Abstract
The hypothesis that wound repair is augmented by delivery of platelet-derived growth factor (PDGF) from platelets and macrophages is an attractive extrapolation from the known activities of PDGF in cell culture and in vivo. To test this hypothesis in mice, we prepared hematopoietic chimeras, in which the hematopoietic system of a normal adult mouse was replaced by the hematopoietic system of a PDGF B-chain −/− or +/+ donor. We initiated local granulation tissue formation either by implanting small surgical sponges to elicit a foreign body granulation tissue response, or by ligating the left common carotid to form an organized thrombus. We found that the absence of hematopoietic PDGF B-chain did not decrease the extent of granulation tissue or vascular lesion formation, and that the vascularization of both lesions increased by ∼100%. We conclude that PDGF B-chain from cells of hematopoietic origin, including platelets and macrophages, is not important for granulation tissue formation, and that it reduces vascularization of granulation issue, probably through disabling of the short-range chemotactic gradients of PDGF that are important for recruiting pericytes/smooth muscle cells to the endothelium of new vessels.
Platelet-derived growth factor (PDGF) is a family of disulfide-bonded homo- or heterodimers of four possible subunits (A-chain, B-chain, C-chain, and D-chain) that act on cells via binding to homo- or heterodimers of one the two known PDGF receptor proteins: PDGFRα and PDGFRβ. PDGF A-chain and PDGF B-chain were identified almost 20 years ago, and much is known about their binding specificities and activities. PDGF B-chain binds to PDGFRα and PDGFRβ, whereas PDGF A-chain can bind only to PDGFRα. 1 PDGF-C and PDGF-D have been identified only recently, and much less is known about their sources and roles. 2-4 In binding specificity, PDGF-C seems most similar to PDGF-A in that it binds to PDGFRα 5 and PDGF-D is most similar to PDGF-B, in that it binds to PDGFRβ, but unlike PDGF B-chain, does not bind to PDGFRα. 2,3
PDGF-A chain plays an important role in the development of specific populations of mesenchymal cells involved in epithelial-mesenchymal interaction as well as of neural crest-derived mesenchyme and glial cells of the oligodendrocyte lineage. 6 Little is known about the roles of PDGF-C and PDGF-D. In adult animals, PDGF B-chain is highly expressed by megakaryocytes/platelets, endothelial cells (ECs), and macrophages. 7-9 It has long been recognized as a potent mitogen and chemotactic agent for connective tissue and stromal cells. 10 This suggested the following simple and elegant hypothesis. At sites of injury, PDGF sequestered in the secretory granules of platelets, and PDGF synthesized by activated macrophages, is released and stimulates local connective tissue cells to proliferate and/or immigrate into the damaged area as part of the wound repair process. 9 In pathological situations, including chronic injury in cardiovascular disease, and in inappropriate secretion by tumor cells, this connective tissue proliferation could be excessive and deleterious.
The response to injury hypothesis has considerable support from experiments in which exogenous PDGF was shown to be able to promote aspects of wound repair. 11-13 Many reports have also demonstrated that exogenous PDGF can enhance angiogenesis. In mice, implantation of PDGF B-chain-transfected melanoma cells resulted in increased fibrovascular stroma development relative to untransfected cells. 14 Administration of PDGF B-chain to the chick chorioallantoic membrane resulted in increased numbers of vessels with little inflammation. 15,16 In vitro studies similarly suggested that PDGF B-chain could be proangiogenic under some conditions, and that its target could be either ECs, or vascular smooth muscle cells (SMCs), or both. Most ECs, in culture or in vivo, do not express detectable PDGFRβ and do not respond to PDGF. However, some in vitro models of angiogenesis demonstrate that PDGF B-chain is capable of stimulating EC proliferation, movement, and tube formation. 17-19 PDGF also acts on vascular support cells, including pericytes, vascular SMCs, and adventitial fibroblasts. In vitro, PDGF B-chain from ECs can recruit perivascular cells via chemotaxis 20,21 and by stimulating proliferation. The addition of PDGF B-chain to aortic organ cultures results in an outgrowth of fibroblast-like cells followed by numerous small vascular outgrowths. 19
The studies above demonstrate that experimentally added PDGF is sufficient, under at least some circumstances, to promote connective tissue formation and/or vascularization. It has been more difficult to test the hypothesis that endogenous PDGF plays an important role in response to injury. Homozygous deletion of either PDGF B-chain or PDGFRβ in mice results in perinatal lethality. The most prominent defect in both the PDGF B-chain−/− and PDGFRβ−/− mice is abnormal blood vessels and renal glomeruli. 22-24 The primary defect seems to be in pericytes rather than ECs. The pericytes express PDGFRβ and are likely targeted by PDGF B-chain expressed by the ECs. 24 Pericytes are reduced in number in both knockouts, 24,25 whereas ECs are increased in number. 26 The vessels are dilated and hyperpermeable, leading to edema and hemorrhage.
