Abstract
Hepatocytes and other cellular elements isolated by collagenase perfusion of the liver and maintained in defined culture conditions undergo a series of complex changes, including apoptosis and cell proliferation, to reconstruct tissue with specific architecture. Cultures in collagen-coated pleated surface roller bottles, with hepatocyte growth medium medium and in the presence of hepatocyte growth factor (HGF) and epidermal growth factor (EGF), form characteristic and reproducible tissue architecture composed of a superficial layer of biliary epithelial cells, an intermediate layer of connective tissue and hepatocytes, and a basal layer of endothelial cells. Dexamethasone, EGF, and HGF are required for the complete histological organization. Analysis of the structures formed demonstrates that the receptor tyrosine kinase ligands HGF and EGF are required for the presence, growth, and phenotypic maturation of the biliary epithelium on the surface of the cultures and for the formation of connective tissue in the cultures. Dexamethasone, in the presence of HGF and EGF, was required for the phenotypic maturation of hepatocytes. The results demonstrate the role of these molecules for the formation and phenotypic maturation of specific histological elements of the liver and suggest roles for these signaling molecules in the formation and structure of the in vivo hepatic architecture.
Recent studies from this and other laboratories 1,2 have demonstrated that hepatocytes in monolayer cultures kept under conditions of sustained proliferation can form complex structures composed of hepatocytes and mesenchymal cells, the latter probably derived from stellate cell contaminants of the isolation procedure. 3 In subsequent studies we also demonstrated that the same cultures in roller bottles on polystyrene beads develop elements of histological organization, which included hepatocytes and fenestrated endothelium. 4 Organized biliary epithelium, however, was not found to be a component of those cultures. In this system, we eliminated any support structures such as polystyrene beads and examined the behavior of cells isolated from collagenase perfusion of the rat liver in collagen-coated roller bottles with a pleated surface, aimed to enhance cellular attachment. In our current study we demonstrate that, in the absence of artificial three-dimensional substrates, hepatocytes and contaminating mesenchymal and epithelial cells undergo a series of complex changes and evolve from monolayers to histological structures. The final tissue covers the surface of the roller bottle flask and is composed of all hepatic cellular elements, including a basal layer of endothelial cells, a middle layer of hepatocytes plus stellate cells with connective tissue, and a surface layer of biliary epithelium. These findings demonstrate that structured histological formations bearing resemblance to hepatic histological organization can form from totally dissociated hepatic cellular elements.
The distinct presence of biliary epithelium, hepatocytes, and stellate cells allows use of these cultures to identify and study factors essential for the phenotypic maturation of these histological elements. Substances such as dexamethasone (nonmetabolizable corticosteroid), epidermal growth factor (EGF), and hepatocyte growth factor (HGF) cannot be easily studied in mouse genetic models of gene deletion. Homozygous deletion of HGF, or its receptor, c-met, results in embryonic lethality. Livers have been described as smaller than normal, with small immature hepatocytes. No other details of histological abnormalities have been provided and it is not clear whether the hepatic abnormalities are secondary to the placental defects observed in the embryos. 5-7 Homozygous deletions of EGF receptor or its multiple ligands have had no impact on hepatic development, probably because of functional overlap with other receptors of the same family. 8,9 Given the difficulty of performing adrenalectomy in rodent embryos and the multiplicity of sites synthesizing corticosteroids in embryonic development, evaluation of the role of corticosteroids in hepatic tissue development by in vivo tissue ablation or genetic models is practically impossible. The same applies to other cytokines and hormones, such as insulin, triiodothyronine, and so forth. The model described in this article results in standard, reproducible, and stereotypic histology, described above. Although this histology is not the same as the typical liver histology, the reproducibility of the in vitro structures as well as the easily observable defects in cellular phenotype and histology on elimination or addition of specific components allow another opportunity to study the role of different molecules in hepatic tissue formation. The results of these studies and their implications for hepatic biology are presented below.
Materials and Methods
Animals
Male Fischer 344 rats from Charles River (Wilmington, MA) were used for the studies described. All animals were treated according to protocols approved by the animal care institutional review board
Materials
EGF was obtained from Collaborative Biomedical (Waltham, MA). Collagenase for hepatocyte isolation was obtained from Boehringer Mannheim (Mannheim, Germany). Vitrogen (Celtrix Labs., Palo Alto, CA) was used for collagen coating of roller bottles. General reagents were obtained from Sigma Chemical Co. (St. Louis, MO). EGF was purchased from BD Pharmingen (San Diego, CA). HGF used for these studies was the Δ5 variant and was kindly donated by Snow Brand Co. (Toshigi, Japan). Antibodies were obtained from the following sources: proliferating cell nuclear antigen (PCNA) from Signet Laboratories (Dedham, MA); Ki-67 from Santa Cruz Biologicals (Santa Cruz. CA); desmin, cytokeratin 19, HEPPAR, and factor VIII from DAKO Corp (Carpinteria, CA).
