Abstract
Carpenter syndrome is a pleiotropic disorder with autosomal recessive inheritance, the cardinal features of which include craniosynostosis, polysyndactyly, obesity, and cardiac defects. Using homozygosity mapping, we found linkage to chromosome 6p12.1-q12 and, in 15 independent families, identified five different mutations (four truncating and one missense) in RAB23, which encodes a member of the RAB guanosine triphosphatase (GTPase) family of vesicle transport proteins and acts as a negative regulator of hedgehog (HH) signaling. In 10 patients, the disease was caused by homozygosity for the same nonsense mutation, L145X, that resides on a common haplotype, indicative of a founder effect in patients of northern European descent. Surprisingly, nonsense mutations of Rab23 in open brain mice cause recessive embryonic lethality with neural-tube defects, suggesting a species difference in the requirement for RAB23 during early development. The discovery of RAB23 mutations in patients with Carpenter syndrome implicates HH signaling in cranial-suture biogenesis—an unexpected finding, given that craniosynostosis is not usually associated with mutations of other HH-pathway components—and provides a new molecular target for studies of obesity.
Carpenter syndrome (MIM %201000), also known as “acrocephalopolysyndactyly type II,” is a rare autosomal recessive disorder characterized by craniosynostosis, obesity, polydactyly, and soft-tissue syndactyly (fig. 1).1–3 Unlike other inherited craniosynostoses, which most commonly affect the coronal sutures, fusion of the midline (metopic and sagittal) sutures is typical in Carpenter syndrome; severe cases have cloverleaf skull. Other well-recognized features include brachydactyly with shortening or absence of the middle phalanges, molar agenesis, genu valgum, hypogenitalism, congenital cardiac defects, umbilical hernia, and learning disability.3–5 Although the molecular basis of many craniosynostosis syndromes is now well described, with mutations of fibroblast growth-factor receptors, ephrins, or the transcription factor TWIST most frequently identified,6 the etiology of Carpenter syndrome has been elusive.
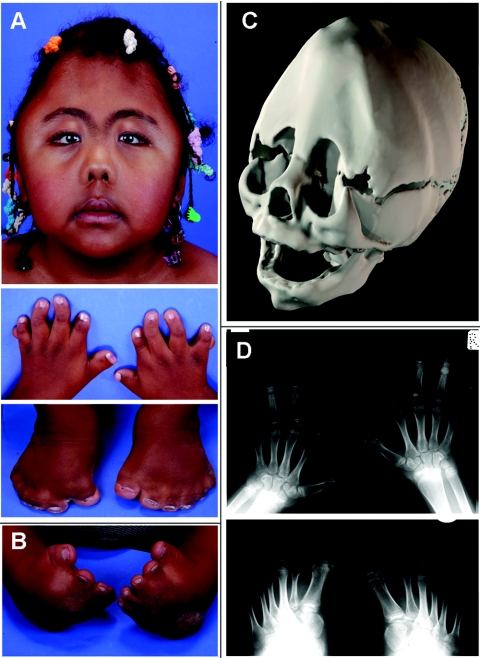
Figure 1. .
Clinical features of Carpenter syndrome. A, Affected sister of subject 4009, aged 6 years. Note metopic ridge and temporal bulging secondary to multisuture synostosis, arched eyebrows, epicanthic folds, anteverted nares, and broad thumbs and halluces with syndactyly, brachydactyly, clinodactyly, and polydactyly (postaxial in hands, central in feet). B, Severe bilateral clubfoot in subject 4009. C, Three-dimensional CT skull reconstruction of subject 3541, aged 4 wk, showing complete synostosis of the metopic, sagittal, and coronal sutures. D, Preoperative radiographs of the hands and feet of subject 3734, aged 11 years. Note characteristic longitudinally split epiphyses at bases of several proximal phalanges, central polydactyly of the feet, and biphalangeal digits II of the hands and II and III of the feet.
We mapped the disease locus, using a large family (family 1; subjects 3541, 3589, and 3593) from the United States.7 After approval from the Oxfordshire Research Ethics Committee and the local institutional review board, DNA was collected with informed consent from six siblings (three affected and three unaffected) and both parents. The parents were not known to be related, but, of their eight grandparents, three of the father’s and two of the mother’s had been born in the same city, raising the possibility of a distant consanguineous loop. We also analyzed an affected male (family 2; subject 3624) whose Danish parents were first cousins; in addition to the classic features of Carpenter syndrome, subject 3624 had a lumbar myelomeningocele. Using the GeneChip Human Mapping 50K Array Hind240 (Affymetrix), we undertook genomewide SNP genotyping and performed parametric multipoint linkage analysis, allowing for heterogeneity, using GENEHUNTER-MODSCORE software.8 In the absence of consanguinity in family 1, no significant linkage was obstained (the maximum possible heterogeneity LOD [HLOD] score of 2.7 was found for three different regions of the genome); however, when a consanguineous loop (conservatively assigned as second-cousin parents) was introduced, the HLOD score increased dramatically for just one of these regions, to a maximum of 4.8, for families 1 and 2 combined, at chromosome 6p12.1-q12 (fig. 2A). The flanking heterozygous SNPs in family 1 were rs7766181 and rs10498828, and the affected individual in family 2 was homozygous throughout this region.
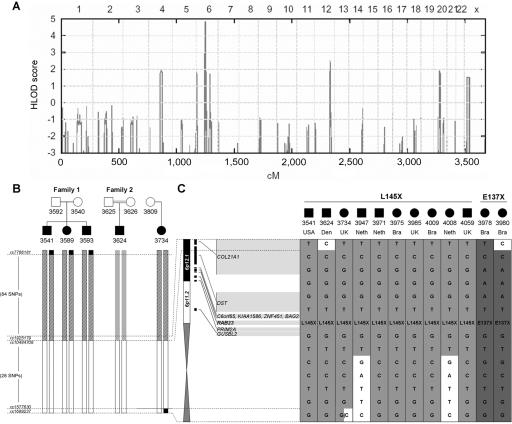
Figure 2. .