Because PDGF B-chain and PDGFRβ null mice do not survive to adulthood, they cannot be used to assess the roles of PDGF in pathological processes or in normal wound repair. To circumvent this limitation, and to obtain quantitative information about the role of the PDGF B-chain/PDGFRβ system in cell proliferation and migration, we have previously used a form of quantitative chimera analysis. In that approach, we prepared chimeric mouse embryos by fusing embryos from wild-type and genomically-marked PDGFRβ−/− lines. As these chimeric embryos develop, we could use changes in the relative abundance of wild-type and mutant cells to calculate the selective advantage conferred by PDGFRβ expression in different cell lineages during growth and differentiation. This technique demonstrated that PDGFRβ plays a role in the development of SMCs, but not ECs or fibroblasts. 27 By contrast, PDGFRβ expression was very important for EC and fibroblast participation in the formation of granulation tissue in an adult model of response to injury. 28 In that report, we speculated that the importance of PDGFRβ for ECs and fibroblasts in granulation tissue formation reflected an important role for PDGF B-chain delivered by platelets and macrophages to the injured/inflamed tissue.
Although quantitative chimera analysis of PDGFRβ function in aggregation chimeras is able to demonstrate a requirement for PDGF in granulation tissue formation, it is not able to discern the source of this PDGF. In this report, we prepared a different kind of chimeric mouse to test the hypothesis that the PDGF that drives wound repair in injured adult tissue is primarily derived from platelets and macrophages. Because these cells are of hematopoietic origin, we could specifically eliminate their ability to provide PDGF by creating hematopoietic chimeras, in which the hematopoietic system of a wild-type mouse was ablated by irradiation and replaced by hematopoietic cells from PDGF B-chain −/− mice. 29 We were surprised to find that the lack of PDGF B-chain of hematopoietic origin did not reduce the granulation tissue that formed in two models of response to injury: implantation of a sponge and formation of an organized intravascular thrombus. In fact, the absence of PDGF B-chain from hematopoietic cells actually increased the extent of vascularization of these lesions. We conclude that delivery of PDGF B-chain by platelets and macrophages is not important for granulation tissue formation, at least in these situations, and discuss two possible mechanisms through which disruption of normal spatial-temporal patterns of PDGF delivery could result in reduced vascularity of the tissue.
Materials and Methods
Creation of Hematopoietic Chimeras
B6.SJL Ly5a mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or bred at the Fred Hutchinson Cancer Research Center (Seattle, WA). 129SV-C 57BL/6J Ly5b mice heterozygous for PDGF B-chain 22 were bred in the Department of Medical Biochemistry, University of Goteborg (Goteborg, Sweden). Hematopoietic chimeras lacking PDGF B-chain expression in cells of hematopoietic origin (hematopoietic B−/− chimeras) were created as previously described. 29 Briefly, 129SV-C57BL/6J PDGF B-chain +/− heterozygotes were mated and E16.5 embryos collected and genotyped. Livers from PDGF B-chain +/+ and −/− embryonic littermates were disaggregated as a source of hematopoietic progenitors. These were injected into the tail vein of wild-type 6-to 12-week-old host B6.SJL Ly5a mice that had been exposed to 14 Gy total body irradiation 1 day before transplantation to eliminate the host hematopoietic system. Recipients were transferred to conventional housing 6 to 8 weeks after transplantation. Complete replacement of host with donor hematopoietic cells had occurred by 3 months 29 and all experiments were performed after this time.
Surgical Implantation of Sponges
Mice were anesthetized by intraperitoneal injection of 100 mg/kg of ketamine and 8 mg/kg of xylazine. Four sterile 5 mm in diameter, 3-mm high polyvinyl alcohol sponges (IVALON; Unipont Industries, Thomasville, NC) were implanted subcutaneously into each mouse via small dorsal midline incisions over the cranial thoracic area and over the lumbar area. One sponge was implanted on each side of the incisions and the incisions were closed using Clay Adams 9-mm wound clips (Becton Dickinson, Sparks, MD). At 6 and 8 weeks after implantation, three mice of each hematopoietic genotype were humanely euthanized and the sponges harvested.
Carotid Ligation
Mice were anesthetized by intraperitoneal injection of 100 mg/kg of ketamine and 8 mg/kg of xylazine. A midventral cervical incision was made followed by blunt dissection to isolate the left common carotid artery that was ligated with 6-0 silk just proximal to the bifurcation into external and internal branches. The skin incision was closed using Michel wound clips. At 4 weeks after ligation, six mice of each hematopoietic genotype were humanely euthanized and carotids harvested.
Vessel Identification and Quantitation
Excised sponges were fixed in methyl Carnoy’s fixative, embedded in paraffin, and cut into 5-μm sections. Vessels within the sponge-induced granulation tissue were identified using four techniques: 1) lumens surrounded by flattened cells visualized after staining with Masson’s trichrome stain; 2) vascular basement membrane profiles immunostained using rabbit anti-mouse laminin (Collaborative Biomedical Products, Bedford, MA) followed by biotinylated goat anti-rabbit IgG then Vectastain elite ABC peroxidase (Vector Laboratories, Burlingame, CA) and visualized using diaminobenzidine with methyl green nuclear counterstain; 3) histologically recognizable vessels associated with smooth muscle α-actin-positive cells identified by immunostaining using monoclonal anti-α-actin conjugated to peroxidase (catalog no. U7033; DAKO, Carpinteria, CA) and visualized using diaminobenzidine with methyl green nuclear counterstain; and 4) histologically recognizable vessels associated with desmin-positive cells identified by immunostaining with monoclonal anti-desmin conjugated to peroxidase (catalog no. U7023, DAKO) and visualized using diaminobenzidine with methyl green nuclear counterstain.