Immunohistochemistry
Tissues from the cultures were harvested and fixed in 10% formalin. Tissues were paraffin-embedded, sectioned at 4 to 5 μm, and affixed to charged slides (Superfrost/Plus; Fisher Scientific, Pittsburgh, PA). Immunohistochemistry was performed using the Vectastain ABC Elite kit (Vector Laboratories, Inc., Burlingame, CA). PCNA antibody was used at a concentration of 1:100 on sections that were microwaved in citrate buffer. Ki-67 antibody was used at a concentration of 1:200 and sections were heated under pressure in citrate buffer. Desmin antibody was used at a concentration of 1:100. Cytokeratin 19 antibody was used at 1:10 in sections microwaved in citrate buffer. HEPPAR antibody was used at a concentration of 1:25 in sections microwaved in citrate buffer. Factor VIII antibody was used at 1:400 sections that were treated with pepsin. Secondary antibodies used for this project were goat anti-rabbit, goat anti-mouse, and donkey anti-goat (Chemicon, Temecula, CA) all used at a 1:500 dilution.
Isolation and Culture of Hepatic Cell Populations
Hepatocytes
Rat hepatocytes were isolated by an adaptation of Seglen’s calcium two-step collagenase perfusion technique 10 as previously described from our laboratory. 4 Hepatocytes isolated from collagenase perfusion of rat liver were added at a concentration of 210,000,000 hepatocytes per 250 ml of medium. As previously described, these preparations are known to contain contaminant small numbers of other hepatic cellular elements, including stellate cells, Kupffer cells, and very few bile duct epithelial cells. The latter typically do not comprise >0.05% of the inoculated cell population. 10 By hematoxylin and eosin (H&E) stain of smears of the isolated hepatocyte pellet, small cells arranged in a ductular configuration were occasionally noted. Although precise calculations were difficult to obtain given the random distribution of these clusters, their number seemed to be even less than the range for ductular cell contamination previously described.
Nonparenchymal Cell Fraction
The supernatant of the first low-gravity centrifugation used to prepare hepatocytes was subjected to a 1000 × g centrifugation for 3 minutes. This fraction primarily contains stellate cells, bile duct cells, and endothelial cells. Small hepatocytes are also present in this fraction, typically comprising ∼5% of the cells.
Roller Bottle Cultures
Freshly isolated hepatocytes were added to roller bottles (850 cm 2 surface) obtained from Falcon (Franklin Lakes, NJ). Each bottle contained 210,000,000 freshly isolated hepatocytes in 250 ml of HGM medium 1 supplemented with HGF (20 ng/ml) and EGF (10 ng/m). The bottles were rotated at a rate of 2.5 rotations per minute and kept in an incubator maintained at 37°C, saturated humidity, and 5% CO2.
Composition of the HGM Cell Culture Medium
HGM medium was prepared as previously described. 1 Dulbecco’s modified Eagle’s medium powder, HEPES, glutamine, and antibiotics were purchased from Life Technologies, Inc., Grand Island, NY. ITS mixture (insulin, transferrin, selenium) was purchased from Boehringer Mannheim. All other additives were cell-culture grade (Sigma). Unless otherwise indicated for specific experiments, the basal HGM consisted of Dulbecco’s modified Eagle’s medium supplemented with purified bovine albumin (2.0 g/L), glucose (2.0 g/L), galactose (2.0 g/L), ornithine (0.1 g/L), proline (0.030 g/L), nicotinamide (0.305 g/L), ZnCl2 (0.544 mg/L), ZnSO4:7H2O (0.750 mg/L), CuSO4:5H2O (0.20 mg/L), MnSO4 (0.025 mg/L), glutamine (5.0 mmol/L), and dexamethasone (10−7 mol/L). Penicillin and streptomycin were added to the basal HGM at 100 mg/L and 100 μg/L, respectively. The mixed basal HGM was sterilized by filtration through a 0.22-μm low-protein-binding filter system, stored at 4°C, and used within 4 weeks. ITS (1.0 g/L) (rh-insulin 5.0 mg/L, human transferrin 5.0 mg/L, 30% diferric iron saturated, and selenium 5.0 μg/L) was added after filtration immediately before use. The growth factors, as required, were added to HGM fresh at the specified concentrations every time the medium was changed.
Transmission Electron Microscopy
Samples for transmission electron microscopy were washed once in phosphate-buffered saline (PBS) with 1 mmol/L MgCl2, 0.5 mmol/L CaCl2, then fixed overnight at 4°C in 2.5% glutaraldehyde in PBS. Samples were washed three times with PBS then postfixed in 1% OsO4, 1% KFe(CN)6 in PBS for 1 hour at room temperature. Samples were washed three times in PBS, then dehydrated through graded series (30 to 100%) of ethanol. After three changes of 100% ethanol, samples were infiltrated with several changes of Polybed 812 resin (Polysciences, Warrington, PA) at room temperature, with a change overnight at 4°C. Thick sections (300 μm), obtained using a Reichert (Vienna, Austria) ultramicrotome fitted with a diamond knife, were heated onto glass slides, stained with 1% Toluidine blue, and rinsed with water. Ultrathin sections (60 nm) were collected on Formvar-coated (Fullam, Schenectady, NY) grids and stained with 2% uranyl acetate in 50% methanol for 10 minutes, then 1% lead citrate for 7 minutes. Sections were analyzed and photographed on a JEOL JEM 1210 transmission electron microscope at 80 kV.
