slices reveals that they possess minimal capacity to directly resorb mineralized bone. 43,44 Similar studies with synovial fibroblasts have not yet been reported. Our observations provide additional evidence that the contribution of these and other cell types to focal bone erosion is limited in the absence of osteoclasts.
This study and the previously reported OPG treatment of mice with adjuvant arthritis 30 demonstrate the critical role of osteoclasts in the pathogenesis of focal bone erosion. In both studies, however, osteoclasts are essentially absent from the time of arthritis onset. In this study, TRANCE/RANKL is absent leading to a complete inability to generate osteoclasts, and in the latter study, OPG treatment was initiated at the onset of arthritis, blocking osteoclastogenesis. These studies do not directly address the possibility that other cell types could contribute to bone erosion in concert with osteoclasts. It has been suggested that activated macrophages, synovial fibroblasts, and other cell types capable of producing matrix-degrading proteases contribute to bone erosion. 4,45-47 For example, synovial fibroblasts and monocyte/macrophages have been shown to produce mRNA for cathepsin K, 4,44 a cysteine protease active in the degradation of the organic matrix of bone. Studies addressing the possible cooperative interactions of osteoclasts and other cell types in bone erosion in arthritis will be of great interest.
An important observation from studies in this arthritis model is that the differentiation and activation of osteoclasts can occur in the arthritic context in the absence of T-cell input. Kong and colleagues 30 proposed that in the adjuvant arthritis model TRANCE/RANKL produced by activated T-cells in the arthritic lesion was responsible for osteoclast activation and focal bone destruction. In serum transfer arthritis expression of TRANCE/RANKL by activated T-cells does not seem to be required for the generation of focal bone erosion because RAG knockout mice that lack mature lymphocytes develop bone erosions (data not shown). 33 Recent results show that the synovitis in the K/B×N model is dependent on complement and Ig-FcR receptors (Ji et al, submitted). Once generated, osteoclasts may be activated by signals mediated through these pathways or, perhaps more likely, respond to indirect signals from activated synoviocytes or other cell types. Synovial fibroblasts and cells of the osteoblast lineage have been demonstrated to express TRANCE/RANKL, 3,19,29 and TRANCE/RANKL may be the molecular mediator of this interaction. It will be of great importance to further elucidate these mechanisms.
In contrast to the findings in bone, definite cartilage destruction is present in both Ctl and TRANCE/RANKL KO mice with serum transfer arthritis, demonstrating that the mechanisms of cartilage destruction are distinct from those of bone erosion and that TRANCE/RANKL is not required for cartilage destruction in this model. Cartilage damage was assessed by analysis of areas of direct pannus invasion into cartilage and by evaluation of cartilage matrix degradation by toluidine blue staining in sites remote from pannus. Scoring of cartilage loss in both cases demonstrated a trend toward milder cartilage damage in the TRANCE/RANKL KO mice. This effect was due at least in part to the presence of large subchondral bone erosions with complete loss of subchondral bone and associated dissolution of cartilage in arthritic control mice. Subchondral bone erosions with associated cartilage loss were absent in the TRANCE/RANKL KO mice. These results differ from results previously reported in mice with adjuvant arthritis treated with OPG. In that model, significant protection of cartilage matrix was noted in OPG-treated mice, although modest focal cartilage damage was seen in areas of cartilage in direct contact with pannus. As suggested by Kong and colleagues, 30 the cartilage protection demonstrated in their study may also be due, in part, to the protection of scaffolding subchondral bone because subchondral bone erosion is a prominent feature of the adjuvant arthritis model. The differences in cartilage protection in these two studies may also reflect differences in the degree of primary cartilage damage intrinsic to these arthritis models. 41 Although TRANCE/RANKL may play a modulatory role in cartilage damage our results do not support a requirement for TRANCE/RANKL in cartilage destruction in this serum transfer model of arthritis independent of its effects on associated bone erosion.
An interesting feature of the serum transfer model of arthritis is new bone formation. 33 In this model the deposition of new woven bone occurred on cortical surfaces at reproducible anatomical locations including the anterior tibia, the volar surface of the calcaneus, and the dorsal surfaces of forefoot bones. The only notable difference in new bone formation between the arthritic TRANCE/RANKL KO and arthritic Ctl mice was the presence of TRAP+ multinucleated osteoclast-like cells in areas of new bone formation in Ctl mice, suggesting ongoing remodeling of new bone in these mice. These observations demonstrate that in this model of arthritis, TRANCE/RANKL is not required for the formation of new bone.