Excised carotid arteries were fixed in formalin, paraffin embedded, serially sectioned at 5 μm, and stained with hematoxylin and eosin. Vessels within the organized thrombus were identified by histology.
To evaluate vessel density, vessels identified using each of the above methods were counted using an intraocular grid. To ensure that only newly formed vessels were included in the data, only vessels at least 0.5-mm interior to the sponge margin, or inside the internal elastic lamella of the carotid, were counted. To determine the fractional area occupied by vessels, a 121-point grid was placed over a video monitor onto which ×200 images of the sponge were displayed from a Hamamatsu charge-coupled device camera attached to the microscope. The number of points on the grid that fell within a vessel lumen or on the endothelium were counted as a measure of vascular area. All analyses were done blinded to tissue source.
Quantitation of PDGF B-Chain Transcript Expression in Fixed Sponge Granulation Tissue
RNA was isolated from paraffin-embedded sponge granulation tissue by cutting two 50-μm thick sections and incubating at room temperature in xylene for 10 minutes with gentle mixing, followed by centrifugation at 14,000 rpm for 5 minutes, and removal of the supernatant. Ethanol (100%) was added and the tissue sections resuspended, microfuged at 14,000 rpm for 5 minutes, the ethanol aspirated off, and the sections air-dried for 5 minutes. The sections were solubilized by the addition of 1 ml of Trizol, vortexing for 1 minute, and passage through 22- and 25-gauge needles. Four μl of molecular biology grade glycogen, 20 mg/ml, was added to each sample, mixed, and incubated at room temperature for 5 minutes. Two hundred μl of CHCl3 was added to each sample, followed by 15 seconds of vigorous shaking, a 5-minute room temperature incubation, and 15 minutes of microfugation at 12,000 rpm at 4°C. The upper aqueous phase was transferred to a new tube and an equal volume of isopropanol was added, mixed, and incubated at room temperature for 10 minutes followed by centrifugation at 10,000 rpm, 4°C, for 10 minutes. The supernatant was removed and discarded and the RNA washed by adding 1 ml of −20°C 75% ethanol, vortexing, and centrifugation at 8000 rpm, 4°C, for 5 minutes. The supernatant was discarded and the RNA dried at room temperature for 5 minutes and resuspended in 10 μl of diethyl pyrocarbonate water. cDNA primed by random hexamers was made from the extracted RNA using the Gibco BRL Superscript Preamplification System.
Transcript levels were quantitated by real time polymerase chain reaction. Standard 18s primers and TaqMan probe and custom-made PDGF B-chain primers and TaqMan probe were obtained from PE Biosystems (Foster City, CA). PDGF B-chain forward primer: tccggagtcgagttggaaag; reverse primer: ggcgattacagcaggctctg; probe: FAM-tcgagggaggaggagccta-TAMRA. Thermocycling was performed on the GeneAmp 5700 Sequence Detection System (Perkin Elmer) using the following parameters: 50°C for 2 minutes, 95°C for 10 minutes, then alternating 40 times between 95°C for 20 seconds and 50°C for 60 seconds. Threshold (CT) values were calculated by the GeneAmp 5700 SDS Detector software. Each sample was analyzed in triplicate polymerase chain reaction reactions accompanied by a standard curve and two no-template control reactions.
Results
PDGF B-Chain Levels Are Significantly Reduced in Granulation Tissue of Hematopoietic B−/− Chimeras
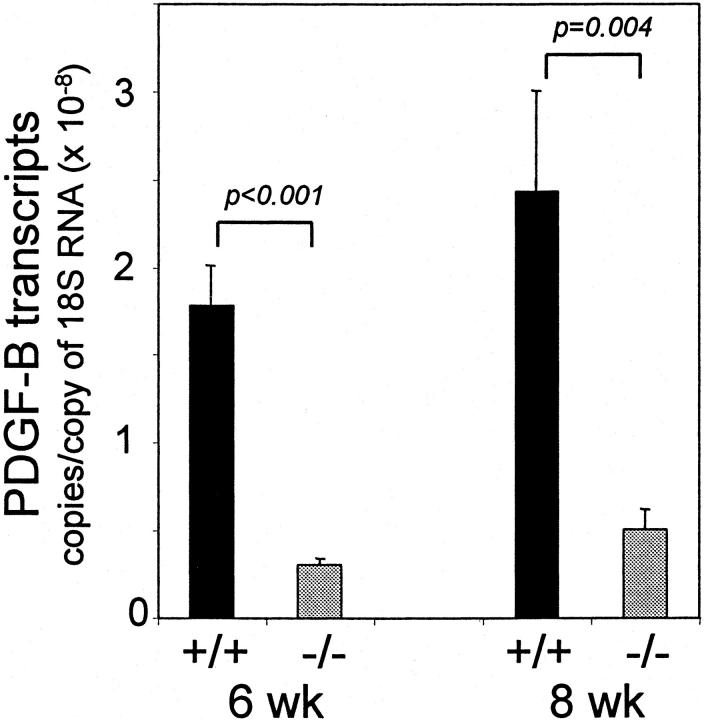