Analysis of Gene Expression by Northern Blots
Extraction of Total RNA and mRNA from Cultures
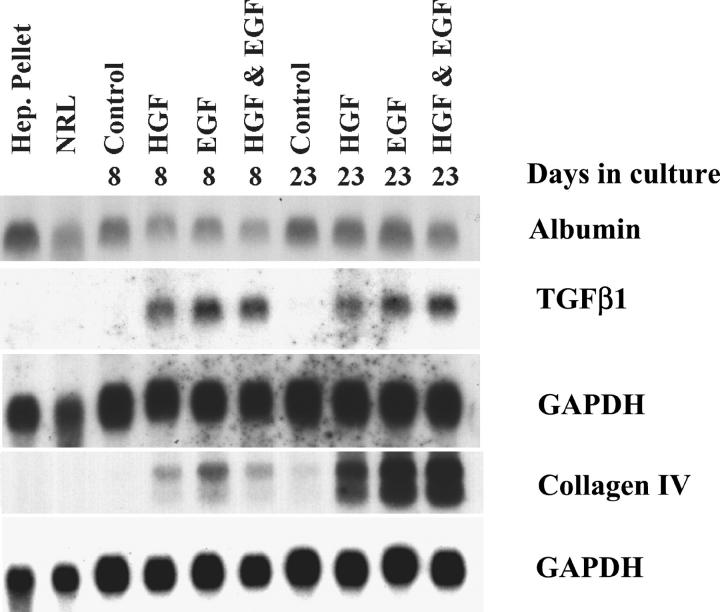
Total RNA was extracted by use of RNAzol B (BioTECX, Houston, TX). RNA extraction from roller-bottle cultures was performed by mixing 1 volume (pelleted) of scraped tissues with three volumes of RNAzol. RNA was purified according to the manufacturer’s guidelines. RNA concentration and purity were determined by routine spectrophotometry. Size separation of 20 μg of RNA per lane was completed on denaturing 1% agarose gels and transferring to nylon membranes (Amersham, Piscataway, NJ) by the capillary method. After cross-linking under ultraviolet light, membranes were hybridized overnight with specific complementary DNA (as indicated in Figure 8 ▶ ) that had been labeled with a [32P]dCTP using an Amersham random primer kit. Membranes were subsequently washed under high stringency conditions and exposed to R film (photographic film) (Eastman-Kodak, Rochester, NY) for 1 to 3 days. Quantification of the RNA hybridization bands was performed by laser densitometry.
Figure 8.
Expression of albumin, TGF-β1 and collagen type IV in cultures at different days, maintained in the presence of either HGF or EGF or both. Control cultures had neither HGF nor EGF supplementation. Hepatocyte pellet isolated at the end of collagenase perfusion as well as whole normal rat liver tissue (NRL) were also examined for comparison. Analysis of extracted RNA was conducted by Northern gels. The upper GAPDH is used as a normalizing control for albumin and TGF-β1 whereas the lower GAPDH was used for the normalization of the data on collagen type IV, because the corresponding RNA were run on two separate gels. EGF was a stronger inducer of both TGF-β1 and collagen type IV at day 8, compared to HGF.
Sources of Complementary DNA Probes
Collagen probes were obtained from ATCC (Rockville, MD). Rat albumin probe was obtained from Dr. Mark Zern; transforming growth factor (TGF)-β1 human probe from Dr. Derynck; Cytochrome P-450 IIB1 (mouse) from Dr. Negishi; collagen IV (mouse) from ATCC.
Results
Culture Conditions and Basic Histology
The surface of the pleated roller bottles was coated with collagen type I before inoculation of cells, as previously described. 11 The culture medium HGM was supplemented with HGF and EGF unless otherwise indicated for specific experiments. The inoculated cells attach to the surface of the culture bottle within ∼24 hours. Approximately 50% of the hepatocytes enter into apoptosis in the first 5 days of the culture. The apoptotic cells gradually disappear from the mix later on as connective tissue develops. By day 18 to 20 of the cultures, the organization of the cellular elements acquires its typical configuration. Sheets of tissue of gray-brown coloration cover the surface of the roller bottle, being more prominent in the grooves of the internal surface. Approximately 2 to 4 g of tissue can be recovered from a roller bottle at 30 days in culture. The sheets of tissue were scraped from the surface of the roller bottles, pelleted, and processed as necessary for histological and biochemical evaluations. The observed histology is standard and highly reproducible. Figure 1A ▶ is a low-power (×20) view of the histological appearance of the many ribbons of tissue removed by scraping from the roller bottle. A higher power view (×200) is shown in Figure 1B ▶ . Each ribbon is composed of the same standard histology. On the surface facing the medium there is a continual monolayer of cuboidal biliary epithelium. Below the biliary layer there is a 5 to 10 cell layer composed of hepatocytes embedded in connective tissue elements. There is a variable amount of connective tissue separating hepatocytes from the biliary layer, from complete absence to a thick layer separating the two cell types (as shown in Figure 1, A and B ▶ ). Hepatocytes have a variable nuclear and nucleolar structure, suggesting different degrees of ploidy. Attached to the substrate and underlying the hepatocytes and connective tissue is a layer of endothelial cells. This typical morphology is seen when the hepatocyte cell fraction from the collagenase perfusion is placed in culture. When the nonparenchymal cell pellet (containing endothelial cells, stellate cells, and occasional small hepatocytes) is put in culture under similar conditions, no growth was observed (data not shown).
Figure 1.

