Abstract
At the invasion front of well-differentiated colorectal adenocarcinomas, the oncogene β-catenin is found in the nuclear compartment of tumor cells. Under these conditions, β-catenin can function as a transcription factor and thus activate target genes. One of these target genes, cyclin D1, is known to induce proliferation. However, invasion front of well-differentiated colorectal adenocarcinomas are known to be zones of low proliferation and express the cell cycle inhibitor p16INK4A. Therefore, we investigated the expression profiles of nuclear β-catenin, cyclin D1, p16INK4A , and the Ki-67 antigen, a marker for proliferation, in serial sections of well-differentiated colorectal adenocarcinomas. Invasion fronts with nuclear β-catenin were compared with areas from central parts of the tumors without nuclear β-catenin, for the expression of cyclin D1, p16INK4A, and Ki-67. It was observed that expression of nuclear β-catenin, cyclin D1, and p16INK4A at the invasion front are significantly correlated. Such areas exhibit low Ki-67 expression indicating a low rate of proliferation. Thus, in colorectal carcinogenesis the function of β-catenin and its target gene cyclin D1 does not appear to be the induction of tumor cell proliferation. In particular, the function of cyclin D1 should be reconsidered in view of these observations.
Most well-differentiated human colorectal adenocarcinomas show genetic alterations in the Wnt/wg pathway. In most cases, two elements of the Wnt/wg pathway are targets for mutations. The tumor suppressor gene APC (adenomatous polyposis coli) is mutated in the vast majority of cases (60 to 80%) 1 and the oncogene β-catenin in some of the remaining cases (12 to 48%). 2 As a consequence, β-catenin is not fed into the 28 S proteasome degradation machinery and persists at a high concentration in affected tumor cells. 2 β-catenin has two major functions. First, it is an integral part of adherens junctions, connecting E-cadherin to the actin skeleton via α-catenin. 3 Thus, it helps to maintain the epithelial phenotype. Second, dimers of β-catenin and one of the members of the TCF/LEF (T cell factor/lymphoid enhancer factor) family function as a transcription factor when localized in the nucleus. In the colon, the relevant TCF/LEF family member appears to be TCF-4. 4,5 Moreover, LEF-1 expression has been shown to be induced by β-catenin in colorectal carcinomas. 6 Many other β-catenin target genes have been described in a variety of species, covering many cellular functionalities (Stanford University: http://www.stanford.edu/∼rnusse/pathways/targets.html). However, a correlation between nuclear β-catenin accumulation and expression in human tissues has been shown only for a minority of these genes. Interestingly, in well-differentiated colorectal adenocarcinomas with APC mutations, β-catenin is not localized in the nuclei of the cells by default. Instead, a pattern can be seen; at the invasion front in most of these tumors, β-catenin is localized in the nucleus, whereas in central parts of the tumor, distant from the invasion front, it is found at the plasma membrane or in the cytoplasm. 7,8 Consequently, β-catenin target genes such as c-myc, 9 matrilysin 10,11 or fibronectin 12 are expressed in tumor cells with nuclear β-catenin. 8,10,13 Another β-catenin target gene is cyclin D1. 14,15 D-type cyclins (D1, D2, and D3) are important in overcoming the restriction point (R) at the G1-S transition during the cell cycle by activating cyclin D-dependent kinases, which in turn phosphorylate the retinoblastoma protein. 16 Additionally, D-type cyclins can sequester the cell cycle inhibitor p27Kip-1 allowing cyclin E to become fully active. 16 Thus, cyclin D1 is an up-regulator of proliferation, and its gene is amplified in a variety of tumors. 17 As a β-catenin target gene, one would expect high expression of cyclin D1, and thus proliferation, at the invasion front of well-differentiated colorectal adenocarcinomas. However, we have shown previously that proliferation of colorectal carcinomas is reduced at the invasion front 18,19 concomitantly with pronounced expression of the cell cycle inhibitor p16INK4A. 20 Furthermore, the expression of c-myc, another β-catenin target gene, 9 also an accepted marker of proliferation, is associated not with proliferation in large adenomas but with nuclear β-catenin expression. 13 Therefore, we wanted to assess the distribution of β-catenin, its target gene cyclin D1, and p16INK4A with respect to proliferation. We document that the invasion front of colorectal adenocarcinomas displays low proliferative activity with highly significant nuclear co-localization of β-catenin, cyclin D1, and p16INK4A.
