Abstract
Although changes in gene regulation may play an important role in adaptive evolution, there have been few attempts to investigate the molecular mechanisms responsible for adaptively significant variation in gene expression. Here we describe the mechanism underlying an adaptive difference in the expression of the lactate dehydrogenase-B gene (Ldh-B) between northern and southern populations of the fish Fundulus heteroclitus. Ldh-B regulatory sequences from northern and southern individuals, coupled to a luciferase reporter gene, were introduced into the livers of live fish. Deletion studies indicated that sequence changes between 400 and 500 bp upstream of the transcription start site resulted in a 2-fold difference in reporter gene transcription. These sequence changes can account for the previously observed 2-fold difference in Ldh-B transcription between populations. Variation in transcription factors did not play an important role. Sequences within the functionally important region resemble a mammary tumor virus glucocorticoid responsive element (MTV-GRE) in southern alleles, whereas northern alleles differ from the consensus by 1 bp. To test the hypothesis that this element is involved in the variation between populations of F. heteroclitus, we exposed transiently transgenic fish containing Ldh-B regulatory sequence/reporter gene constructs to handling stress or injected cortisol. Both treatments increased reporter gene transcription driven by southern alleles but not northern alleles, as expected if an MTV-GRE sequence were involved. This finding suggests that sequence variation in a GRE is the cause of the adaptive differences in Ldh-B gene expression between populations and demonstrates that small changes in gene regulatory sequences can have important evolutionary consequences.
Populations of Fundulus heteroclitus are found along the East Coast of North America from Newfoundland to mid-central Florida. At the extremes of the species' range, fish experience temperatures that differ on average by over 10°C. Coincident with this thermal gradient, physiological performance differs between northern and southern populations of F. heteroclitus, such that fish with the northern genotype are superior at low temperatures, whereas fish with the southern genotype are superior at high temperatures (1–3). This suggests that these populations have undergone local adaptation, a hypothesis supported by the results of laboratory selection experiments (4). Previous work has shown that these performance differences are associated with allelic variation at the lactate dehydrogenase-B (Ldh-B) locus (4). The expression of Ldh-B also differs between populations, with northern fish expressing 2-fold more LDH-B in the liver and heart than southern fish, exactly as would be expected for thermal compensation (5). This difference in Ldh-B expression is maintained even after long-term acclimation to a common temperature of 20°C, suggesting that the difference in enzyme activity is the result of genetic changes between the populations, rather than reversible acclimation effects (5). Elevated levels of LDH-B are observed in many cold-water endemic taxa within the genus Fundulus (6). Comparative phylogenetic methods have been used to show that this pattern is not the result of phylogenetic relatedness among the groups, but rather is best explained as an adaptation to environmental temperature (6). Therefore, Ldh-B expression in Fundulus is one of the few examples for which the adaptive significance of variation in gene regulation between natural populations has been clearly demonstrated.
In F. heteroclitus, the difference in LDH-B enzyme activity can be accounted for by variation at the level of transcription (7), rather than changes in gene copy number, or effects at other levels of regulation. To investigate the molecular basis of this difference, we have cloned and sequenced several kb of DNA immediately 5′ of the Ldh-B transcription start site (8, 9). The distribution of sequence variation within the 5′ regulatory sequences of Ldh-B among populations of F. heteroclitus suggests that these sequences have been subject to directional selection (9, 10). However, functional analyses in heterologous cell culture have yielded conflicting results, making it difficult to determine the molecular basis for the variation in gene expression (8–10).
Our experiments (9) implicated a cis-acting sequence approximately 500 bp upstream of the start site of transcription as important in setting the difference in transcription between populations. In this region, there is a 7-bp site identical to a mouse mammary tumor virus glucocorticoid responsive element (MTV-GRE) repressor (11) in southern alleles, and this site differs from the mammalian sequence by 1 bp in the northern alleles. In mammals, the MTV-GRE repressor inhibits transcription in the absence of stress hormones. In contrast, when stress hormone levels are high, the repression is removed and transcription increases (11). We reasoned that the putative element within the F. heteroclitus Ldh-B gene might behave in a similar way. This hypothesis allowed us to generate several testable predictions about the behavior of the Ldh-B gene in liver:
(i) LDH-B enzyme activity in the liver of F. heteroclitus from southern populations should be low at rest and increase in response to stress. In contrast, LDH-B activity should be 2-fold higher in unstressed fish from northern populations and should not change in response to stress.