Linkage and haplotype mapping of Carpenter syndrome to chromosome 6p12.1. A, Genomewide HLOD scores from 50K microarray data for families 1 and 2 combined, with the assumption of a consanguineous loop (second-cousin parents) in family 1. HLOD scores are given along the Y-axis, relative to genomic position (cM) on the X-axis. Note the significant peak (HLOD 4.8) in the centromeric region of chromosome 6. B, Representation of 50K SNP haplotypes (vertical bars) for chromosomes of affected individuals in families 1 and 2 and an additional sporadic case (subject 3734), all of whom are homozygous for the 434T→A (L145X) mutation in RAB23. Distinct haplotypes are represented by different shaded bars. On the basis of homozygosity, the critical region on chromosome 6 is defined by heterozygosity for SNPs rs7766181 (family 1) and rs1689237 (subject 3734). Within this, a smaller region (white bars) is identical in all affected individuals for 30 consecutive SNPs, suggesting a common ancestral origin of the L145X mutation. C, Genotyping of 13 selected SNPs spanning this identical segment in 10 individuals homozygous for the L145X mutation and 2 individuals homozygous for E137X. At left, the position of these SNPs is shown in relation to RAB23 and 8 additional genes within the 6p12.1-q11 region. Note that all patients with the L145X mutation share a common haplotype for seven consecutive SNPs; this is interrupted proximally in two Dutch patients, probably because of a shared recombination. From top to bottom, the genotyped SNPs are rs1925179, rs2397214, rs9296842, rs1547625, rs6927258, rs6906792, rs3904827, rs6934928, rs1343391, rs1224703, rs1850417, rs2343013, and rs1689237. Bra=Brazil; Den=Denmark; Neth=The Netherlands.
The interval of homozygosity shared by the two families contained 24 annotated genes (Ensembl Genome Browser). Initially, we considered BMP5 a candidate, because mutation of murine Bmp5 causes a range of skeletal defects resulting in the short ear phenotype9; however, no mutations were found. We next analyzed RAB23; recessive nonsense mutations in the orthologous murine Rab23 gene cause neural-tube defects, abnormal somites, polydactyly, and poorly developed eyes (opb [open brain] locus).10–12 With use of the primers listed in table 1, direct sequencing of the seven exons and surrounding intronic regions of RAB23 revealed an identical homozygous 434T→A transversion encoding an L145X nonsense mutation (fig. 3A) in the four affected individuals from families 1 and 2, as well as in a further sporadic case (subject 3734). These five subjects were all identically homozygous for 30 fully genotyped consecutive SNPs on the Affymetrix 50K array (from rs10484709 to rs1577630) (fig. 2B), indicating that they were very likely to share a single ancestral mutation (see below).
Table 1. .
Primers Used for PCR Amplification of RAB23[Note]
| Primer Sequence(5′→3′) |
||||
| Primer | Forward | Reverse | Product Size (bp) |
Wave Temperature(s) (°C) |
| RAB23_1 | CTCCACCCTGGCATTTAGAC | AACAGCCCTTTTCAGACCCT | 270 | 59 |
| RAB23_2 | CCACAGATTTGAGAGGGAAGA | AGTTGCCACACCTCGAAATC | 333 | 56.4 |
| RAB23_3 | TTACCAAAAACATTTTCCTTTACA | GCCAAAATAATATGCCCAAA | 188 | 54 |
| RAB23_4 | TGTTAATGTAAATACCTTGATTGATTG | TATAGAATTACTGTCCCTCCTTCCC | 250 | 56.5 and 58.5 |
| RAB23_5 | AAACAAGCTATCAGAAGGCACC | CAACACAATTTTAAAAGCGCA | 207 | 54.5 |
| RAB23_6 | ATCATTGACCTGGTTCTGGG | TCACTTTTAAATCACATTTCTGAAAGA | 228 | 55 |
| RAB23_7 | TAACTCAGGCGTGTCAGTGG | ATGACAGCTGGATGGGTTTC | 256 | 57 |
Note.— DNA was obtained from whole-blood samples by phenol-chloroform extraction and was amplified in a total volume of 25 μl containing 15 mM TrisHCl (pH 8.0), 50 mM KCl, 2.5 mM MgCl2, 100 μM each deoxynucleoside triphosphate, 0.5 μM primers, and 0.75 units of Amplitaq Gold polymerase (Applied Biosystems). All PCRs were performed using an annealing temperature of 60°C. Cycling conditions consisted of an 8-min denaturation step at 94°C, followed by 35 cycles at 94°C for 30 s, at 60°C for 30 s, and at 72°C for 60 s, with a final extension at 72°C for 10 min.
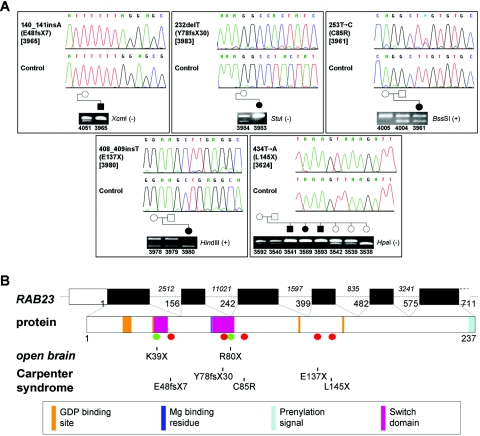
Figure 3. .
RAB23 mutations in Carpenter syndrome. A, Sequence chromatograms and confirmatory restriction digests for the five pathogenic mutations identified. Note that patient 3961 is a compound heterozygote for the C85R and L145X mutations. B, top, The exon/intron organization of RAB23, with the coding part of the cDNA (GenBank [accession number NM_183227.1]; Ensembl Genome Browser [reference OTTHUMG00000014918]) in black and the UTRs in white (alternatively spliced 5′ noncoding exons omitted). Plain numbering refers to the first nucleotide of each exon, starting from the initiation codon, and italic numbering indicates the length of introns. Bottom, Functional domains in the 237-aa protein,13,14,17 color coded, with the location of human mutations causing Carpenter syndrome and those found in open brain mice12 indicated by red and green dots, respectively. GDP=guanosine diphosphate.