Materials and Methods
Patient Data
Well-differentiated, sporadic colorectal adenocarcinomas from 56 patients were investigated. All cases were drawn from the archives of the Institute for Pathology in Erlangen, Germany. Ages ranged from 44 to 88 years (mean 66.4 years). There were 28 women and 28 men. The distribution according to the TNM classification was as follows: 2 T1, 15 T2, 31 T3, and 8 T4 cases. 26 of the patients (46.4%) had lymph node metastases (N1, N2). 12 patients (21.4%) presented with distant metastases (M1, M2) in the liver (9) or lungs (3). According to the Unio Internationalis Contra Cancrum (International Union against Cancer, UICC) classification, 21 15 patients each (26.8%) were staged UICC I or II and 13 patients each (23.2%) were classified UICC III or IV, respectively.
Immunohistochemistry
Tissue specimens were fixed in 10% buffered formaldehyde and embedded in paraffin according to routine methods. Sections 5 μm thick were mounted on Biogenex slides (Christian Sartori GmbH, Hamburg, Germany), deparaffinized, and hydrated using graded ethanols following routine protocols. Antigen retrieval and antibody dilutions were optimized individually as follows. A 1:750 dilution of a polyclonal rabbit serum specific for β-catenin (C2206; Sigma, Deisenhof, Germany) or a 1:50 dilution of a mouse monoclonal antibody specific for the Ki-67 antigen (clone MIB-1 7240, DAKO, Hamburg, Germany) were applied overnight to serial sections after microwave treatment twice for 10 minutes in citrate buffer (10.5 g/L citric acid trihydrate-NaOH, pH 6.0) at 600 W and again at 450 W. For cyclin D1 (clone DCS-6; DAKO), sections were heated as described above in citrate buffer, pH 6.0 (DAKO), treated for 10 minutes in 3% (v/v) H2O2 at room temperature, and incubated overnight with a 1:750 dilution of the DCS-6 mouse monoclonal antibody. Developing was initiated by incubating the sections for 30 minutes with a 1:50 dilution of biotinylated goat anti-rabbit or rabbit anti-mouse secondary antibody (DAKO). For cyclin D1, an additional tyramide enhancement step was introduced by further incubating for a further 10 minutes with biotin-tyramide enhancer (0.000375% (w/v) tyramide, 0.00125% (w/v) biotin, 0.2% (v/v) H2O2 in Tris-HCl, pH 8.0). 22 After 30 minutes of incubation with Strep-AB-complex (DAKO) and subsequent staining for 15 minutes with Fast Red (Sigma, Deisenhof, Germany), sections were counterstained with hemalaun (Merck, Darmstadt, Germany). p16INK4A staining was performed as described previously. 20 Briefly, sections were microwave-treated in citrate retrieval buffer, pH 6.0 (Ventana Inc., Tucson, AZ). Staining was performed with a 1:25 dilution of mouse monoclonal antibody (clone G174–405, Pierce, Rockford, IL) and the Ventana DAB staining system using a Ventana semiautomated staining machine (all Ventana), following the manufacturer’s recommendations. For quality control of staining, positive and negative controls were included in each staining run. For cyclin D1, p16INK4A and Ki-67, sections of tonsils were used as positive controls. The cyclin D1 antibody strongly stained stratified squamous epithelium cells, whereas endothelial cells and histiocytes were stained weakly. 23 p16INK4A antibody also stained suprabasal cells of the stratified squamous epithelium, but these were situated more apically. Lymphocytes were not stained. Ki-67 antibody reacted strongly with germinal center lymphocytes, whereas cells of the mantle zone remained negative. Sections of colorectal tumors served as positive controls for β-catenin, with strong staining of epithelial cells but no staining of stroma cells. Staining in the absence of primary antibodies served as negative controls, in which no staining reaction was seen.