Sections of tissue from organoid cultures at day 20. Cultures were maintained in HGM medium with HGF and EGF. A: H&E stain of sections of tissue ribbons scraped from the interior of the roller bottles. Original magnification, ×20. B: Tissue organization of the ribbons shown in A. The surface is covered by cuboidal biliary epithelium. A layer of connective tissue with interspersed nests of hepatocytes underlies the biliary epithelium. Endothelial cells are at the bottom surface of the ribbons, attached to the plastic of the substratum. Original magnifications, ×200.
By electron microscopy, all typical features of the cellular elements present are easily identified. Figure 2A ▶ shows a binucleate hepatocyte. Details of cytoplasmic organization including mitochondria, rough endoplasmic reticulum, bile canaliculi, tight junctions, and so forth, are shown in Figure 2B ▶ . Figure 3 ▶ shows the cellular ultrastructure of other cellular elements of the organoid cultures. The biliary epithelium (Figure 3A) ▶ displays typical cerebriform nuclei and surface microvilli. A dense network of collagen fibrils underlies the surface epithelium. Stellate-like cells with small lipid droplets are shown embedded in the connective tissue matrix in Figure 3B ▶ . Endothelial cells at the basal layer also display typical subcellular architecture for the cell type (Figure 3C) ▶ . We did not detect presence of fenestrated endothelium. Occasional macrophages were also seen.
Figure 2.

Electron microscopy of hepatocytes embedded in the tissue of the cultures. Left: Binucleate hepatocyte embedded within the organoid, containing vacuolar inclusions (V) surrounded by collagenous matrix (Col). Note the round nuclei (N) indicating differentiated hepatocytes. Right: Higher magnification of areas of cell-cell contact between differentiated hepatocytes. Bile canaliculus (BC) with luminal microvilli is bounded by both desmosomes (D) and tight junctions (TJ). Glycogen (Gly), mitochondria (Mt), and rough endoplasmic reticulum (RER) are abundant within the hepatocytes.
Figure 3.
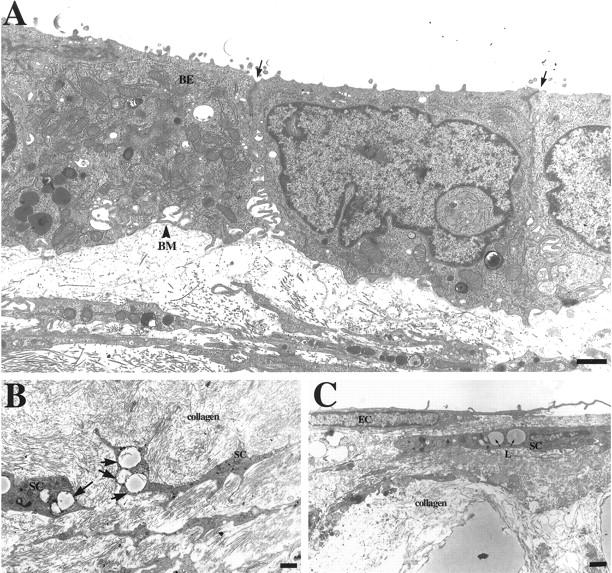
Organoids isolated from 30-day cultures were fixed and processed for transmission electron microscopy to examine ultrastructural characteristics of the tissue. A: Biliary epithelium (BE) present on the surface of the organoids displays characteristic cuboidal epithelial monolayer structure and expresses tight junctions and desmosomes at cell-cell contacts (arrows) as well as highly interdigitated lateral membrane domains. Monolayers produce basement membrane (BM) extracellular matrix at their basolateral domain. B: Stellate cells (SC) with lipid droplet inclusions (arrows) are observed embedded within the collagenous matrix. C: Ultrastructure of endothelial cell (EC) layer found on the surface of the organoid containing highly articulated epithelial-type cells. A stellate cell is visible in the collagenous matrix just underneath the EC and contains two lipid droplets (L) within its cytoplasm. Scale bars: 1 μm (A and C), 2 μm (B).
Histochemistry
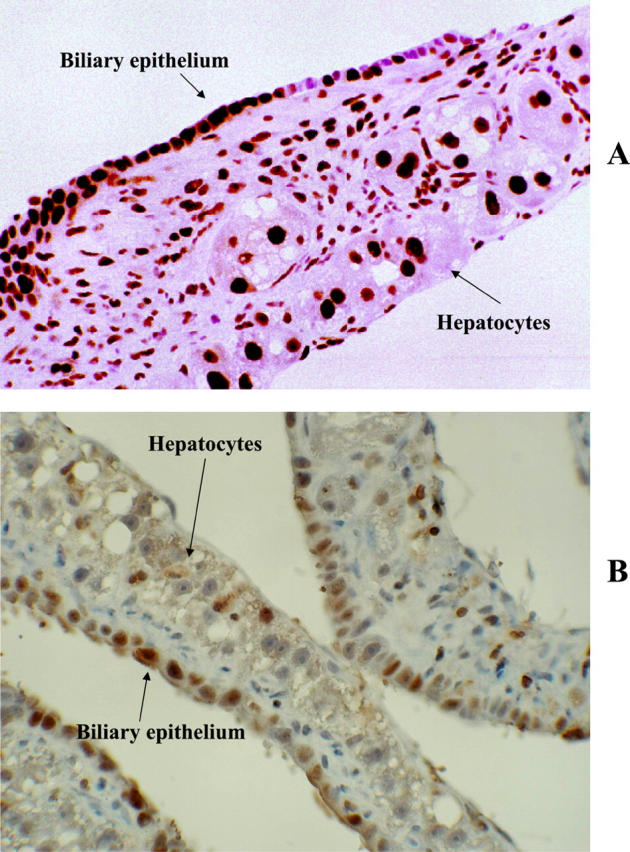
Results are shown in Figure 4 ▶ . The superficial biliary epithelial cells were positive for cytokeratin 19, as expected and they appear as a linear brown staining on low power (Figure 4A) ▶ . Desmin, typically present in myofibroblasts and stellate cells, was seen in mesenchymal cells embedded in the connective tissue matrix and associated with presence of collagen bundles (Figure 4B) ▶ . HEPPAR antibody 12 as well as antibody to cytochrome P-450 IIB1 stained hepatocytes positive, with occasional biliary epithelial cells also staining positive for the markers (Figure 4, C and E ▶ , correspondingly). The endothelial cells in the basal surface were positive for factor VIII (Figure 4D) ▶ . Canaliculi stained positive for Mg++ ATPase 13 (Figure 4F ▶ , see arrows).