To expand the mutation spectrum in RAB23, we ascertained 12 additional unpublished Carpenter syndrome cases unrelated by family history. Including the three families described above, the 15 independent families included 17 affected individuals from whom DNA was available; 6 cases arose from known consanguineous unions. The families originated from the United Kingdom (five), Brazil (five), The Netherlands (three), and Denmark and the United States (one each). The clinical features of these 17 cases, which represent the largest series of subjects with Carpenter syndrome to date, are presented in table 2. Craniosynostosis was present in all individuals, with the sutures affected with relative frequency sagittal>metopic>coronal>lambdoid. Abnormalities of the hands included postaxial polydactyly (9 of 17 cases), broad or bifid thumbs (6 of 17), cutaneous syndactyly (12 of 17), and absent middle phalanges (9 of 11). In the feet, preaxial or central polydactyly (16 of 17) and syndactyly (17 of 17) were nearly always present. High birth weight (9 of 9) and obesity (9 of 10) were prevalent. Other significant complications included umbilical hernia (8 of 17), congenital heart disease (3 of 17), deformities of the knees (4 of 14) or ankles (4 of 17), and cryptorchidism or hypoplastic testes in males (6 of 8). Brain imaging showed abnormalities in 7 of 10 subjects; 3 of 16 had hydrocephalus requiring insertion of a shunt. Significant learning disability was present in 6 of 13 individuals. The occurrence of an open neural-tube defect (family 2; subject 3624), although not previously described in Carpenter syndrome, is unlikely to be coincidental, because this is a cardinal feature of Rab23 mutation in the mouse; the mother of subject 3624 had taken periconceptional folic acid supplements.
Table 2. .
Clinical Features of Patients with Mutations in RAB23[Note]
| Polydactyly(L/R) | |||||||||||||||||||
| ID | Sex | Genotype | Birth Weight(g) | Cranial Suture Fusiona | Hydrocephalus Requiring Shunt | CNS Malformation(s) | Postaxial Hand | Footb | Cutaneous Syndactylyc(H/F) | Biphalangeal Digitsc (H/F) | Talipes/Clubfoot | Umbilical Hernia | Cardiac Malformation | Urogenital Malformation | Other | Height(m) | Weight(kg) | Age (years) | Neurodevelopment |
| 3965 | M | p.[E48fsX7]+[E48fsX7] | 604d | M, S, and BC | Ventriculomegaly and hypoplastic corpus callosum | +/+ | PR/PR | H and F | H | + | − | − | − | Meckel diverticulum | |||||
| 3983 | F | p.[Y78fsX30]+ [Y78fsX30]e | 4,180 | MU | − | +/+ | +/+ | F | H | − | + | − | − | − | .74 | Obese | 1.1 | Not delayed at age 1.1 year | |
| 3961 | F | p.[C85R]+[L145X] | 4,260 | M and S | − | − | +/+ Broad R thumb | PR/PR | H and F | H | − | + | ASD/PDAf | − | 1.07 | 37.8 | 4.2 | Mild delay | |
| 3978 | F | p.[E137X]+[E137X] | 4,200 | M, S, BC, and UL | − | −/+ Thumb duplication | PR/PR | F | − | + | Heart murmur | − | Cleft palate | .83 | 14.0 | 2.6 | Grossly normal | ||
| 3980 | F | p.[E137X]+[E137X] | ∼5,000 | S and BC | − | +/+ Broad thumbs | C/C | F | − | − | − | − | 1.56 | 102 | 19 | Mild learning disability | |||
| 3593 (Family 1) | M | p.[L145X]+[L145X] | 5,500 | M, S, and BC | − | Enlarged 3rd and lateral ventricles | −/− | PR/PR | H and F | − | − | − | Bilateral cryptorchidism | Moderate conductive hearing loss | Intelligence quotient 68 at age 3 years | ||||
| 3589 (Family 1) | F | p.[L145X]+[L145X] | M, S, and BC | − | Moderate ventricular dilatation | −/+ | PR/PR | H and F | − | − | − | − | Moderate conductive hearing loss | Obese | Mild delay | ||||
| 3541 (Family 1) | M | p.[L145X]+[L145X] | M, S, BC, and BL | − | − | −/− | PR/PR | H and F | − | Periumbilical infection | Tetralogy of Fallot | − | |||||||
| 3624 (Family 2) | M | p.[L145X]+[L145X] | 5,000 | MU | + | Lumbar myelomeningocele, Chiari malformation, and hypoplasia of corpus callosum | −/− Broad thumbs | PR/PR | F | H | + | − | − | Bilateral cryptorchidism | Tibial bowing | Grossly normal | |||
| 3734 | F | p.[L145X]+[L145X]e | MU | − | Moderate ventricular dilatation | −/− Broad thumbs | C/C | F | HII and FII, III | − | + | Tetralogy of Fallot | − | − | 73.6 | 13.1 | |||
| 3947 | M | p.[L145X]+[L145X] | M and S | − | +/+ | PR/PR | H and F | H II and F | − | +g | − | Bilateral cryptorchidism | Hiatus hernia and genu valgum | Grossly normal | |||||
| 3971 | M | p.[L145X]+[L145X] | 6,240 | S | + | Frontotemporal hypoplasia and midline cyst | +/+ Bifid thumbs | PR/PR | H and F | H | + | − | − | Hypogonadism and low testosterone | Unerupted molars | 1.84 | 93 | 26 | Developmental quotient <69 |
| 3975 | M | p.[L145X]+[L145X] | Dolichocephaly | − | −/− | −/− | H and F | − | + | − | Inguinal hernia and lateral patellae | Normal | |||||||
| 3985 | F | p.[L145X]+[L145X] | 4,000 | M, S, and BC | − | − | −/− | C/C | H and F | − | − | + | − | − | − | 140.5 | 53.7 | 10.8 | Normal |
| 4008 | F | p.[L145X]+[L145X] | S | − | Large 3rd and lateral ventricles | −/− | PR/PR | H and F | − | − | − | − | − | Genu valgum and dental agenesis (2 premolar and 1 molar) | Obese | Grossly normal | |||
| 4009 | M | p.[L145X]+[L145X] | MU | + | −/− | C/PR | H and F | H | + | + | − | Bilateral cryptorchidism | − | Mild learning disability | |||||
| 4059 | M | p.[L145X]+[L145X] | 4,900 | M and BC | − | +/+ | PR/PR | H and F | H | − | − | − | R cryptorchidism and L inguinal testis | Genu valgum and subluxed patellae | Obese | Normal | |||
Note.— A blank cell indicates that information was not recorded.