Scoring of Immunohistochemical Staining
Serial sections were used to analyze identical areas for the expression and subcellular localization of β-catenin, cyclin D1, Ki-67 and p16INK4A. Tumor cells in 10 fields at high power magnification (400×) were counted independently by two observers (A. J. and M. S.).
As the functions of cyclin D1, p16INK4A, β-catenin as transcription factor and of the Ki-67 antigen are associated with nuclear localization, the percentage of cells showing nuclear staining was scored and rounded up or down to the nearest 5%. The results of both observers were comparable, and in two cases with conflicting results (deviation > 5%), the final result was determined jointly. Initially, sections were examined for expression of β-catenin, cyclin D1, and Ki-67 antigen only (set 1, represented in Figure 1 ▶ ). For analysis of p16INK4A expression, a second series of sections was subsequently cut from the same tissue blocks (set 2, represented in Figure 2 ▶ ). This set was also stained for β-catenin for comparison. Both sets showed virtually identical results with regard to β-catenin expression (Figure 1G ▶ and Figure 2I ▶ ), demonstrating the high consistency of staining and analysis.
Figure 1.
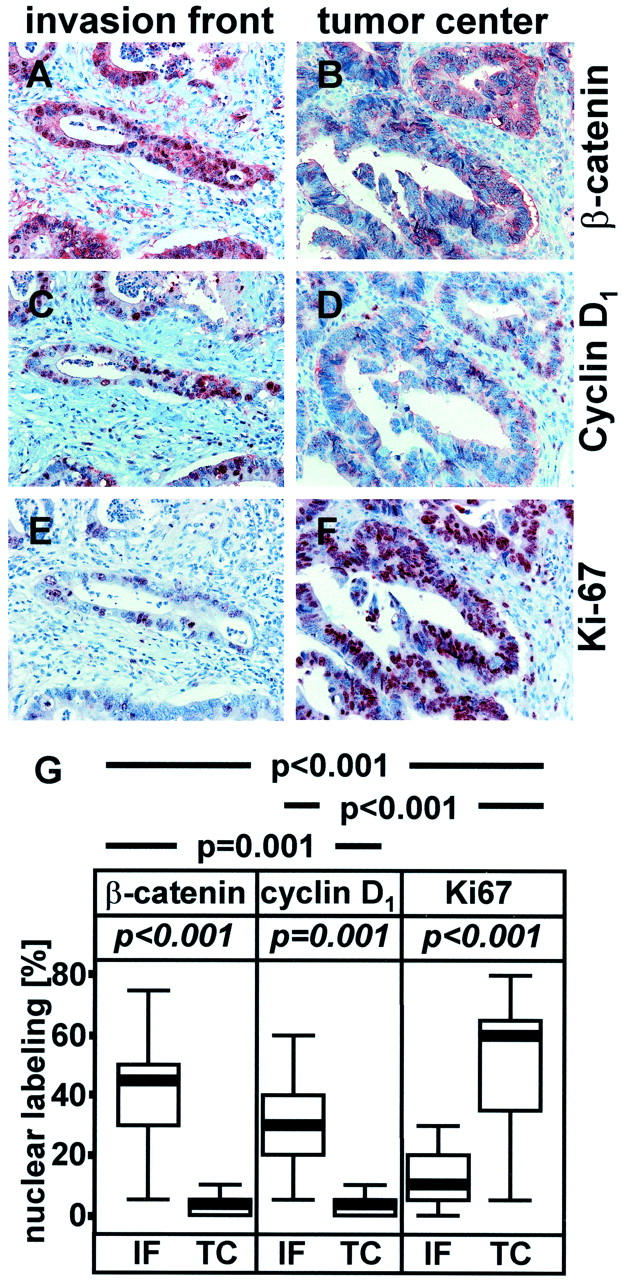
Areas taken from the invasion front (A, C, E) and central parts of the tumor (B, D, F) from the same tumor stained immunohistochemically with antibodies directed against β-catenin (A, B), cyclin D1 (C, D), or Ki-67 (E, F). As serial sections were used comparable tumor regions are shown. G: Statistical analysis of immunohistochemical staining data. Nuclear labeling: rounded percentage value of cells showing nuclear localization (rounding was done in 5% steps); IF, invasion front; TC, central parts of the tumor; p-value (Mann-Whitney test) for correlating location (IF versus TC) with expression of β-catenin, cyclin D1, or Ki-67; p-value, χ 2 test (Fisher’s exact test) for correlating co-localization of antigen pairs: β-catenin/Ki-67, cyclin D1/Ki-67, β-catenin/cyclin D1.