(ii) If the putative GRE is involved in this difference between populations, then the functionally important site should map as a repressor located ≈500 bp upstream of the start site of transcription in southern alleles when tested in F. heteroclitus liver. In contrast, such a repressor should be absent from northern alleles.
(iii) If transiently transgenic fish containing Ldh-B regulatory sequence/reporter gene constructs are exposed to stress, reporter gene activity should increase in fish carrying regulatory constructs derived from the southern allele. In contrast, reporter gene activity in fish containing regulatory constructs derived from the northern allele should be higher at rest and unaffected by stress.
To test these predictions, it is critical that a method be developed for assessing gene regulation in F. heteroclitus liver, the tissue in which Ldh-B normally is expressed. Heterologous cells such as mammalian cell lines, cell lines from other species of fish, or even F. heteroclitus muscle, which have previously been used to assess Ldh-B regulatory constructs (8–10), may or may not contain appropriate transcription factors or stress-responsive signaling pathways. This makes results generated in these heterologous systems open to question. Unfortunately, it is technically difficult to perform tests in F. heteroclitus livers because no cell cultures are available, and the 1-year generation time of the species makes the creation of stable transgenics impractical. Taking advantage of advances in human gene therapy, we developed a method for the injection of supercoiled plasmid directly into the muscle of living F. heteroclitus (9). Injection of naked plasmid DNA has been shown to result in moderately high levels of reporter gene expression in the muscle tissue of a variety of species, including fish (12–14), but the transfection of liver by direct injection has proven difficult in mammalian systems (15). In this report, we adapt our method of in vivo injection of plasmid DNA into skeletal muscles (9) for use in the liver of F. heteroclitus. This allowed us to perform direct tests of the hypothesis that a stress-responsive element is responsible for the adaptive differences in Ldh-B gene expression between populations of F. heteroclitus.
Materials and Methods
Animal Care.
F. heteroclitus from Whitney Island, FL, and Hampton, NH, were acclimated to aquaria containing fully aerated, dechlorinated fresh water mixed with seawater to a salinity of 20 parts per thousand, at a temperature of 20 ± 1°C. Fish were maintained on a 12:12 light cycle and fed once daily to satiation with Tetramin flake food. They were acclimated to these conditions for at least 3 months before the onset of experimentation.
Stress Experiments.
Animals were transferred from their holding tanks into experimental aquaria 2 weeks before experimentation to allow them to recover from any stress associated with transfer. During experimentation, animals were exposed to mild handling stress by catching and holding them under water in a net three times daily for 7 days. Handling stress of this type causes glucocorticoid stress hormone levels to increase in F. heteroclitus (16). Fish were removed from the experimental tanks and rapidly decapitated, and their livers were removed, immediately frozen in liquid nitrogen, and stored at −80°C until use. Frozen livers were placed in ice-cold homogenization buffer (100 mM Hepes/2 mM EDTA/0.2 mM PMSF/0.1 mM DTT) and immediately homogenized by using a Powergen 125 tissue homogenizer. Samples were centrifuged twice at 2,500 × g (4°C) for 10 min, and the clarified supernatant was removed and assayed spectrophotometrically for LDH-B activity and total protein. These experiments were performed under University of Waterloo animal care protocol #9710.
Plasmids.
Portions of the Ldh-B 5′ regulatory sequence were obtained by PCR from cloned F. heteroclitus Ldh-B regulatory sequences (9). The following alleles were used in this study: FL1, from Florida (GenBank accession no. U59833); ME1, from Maine (GenBank accession no. U59845); GA6, from Georgia (GenBank accession no. U59843); and NJ3, from New Jersey (GenBank accession number U59853). Note that New Jersey populations contain alleles of both northern and southern type. NJ3 was previously identified as a southern allele, based on phylogenetic analyses (9).
PCR primers were designed in conserved regions of the regulatory sequence ≈1,000, 500, or 400 bp upstream of the primary transcription start site of the gene (primer 1,000: AGA TAT ATT CC AGT TT; primer 500: ATA ATG AAA GTT TGT GCT G; primer 400: CCT TGG CAC CCT CAT TTA T). Each primer was used in combination with a primer at the extreme 3′ end of the first exon of F. heteroclitus Ldh-B (AGA TCA GAA GAA AAG TCT GGG ATC AGA GAC TGA G) to generate products containing ≈1,000, 500, or 400 bp of regulatory sequence plus the Ldh-B 5′ untranslated region up to the boundary of the first intron (7 bp upstream of the start codon). Thus, no protein coding regions were included in the constructs. All PCR products were blunt-end cloned into the EcoRV site of pBluescript KS− (Stratagene) and sequenced completely in both directions to ensure that no PCR errors were incorporated. These inserts then were subcloned in the appropriate orientation into the BamHI and HindIII sites of the firefly luciferase reporter gene plasmid pLuc (kindly supplied by Manfred Schartl, Biozentrum der Universität, Würzburg, Germany). Endotoxin-free supercoiled plasmid was prepared in milligram quantities from Escherichia coli strain XL1-Blue (Stratagene) by Bio101. A sea pansy luciferase reporter gene plasmid containing the cytomegalovirus (CMV) early promoter (pRL-CMV, Promega) was used as a control for transfection efficiency.