M=metopic; S=sagittal; C=coronal; L=lambdoid; B=bilateral; U=unilateral; MU=multiple sutures.
PR=preaxial; C=central; PO=postaxial polydactyly.
H=hand; F=foot; roman numerals indicate specific digits affected.
Pregnancy terminated at 21 wk gestation.
Complete deletion of one allele was not formally excluded.
ASD=atrial septal defect; PDA=patent ductus arteriosus. Both closed spontaneously.
Large epigastric hernia.
Pathogenic sequence variants were found in all individuals with the classic phenotype (table 3 and fig. 3A), showing that mutations of RAB23 are the major cause of Carpenter syndrome. We identified five different mutations, all of which predict a loss of function. Four (E48fsX7, Y78fsX30, E137X, and L145X) of the five alleles are nonsense or frameshifting mutations that would generate truncated proteins. We identified a single missense mutation, C85R, encoding a nonconservative substitution from an uncharged to a charged amino acid; this residue is involved in β-sheet formation and is completely buried in the core of the protein (fig. 4),13,14 suggesting that this substitution would disrupt normal folding of RAB23. This mutation was present in individual 3961, who was a compound heterozygote for the C85R and L145X alleles; all other affected individuals appeared homozygous for their particular mutation. When samples were unavailable from both parents but we had sufficient proband DNA, we excluded the possibility that one allele harbored a deletion (table 3) by multiplex ligation-dependent probe-amplification (MLPA) analysis using synthetic oligonucleotide probes to RAB23 exons 1, 3, and 7 (MRC-Holland). All mutations were absent in ⩾292 control chromosomes, as assessed by diagnostic restriction digests (fig. 3A and table 4).
Table 3. .
RAB23 Mutations in Patients with Carpenter Syndrome[Note]
| Mutation at Allele |
|||||||||
| Maternal |
Paternal |
||||||||
| Subject | Sex | Parental Consanguinity | Country of Origin | Ethnicity | Sample(s) Analyzeda | DNA | Protein | DNA | Protein |
| 3965 | M | − | United Kingdom | White | C and M | 140_141insA | E48fsX7 | 140_141insA | E48fsX7 |
| 3983 | F | 1st cousin | Brazil | African/white | C and M | 232delT | Y78fsX30 | Sameb | − |
| 3961 | F | − | United Kingdom | White | C, M, and F | 434T→A | L145X | 253T→C | C85R |
| 3978 | F | − | Brazil | African/white | C and M | 408_409insT | E137X | 408_409insT | E137X |
| 3980 | F | 1st cousin once removed | Brazil | White | C, M, and F | 408_409insT | E137X | Same | − |
| 3541 (Family 1) | M | − | United States | White | C, M, and F | 434T→A | L145X | 434T→A | L145X |
| 3589 (Family 1) | F | − | United States | White | C, M, and F | 434T→A | L145X | 434T→A | L145X |
| 3593 (Family 1) | M | − | United States | White | C, M, and F | 434T→A | L145X | 434T→A | L145X |
| 3624 (Family 2) | M | 1st cousin | Denmark | White | C, M, and F | 434T→A | L145X | Same | − |
| 3734 | F | − | United Kingdom | White | C and M | 434T→A | L145X | 434T→Ab | L145X |
| 3947 | M | − | Netherlands | White | C, M, and F | 434T→A | L145X | 434T→A | L145X |
| 3971 | M | 1st cousin | Netherlands | White | C, M, and F | 434T→A | L145X | Same | − |
| 3975 | M | 1st cousin | Brazil | White | C, M, and F | 434T→A | L145X | Same | − |
| 3985 | F | − | United Kingdom | White | C, M, and F | 434T→A | L145X | 434T→A | L145X |
| 4008 | F | − | Netherlands | White | C | 434T→A | L145X | 434T→A | L145X |
| 4009 | M | 1st cousin | Brazil | African | C, M, and F | 434T→A | L145X | Same | − |
| 4059 | M | − | United Kingdom | White | C | 434T→A | L145X | 434T→A | L145X |
Note.— Nucleotide numbering of RAB23 cDNA is based on GenBank sequence NM_183227.1 but starts from the first base of the initiation codon. For cases in which the two parental alleles are very unlikely to be independent (due to known consanguinity), the paternal allele is denoted “Same.”
C=child; M=mother; F=father.
Complete deletion of one allele was not formally excluded by MLPA analysis.
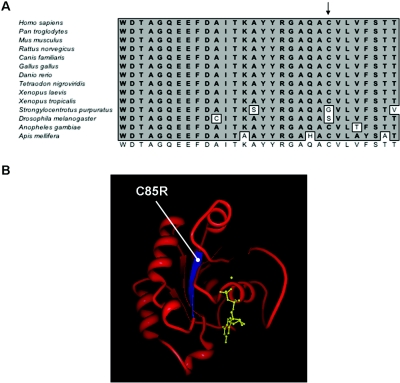
Figure 4. .