Figure 2.
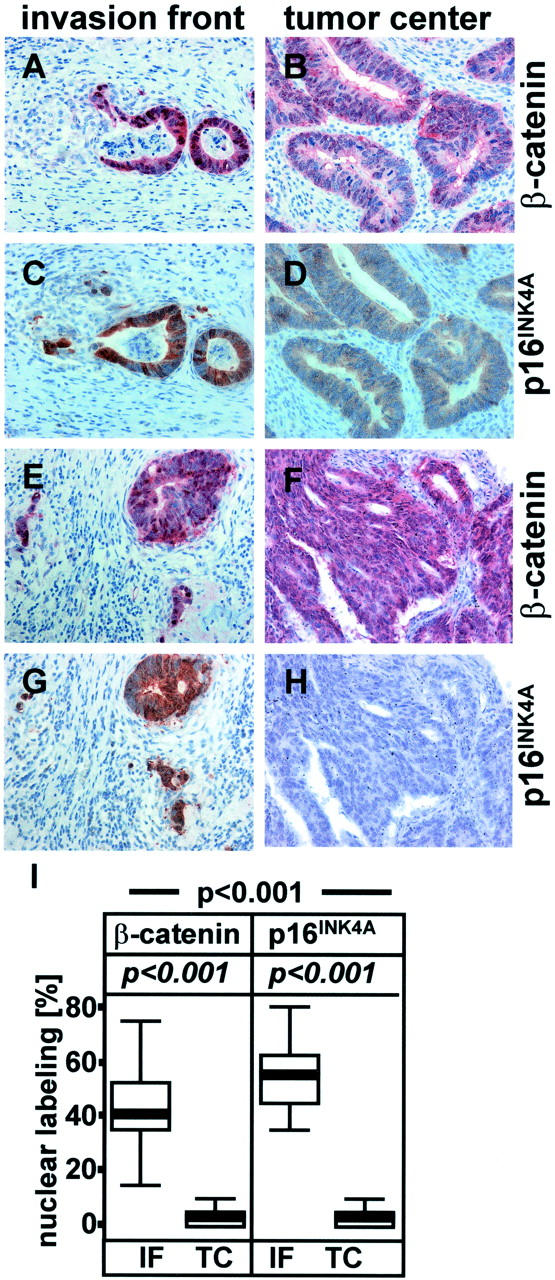
Areas taken from the invasion front (A, C, E, G) and central parts of the tumor (B, D, F, H) from the same tumor (tumor 1, A–D; tumor 2, E–H) stained immunohistochemically with antibodies directed against β-catenin (A, B, E, F) and p16INK4A (C, D, G, H). I: Statistical analysis of immunohistochemical staining data. p-value (Mann-Whitney test) for correlating location (IF versus TC) with expression of β-catenin or p16INK4A; p-value, χ 2 test (Fisher’s exact test) for correlating co-localization of β-catenin with p16INK4A. For a more detailed description see Figure 1 ▶ .
Statistics
All statistical analyses were performed using SPSS version 10.0.5 software (SPSS Inc., Chicago, IL). The patient data were analyzed for frequencies of age, gender, TNM status and UICC stage (see Patient Data). Data are presented using box plots, a descriptive form of statistical analysis. The box represents the interquartile range (75% of all cases) and includes the median (bar in the box). The T-bars represent so-called outliers (values deviating more than 1.5-fold from the interquartile range), thus defining the total range of variation of all values (100%). Correlations between parameters and resulting p-values were calculated by applying the nonparametric Mann-Whitney test or the χ 2 test (Fisher’s exact test).