We created hematopoietic chimeras by high-dose irradiation of wild-type donor mice followed by intravenous injection of hematopoietic progenitors from donor PDGF B-chain−/− embryos, or from PDGF B-chain +/+ embryos as controls (see Materials and Methods). 29,30 We have already found that lack of PDGF B-chain expression by hematopoietic cells and their progenitors does not affect the establishment of a normal hematopoietic system in the host mice, with platelets and all leukocyte cell types present at their normal levels. 29 The hematopoietic B−/− chimeras are expected to show no PDGF B-chain expression in platelets, macrophages, or other hematopoietic derivatives, but expression of PDGF B-chain in nonhematopoietic cell types, including ECs, should be normal. To verify the absence of PDGF B-chain from hematopoietic cells, we isolated peritoneal macrophages and evaluated B-chain transcript levels by semiquantitative reverse transcriptase-polymerase chain reaction. Stimulated peritoneal macrophages from hematopoietic B+/+ chimeras produced abundant PDGF B-chain transcripts whereas stimulated peritoneal macrophages from hematopoietic B−/− chimeras did not produce detectable PDGF B-chain transcripts (data not shown). To evaluate PDGF B-chain expression by other cell types within granulation tissue, we isolated mRNA from the sponge-induced granulation tissue (see below). Figure 1 ▶ shows that PDGF B-chain transcript levels in hematopoietic B−/− chimeras are 18% (at 6 weeks) and 21% (at 8 weeks) of the levels in hematopoietic B+/+ chimeras. This demonstrates that the majority of PDGF B chain transcript within granulation tissue is of hematopoietic origin, but that a substantial portion is of nonhematopoietic origin, probably from ECs. It should be noted, however, that because the great majority of the PDGF B-chain protein present in platelets was synthesized in the megakaryocyte precursors of platelets, the contribution of PDGF B-chain protein from platelets will be underestimated by measurement of transcript levels.
Figure 1.
PDGF B-chain transcript levels in granulation tissue. RNA was extracted from thick sections of sponge granulation tissue at 6 and 8 weeks after implantation. PDGF B-chain transcript levels were determined by TaqMan real-time reverse transcriptase-polymerase chain reaction and normalized to level of 18s RNA. Results are the mean ± SEM of triplicate determinations of three to four sponges from each mouse, three mice/genotype.
PDGF B-Chain of Hematopoietic Origin Is Not Necessary for Foreign Body-Induced Granulation Tissue Formation and Its Absence Promotes Vascularization
As a model system in which to evaluate granulation tissue formation and vascularization, we implanted polyvinyl alcohol sponges subcutaneously into the hematopoietic chimeric mice. A highly vascularized granulation tissue forms in and around the sponge matrix. Because the sponge is initially devoid of cells, any tissue found inside the sponge can be considered newly formed, and this is where we focused our evaluation. The granulation tissue in the hematopoietic B−/− chimeras contained a noticeably greater density of blood vessels than did hematopoietic B+/+ chimeras. We quantitated the density of vessels within the sponge at 6 and 8 weeks after implantation. We identified vessels by combining morphology with one of the following: 1) Masson’s trichrome, a connective tissue stain with which vessels are easily identified (Figure 2, A and D) ▶ ; 2) anti-smooth muscle α-actin antibody, which delineates a subset of vessels surrounded by α-actin-positive cells (SMCs and pericytes); 3) anti-desmin antibody, which delineates a subset of vessels surrounded by desmin-expressing cells (SMCs and pericytes); and 4) anti-laminin antibody, which clearly stains vascular basement membranes (Figure 2, B and E) ▶ . As shown in Figure 3 ▶ , regardless of the method used to identify vessels, there are significantly more vessels within the granulation tissue of hematopoietic B−/− chimeras at both 6 and 8 weeks after implantation. Depending on the method used to assess vascularity, and the time point selected, vascularity was increased by 1.7-fold to 2.5-fold and, on the average, it doubled the vascularity of the sponge granulation tissue. Because the vascularity of an area can also be described by the size of the vessels, we compared the vascular cross-sectional area of the two groups of mice. This analysis showed increased vascular cross-sectional area in hematopoietic B−/− chimeras when compared to hematopoietic B+/+ chimeras (data not shown). The increase was statistically significant at 8 weeks (1.7-fold, P = 0.02) but not at 6 weeks (1.4-fold, P = 0.12).
Figure 2.