Figure 4.

All sections were taken from 20-day-old cultures maintained in complete medium with dexamethasone, HGF, and EGF. A: Immunohistochemical stain of a frozen section, for cytokeratin 19. The superficial bile duct epithelial layer stains positive for the stain (linear brown areas). B: Immunohistochemistry for desmin demonstrates desmin-positive cells accompanying collagen fibrils interspersed between hepatocytes. C: Immunohistochemical stain with the hepatocyte-specific HEPPAR antibody. Hepatocytes are positive (brown color). Occasional biliary epithelial cells are positive as well. D: Immunohistochemical stain against coagulation factor VIII demonstrates the endothelial cells on the basal surface of the ribbons. H, hepatocytes; B, biliary epithelium. E: Immunohistochemistry against cytochrome P-450 IIB1. Large hepatocytes are positive (brown color). F: Histochemical stain for Mg++ ATPase. 13 Frozen section. Positive canaliculi containing the enzyme are seen as thin brown lines.
Cellular Kinetics
In the presence of HGF and EGF, most cells (>70% for each type) stained positive for PCNA (Figure 5A) ▶ . This indicates that most of the cells in the cultures are in the cell cycle. The antigen Ki-67 is typically expressed in cells actually in S phase. Less than 5% of the hepatocytes in the cultures stained positive for Ki-67 whereas >60% of the biliary epithelial cells were positive (Figure 5B) ▶ . A higher (>80%) PCNA labeling and a higher Ki-67 labeling were noted in all systems in which dexamethasone was not present (see below).
Figure 5.
A: PCNA stain of an organoid ribbon (20-day-old cultures). More than 80% of the surface biliary epithelium, connective tissue cells, and hepatocytes have positive nuclei, indicating that the cells are in the cell cycle. B: Immunohistochemical stain for Ki-67, intended to identify cells actively synthesizing DNA (in S phase). Positive nuclei (dark areas) are seen in <5% of hepatocytes, whereas >60% of the biliary epithelial cells stained positive for this nuclear protein. Original magnifications, ×200.
Influence of Growth Factors and Hormones on Tissue Organization
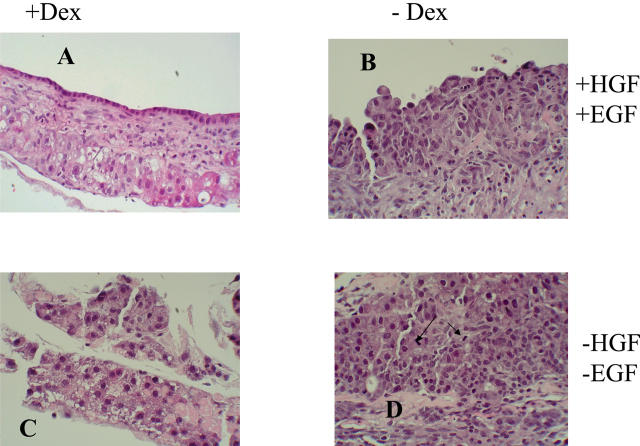
The results of these studies are shown in Figure 6 ▶ (H&E stains) and Figure 7 ▶ (cytokeratin 19 stain, as a marker for the biliary epithelium). The typical histology described above was seen in cultures maintained in the presence of dexamethasone, HGF, and EGF (Figures 6A and 7A) ▶ ▶ (please note that Figures 1B and 6A ▶ ▶ are identical, for comparison purposes). The histology of the cultures however was very much affected by selective elimination of these components.
Figure 6.
H&E stains of organoid cultures at day 25 maintained under different conditions of growth. The supplementations of dexamethasone (Dex), and HGF+EGF are shown on the side. Typical morphology is shown in A, with Dex, HGF, and EGF present (please note: the photo used is identical to that of Figure 1B ▶ ). In B [minus Dex, plus (HGF + EGF)], there are epithelioid cells with primitive characteristics, with very few cell distinguishable as hepatocytes. Less than 15% of these cells were positive for HEPPAR or cytochrome P-450 IIB1 (data not shown). In C [plus Dex, minus (HGF + EGF)], hepatocytes remain small, HEPPAR-negative, with several apoptotic bodies, no surface biliary epithelium, and no connective tissue. In D [minus Dex, minus (HGF + EGF)] there is no surface biliary epithelium and hepatocytes are small or have features of oval cells. Two mitoses are seen in the center of the photo (arrows). Original magnifications, ×200.