Sequence conservation and structural context of C85R substitution. A, Amino acid sequence comparison of the Switch 2 region of human RAB23 (top) with 13 other species. The consensus sequence is shown at the bottom, and the position of the mutated C85 residue is indicated with an arrow. B, Structure of human RAB2314 (Protein Data Bank [number 1Z22]), showing the C85 residue located in a β-strand (blue) and completely buried in the core of the protein. The bound Mg-GDP is shown in yellow. The structure was modeled using the Protein Workshop tool (Protein Data Bank).
Table 4. .
Primers and Restriction Enzymes Used for Confirmation of RAB23 Mutations[Note]
| Mutation and Primer | Primer Sequence (5′→3′)a | Product Size (bp) |
Restriction Enzyme |
| E48fsX7: | 157 | XcmI (−) | |
| E48fsXdigF | AAAGACTACAAGAAAACCATTGCCATTG | ||
| RAB23_2R | AGTTGCCACACCTCGAAATC | ||
| Y78fsX30: | 188 | StuI (−) | |
| RAB23_3F | TTACCAAAAACATTTTCCTTTACA | ||
| RAB23_3R | GCCAAAATAATATGCCCAAA | ||
| C85R: | 156 | BssSI (+) | |
| C85RdigF | TTTGAATGGATAAAAGTTGCCC | ||
| C85RdigR | TTCCCTATCTGTGGTAGAGAACTCGAGC | ||
| E137X: | 207 | HindIII (+) | |
| RAB23_5F | AAACAAGCTATCAGAAGGCACC | ||
| RAB23_5R | CAACACAATTTTAAAAGCGCA | ||
| L145X: | 120 | HpaI (−) | |
| RAB23_5F | AAACAAGCTATCAGAAGGCACC | ||
| RAB23_L145XdigR | TTCTTTCACTGATGTTCTGTAGAATGTT |
Note.— PCRs were performed using the same conditions as described in table 1.
Nucleotides shown in bold represent mismatches incorporated into primers to engineer diagnostic restriction sites.
The L145X mutation was apparently homozygous in 10 probands (3 each from the Netherlands and the United Kingdom, 2 from Brazil, and 1 each from the United States and Denmark), 3 of whom had been shown by the Affymetrix SNP analysis to share a common haplotype around the mutation (fig. 2B). To check whether any of the other cases had arisen from an independent mutation, we genotyped them for a subset of 13 SNPs around RAB23 (table 5). All 10 patients shared a common haplotype comprising 7 SNPs and spanning 2.2 Mb. This haplotype—which, according to HapMart (International HapMap Project), is present in only 11 of 120 Utah-CEPH chromosomes from HapMap16—was delimited by ancestral recombinations, distally in the Danish family and proximally in two Dutch families, and contains only eight genes in addition to RAB23 (fig. 2C). These data indicate that a founder effect, rather than a recurrent mutation, underlies Carpenter syndrome in patients of northern European descent and does not support the possibility that the L145X mutation has particular functional consequences (see below). Two patients from eastern Brazil both appeared homozygous for the E137X mutation, which resides on a shared haplotype spanning at least 5.8 Mb (fig. 2C). The E48fsX7 and Y78fsX30 mutations were each found in one patient only.
Table 5. .
Primers and Restriction Enzymes Used for Haplotype Analysis[Note]
| Primer | Primer Sequence (5′→3′) |
Product Size (bp) |
Assaya |
| rs1925179F | GGCAGCTTTCTTCCTGACTG | ||
| rs1925179R | TTACATTTCAAAGGGGTGGC | 188 | Hpy188I |
| rs2397214F | GGCCTGTGATTTGAGTGGTT | ||
| rs2397214R | TCTCTTGTGACCAGATGCCA | 165 | MnlI |
| rs9296842F | CTGAAGTTGCATTCTTGCCA | ||
| rs9296842R | AAATTAAAAATCAGTGTCCTGCAA | 165 | BtsCI |
| rs1547625Fseq | CCTGGCCTCATCCTACCATA | ||
| rs1547625Rseq | TGCCTGAGAAAAATGAGGCT | 154 | Seq |
| rs6927258F | TTCTGATCATGATGTAGTGCCA | ||
| rs6927258R | GGGCTTGGCATCTCTGAGTA | 218 | MboI |
| rs6906792Fseq | CAGAAACTTGGCAACAAAATG | ||
| rs6906792Rseq | TTCCTGGAATTTAAAAGGTAGCA | 182 | Seq |
| rs3904827F | ACTGCATACCGCTTACCAAA | ||
| rs3904827R | TCCAGACAAACAAAGGCTGA | 275 | Tsp509I |
| rs6934928F | AAGTGGCTTATTTCCTCCAAGA | ||
| rs6934928R | GCCCAAATCCATGTAACTTCT | 180 | RsaI |
| rs1343391Fseq | AAGGAGAGGGAGAGACCGAG | ||
| rs1343391Rseq | AGCACATGATATGCCCACTT | 187 | Seq |
| rs1224703F | AATAGGGCAGAAGGGTGCTC | ||
| rs1224703R | ACCCACCAAAGAGACGTGAG | 195 | Tsp509I |
| rs1850417F | CTTTAACTCCATTTTAAGGGACAG | ||
| rs1850417R | AACAAAGCTTGGAGAAGCAAA | 190 | EarI |
| rs2343013F | TGTGTTCCCAAACTGCTGAA | ||
| rs2343013R | CATCTCCCCCGGTTAAACTT | 221 | MwoI |
| rs1689237Fseq | TGAGGGATCTGGGATGCTAC | ||
| rs1689237Rseq | TTTAGCTCTCACTGCATGGC | 157 | Seq |
Note.— PCRs were performed using the same conditions as described in table 1.
Seq=analysis by DNA sequencing.