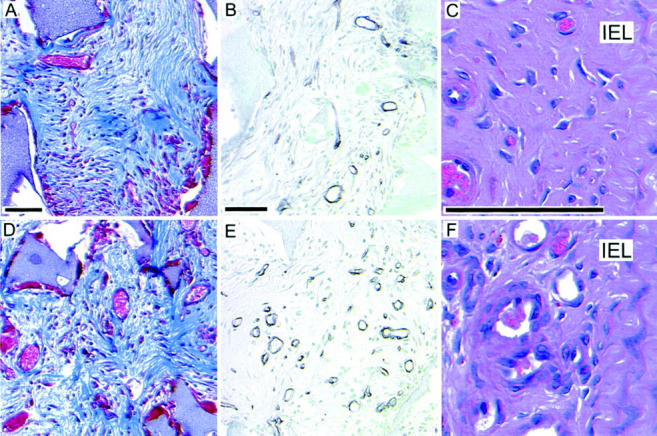
Granulation tissue formation in hematopoietic chimeras. The above representative micrographs are taken from implanted sponges at 8 weeks (A, B, D, and E) or ligated carotid arteries at 4 weeks (C and F). A, B, and C are from hematopoietic B+/+ chimeras. D, E, and F are from hematopoietic B−/− chimeras. A and D are stained with Masson’s trichrome. B and E are immunostained with anti-laminin with methyl green nuclear counterstain. C and F are stained with H&E.
Figure 3.
Vascularity of sponge-induced granulation tissue is increased by elimination of PDGF B-chain expression in hematopoietic cells. Small surgical sponges were implanted subcutaneously and removed after 6 and 8 weeks for fixation, sectioning, and analysis. Vessels within the sponge-induced granulation tissue were identified using four techniques: 1) lumens surrounded by flattened cells visualized after staining with Masson’s trichrome stain; 2) histologically recognizable vessels associated with smooth muscle α-actin-positive cells identified by immunostaining with anti-smooth muscle α-actin antibody; 3) histologically recognizable vessels associated with desmin-positive cells identified by immunostaining with anti-desmin antibody; and 4) vascular basement membrane profiles immunostained with anti-laminin antibody. To evaluate vessel density, vessels identified using each of the above methods were counted using an intraocular grid in two complete nonsequential cross sections from each sponge. Results are presented as the mean ± SEM of determinations from three to four sponges from each mouse, three mice/genotype/time point.
PDGF B-chain is a mitogen for cultured fibroblasts, and we anticipated that the hematopoietic B−/− chimeras would show diminished fibrosis and connective tissue matrix deposition. As a general survey of connective tissue formation in implanted sponges, we stained with Masson’s trichrome stain (Figure 2, A and D) ▶ . Apart from the difference in vascularity noted above, we observed no obvious differences between granulation tissue formed in hematopoietic B−/− versus B+/+ chimeras. To compare the two genotypes more rigorously, we blindly (with respect to genotype) ranked sections from each time point (11 to 12 from each genotype) in order of increasing cellularity/fibrosis and compared the rankings using the nonparametric Mann-Whitney test. Although the mean numerical ranking of the hematopoietic B−/− chimeras was higher at both time points, suggesting increased cellularity/fibrosis within the sponges of PDGF B-chain null mice, the differences were statistically insignificant (6 weeks, P = 0.62; 8 weeks, P = 0.18). We also compared the extent of fibrosis by assigning a score of 1 to 4 for capsule thickness and intrasponge fibrosis in each section. Again, the hematopoietic B−/− chimeras showed more fibrosis than hematopoietic B+/+ chimeras, but the differences were not statistically significant.
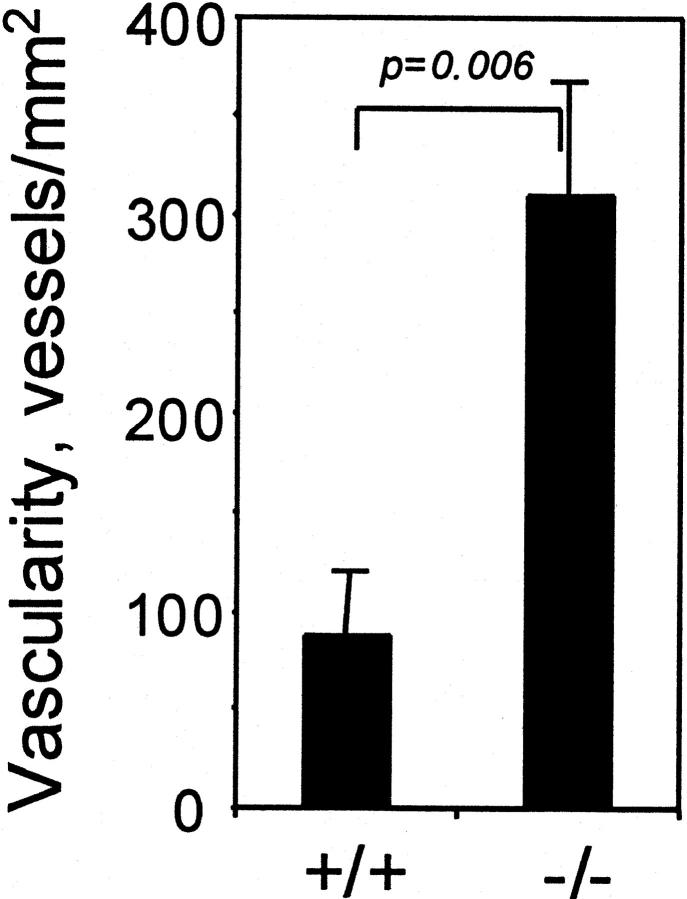
PDGF B-Chain of Hematopoietic Origin Is Not Necessary for Formation of an Intra-Arterial Organized Thrombus, and Its Absence Promotes Vascularization
To determine whether the effects of hematopoietic B-chain elimination observed in foreign body-induced granulation tissue were generalizable to granulation tissue formed under other circumstances, we evaluated the role of hematopoietic PDGF B-chain in development of a lesion referred to as an “organized thrombus.” When a thrombus forms at a site of vascular damage, for example at the surface of an atherosclerotic lesion, it is often organized by in-growth of capillaries and other connective tissue cells, in a process that is analogous to granulation tissue formation. When the left common carotid artery of a mouse is ligated to block blood flow, the ligated vessel remodels 31,32 and the portion of the vessel near the ligature becomes filled with tissue that resembles organized thrombus, with numerous small vessels. We ligated the carotid arteries of hematopoietic chimeras and evaluated the histology and vascularity of the organized thrombus that formed (Figure 2, C and F) ▶ . As observed in the sponge-induced granulation tissue, the most obvious difference between the lesions in mice with or without hematopoietic PDGF B-chain, was that vessel density was substantially greater in the hematopoietic B−/− chimeras (Figure 4) ▶ . As evaluated by serial sectioning, there was no statistical difference in the length (P = 0.81) or volume (P = 0.66) of the carotid lesions in B+/+ versus B−/− chimeras (data not shown).