Results
β-Catenin
The subcellular distribution of β-catenin was found to be heterogeneous throughout each tumor, as described. 7 At the invasion front, single cells and small cell clusters showed strong nuclear staining (Figure 1A ▶ ; Figure 2, A and E ▶ ) in varying numbers of cells (Figure 1G ▶ ; 5 to 75%, median 45%; Figure 2I ▶ ; 15 to 75%, median 40%). Cells from central parts of the tumor rarely showed nuclear staining (Figure 1G ▶ , Figure 2I ▶ ; 0 to 10%, median 5%). Here, the cytoplasm or the plasma membrane were stained prominently in some areas of the tumor (Figure 1B ▶ ; Figure 2, B and F ▶ ). This subcellular localization of β-catenin and the spatial distribution within the tumors correlated with high significance (Figure 1G ▶ ; Figure 2I ▶ ; set 1 and 2: Mann-Whitney test: P < 0.001).
Cyclin D1
As expected, in all cases, strong nuclear expression of cyclin D1 (Figure 1C) ▶ was observed in those areas displaying nuclear β-catenin, so that mainly cells at the invasion front were positive (Figure 1G ▶ ; 5 to 60%, median 30%). In contrast, central parts of the tumor rarely showed cyclin D1 staining (Figure 1, D and G ▶ ; 0 to 10%, median 5%). Nuclear localization of cyclin D1 at the invasion front correlated with high significance (Figure 1G ▶ ; Mann-Whitney test: P = 0.001), as did co-localization of nuclear β-catenin and nuclear cyclin D1 (Figure 1G ▶ ; χ 2 test: P = 0.001).
Proliferation (Ki-67 Antigen)
Taking cyclin D1 expression as a marker for proliferation, one would expect proliferation mainly at the invasion front. The Ki-67 antigen is expressed in all phases of the cell cycle except G0 and is thus a generally accepted marker of proliferation. 24 Unexpectedly, in our collection of cases, nuclear Ki-67 staining was prominent mainly in central parts of the tumor, mostly in a large proportion of cells (Figure 1, F and G ▶ ; 5 to 80%, median 60%). In contrast, Ki-67 expression, and thus proliferation, were much lower at the invasion front (Figure 1, E and G ▶ ; 0 to 30%, median 10%). Ki-67 expression and localization in central parts of the tumor correlated strongly (Figure 1G ▶ ; Mann-Whitney test: P < 0.001). In support of this, cyclin D1 and β-catenin showed a negative correlation with Ki-67 expression, which was statistically highly significant for both pairs (Figure 1G ▶ ; χ 2 test: P < 0.001).
p16INK4A (Inhibitor of Kinase 4)
As the statistically significant negative correlation between cyclin D1 and Ki-67 staining was unexpected, we looked for expression of p16INK4A, as we had previously shown that this cell cycle inhibitor is up-regulated at the invasion front of colorectal adenocarcinomas. 20 Strong nuclear expression of p16INK4A was seen in areas displaying nuclear β-catenin (Figure 2, A and E) ▶ , so that cells mostly at the invasive front were positive (Figure 2, C, G, I ▶ ; 35 to 80%, median 55%). Central parts of the tumor with cytoplasmic or membranous β-catenin staining (Figure 2, B and F) ▶ on the contrary rarely showed nuclear p16INK4A staining (Figure 2, D, H, I ▶ ; 0 to 10%, median 5%). Notably, many of the tumors (∼60%) showed residual p16INK4A expression in the cytoplasm of cells in central parts of the tumors (Figure 2D) ▶ , whereas the rest (∼40%) showed only minimal or no staining at all (Figure 2H) ▶ . The absence of staining was not due to methodological problems, as the same tumors displayed a strong p16INK4A reactivity at the invasion front (Figure 2G) ▶ .