These studies highlight osteoclasts as important targets for therapeutic intervention to block focal bone erosion in inflammatory arthritis. Data from other animal models of inflammatory arthritis showing that bisphophonates prevent focal bone resorption lend support to this hypothesis. 48 However, in patients with RA, treatment with anti-resorptive therapies alone, including bisphosphonates or calcitonin, have not prevented the progression of focal bone erosions. 49,50 This could be because of inadequate dosing of these drugs. Alternatively, in the case of bisphosphonates, lack of efficacy may be because of a limited ability to concentrate the drug at sites of inflammation. The results reported here support the hypothesis that therapies interfering with osteoclast differentiation or activity, including agents that block TRANCE/RANKL, may prove useful in the treatment of RA.
Acknowledgments
We thank Drs. Sandy C. Marks, Jr., and Paul R. Odgren for input on the TRANCE/RANKL osteopetrotic bone phenotype, and Alfie Tsay and Dr. Koichiro Ohmura for technical assistance.
Footnotes
Address reprint requests to Ellen M. Gravallese, M.D., Harvard Institutes of Medicine, 4 Blackfan Circle, Boston, MA 02115. E-mail: egravall@caregroup.harvard.edu.
Supported by the Arthritis Foundation of Australia and the National Health and Medical Research Council (to A. R. P.), an M. E. Müller Professorship in Bioengineering (to R. M.), and the National Institutes of Health (grant RO1-DK46773 to S. R. G.).
A. R. P. and H. J. contributed equally to this work.
References
- 1.Leisen JCC, Duncan H, Riddle JM, Pitchford WC: The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J Rheumatol 1988, 15:17-22 [PubMed] [Google Scholar]
- 2.Bromley M, Woolley DE: Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 1984, 27:968-975 [DOI] [PubMed] [Google Scholar]
- 3.Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR: Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 2000, 43:250-258 [DOI] [PubMed] [Google Scholar]
- 4.Hummel KM, Petrow PK, Franz JK, Muller-Ladner U, Aicher WK, Gay RE, Bromme D, Gay S: Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J Rheumatol 1998, 25:1887-1894 [PubMed] [Google Scholar]
- 5.Hattersley G, Chambers TJ: Generation of osteoclastic function in mouse bone marrow cultures: multinuclearity and tartrate-resistant acid phosphatase are unreliable markers for osteoclastic differentiation. Endocrinology 1989, 124:1689-1696 [DOI] [PubMed] [Google Scholar]
- 6.Kurihara N, Gluck S, Roodman GD: Sequential expression of phenotype markers for osteoclasts during differentiation of precursors for multinucleated cells formed in long term human marrow cultures. Endocrinology 1990, 127:3215-3221 [DOI] [PubMed] [Google Scholar]
- 7.Faust J, Lacey DL, Hunt P, Burgess TL, Scully S, Van G, Eli A, Qian Y, Shalhoub V: Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem 1999, 72:67-80 [DOI] [PubMed] [Google Scholar]
- 8.Suda T, Takahashi N, Martin TJ: Modulation of osteoclast differentiation. Endocr Rev 1992, 13:66-80 [DOI] [PubMed] [Google Scholar]
- 9.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR: Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998, 152:943-951 [PMC free article] [PubMed] [Google Scholar]
- 10.Hattersley G, Chambers TJ: Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology 1989, 125:1606-1612 [DOI] [PubMed] [Google Scholar]
- 11.Lee SK, Goldring SR, Lorenzo JA: Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology 1995, 136:4572-4581 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Nishikaku F, Nakatuka M, Koga Y: Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol 1998, 25:1154-1160 [PubMed] [Google Scholar]
- 13.Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakasima A, Iijima T: Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathology 1998, 13:751-759 [DOI] [PubMed] [Google Scholar]
- 14.Romas E, Bakharevski O, Hards DK, Kartsogiannis V, Quinn JMW, Ryan PFJ, Martin J, Gillespie MT: Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum 2000, 43:821-826 [DOI] [PubMed] [Google Scholar]
- 15.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 [DOI] [PubMed] [Google Scholar]
- 16.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki SI, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T: Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998, 95:3597-3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y: TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 1997, 186:2075-2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L: A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390:175-179 [DOI] [PubMed] [Google Scholar]
- 19.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL: The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 2000, 15:2-12 [DOI] [PubMed] [Google Scholar]
- 20.Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S: Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun 1998, 250:776-781 [DOI] [PubMed] [Google Scholar]
- 21.Horwood NJ, Elliott J, Martin TJ, Gillespie MT: Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 1998, 139:4743-4746 [DOI] [PubMed] [Google Scholar]
- 22.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K: A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25:109-113 [DOI] [PubMed] [Google Scholar]