In Vivo Reporter Gene Expression.
Between 10 and 60 μg of experimental plasmid (depending on the experiment) and 5 μg of pRL-CMV control plasmid were resuspended in 1× physiological saline with 2 μl of artist's tempera paint. Fish were anesthetized in 3-aminobenzoic acid ethyl ester (MS-222, Sigma), and a small surgical incision was made in the peritoneal cavity to expose the liver. Plasmid solution was slowly injected into the exposed liver with a 50-μl Hamilton syringe. Fundulus livers are small (100–500 mg) and have only moderate structural integrity, which makes injection into this tissue difficult. The inclusion of tempera paint was critical in visualizing the progress of the injection to prevent damage to the liver and to ensure that the fluid did not leak out into the peritoneal cavity. Preliminary experiments using injection into muscle tissue (data not shown) indicated that the paint does not interfere with DNA uptake or expression. Seven days after injection, fish were killed by rapid decapitation and their livers were excised and immediately homogenized in lysis buffer (Dual Luciferase Assay System, Promega) by using a Polytron tissue homogenizer. The homogenate was clarified by microcentrifugation twice for 15 min at 10,000 × g and stored frozen for 24 h before assay. Firefly luciferase activity driven by the Ldh-B regulatory sequences and sea pansy luciferase activity driven by the CMV promoter were assessed by using the Dual Luciferase Assay System (Promega). Light emission was detected by using a Turner Designs TD20/20 luminometer. These experiments were conducted under Stanford University Animal Care Protocol #3622.
Stress and Reporter Gene Expression.
Four treatment groups of fish were injected with 40 μg of reporter gene plasmid and 5 μg of control plasmid as described above. After injection, one group was fed daily, but not otherwise disturbed, while a second group was exposed to handling stress as described previously. A third group was injected i.p. with 1 ml of melted coconut oil, while the fourth group was i.p. injected with 1 ml of coconut oil containing 2.5 mg of cortisol (Sigma) as described (17). After 7 days, the fish were killed and their livers were assayed for reporter gene activity as described above.
Results
Stress Experiments.
To determine whether Ldh-B in southern F. heteroclitus is responsive to stress, we exposed fish to 7 days of mild handling stress and measured LDH-B specific activity in the liver. LDH-B activity increased in response to stress in southern fish, but not in northern fish (Table 1). This is consistent with the first prediction of our hypothesis.
Table 1.
LDH specific activity in response to stress in F. heteroclitus liver tissue
| Genotype | No handling stress | Exposure to handling stress
|
||
|---|---|---|---|---|
| 1 day | 2 days | 7 days | ||
| Northern | 1.89 ± 0.26 | 2.29 ± 0.47 | 1.96 ± 0.32 | 2.37 ± 0.30 |
| Southern | 0.91 ± 0.29 | 1.18 ± 0.36 | 0.89 ± 0.21 | 2.16 ± 0.19* |
Specific activity: units/mg total protein (mean ± SD), n = 5 per group; *, significantly different from unstressed group (P < 0.05).
Development of in Vivo Reporter Gene Assays.