Discussion
Our observation that β-catenin and cyclin D1 are co-localized in the nuclei of isolated cells or in small cell clusters at the invasion front of well-differentiated colorectal adenocarcinomas strongly supports in vitro data showing that cyclin D1 is a target gene for β-catenin. 14,15 In particular, the highly significant co-localization of cyclin D1 with nuclear β-catenin in serial sections strongly suggests that cyclin D1 is up-regulated by β-catenin in human colorectal adenocarcinomas. However, this region displays low proliferative activity as identified by Ki-67 expression. In central parts of the tumor, the opposite is the case, ie, low or absent nuclear expression of β-catenin and cyclin D1, but strong expression of the Ki-67 antigen. This is in agreement with our previous observations, namely that the invasion front of human colorectal cancers displays low proliferative activity. 18-20
Our observation that cyclin D1 is predominantly expressed in non-proliferating cells at the invasion front but not in proliferating cells in central parts of colorectal adenocarcinomas was unexpected. In elegant experiments, Tetsu and McCormick 14 showed that the human colorectal cancer cell line, HCT 116 (mutant β-catenin, wild type APC), ceased proliferation after transduction with retroviral expression vectors encoding dominant negative TCF-4. Dominant negative TCF-4 leads to suppression of β-catenin target genes, and consequently of cyclin D1. Co-infection with a cyclin D1-encoding retroviral expression vector restored proliferation suggesting that cyclin D1 is causative for proliferation in human colorectal cancer. 14 Our results indicate, that within an intact histological context, a different scenario may apply. This may relate to the fact that the cell cycle inhibitor p16INK4A is expressed in tumor cells at the invasion front together with cyclin D1 and β-catenin. Incidentally, we have data showing that p16INK4A is another target gene for β-catenin (Wassermann S, Brabletz T, Palmqvist R, Frank P, Kirchner T, Jung A, manuscript in preparation), which are in support with the high correlation of β-catenin and p16INK4A co-expression. However, at least some cultured colorectal carcinoma cell lines do not express p16INK4A 25 and show, like 37 to 55% of human colorectal cancers, methylation of CpG islands in the p16INK4A promoter/exon 1. 25,26 Our observation that around 40% of investigated tumors do not express p16INK4A in central parts of the tumors (Figure 2H) ▶ but re-express it at the invasion front (Figure 2G) ▶ may be due to promoter methylation, which is thought to shut off gene expression. 26 Thus, it will be interesting to investigate first, the methylation status of tumor cells in central parts and second, whether changes in the methylation status of the p16INK4A promoter occur in colorectal tumor cells at the invasion front.
Our observations raise questions regarding the possible function of cyclin D1 expression at the invasion front, and the role of the invasion front during the process of carcinogenesis of well-differentiated colorectal tumors. It could be envisaged that co-expression of cyclin D1 and p16INK4A maintains tumor cells in a non-proliferating state. However, at the same time this might allow tumor cells to react rapidly to environmental signals and to enter the cell cycle after degradation of p16INK4A. Moreover, it seems that cells displaying nuclear β-catenin undergo a mesenchymal-like transdifferentiation, or possibly dedifferentiation, as suggested by changes in morphology 8 and gene expression profile. 8,10,11 If proliferation and differentiation are generally mutually exclusive functionalities of cells, and if co-expression of cyclin D1 and p16INK4A keeps cells at the invasion front of colorectal adenocarcinomas in a non-proliferating state, then such zones would allow cells to undergo differentiation and proliferation in close vicinity. Consequently, tumors could expand, and at the same time remodel the stroma, giving rise to highly regulated invasion. More work must be done comparing cells from the invasion front and central parts of the tumor, and developing in vitro systems reflecting the heterogeneity of these two areas in well-differentiated colorectal adenocarcinomas.
Acknowledgments
We thank Mrs. Kerstin Näslund for skillful technical assistance and Stephen Köver for criticism and proofreading the manuscript.
Footnotes
Address reprint requests to Andreas Jung, Pathologisches Institut, Molekulare Pathologie, Krankenhausstrasse 8–10, D-91054 Erlangen, Germany. E-mail: andreas.jung@patho.imed.uni-erlangen.de.