To explore whether RAB23 mutations play a more general pathological role either in craniosynostosis or in limb malformations, we screened respective patient panels by a combination of Wave denaturing high-performance liquid chromatography (Transgenomic) and diagnostic restriction digests for mutations identified in Carpenter syndrome. DNA from 256 patients with craniosynostosis (negative for the common mutations in the FGFR1, FGFR2, FGFR3, and TWIST1 genes),6 202 patients with limb malformations requiring plastic surgery, and 163 control individuals was analyzed using the assays detailed in tables 1, 4, and 6. None of the Carpenter syndrome mutations (table 3) were identified in any of these cohorts. Although six novel alleles—including an amino acid substitution, an amino acid deletion, and a nonsense mutation (all in the heterozygous state)—were found in the patient groups (table 6), none appeared related to the clinical phenotype. An additional common nonsynonymous SNP c.619G→A (p.G207S; rs1040461) was present, for which all three genotypes were identified in unaffected individuals in the HapMap panel.16 These results show that RAB23 mutations do not frequently contribute either to craniosynostosis or to limb malformations.
Table 6. .
RAB23 Variants Identified in Patients with Craniosynostosis and Limb Malformations[Note]
| No. of Heterozygotes |
||||||||
|
dbSNP Accession Number |
Nucleotide | Amino Acid | Exon (Intron) | With Craniosynostosis (N=256) |
With Limb Malformation (N=202) |
Controls (N=163) |
Present in Unaffected Parenta | Restriction Digest |
| ss69357975 | c.1–49C→G | − | (1) | 2 | 0 | 0 | NT | Hpy188III (−) |
| ss69357972 | c.39_41del | p.V13del | 2 | 1 | 0 | 0 | NA | XcmI (−) |
| ss69357968 | c.119A→G | p.K40R | 2 | 0 | 0 | 1 | − | NT |
| ss69357974 | c.155+44C→A | − | (2) | 0 | 0 | 1 | − | NT |
| ss69357973 | c.242–15_-12del | − | (3) | 0 | 1 | 0 | NA | NT |
| ss69357971 | c.247C→T | p.Q83X | 4 | 1 | 0 | 0 | NA | BfaI (+) |
| ss69357970 | c.301T→G | p.S101A | 4 | 5 | 2 | 2 | 2 (5 NA) | MwoI (+) |
| ss69357969 | c.574+28G→A | − | (6) | 0 | 1 | 1 | NA | NT |
Note.— NT=not tested.
NA=one or both parents unavailable for testing.
Rab23, first isolated from the mouse in 1994,17 belongs to the RAB family of >60 small guanosine triphosphatases (GTPases) that regulate intracellular trafficking of membrane-associated proteins13–15; other family members for which germline mutations cause human disorders are RAB7 (Charcot-Marie-Tooth disease type 2B, dominant inheritance [MIM #600882])18 and RAB27A (Griscelli syndrome type 2, recessive inheritance [MIM #607624]).19 Our finding of RAB23 mutations in Carpenter syndrome is unexpected, because similar nonsense mutations of the orthologous murine Rab23 gene (encoding K39X and R80X) in opb mice cause recessive embryonic lethality with exencephaly.10–12 It is unlikely that the human RAB23 mutations represent partial loss-of-function alleles of lesser severity than do the murine ones, for two reasons. First, prenylation at a consensus site in the C-terminus of RAB proteins by RAB geranylgeranyl transferase is essential for their correct membrane targeting,15 predicting that all truncating mutations should result in complete loss of function. Second, two of the human truncating mutations (E48fsX7 and Y78fsX30) occur upstream of the opb mutation R80X (fig. 3B),12 yet neural-tube defects were absent in the affected individuals. Consistent with this, we did not find any clear genotype-phenotype correlation for the human mutations, although the fetus 3965, with the most N-terminal truncation (E48fsX7), was the only subject terminated antenatally and might represent a more severe phenotype (table 2).
The original identification of Rab23 mutations in opb mice was driven by genetic studies to identify modifiers of hedgehog (HH) signaling in the neural tube. In mammals, there are three paralogous HH genes—Shh, Ihh, and Dhh—encoding the sonic, Indian, and desert HH proteins, respectively.20,21 The opb locus was initially described in a sporadically occurring mouse mutant with exencephaly10 (a variably expressed phenotype observed on both C57Bl/6 and C3H backgrounds).10,11 Subsequently, a second allele was isolated in an ethylnitrosourea mutagenesis screen for recessive embryonic lethal mutations22; nonsense mutations of Rab23 were demonstrated in both strains.12 Homozygosity for these opb mutations rescues many of the morphological defects in Shh−/− mice, with Shh/opb double-mutant mice largely resembling opb single mutants, indicating that the Rab23 mutations bypass the requirement for Shh.12 In the neural tube, mutation of Shh has opposite consequences to mutation of Rab23, causing loss (Shh) and expansion (Rab23) of ventral markers; Patched1, a transcriptional target of Shh signaling, is activated in opb mice, showing that Rab23 is a negative regulator of HH signaling.11,12 Genetic analysis of epistatic relationships shows that Rab23 acts downstream of the key HH signaling intermediate Smoothened but upstream of both the effector transcription factors Gli2 and Gli323 and the intraflagellar transport proteins (such as those encoded by Ift88/polaris and Ift172/wim), required for their capacitation.24 As such, Rab23 is one of a number of genes (including iguana, talpid3, Fkbp8, and Ift family members) implicated in the regulation of Gli transcription–factor processing specifically in vertebrates21,25 and is the first of these implicated in a human disorder. Rab23 localizes to membranes26,27 and is expressed at multiple sites in the mouse, including embryonic neural tube, limb bud, branchial arches, tooth and palate,12,28,29 and adult brain27; however, its precise membrane-transport activity has not been defined.