Figure 4.
Vascularity of organized thrombus is increased by elimination of PDGF B-chain expression in hematopoietic cells. We identified vessels histologically in H&E-stained sections. From each lesion we counted all vessels within the area delimited by the internal elastic lamina in four to seven sections spanning 300 μm beginning at the ligature and extending toward the heart. Results are plotted as mean ± SEM of determinations from lesions from six mice/genotype at 4 weeks.
PDGF B-Chain of Hematopoietic Origin Reduces Investment of Vessels by SMCs
In the studies above, we did not distinguish between different types of vessels in the granulation tissue. To determine the fraction of vessels that included associated pericytes/SMCs, we stained for laminin (to identify all vessels) and for either desmin or smooth muscle α-actin to identify associated SMC-like cells. There is no well-characterized marker that recognizes all cells that may function as pericytes, but desmin seems to identify the largest fraction of such cells. 25 In the sponge-induced granulation tissue, the fraction of laminin-positive vessel profiles that included associated desmin-positive cells is greater in the hematopoietic B−/− chimeras than in the hematopoietic B+/+ chimeras at both 6 and 8 weeks (Figure 5) ▶ . The ratio of smooth muscle α-actin-positive vessels to laminin-positive vessels showed the same trend, but the differences were not statistically significant (data not shown).
Figure 5.
Elimination of hematopoietic PDGF B-chain increases the fraction of vessels that are invested by pericytes/SMCs. Vessels in sections of sponge removed after 6 or 8 weeks were identified using anti-laminin immunostaining, to identify all vessels with a well-established basement membrane, and by anti-desmin immunostaining, to identify vessels associated with smooth muscle-like cells. Results are plotted as mean ± SEM of the ratios of desmin-positive to laminin-positive vessels for three to four sponges from each mouse, three mice/genotype/time point.
Discussion
PDGF can stimulate the migration and proliferation of many connective tissue cell types. One of the earliest, and most attractive, hypotheses for the pathophysiological function of PDGF was that it stimulates repair of connective tissue damage via local release from degranulating platelets and via secretion by activated macrophages. This hypothesis was based primarily on the observations that PDGF is abundant in platelets and in activated macrophages, and on experiments demonstrating that addition of exogenous PDGF is capable of stimulating connective tissue cell proliferation. One method for directly testing the role of endogenous PDGF in disease processes in vivo is quantitative aggregation chimera analysis using PDGFRβ-deficient cells. 27 Quantitative chimera analysis demonstrated that PDGFRβ expression by ECs, fibroblasts, and SMCs is critical for their participation in formation of sponge-induced granulation tissue. 28 This demonstrates that signaling through PDGFRβ plays an important role in granulation tissue formation, but it did not provide insight into the identity or source of the ligand for PDGFRβ during granulation tissue formation. We had speculated that the cause of the much greater role for PDGF in fibroblast and EC participation in granulation tissue formation was that granulation tissue formation involved an additional source PDGF: macrophages and platelets.