Supported by Wilhelm-Sander-Stiftung, AZ. Grant 99.065.1 to A.J., T.B., and T.K.; The Swedish Cancer Society (2520-B99-13XAC), the Lion’s Cancer Research Foundation Umeå, and the Medical Faculty of Umeå University to R.P.
M. Schrauder and U. Oswald contributed equally to this work.
References
- 1.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 2.Polakis P: Wnt signaling and cancer. Genes Dev 2000, 14:1837-1851 [PubMed] [Google Scholar]
- 3.Hirohashi S: Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H: Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998, 19:379-383 [DOI] [PubMed] [Google Scholar]
- 5.Barker N, Huls G, Korinek V, Clevers H: Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am J Pathol 1999, 154:29-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML: β-Catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 2001, 28:53-57 [DOI] [PubMed] [Google Scholar]
- 7.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T: Nuclear overexpression of the oncoprotein β-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 1998, 194:701-704 [DOI] [PubMed] [Google Scholar]
- 8.Kirchner T, Brabletz T: Patterning and nuclear β-catenin expression in the colonic adenoma-carcinoma sequence: analogies with embryonic gastrulation. Am J Pathol 2000, 157:1113-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]
- 10.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T: β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999, 155:1033-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM: The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18:2883-2891 [DOI] [PubMed] [Google Scholar]
- 12.Gradl D, Kuhl M, Wedlich D: The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol Cell Biol 1999, 19:5576-5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T: Expression of nuclear β-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol 2000, 156:865-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tetsu O, McCormick F: β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398:422-426 [DOI] [PubMed] [Google Scholar]
- 15.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A: The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 1999, 96:5522-5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherr CJ: The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 2000, 60:3689-3695 [PubMed] [Google Scholar]
- 17.Weinstein IB: Relevance of cyclin D1 and other molecular markers to cancer chemoprevention. J Cell Biochem Suppl 1996, 25:23-28 [PubMed] [Google Scholar]
- 18.Palmqvist R, Oberg A, Bergstrom C, Rutegard JN, Zackrisson B, Stenling R: Systematic heterogeneity and prognostic significance of cell proliferation in colorectal cancer. Br J Cancer 1998, 77:917-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmqvist R, Sellberg P, Oberg A, Tavelin B, Rutegard JN, Stenling R: Low tumour cell proliferation at the invasive margin is associated with a poor prognosis in Dukes’ stage B colorectal cancers. Br J Cancer 1999, 79:577-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmqvist R, Rutegard JN, Bozoky B, Landberg G, Stenling R: Human colorectal cancers with an intact p16/Cyclin D1/pRb pathway have up-regulated p16 expression and decreased proliferation in small invasive tumor clusters. Am J Pathol 2000, 157:1947-1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TNM Classification of Malignant Tumors. Edited by JH Sorbin, C Wittekind. New York, Wiley-Liss, Inc., 1997
- 22.Merz H, Malisius R, Mannweiler S, Zhou R, Hartmann W, Orscheschek K, Moubayed P, Feller AC: ImmunoMax: a maximized immunohistochemical method for the retrieval and enhancement of hidden antigens. Lab Invest 1995, 73:149-156 [PubMed] [Google Scholar]
- 23.Chan JK, Miller KD, Munson P, Isaacson PG: Immunostaining for cyclin D1 and the diagnosis of mantle cell lymphoma: is there a reliable method? Histopathology 1999, 34:266-270 [DOI] [PubMed] [Google Scholar]
- 24.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 25.Guan RJ, Fu Y, Holt PR, Pardee AB: Association of K-ras mutations with p16 methylation in human colon cancer. Gastroenterology 1999, 116:1063-1071 [DOI] [PubMed] [Google Scholar]
- 26.Esteller M, Corn PG, Baylin SB, Herman JG: A gene hypermethylation profile of human cancer. Cancer Res 2001, 61:3225-3229 [PubMed] [Google Scholar]