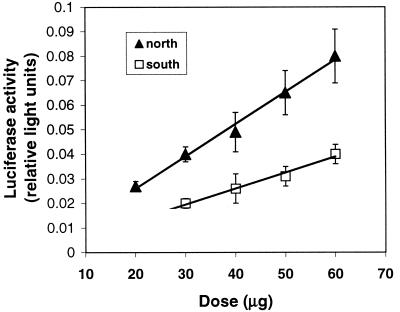
To test the second prediction of our hypothesis, that a repressor is located between 400 and 500 bp upstream of the major transcription start site, it was first necessary to develop a quantitative method for assessing reporter gene expression in vivo in Fundulus livers. Preliminary experiments indicated that high levels of DNA must be injected to generate detectable expression, and that optimal expression levels were obtained 7 days after injection. Relative reporter gene activity (normalized to control luminescence) increased linearly as increasing amounts of experimental plasmid were injected into F. heteroclitus liver (Fig. 1; r2 > 0.98 for each line, no significant difference between slopes of the lines), indicating that DNA injection into F. heteroclitus liver is an effective way to quantify gene expression. A construct containing 1 kb of Ldh-B regulatory sequence from a typical northern individual promoted approximately 2-fold greater reporter gene activity than an equivalent construct containing regulatory sequence from a typical southern individual at all doses tested (significantly different P < 0.05; Fig. 1). These results indicate that sequence differences between constructs are sufficient to cause the previously observed 2-fold differences in Ldh-B transcription, mRNA, and protein concentration between populations (7).
Figure 1.
Reporter gene activity in F. heteroclitus liver as a function of plasmid dose injected. The northern construct (▴) contains ≈1,000 bp of Ldh-B 5′ regulatory sequence from a fish from Maine (ME1). The southern construct (□) contains ≈1,000 bp of Ldh-B 5′ regulatory region from a fish from Florida (FL1). Activity is light units generated by Ldh-B firefly luciferase constructs relative to light units generated by prL-CMV (sea pansy luciferase) constructs (mean ± SE; n = 7). For each dose, the northern construct promoted significantly higher transcription than the southern construct (P < 0.05, ANOVA followed by GT-2 multiple comparison of means).
Effects of Within-Population Sequence Variation.
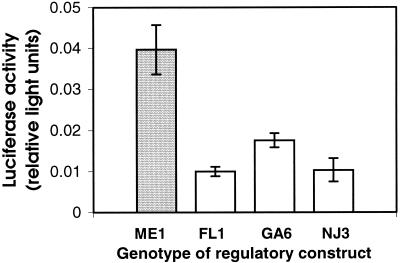
Ldh-B regulatory sequences vary within populations, as well as between populations (9), and this intrapopulational variation also might have functional consequences. Within-population sequence variation is found almost exclusively in southern populations (9). Therefore, we tested several of the most divergent Ldh-B regulatory sequence alleles from southern populations for their ability to promote reporter gene expression in vivo in F. heteroclitus liver. All southern alleles promoted significantly lower reporter gene transcription than the typical northern allele (P < 0.05, Fig. 2), and no statistically significant differences were observed between southern alleles. This indicates that although there are substantial sequence differences among alleles in the southern population, these differences do not have detectable functional consequences.
Figure 2.
Effect of sequence variation within southern populations on reporter gene activity in vivo. All constructs tested contain ≈1,000 bp Ldh-B 5′ regulatory sequence. Alleles are drawn from populations as follows: ME1, Maine; GA6, Georgia; FL1, Florida; NJ3, New Jersey. Activity is light units generated by Ldh-B firefly luciferase constructs relative to light units generated by prL-CMV (sea pansy luciferase) constructs (mean ± SE; n = 5 for each group). All southern (GA6, FL1, NJ3) constructs (open bars) promoted significantly lower expression than the northern (ME1) construct (shaded bar) and did not differ significantly from each other (P < 0.05 ANOVA followed by GT-2 multiple comparison of means).
Deletion Analyses.
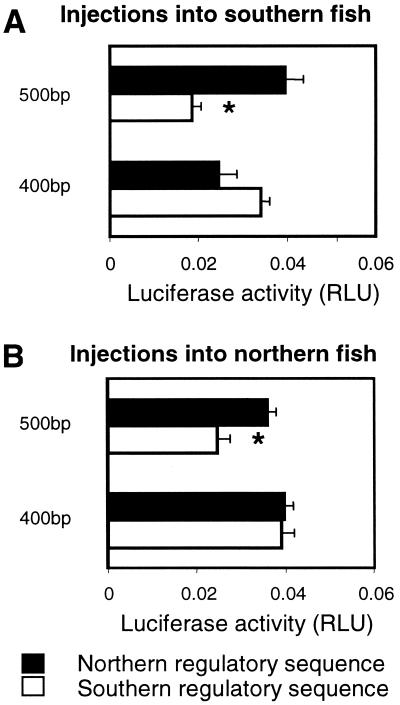
To further localize the sequences responsible for differences in reporter gene expression between alleles, we prepared deletions containing either 500 or 400 bp of the regulatory sequence from a typical northern and southern allele. Significant differences in reporter gene expression were observed between northern and southern alleles for the 500-bp constructs, but not the 400-bp constructs (Fig. 3A). This finding indicates that the functionally important differences between alleles are located between 400 and 500 bp upstream of the major transcriptional start site. This is consistent with our hypothesis, which postulates a functional role for a putative GRE located in this region. In addition, the 500-bp southern construct promoted lower levels of expression than the 400-bp southern construct (Fig. 3A). Therefore, the region between 400 and 500 bp upstream of the major transcription site must contain a repressor in the southern allele. In contrast, there is no evidence of a similar repressor in the northern allele, again consistent with the predictions of our hypothesis.