Figure 7.
Cytokeratin 19 stains of the organoid cultures at day 25 maintained under similar conditions of growth as described in Figure 6 ▶ . In A, cytokeratin 19 is seen staining the biliary epithelium in the organoid cultures, with Dex, HGF, and EGF present. In B [minus Dex, plus (HGF + EGF)], surface epithelium stains positive for cytokeratin 19. A weak stain seen in C [plus Dex, minus (HGF + EGF)] reflects uptake of the secondary antibody by the apoptotic cells. Note in D [minus Dex, minus (HGF + EGF)], the lack of cytokeratin 19-positive biliary epithelium. Original magnifications, ×200.
Removal of EGF and HGF, Presence of Dexamethasone (Figures 6C and 7C) ▶ ▶
Combined removal of these two growth factors resulted in elimination of the biliary epithelium in day 20 cultures. Hepatocytes were recognizable but small and remained negative for the HEPPAR and cytochrome P-450 IIB1 antigens (data not shown). Many apoptotic hepatocytes were embedded in the histology of the cultures. No connective tissue development was noted.
Removal of Dexamethasone, Presence of HGF and EGF
There was an overall arrest in phenotypic maturation of hepatocytes. The cells resembled oval cells seen in rat liver in vivo. Some immature hepatocytes (<15% of the total) were positive for HEPPAR and cytochrome P-450 IIB1. Although cytokeratin 19 strongly labeled only the surface epithelium (Figure 7B) ▶ , there was no clear demarcation between the surface biliary epithelium and the underlying hepatocytes in H&E stains (Figure 6B) ▶ . There were no canalicular structures as demonstrable by Mg++ ATPase or electron microscopy (data not shown). Connective tissue was present. Ki-67 labeling index was ∼10%.
Removal of Dexamethasone, HGF, and EGF
The surface biliary epithelium was absent (Figure 7D) ▶ . Hepatocytes (Figure 6D) ▶ appeared immature, similar to those seen in Figure 6B ▶ . Some immature hepatocytes (<35% of the total) were positive for HEPPAR and cytochrome P-450 IIB1. Surprisingly, several mitoses and a high PCNA (>90%) and Ki-67 (∼25%) labeling index for hepatocytes were seen in these cultures. Connective tissue was present.
The combined results suggest that dexamethasone is required for the formation of fully mature, histologically recognizable, hepatocytes, distinct from the biliary layer. This is more apparent by simple histological analysis when HGF and EGF are present (compare Figure 6, A and B ▶ ). When dexamethasone alone is added, it inhibits cell proliferation and is associated with smaller atrophic hepatocytes. Thus, although dexamethasone is a modulator of hepatocyte differentiation, its effects vary depending on HGF, EGF, and perhaps other components of the medium. HGF and EGF are required for the appearance, maintenance, or growth of the biliary epithelium. Addition of either HGF or EGF alone restored formation of the biliary epithelium, but not to the full extent as seen when both growth factors were present. Connective tissue formation also depends on the presence of HGF and EGF. The mechanisms of this are not clear. As mentioned above, when the nonparenchymal fraction isolated from collagenase perfusion of the rat liver was placed in culture in the absence of hepatocytes, and with the full complement of the HGF medium plus dexamethasone, HGF, or EGF, no growth of connective tissue elements or any tissue formation was noted. EGF or HGF alone restored some connective tissue formation in these cultures. EGF appeared more efficient in restoring connective tissue formation. The histological findings paralleled results from analysis of gene expression. Figure 8 ▶ demonstrates expression of collagen type IV in cultures maintained in the presence of no growth factors (control), EGF alone, HGF alone, and EGF plus HGF. The strongest expression of collagen IV gene is seen in cultures maintained in the presence of EGF (alone or in combination with HGF). HGF alone also increased expression of type IV collagen above the control values at both day 8 and day 23 in culture, but to a lesser extent than EGF. Both growth factors however were equally efficient in inducing expression of TGF-β. In contrast, there were no apparent differences related to growth factors for albumin expression.
Discussion
The findings of this work provide information on the role of specific signals in formation of hepatic histology and point out to ways that hepatic tissue and other complex tissues may be at some point assembled in culture. Histological patterns giving rise to mature tissues are created in embryogenesis. Portal triads and the adjacent arrangement of hepatocytes into plates lined by sinusoids are hallmarks of hepatic histology. Triads contain vascular endothelial cells and biliary epithelium organized into complete vascular and ductular structures. The tissues forming in these cultures most resemble a linearized portal triad architecture, where instead of round ducts and vessels, the structures are flattened out. This is probably because of the influence exerted by the geometry of the flat substrate from which the tissue has to grow. It should be mentioned however that round ductules, often of irregular shape, were also noted, although it was not clear whether these structures represented invaginations of the surface epithelium.