The results of the current study demonstrate that this explanation was not correct. Specific ablation of PDGF B-chain expression in hematopoietic cells, including platelets and monocytes/macrophages, did not diminish granulation tissue formation in the two models that we evaluated: induced by a foreign body or by an intravascular thrombus. This means that PDGF B-chain from platelets and macrophages is not important for granulation tissue formation. Either the PDGFRβ ligand required for fibroblast, EC, and SMC participation in granulation tissue formation is PDGF D-chain, or the necessary PDGF B-chain must derive from a nonhematopoietic source. Consistent with the latter possibility, we found that granulation tissue in hematopoietic B−/− chimeras still expressed PDGF B-chain transcripts at ∼20% of the level in hematopoietic B+/+ chimeras (Figure 1) ▶ . This remaining PDGF B-chain is probably derived from ECs. 33 In addition to this nonhematopoietic PDGF B-chain, it is possible that PDGF D-chain serves as a significant additional source of PDGFRβ ligand. PDGF D-chain is not known to be present in macrophages, but it is expressed at low levels in peripheral blood leukocytes 3 and relatively high levels by adventitial fibroblasts and cultured ECs. 4
There is considerable evidence (see Introduction) that PDGF B-chain can stimulate proliferation and migration of the cell types involved in angiogenesis and granulation tissue formation. However, we found that granulation tissue was substantially less highly vascularized in mice in which platelets and macrophages contained normal levels of PDGF B-chain, than in mice in which hematopoietic sources of PDGF B-chain had been eliminated. Our studies do not provide a unique explanation for this surprising result, but there are at least two mechanisms through which changes in the source and timing of PDGF delivery could affect vascularization. These two mechanisms are based on reports that pericytes can act either as inhibitors of EC migration/proliferation (when present early) or as stabilizers of capillaries (when present later). The first possible mechanism is that, in the absence of PDGF B-chain from platelets and macrophages, PDGF B-chain−/− chimeras have reduced early recruitment of pericytes into the area in which granulation tissue is developing. Because pericytes have been shown to inhibit the early proliferation and migration of ECs, 34,35 the reduced pericyte population could allow increased expansion of capillaries. This hypothesis accounts for the increased vascularization observed in the −/− animals but does not explain why they have higher ratios of pericytes/vessel.
A second possible explanation is that PDGF B-chain delivered by platelets and macrophages may partially obscure the short-range chemotactic gradients of PDGF B-chain produced by vascular ECs. This short-range gradient is thought to be involved in recruiting the pericytes/SMCs that are important for stabilizing new vessels, so the vessels formed in the presence of hematopoietic PDGF B-chain may be less efficiently invested by pericytes, less stable and, ultimately, less abundant. The following observations support this possibility. 1) Newly formed vessels are relatively unstable until they are invested by pericytes/SMCs. 36 The recruitment of pericytes/SMCs is mediated in part via PDGF B-chain secreted by ECs. This conclusion is supported by the pattern of expression of PDGF B-chain and PDGFRβ 25,37 and by the phenotype of PDGF B-chain 22,23 or PDGFRβ-null mice. 22,23 In both null phenotypes, there is a deficiency of mesangial cells, the pericytes of the glomerulus, resulting in dilated capillary loops 22,23 and there is a deficiency of pericytes in other capillary beds, resulting in microvascular instability, dilation, and hemorrhage in the dermis. 22-24 2) Responses to PDGF and other chemotactic agents are biphasic. Excess ligand can saturate receptors and block chemotaxis. High concentrations of PDGF have been demonstrated to inhibit SMC chemotaxis. 38,39 When exogenous PDGF B-chain is administered to the eye, pericytes are drawn away from newly formed vessels resulting in vascular destabilization and regression. 40 The granulation tissue in our models contains large numbers of inflammatory cells and these may secrete enough PDGF B-chain to partially mask the chemotactic gradient toward ECs, thereby partially disrupting the ability of the pericytes/SMCs to find and/or invest the newly formed EC tubes. 3) Our data (Figure 5) ▶ show that the ratio of desmin-positive vessels (vessels that have adjacent pericytes/perivascular SMCs) to laminin-positive vessels, is higher in the hematopoietic B−/− chimeras than in hematopoietic B+/+ chimeras (Figure 5) ▶ , suggesting that the presence of hematopoietic PDGF B-chain may reduce pericyte/SMC investment of new vessels.
We conclude that PDGF B-chain from cells of hematopoietic origin, including platelets and macrophages, is not necessary for granulation tissue formation elicited by at least two stimuli, and that it plays an unexpected inhibitory role in vascularization of that tissue. The finding that vascularization is actually less in mice with wild-type hematopoietic systems than in mice deleted for hematopoietic PDGF B-chain, suggests that, in this situation, the hematopoietic PDGF may be interfering with the timing or strength of the chemotactic gradients of PDGF that are important for recruiting pericytes/SMCs to the endothelium of new vessels. It is possible that in tissue processes that involve relatively few macrophages and platelets, the contribution of PDGF B-chain from hematopoietic cells will not be sufficient to interfere with vessel investment, and may be sufficient to stimulate small local increases in proliferation or function of PDGF responsive connective tissue cells. In either case, the possibility that elevated concentrations of PDGF may inhibit vascularization must be considered when addition of exogenous PDGF is considered as a possible therapy to promote wound healing.
Footnotes
Address reprint requests to Daniel F. Bowen-Pope, Ph.D., University of Washington, Dept. of Pathology, Box 357470, Seattle, WA 98195-7470. E-mail: bp@u.washington.edu.
Supported by National Institutes of Health grants HL03174 (to D. F. B.-P.) and HL18645 (to E. W. R.), and Deutsche Forschungsgemeinschaft grant Kal078/1 (to W. E. K.).