Figure 3.
In vivo reporter gene activity promoted by 500- and 400-bp fragments of Ldh-B 5′ regulatory sequence. Open bars are constructs generated from a representative southern allele (FL1) containing either 400 or 500 bp of 5′ regulatory sequence. Filled bars are constructs generated from a representative northern allele (ME1) containing either 400 or 500 bp of 5′ regulatory sequence. (A) Injected into southern fish (from Florida). (B) Injected into northern fish (from New Hampshire). Activity is light units generated by Ldh-B firefly luciferase constructs relative to light units generated by prL-CMV (sea pansy luciferase) constructs (mean ± SE; n = 5). The 500-bp southern construct (indicated by *) is significantly different from all other constructs injected into southern or northern fish, respectively (P < 0.05, ANOVA followed by GT-2).
When constructs were injected into fish from either northern or southern populations, a similar pattern was observed in each case (compare Fig. 3 A and B). There are two relatively minor differences between Fig. 3 A and B. First, there is an apparent difference in the ratio of expression of the northern and southern 500-bp constructs when injected into fish from different populations (2-fold in southern fish vs. 1.6-fold in northern fish). However, there is no significant difference in activity for either the southern or northern 500-bp construct when injected into northern vs. southern fish. Therefore, the difference in ratio between Fig. 3 A and B is more apparent than real. The only significant difference between constructs of the same length injected into different genetic backgrounds is between the 400-bp northern regulatory sequence when injected into northern and southern fish. In this case, expression is marginally greater (P < 0.06) when the northern construct is injected into northern fish. This finding indicates that there may be a transcription factor interacting with the 400-bp region that differs between the populations. However, this region is not responsible for the 2-fold difference in expression between populations because there is no significant difference between the activities of northern and southern 400-bp regulatory constructs, when injected into either genetic background. Overall, there is clear evidence that the 2-fold difference in Ldh-B gene expression is the result of a repressor element present in the southern alleles. Because this repressor element can be detected when tested in either northern or southern fish, the cognate transcription factor is likely to be present in both populations. Therefore, the 2-fold difference in reporter gene transcription is the result of differences between populations in cis-acting factors (i.e., regulatory sequences) and not trans-acting factors (i.e., transcription factors or differences in the overall physiology of the fish from different populations).
Effect of Stress on Reporter Gene Expression.
To test the hypothesis that this putative GRE is stress-regulated, we injected fish with either the southern or the northern 500-bp regulatory sequence, coupled to a reporter gene. This is the minimum construct that contains the putative MTV-GRE in southern alleles. We then exposed the fish to handling stress. Reporter gene transcription increased in the stressed fish injected with the southern but not the northern construct (Table 2). Therefore, the southern alleles contain an element that is responsive to stress, whereas the northern alleles do not. To test whether this stress-regulated change in gene expression was a response to changes in glucocorticoid stress hormones and not some secondary effect of handling stress, fish were rendered transiently transgenic, as above, and injected with a pure preparation of cortisol. The results of this experiment exactly mimicked the effect of handling stress (Table 2). Therefore, elevated stress hormones, whether induced by a handling stress or by injection of exogenous cortisol, increase transcription from the southern regulatory sequences but do not affect transcription from the northern sequences.
Table 2.
Reporter gene expression in fish injected with a plasmid containing Ldh-B regulatory sequence, at rest, exposed to handling stress, or injected with exogenous cortisol
| Genotype of injected regulatory sequence | Luciferase activity, RLU
|
|||
|---|---|---|---|---|
| Unstressed | Stressed | Sham injected | Cortisol injected | |
| Northern | 0.047 ± 0.007 | 0.048 ± 0.009 | 0.043 ± 0.007 | 0.039 ± 0.005 |
| Southern | 0.017 ± 0.002 | 0.032 ± 0.004* | 0.023 ± 0.004 | 0.042 ± 0.008* |
RLU = relative light units (mean ± SE, n = 10/group); *, significantly different from unstressed group (P < 0.05). Injections were performed in fish from Florida.