The origin of the biliary epithelium in these cultures is intriguing. As mentioned above, studies from other investigators have shown that the original cell inoculum of the hepatocyte fraction isolated from liver perfusion by the two-step collagenase perfusion contains a very small number (<0.05%) of biliary epithelial cell. 10 We performed cytokeratin 19 stains on smears of cell isolates directly from collagenase perfusion and the number of cytokeratin 19-positive cells was in the same range as previously described (data not shown). No biliary epithelium is seen in cultures in the absence of EGF and HGF. Each of these two growth factors added alone induced appearance of biliary epithelium to a degree that was much less when the two were combined. This does not imply that these two growth factors are the only regulating molecules for biliary epithelium development. Recent studies by Auth and colleagues 14 suggest that additional factors derived from hepatocytes may also be involved. The cellular origin of the biliary epithelium in these cultures is not clear. Two possibilities exist: 1) biliary epithelium is derived from contaminating biliary epithelial cells at the time of cell isolation. Despite the fact that exceedingly small numbers of biliary epithelial cells are present in the original cell isolate, it is possible that these cells are the precursors of the biliary epithelium we see in our cultures. At the published maximum of 0.05% biliary epithelial cells present in the original cell isolation, inoculation of 250,000,000 hepatocytes may include up to 125,000 biliary epithelial cells. Given the loss of hepatocytes in the first 5 to 10 days of the cultures, the percentage of biliary cells may rise if biliary epithelial cells have a selective advantage in survival under the same conditions. 2) Biliary epithelium is derived from hepatocytes undergoing dedifferentiation and redifferentiation. Histological analysis of the cultures at early stages does not demonstrate presence of biliary epithelium. It appears in cultures between 6 to 8 days. The appearance of the biliary epithelium is paralleled by expression of cytokeratin 19. The cells on the surface of the cultures before day 6 look like immature small hepatocyte precursors and are indistinguishable from the other cells within the tissue. The emergence of the cytokeratin 19-positive surface biliary epithelium in these cultures is best explained by the redifferentiation of the immature hepatocytes that happen to be on the surface of the tissue at that time. The possibility that the biliary epithelium in these cultures may be derived from undifferentiated hepatocytes can only be established by selective in vivo tagging of hepatocytes before collagenase perfusion. We have attempted to do such tagging using adenoviral constructs containing β-galactosidase (data not shown). We discovered however biliary cells were also tagged by the adenovirus (data not shown). Further studies with selective tagging of hepatocytes need to be performed to conclusively test this possibility.
Intriguing is the difference in proliferation rates between biliary epithelium and hepatocytes. The percentage of PCNA-stained nuclei was very high and comparable for both cell types, indicating that the same percentage of cells in both types is in the cell cycle. The percentage of nuclei positive for Ki-67 (indicator of the S phase of the cycle), however, was much larger for the biliary epithelium (60%) versus hepatocytes (5%). This suggests that hepatocytes enter into the cycle in large numbers but become arrested in G1, before entering the S phase. The mechanisms or the implications of this are not clear at this point.
The mesenchymal cells seen in the cultures (stellate cells, endothelial cells, and so forth) are probably derived from the small percentage of cellular contaminants present in the pellet of hepatocytes after collagenase perfusion. The mechanisms leading to localization of the endothelial cells into the basal portion of the structures are not clear. Complete round spaces lined by these endothelial cells and resembling vascular spaces were also seen. It is not clear why there are so few of these structures and whether they formed from migration of endothelial cells from the bottom portion of the tissue. Stellate cells have been shown to be the cell of origin of the fibroblasts seen in older primary cultures of hepatocytes. 3 Our study also shows that the expansion of the mesenchymal stromal cells is clearly dependent on the presence of unknown functions provided by hepatocytes. There was no growth seen when the nonparenchymal cell pellet isolated from collagenase perfusion was put in culture under similar conditions (HGM medium with HGF plus EGF in the roller bottles) but in the absence of hepatocytes. Hepatocytes synthesize fibroblast growth factor-1, vascular endothelial growth factor, and TGF-α during liver regeneration after partial hepatectomy. 15 Combined addition of these growth factors in nonparenchymal cell cultures in the absence of hepatocytes also did not result in growth of connective tissue (data not shown). The nature of the interactive stimuli between hepatocytes and the nonparenchymal mesenchymal elements forming the connective tissue seen in the cultures is not clear and it seems to involve stimuli not as yet identified. Regardless of the nature of the factors involved the data underscore that the mature hepatocyte is a key essential cellular element required for building of hepatic tissue in culture. Our findings also demonstrate the important role of EGF (or EGF receptor ligands) for the formation of hepatic histology. EGF was a more potent inducer of the appearance of the biliary epithelium and the connective tissue formation than HGF.
In addition to its value as a model for building tissue structures, the system described also allows an in vitro embryology approach to study the effects of different growth factors in liver tissue organization. Obviously any extrapolation from in vitro to in vivo has innate limitations. On the other hand, several studies may be performed with this system, which are impossible to perform in the whole animal (adult or embryo). Although homozygous deletions of specific genes in mice remain a highly valuable tool to explore the role of specific signals, they are often of limited usefulness in conditions resulting in embryonic lethality (such as deletion of HGF and its receptor 5-7,16 ). Gene knockout mice are also not of much help in determining the effects of small hormones such as corticosteroids and triiodothyronine. Specific elimination of genes involved in the biosynthesis of these hormones (as seen in may spontaneously occurring endocrine syndromes in humans) usually results in accumulation of precursor metabolites upstream from the enzymatic block, thus complicating interpretation of results. Equally difficult is the combined elimination of groups of growth factors (for example HGF plus EGF). The system presented in this study can be used to provide such information. Previous studies have shown that HGF and EGF are mitogens for biliary epithelial cells. The results obtained in our study, however, suggest that these growth factors are essential for the very appearance and induction of the phenotype of the biliary epithelium. Although the role of corticosteroids is more complex, it seems that they are essential for the full phenotypic maturation of the hepatocytes. It should also be noted that dexamethasone, although essential for the development of the complete histology, had an overall negative effect on proliferation of the epithelial cells. As mentioned above, hepatocytes seem to be essential for the proper growth of the connective tissue stromal elements, because no tissue growth was seen when the nonparenchymal fraction was cultured alone. EGF seems to be a more critical factor in this process than HGF, as shown by the induction of collagen type IV gene expression (Figure 8) ▶ . It is not clear whether the effect of HGF and EGF was direct or mediated through TGF-β1 or which cellular elements (epithelial or mesenchymal cells) were the sources of TGF-β1 production.