Given the evidence that Rab23 regulates the HH pathway, it is not surprising that some aspects of the phenotype of Carpenter syndrome resemble other human disorders associated with disturbed HH signaling. Most notably, the combination of postaxial polysyndactyly of the hands and preaxial polysyndactyly of the feet is very similar to the pattern that occurs in Greig syndrome (MIM #175700), which is due to haploinsufficiency of GLI3,30 and is consistent with the observed reduction in the proportion of Gli3 repressor in Rab23-mutant embryos.23 The brachydactyly present in Carpenter syndrome, characterized by hypoplasia or absence of the middle phalanges, resembles brachydactyly type A1, which is caused by heterozygous missense mutations in IHH that appear to cluster on one surface of the protein and disrupt phalangeal patterning by an unknown mechanism.31 However, the viability of the human RAB23 homozygous mutations in Carpenter syndrome uncovers several phenotypes (table 2), not previously observed in the lethal opb mouse mutants,10–12,22 that are not well recognized features of perturbed HH signaling.20 Particularly interesting are the craniosynostosis and tendency to postnatal obesity, which may provide new clues for dissecting the pathophysiology of these phenotypes.
Relatively little is known about the role of HH signaling in the cranial sutures. In mice, Shh is expressed in the osteogenic fronts of the parietal bones and sagittal sutures only at a relatively late stage of suture development (embryonic day 17),32 and Shh−/− mice die too early to assess the developmental contribution to the cranial sutures. Although endochondral ossification is characteristically deficient in Ihh−/− mice, membranous ossification of the skull vault is maintained33; however, there are no published data on the expression pattern of Ihh in the sutures. Our work should stimulate efforts to identify the active HH ligand(s) and to explore the extent to which the well-documented developmental relationship among HH signaling, twist, and FGF receptors in the limbs34 is recapitulated in the cranial suture. There is a potential pathophysiological link with Antley Bixler syndrome (MIM #207410), another recessively inherited craniosynostosis caused by mutations in P450 oxidoreductase (POR).35 POR is the single flavoprotein involved in electron transfer to all cytochrome P450 enzymes, including several involved in cholesterol biosynthesis, defects of which disrupt HH signaling.36
Although postnatal obesity (which has a central distribution) is practically universal in subjects with Carpenter syndrome, its neuroendocrinological and biochemical bases are not known. Obesity has not been described elsewhere in mammalian disorders of HH signaling20 and was not observed in opb mice, probably because of embryonic lethality. Pharmacological approaches have previously suggested that HH signaling may regulate adipogenesis, but the results have been conflicting as to whether this effect was inhibitory or stimulatory.37 The association of RAB23 mutations with obesity may provide new insight into the role of HH signaling in the control of fat metabolism. Alternatively, this phenotype may reflect either the regulation of RAB23 itself (possibly by bone morphogenetic proteins)12 or downstream HH-independent processes. The possible interaction of RAB23 with cilia21 suggests an overlap with the Bardet-Biedl syndromes, which are ciliopathies that also feature obesity and polydactyly.38
Acknowledgments
We thank all the families for their participation in this study; S. Balci and A. Richieri-Costa for referring patient samples; C. Becker (Nürnberg laboratory) for expert technical assistance in providing the SNP genotype data from Affymetrix microarrays; other members of the Wilkie laboratory for their support, especially D. Furniss for access to samples from patients with limb malformations and I. Taylor for DNA extraction; K. Clark for DNA sequencing; and J. Eggenschwiler and B. St-Jacques for discussions. This work was funded by the E. P. Abraham Cephalosporin Fund (support to D.J.), German Federal Ministry of Sciences and Education through the National Genome Research Network grant 01GR0416 (to P.N.), and the Wellcome Trust (support to A.O.M.W.).
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for SNPs, including rs1040461, rs1925179, rs2397214, rs9296842, rs1547625, rs6927258, rs6906792, rs3904827, rs6934928, rs1343391, rs1224703, rs1850417, rs2343013, and rs1689237)
- Ensembl Genome Browser, http://www.ensembl.org/ (for RAB23 [reference OTTHUMG00000014918])
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human RAB23 cDNA reference sequence [accession number NM_183227.1])
- International HapMap Project, http://hapmart.hapmap.org/BioMart/martview (for HapMart)
- MRC-Holland, http://www.mrc-holland.com/pages/indexpag.html (for information on MLPA reagents and methods)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Carpenter syndrome, Charcot-Marie-Tooth disease type 2B, Griscelli syndrome type 2, Greig syndrome, and Antley Bixler syndrome)
- Protein Data Bank, http://www.rcsb.org/pdb/home/home.do (for RAB23 structure [number 1Z22] and Protein Workshop)
References
- 1.Carpenter G (1901) Two sisters showing malformations of the skull and other congenital abnormalities. Rep Soc Study Dis Child Lond 1:110–118 [Google Scholar]
- 2.Carpenter G (1909) Case of acrocephaly, with other congenital malformations. Proc Roy Soc Med 2:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temtamy SA (1966) Carpenter’s syndrome: acrocephalopolysyndactyly. J Pediatr 69:111–120 10.1016/S0022-3476(66)80368-2 [DOI] [PubMed] [Google Scholar]
- 4.Robinson LK, James HE, Mubarak SJ, Allen EJ, Jones KL (1985) Carpenter syndrome: natural history and clinical spectrum. Am J Med Genet 20:461–469 10.1002/ajmg.1320200307 [DOI] [PubMed] [Google Scholar]
- 5.Cohen DM, Green JG, Miller J, Gorlin RJ, Reed JA (1987) Acrocephalopolysyndactyly type II—Carpenter syndrome: clinical spectrum and an attempt at unification with Goodman and Summit syndromes. Am J Med Genet 28:311–324 10.1002/ajmg.1320280208 [DOI] [PubMed] [Google Scholar]