Discussion
It has been more than 30 years since Britten and Davidson first suggested that changes in gene regulation are likely to be a fundamentally important component of evolutionary change (18). By using comparative phylogenetic methods, it is now possible to determine whether a particular change in gene expression is likely to be of adaptive value (6). Because these approaches are comparative, however, they are seldom applied to “model” organisms. As a result, it can be very difficult to assess the molecular basis for the observed differences in gene regulation because of a lack of molecular tools, well-characterized cell lines, or appropriate transgenic methods for the species of interest. For example, in attempting to understand the basis for an adaptively significant 2-fold difference in the regulation of Ldh-B in populations of F. heteroclitus adapted to different thermal habitats, we and others have used functional analyses in a variety of heterologous cell lines to develop hypotheses about the functional basis of this difference (8–10). However, these analyses have yielded conflicting results, making it difficult to determine the likely basis for the difference in gene expression between populations of F. heteroclitus. To distinguish between these possibilities, it is necessary to test regulatory sequences in the tissue of interest, ideally in vivo, which is a substantial challenge in all but a few model organisms. Our method of in vivo plasmid injection into the liver allowed us to perform these tests.
The data presented here clearly show that there is a repressor element in the regulatory sequence of Ldh-B in F. heteroclitus from southern populations. This repressor is absent in fish from northern populations. The repressor behaves in a stress-responsive fashion, causing transcription to increase in response to handling stress or injection of exogenous cortisol when driven by southern regulatory sequences. Not only was this true in the context of in vivo-injected reporter gene constructs, but we also observed stress-regulated increases in LDH-B enzyme activity in fish that were not subjected to surgery or other manipulations (Table 1). This observation suggests that the behavior of our reporter gene accurately mimics the behavior of the endogenous Ldh-B gene. Therefore, differences in methylation pattern or higher-order chromosome conformation (which are not likely to affect reporter gene expression from the injected plasmid DNA) are not necessary to confer stress responsiveness. This is consistent with the predictions of our hypothesis that variation in a GRE repressor, similar to a mammalian MTV-GRE, is responsible for the adaptive difference in Ldh-B transcription between populations of F. heteroclitus. The known behavior of this GRE in mammals can predict the behavior of the F. heteroclitus regulatory sequence. That is, the element acts as a repressor, and this repression is released in response to stress. Variation in this stress-responsive element is sufficient to account for the previously observed 2-fold difference in LDH-B activity, protein, mRNA, and transcription between populations of F. heteroclitus tested under unstressed conditions (7).
Functional analyses of regulatory sequences have been performed for only one other stress-regulated gene in fish: the prolactin gene of rainbow trout (19). In this study, deletions of the prolactin promoter were tested for their response to applied glucocorticoid hormones in rainbow trout cell cultures. The functionally important region of the trout prolaction promoter (GenBank accession no. X95907) was found to contain several consensus GREs. These GREs are positive regulators, rather than repressors, and differ both functionally and in sequence from the MTV-GRE and the site that we identified in F. heteroclitus. However, when we reanalyzed the trout prolactin gene sequence, we found a previously unidentified element that is identical to the putative MTV-GRE from F. heteroclitus. This element is located in close proximity to the previously identified positive GREs in the trout prolactin gene. It is highly unlikely that this element would be present in stress-regulated genes from F. heteroclitus, rainbow trout, and mammals simply by chance. Strong conservation of this type supports the conclusion that the putative GRE in F. heteroclitus is a functional element.
The functionally important region of the F. heteroclitus Ldh-B regulatory sequence (between 400 and 500 bp upstream of the transcription start site) contains five fixed differences between the Maine and Florida populations (9). Only one of these differences is located within the 7 bp long putative MTV-GRE. It is possible that one of the other sites, or a combination of sites, could be responsible for, or contribute to, the differences in gene expression promoted by the northern and southern alleles, rather than the putative GRE. In fact, all of the other mutations in this region are located within sequences that share some similarities to sequences within the rainbow trout prolactin promoter, particularly some of the positive GREs. However, the highest degree of conservation between species is with the MTV-GRE-like element. This observation combined with the fact that the response is a release from repression, rather than stress activation, is consistent with the hypothesis that the MTV-GRE-like sequence is involved in the observed variation in reporter gene activity. In previous studies in cultured cells, we have shown that a naturally occurring mutation of the MTV-GRE repressor sequence, in a fish from intermediate populations, causes increases in transcription (9). This strongly suggests that the MTV-GRE repressor plays the major role in setting the difference between populations. Although we cannot conclusively rule out the possibility that one or more of the four additional mutations in this 100-bp region also play a functional role, we can conclude that a minimum of a single base pair change in a stress responsive element is required to produce this adaptively important change in the regulation of Ldh-B.