The nature of mitogenic stimuli in cultures in which dexamethasone, HGF, and EGF were omitted is not clear. There was considerable growth of cells resembling hepatocyte precursors (Figures 5D and 6D) ▶ ▶ with several mitoses easily identifiable. Although most of the cells were not identifiable as mature hepatocytes, several groups of them were positive for the HEPPAR antigen. Mesenchymal cells present in the cultures may produce HGF and proliferating hepatocytes are known to produce TGF-α. 17 If this is so, however, the growth factors produced are not at sufficient levels to induce the appearance of the biliary epithelium. Insulin and/or diferric iron-saturated transferrin (standard additives to the HGM medium) may also play a role in this process. 18,19
More studies are required to fully analyze the implications of the results presented in this study. The model however is uniquely amenable for in vitro embryology studies, in which selective blocking agents (antisense RNA, antibodies) to specific components of matrix and growth factors may yield unique information on mechanisms and pathways important for hepatic tissue organization.
Acknowledgments
We thank Mr. Mark A. Ross for fine technical assistance.
Footnotes
Address reprint requests to George K. Michalopoulos, M.D., Ph.D., Professor and Chairman, Dept. of Pathology, Univ. of Pittsburgh School of Medicine, S-410 Biomedical Science Tower, Pittsburgh, Pennsylvania, 15261. E-mail: michalopoulosgk@msx.upmc.edu.
Supported by National Institutes of Health grants CA30241 and CA35373 (Principal Investigator, George K. Michalopoulos) and CA76541 (Principal Investigator, Donna Beer Stolz).
References
- 1.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK: Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol 1996, 132:1133-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y: Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology 1999, 29:111-125 [DOI] [PubMed] [Google Scholar]
- 3.Maher JJ, Bissell DM, Friedman SL, Roll FJ: Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest 1988, 82:450-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC: Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology 1999, 29:90-100 [DOI] [PubMed] [Google Scholar]
- 5.Uehara Y, Mori C, Noda T, Shiota K, Kitamura N: Rescue of embryonic lethality in hepatocyte growth factor/scatter factor knockout mice. Genesis 2000, 27:99-103 [PubMed] [Google Scholar]
- 6.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C: Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373:699-702 [DOI] [PubMed] [Google Scholar]
- 7.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C: Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376:768-771 [DOI] [PubMed] [Google Scholar]
- 8.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC: Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126:2739-2750 [DOI] [PubMed] [Google Scholar]
- 9.Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC: The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 1994, 8:399-413 [DOI] [PubMed] [Google Scholar]
- 10.Seglen PO: Preparation of isolated rat liver cells. Methods Cell Biol 1976, 13:29-83 [DOI] [PubMed] [Google Scholar]
- 11.Strom SC, Michalopoulos G: Collagen as a substrate for cell growth and differentiation. Methods Enzymol 1982, 82:544-555 [DOI] [PubMed] [Google Scholar]
- 12.Fiel MI, Antonio LB, Nalesnik MA, Thung SN, Gerber MA: Characterization of ductular hepatocytes in primary liver allograft failure. Mod Pathol 1997, 10:348-353 [PubMed] [Google Scholar]
- 13.Hendrich S, Campbell HA, Pitot HC: Quantitative stereological evaluation of four histochemical markers of altered foci in multistage hepatocarcinogenesis in the rat. Carcinogenesis 1987, 8:1245-1250 [DOI] [PubMed] [Google Scholar]
- 14.Auth MK, Joplin RE, Okamoto M, Ishida Y, McMaster P, Neuberger JM, Blaheta RA, Voit T, Strain AJ: Morphogenesis of primary human biliary epithelial cells: induction in high-density culture or by coculture with autologous human hepatocytes. Hepatology 2001, 33:519-529 [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos GK, DeFrances MC: Liver regeneration. Science 1997, 276:60-66 [DOI] [PubMed] [Google Scholar]
- 16.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N: Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373:702-705 [DOI] [PubMed] [Google Scholar]
- 17.Mead JE, Fausto N: Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA 1989, 86:1558-1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francavilla A, Starzl TE, Porter K, Foglieni CS, Michalopoulos GK, Carrieri G, Trejo J, Azzarone A, Barone M, Zeng QH: Screening for candidate hepatic growth factors by selective portal infusion after canine Eck’s fistula. Hepatology 1991, 14:665-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohno Y, Shiraki K, Mura T, Ikawa S: Iron-saturated lactoferrin as a co-mitogenic substance for neonatal rat hepatocytes in primary culture. Acta Paediatr 1993, 82:650-655 [DOI] [PubMed] [Google Scholar]