- 6.Morriss-Kay GM, Wilkie AOM (2005) Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat 207:637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlyn CA, Marsh JL (2007) Craniofacial dysmorphology of Carpenter syndrome: lessons from three affected siblings. Plast Reconstr Surg (in press) [DOI] [PubMed] [Google Scholar]
- 8.Dietter J, Mattheisen M, Fürst R, Rüschendorf F, Wienker TF, Strauch K (2007) Linkage analysis using sex-specific recombination fractions with GENEHUNTER-MODSCORE. Bioinformatics 23:64–70 10.1093/bioinformatics/btl539 [DOI] [PubMed] [Google Scholar]
- 9.King JA, Marker PC, Seung KJ, Kingsley DM (1994) BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol 166:112–122 10.1006/dbio.1994.1300 [DOI] [PubMed] [Google Scholar]
- 10.Günther T, Struwe M, Aguzzi A, Schughart K (1994) open brain, A new mouse mutant with severe neural tube defects, shows altered gene expression patterns in the developing spinal cord. Development 120:3119–3130 [DOI] [PubMed] [Google Scholar]
- 11.Eggenschwiler JT, Anderson KV (2000) Dorsal and lateral fates in the mouse neural tube require the cell-autonomous activity of the open brain gene. Dev Biol 227:648–660 10.1006/dbio.2000.9918 [DOI] [PubMed] [Google Scholar]
- 12.Eggenschwiler JT, Espinoza E, Anderson KV (2001) Rab23 is an essential negative regulator of the mouse sonic hedgehog signalling pathway. Nature 412:194–198 10.1038/35084089 [DOI] [PubMed] [Google Scholar]
- 13.Pereira-Leal JB, Seabra MC (2000) The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily J Mol Biol 301:1077–1087 [DOI] [PubMed] [Google Scholar]
- 14.Eathiraj S, Pan X, Ritacco C, Lambright DG (2005) Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature 436:415–419 10.1038/nature03798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira-Leal JB, Seabra MC (2001) Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313:889–901 10.1006/jmbi.2001.5072 [DOI] [PubMed] [Google Scholar]
- 16.The International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437:1299–1320 10.1038/nature04226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olkkonen VM, Peterson JR, Dupree P, Lütcke A, Zerial M, Simons K (1994) Isolation of a mouse cDNA encoding Rab23, a small novel GTPase expressed predominantly in brain. Gene 138:207–211 10.1016/0378-1119(94)90809-5 [DOI] [PubMed] [Google Scholar]
- 18.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, et al (2003) Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet 72:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, et al (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25:173–176 10.1038/76024 [DOI] [PubMed] [Google Scholar]
- 20.Nieuwenhuis E, Hui C-c (2005) Hedgehog signaling and congenital malformations. Clin Genet 67:193–208 10.1111/j.1399-0004.2004.00360.x [DOI] [PubMed] [Google Scholar]
- 21.Huangfu D, Anderson KV (2006) Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133:3–14 10.1242/dev.02169 [DOI] [PubMed] [Google Scholar]
- 22.Kasarskis A, Manova K, Anderson KV (1998) A phenotype-based screen for embryonic lethal mutations in the mouse. Proc Natl Acad Sci USA 95:7485–7490 10.1073/pnas.95.13.7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV (2006) Mouse Rab23 regulates Hedgehog signaling from smoothened to Gli proteins. Dev Biol 290:1–12 10.1016/j.ydbio.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 24.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- 25.Davey MG, Paton IR, Yin Y, Schmidt M, Bangs FK, Morrice DR, Gordon Smith T, Buxton P, Stamataki D, Tanaka M, et al (2006) The chicken talpid3 gene encodes a novel protein essential for Hedgehog signaling. Genes Dev 20:1365–1377 10.1101/gad.369106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C (2003) Rab23, a negative regulator of hedgehog signaling, localizes to plasma membrane and the endocytic pathway. Traffic 4:869–884 10.1046/j.1600-0854.2003.00141.x [DOI] [PubMed] [Google Scholar]
- 27.Guo AC, Wang T, Ng EL, Aulia S, Chong KH, Teng FYH, Wang Y, Tang BL (2006) Open brain gene product Rab23: expression pattern in the adult mouse brain and functional characterization. J Neurosci Res 83:1118–1127 10.1002/jnr.20788 [DOI] [PubMed] [Google Scholar]
- 28.Miletich I, Cobourne MT, Abdeen M, Sharpe PT (2005) Expression of the Hedgehog antagonists Rab23 and Slimb/βTrCP during mouse tooth development. Arch Oral Biol 50:147–151 10.1016/j.archoralbio.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 29.Rice R, Connor E, Rice DPC (2006) Expression patterns of Hedgehog signalling pathway members during mouse palate development. Gene Expr Patterns 6:206–212 10.1016/j.modgep.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Biesecker LG (2004) GLI3 and the Pallister-Hall and Greig cephalopolysyndactyly syndromes. In: Epstein CJ, Erickson RP, Wynshaw-Boris A (eds) Inborn errors of development. Oxford University Press, Oxford, United Kingdom, pp 257–264 [Google Scholar]
- 31.Hellemans J, Coucke PJ, Giedion A, De Paepe A, Kramer P, Beemer F, Mortier GR (2003) Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet 72:1040–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H-J, Rice DPC, Kettunen PJ, Thesleff I (1998) FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 125:1241–1251 [DOI] [PubMed] [Google Scholar]
- 33.St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Rourke MP, Soo K, Behringer RR, Hui C-c, Tam PP (2002) Twist plays an essential role in FGF and SHH signal transduction during mouse limb development. Dev Biol 248:143–156 10.1006/dbio.2002.0730 [DOI] [PubMed] [Google Scholar]
- 35.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, et al (2005) Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA (2003) A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 33:508–513 10.1038/ng1134 [DOI] [PubMed] [Google Scholar]
- 37.Rosen ED (2006) New drugs from fat bugs? Cell Metab 3:1–2 10.1016/j.cmet.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 38.Badano JL, Mitsuma N, Beales PL, Katsanis K (2006) The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7:125–148 10.1146/annurev.genom.7.080505.115610 [DOI] [PubMed] [Google Scholar]