Other regions of the Ldh-B regulatory sequence also have been implicated as having a role in the differences in transcription rates between populations of F. heteroclitus (8, 10). Crawford et al. (10) detected functional differences between 300-bp northern and southern F. heteroclitus regulatory constructs, a region that excludes the putative GRE. However, these experiments were performed in heterologous cell cultures, and varying results were obtained in different cell lines (10). When tested in vivo in fish liver, northern and southern alleles do not differ functionally in this region (Fig. 3, 400-bp construct). These results highlight the importance of assaying regulatory function in the appropriate species and tissue. Crawford et al. (10) also detected strong evidence of directional selection acting on the 300-bp region immediately upstream of the transcription start site of F. heteroclitus Ldh-B (10), which suggests that adaptive evolutionary processes are occurring in this region of the sequence, in addition to those we have detected in the region surrounding the MTV-GRE (9). Directional selection acting on the promoter is difficult to explain given that sequence variation between northern and southern alleles in this region does not appear to have functional effects when tested in F. heteroclitus liver in vivo (see Fig. 3, 400-bp construct). It is likely that interactions between proximal promoter variants and upstream enhancer and repressor elements are important, which could account for the action of selection on the promoter region. However, these interactive effects are likely to be subtle, because enhancers and repressors generally are able to function in the context of a variety of different promoters (20). Perhaps even subtle functional differences have effects that can be the target of natural selection under appropriate conditions. Alternatively, from the in vivo expression data (Fig. 3), there appears to be some evidence of variation in the transcription factors that bind to the promoter region between northern and southern fish (compare the activity of the northern 400-bp construct when injected into northern and southern fish). Although these differences are not statistically significant, it is possible that the evidence of directional selection in the promoter is the result of a need to preserve interactions with these changing transcription factors.
The variation in the response of Ldh-B to stress between populations raises an interesting question as to the potential adaptive significance of such a change in phenotypic plasticity. The physiological consequences, if any, of the difference in the ability of Ldh-B to respond to stress between populations of F. heteroclitus are not clear. Previous experiments have demonstrated that fish carrying alternate alleles at Ldh-B differ in swimming performance and developmental rate (1, 2), but little is known about the relationship between differences in responses to stress and variation in organismal performance in this species.
The Ldh-B gene of a closely related congener, F. grandis, also contains a putative MTV-GRE-type repressor element, identical to that found in the southern alleles of F. heteroclitus, and different from that in the northern fish (9). Therefore, the genotype of the northern populations of F. heteroclitus is likely the derived condition. Population genetic analyses indicate that the regulatory sequences of the northern populations have arisen through strong directional selection. Taken together, this implies that there has been selection for the loss of a function (stress regulation). This apparent contradiction is best explained by the hypothesis that under warm water conditions there is an advantage, possibly quite slight, to the ability to regulate Ldh-B in response to stress. In contrast, under cold-water conditions, this advantage is outweighed by the disadvantage of having low LDH-B under resting conditions. This hypothesis helps to explain another puzzling feature of the data presented here: the relatively long time course over which LDH-B amounts increase in response to stress in the southern population. Under the moderate handling stress applied in the laboratory, LDH-B levels increased sometime between 48 h and 7 days of handling. In contrast, cortisol levels in F. heteroclitus increase very rapidly in response to stress (within minutes, ref. 16). There is likely to be some delay in increases in [LDH-B] because of the need for binding of the hormone to the receptor, binding of the resultant transcription factor to DNA, followed by transcription and translation, but 48 h should be ample for the first evidence of an effect at the protein level. This suggests that the glucocorticoid response in southern fish is a relatively low affinity process that requires either extreme stress, or repeated moderate stress, for activation. We have observed much more rapid increases (within 8 h) in LDH-B levels in response to shipping stress (unpublished results), an extreme stressor. If Ldh-B is only up-regulated in response to extreme stress, the net selective benefit of possessing this element could be quite slight, and thus its loss a relatively minor cost in the northern populations. Additional investigations into the mechanisms of the stress-regulated expression of Ldh-B, particularly the characterization of the kinetics and affinity of the GRE, will provide further insight into this important evolutionary question.