- 23.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Amgen EST, Boyle WT: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309-319 [DOI] [PubMed] [Google Scholar]
- 24.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K: Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998, 139:1329-1337 [DOI] [PubMed] [Google Scholar]
- 25.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM: OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397:315-323 [DOI] [PubMed] [Google Scholar]
- 26.Kim N, Odgren PR, Kim DK, Marks SC, Choi Y: Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA 2000, 97:10905-10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, Rennert PD, Choi Y: Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med 2000, 192:1467-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y: TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med 2000, 191:495-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S: Involvement of receptor activator of nuclear factor kappa-B ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 2000, 43:259-269 [DOI] [PubMed] [Google Scholar]
- 30.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM: Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402:304-309 [DOI] [PubMed] [Google Scholar]
- 31.Horwood NJ, Kartsogiannis V, Quinn JMW, Romas E, Martin TJ, Gillespie MT: Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun 1999, 265:144-150 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto I, Staub A, Benoist C, Mathis D: Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999, 286:1732-1735 [DOI] [PubMed] [Google Scholar]
- 33.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D: From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 1999, 10:451-461 [DOI] [PubMed] [Google Scholar]
- 34.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D: Organ-specific disease provoked by systemic autoimmunity. Cell 1996, 87:811-822 [DOI] [PubMed] [Google Scholar]
- 35.Ruegsegger P, Koller B, Muller R: A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int 1996, 58:24-29 [DOI] [PubMed] [Google Scholar]
- 36.Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, Ruegsegger P: Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone 1998, 23:59-66 [DOI] [PubMed] [Google Scholar]
- 37.Balto K, Muller R, Carrington DC, Dobeck J, Stashenko P: Quantification of periapical bone destruction in mice by micro-computed tomography. J Dent Res 2000, 79:35-40 [DOI] [PubMed] [Google Scholar]
- 38.Muller R, Ruegsegger P: Micro-tomographic imaging for the nondestructive evaluation of trabecular bone architecture. Stud Health Technol Inform 1997, 40:61-79 [PubMed] [Google Scholar]
- 39.Muller R, Hildebrand T, Ruegsegger P: Non-invasive bone biopsy: a new method to analyze and display the three-dimensional structure of trabecular bone. Phys Med Biol 1994, 39:145-164 [DOI] [PubMed] [Google Scholar]
- 40.Glowacki J, Rey C, Glimcher MJ, Cox KA, Lian J: A role for osteocalcin in osteoclast differentiation. J Cell Biochem 1991, 45:292-302 [DOI] [PubMed] [Google Scholar]
- 41.Bendele A, McAbee T, Sennello G, Frazier J, Chlipala E, McCabe D: Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical data. Arthritis Rheum 1999, 42:498-506 [DOI] [PubMed] [Google Scholar]
- 42.Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, Garchon H-J, Degott C, Lathrop M, Benoist C, Mathis D: Genetic influences on the end-stage effector phase of arthritis. J Exp Med 2001, 194:321-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers TJ, Horton MA: Failure of cells of the mononuclear phagocyte series to resorb bone. Calcif Tissue Int 1984, 36:556-558 [DOI] [PubMed] [Google Scholar]
- 44.Higuchi Y, Ito M, Tajima M, Higuchi S, Miyamoto N, Nishio M, Kawano M, Kusagawa S, Tsurudome M, Sudo A, Katou K, Uchida A, Ito Y: Gene expression during osteoclast-like cell formation induced by antifusion regulatory protein-1/CD98/4F2 monoclonal antibodies (MAbs): c-src is selectively induced by anti-FRP-1 MAb. Bone 1999, 25:17-24 [DOI] [PubMed] [Google Scholar]
- 45.Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL: Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem 1997, 272:22053-22058 [DOI] [PubMed] [Google Scholar]
- 46.Kaneko M, Tomita T, Nakase T, Ohsawa Y, Seki H, Takeuchi E, Takano H, Shi K, Takahi K, Kominami E, Uchiyama Y, Yoshikawa H, Ochi T: Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology 2001, 40:247-255 [DOI] [PubMed] [Google Scholar]
- 47.Pap T, Muller-Ladner U, Gay RE, Gay S: Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res 2000, 2:361-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis MD, Hovancik K, Boyce RW: NE-58095: a diphosphonate which prevents bone erosion and preserves joint architecture in experimental arthritis. Int J Tiss Reac 1989, 11:239-252 [PubMed] [Google Scholar]
- 49.Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BAC, Valkema R, Westedt ML, Landman J-O, Pauwels EKJ, Breedveld FC: Increased bone mass with pamidronate treatment in rheumatoid arthritis; results of a three-year randomized double-blind trial. Arthritis Rheum 1996, 39:396-402 [DOI] [PubMed] [Google Scholar]
- 50.Sileghem A, Geusens P, Dequeker J: Intranasal calcitonin for the prevention of bone erosion and bone loss in rheumatoid arthritis. Ann Rheum Dis 1992, 51:761-764 [DOI] [PMC free article] [PubMed] [Google Scholar]