References
- 1.Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF: Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem 1989, 264:8771-8778 [PubMed] [Google Scholar]
- 2.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U: PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 2001, 3:512-516 [DOI] [PubMed] [Google Scholar]
- 3.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS: PDGF-D, a new protease-activated growth factor. Nat Cell Biol 2001, 3:517-521 [DOI] [PubMed] [Google Scholar]
- 4.Uutela M, Lauren J, Bergsten E, Li X, Horelli-Kuitunen N, Eriksson U, Alitalo K: Chromosomal location, exon structure, and vascular expression patterns of the human PDGFC and PDGFD genes. Circulation 2001, 103:2242-2247 [DOI] [PubMed] [Google Scholar]
- 5.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U: PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2000, 2:302-309 [DOI] [PubMed] [Google Scholar]
- 6.Betsholtz C, Karlsson L, Lindahl P: Developmental roles of platelet-derived growth factors. Bioessays 2001, 23:494-507 [DOI] [PubMed] [Google Scholar]
- 7.Shimokado K, Raines EW, Madtes DK, Barrett TB, Benditt EP, Ross R: A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 1985, 43:277-286 [DOI] [PubMed] [Google Scholar]
- 8.Bowen-Pope DF, Hart CE, Seifert RA: Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem 1989, 264:2502-2508 [PubMed] [Google Scholar]
- 9.Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H: Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science 1990, 248:1009-1012 [DOI] [PubMed] [Google Scholar]
- 10.Ross R, Raines EW, Bowen-Pope DF: The biology of platelet-derived growth factor. Cell 1986, 46:155-169 [DOI] [PubMed] [Google Scholar]
- 11.Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK: Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest 1985, 76:2323-2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, Thomason A, Deuel TF: In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med 1988, 167:974-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R: PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990, 136:1235-1246 [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg K, Valyi-Nagy I, Heldin CH, Herlyn M, Westermark B: Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci USA 1993, 90:393-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risau W, Drexler H, Mironov V, Smits A, Siegbahn A, Funa K, Heldin CH: Platelet-derived growth factor is angiogenic in vivo. Growth Factors 1992, 7:261-266 [DOI] [PubMed] [Google Scholar]
- 16.Oikawa T, Onozawa C, Sakaguchi M, Morita I, Murota S: Three isoforms of platelet-derived growth factors all have the capability to induce angiogenesis in vivo. Biol Pharm Bull 1994, 17:1686-1688 [DOI] [PubMed] [Google Scholar]
- 17.Bar RS, Boes M, Booth BA, Dake BL, Henley S, Hart MN: The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology 1989, 124:1841-1848 [DOI] [PubMed] [Google Scholar]
- 18.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M: PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994, 125:917-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicosia RF, Nicosia SV, Smith M: Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 1994, 145:1023-1029 [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschi KK, Rohovsky SA, D’Amore PA: PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 1998, 141:805-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA: Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 1999, 84:298-305 [DOI] [PubMed] [Google Scholar]
- 22.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994, 8:1875-1887 [DOI] [PubMed] [Google Scholar]
- 23.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994, 8:1888-1896 [DOI] [PubMed] [Google Scholar]
- 24.Lindahl P, Johansson BR, Leveen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277:242-245 [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C: Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001, 153:543-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF: Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet 1998, 18:385-388 [DOI] [PubMed] [Google Scholar]
- 28.Crosby JR, Tappan KA, Seifert RA, Bowen-Pope DF: Chimera analysis reveals that fibroblasts and endothelial cells require platelet-derived growth factor receptor-beta expression for participation in reactive connective tissue formation in adults but not during development. Am J Pathol 1999, 154:1315-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaminski WE, Lindahl P, Lin NL, Broudy VC, Crosby JR, Hellstrom M, Swolin B, Bowen-Pope DF, Martin PJ, Ross R, Betsholtz C, Raines EW: Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood 2001, 97:1990-1998 [DOI] [PubMed] [Google Scholar]
- 30.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF: Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 2000, 87:728-730 [DOI] [PubMed] [Google Scholar]
- 31.Bryant SR, Bjercke RJ, Erichsen DA, Rege A, Lindner V: Vascular remodeling in response to altered blood flow is mediated by fibroblast growth factor-2. Circ Res 1999, 84:323-328 [DOI] [PubMed] [Google Scholar]
- 32.Harmon KJ, Couper LL, Lindner V: Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol 2000, 156:1741-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiCorleto PE, Bowen-Pope DF: Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci USA 1983, 80:1919-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orlidge A, D’Amore PA: Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 1987, 105:1455-1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato Y, Rifkin DB: Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol 1989, 109:309-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folkman J, D’Amore PA: Blood vessel formation: what is its molecular basis? Cell 1996, 87:1153-1155 [DOI] [PubMed] [Google Scholar]
- 37.Seifert RA, Alpers CE, Bowen-Pope DF: Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int 1998, 54:731-746 [DOI] [PubMed] [Google Scholar]
- 38.Clunn GF, Refson JS, Lymn JS, Hughes AD: Platelet-derived growth factor beta-receptors can both promote and inhibit chemotaxis in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1997, 17:2622-2629 [DOI] [PubMed] [Google Scholar]
- 39.Grotendorst GR, Chang T, Seppa HE, Kleinman HK, Martin GR: Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol 1982, 113:261-266 [DOI] [PubMed] [Google Scholar]
- 40.Benjamin LE, Hemo I, Keshet E: A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998, 125:1591-1598 [DOI] [PubMed] [Google Scholar]