Very little currently is known about the molecular mechanisms underlying adaptively significant differences in organismal phenotypes. Here we have shown that minor changes in cis-acting DNA regulatory sequences have substantial consequences for gene expression in natural populations, altering resting levels of expression and the ability of the gene to respond to stress. These changes can account for the adaptively significant differences in Ldh-B gene expression between populations of F. heteroclitus, which are thought to play a role in thermal adaptation in this species (6). The in vivo method that we developed, and which allowed us to reach these conclusions, should be applicable to most tissues in any vertebrate species of interest. This should greatly simplify the process of determining the molecular basis of adaptively significant variation in gene expression in nonmodel organisms.
Acknowledgments
This work was supported by National Science Foundation Grant DEB-96–29572 to D.A.P., a Natural Sciences and Engineering Research Council of Canada operating grant to P.M.S., and a Natural Sciences and Engineering Research Council of Canada postdoctoral fellowship to H.C.G.
Abbreviations
- LDH-B
lactate dehydrogenase-B
- GRE
glucocorticoid responsive element
- MTV-GRE
mammary tumor virus GRE
- CMV
cytomegalovirus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.DiMichele L, Powers D A. Nature (London) 1982;296:563–564. doi: 10.1038/296563a0. [DOI] [PubMed] [Google Scholar]
- 2.DiMichele L, Powers D A. Science. 1982;216:1014–1016. doi: 10.1126/science.7079747. [DOI] [PubMed] [Google Scholar]
- 3.DiMichele L, Paynter K T, Powers D A. Science. 1991;253:898–900. doi: 10.1126/science.1876847. [DOI] [PubMed] [Google Scholar]
- 4.Powers D A, Lauerman T, Crawford D, DiMichele L. Annu Rev Genet. 1991;25:629–659. doi: 10.1146/annurev.ge.25.120191.003213. [DOI] [PubMed] [Google Scholar]
- 5.Crawford D L, Powers D A. Proc Natl Acad Sci USA. 1989;86:9365–9369. doi: 10.1073/pnas.86.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce V A, Crawford D L. Science. 1997;276:256–259. doi: 10.1126/science.276.5310.256. [DOI] [PubMed] [Google Scholar]
- 7.Crawford D L, Powers D A. Mol Biol Evol. 1992;9:806–813. doi: 10.1093/oxfordjournals.molbev.a040762. [DOI] [PubMed] [Google Scholar]
- 8.Segal J A, Schulte P M, Powers D A, Crawford D L. J Exp Zool. 1996;275:355–364. doi: 10.1002/(SICI)1097-010X(19960801)275:5<355::AID-JEZ4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Schulte P M, Gomez-Chiarri M, Powers D A. Genetics. 1997;145:759–769. doi: 10.1093/genetics/145.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford D A, Segal J A, Barnett J L. Mol Biol Evol. 1999;16:194–207. doi: 10.1093/oxfordjournals.molbev.a026102. [DOI] [PubMed] [Google Scholar]
- 11.Langer S J, Ostrowski M C. Mol Cell Biol. 1988;8:3872–3881. doi: 10.1128/mcb.8.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J-H, Chan W-K. Mol Mar Biol Biotechnol. 1997;6:98–109. [PubMed] [Google Scholar]
- 13.Rahman A, Maclean N. Mol Mar Biol Biotechnol. 1992;1:286–289. [PubMed] [Google Scholar]
- 14.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgener P L. Science. 1990;247:1456–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 15.Hickman M A, Malone R W, Lehmann-Bruinsma K, Sih T, Knoell R D, Szoka F C, Walzem R, Carlson D M, Powell J S. Hum Gene Ther. 1994;5:1477–1483. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- 16.Leach G L, Taylor M H. Comp Biochem Physiol A Physiol. 1977;56:217–223. doi: 10.1016/0300-9629(77)90188-8. [DOI] [PubMed] [Google Scholar]
- 17.Gamperl A K, Vijayan M M, Boutilier R G. Rev Fish Biol Fisheries. 1994;4:215–255. [Google Scholar]
- 18.Britten R J, Davidson E. Science. 1969;165:349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- 19.Argenton F, Ramoz N, Charlet N, Bernardini S, Colombo L, Bortolussi M. Biochem Biophys Res Commun. 1996;224:57–66. doi: 10.1006/bbrc.1996.0984. [DOI] [PubMed] [Google Scholar]
- 20.Kermekchiev M, Petterson M, Matthias P, Schaffner W. Gene Exp. 1991;1:71–81. [PMC free article] [PubMed] [Google Scholar]