Abstract
Retinoic acid (RA) is a potent teratogen in all vertebrates when tight homeostatic controls on its endogenous dose, location, or timing are perturbed during early embryogenesis. STRA6 encodes an integral cell-membrane protein that favors RA uptake from soluble retinol-binding protein; its transcription is directly regulated by RA levels. Molecular analysis of STRA6 was undertaken in two human fetuses from consanguineous families we previously described with Matthew-Wood syndrome in a context of severe microphthalmia, pulmonary agenesis, bilateral diaphragmatic eventration, duodenal stenosis, pancreatic malformations, and intrauterine growth retardation. The fetuses had either a homozygous insertion/deletion in exon 2 or a homozygous insertion in exon 7 predicting a premature stop codon in STRA6 transcripts. Five other fetuses presenting at least one of the two major signs of clinical anophthalmia or pulmonary hypoplasia with at least one of the two associated signs of diaphragmatic closure defect or cardiopathy had no STRA6 mutations. These findings suggest a molecular basis for the prenatal manifestations of Matthew-Wood syndrome and suggest that phenotypic overlap with other associations may be due to genetic heterogeneity of elements common to the RA- and fibroblast growth factor–signaling cascades.
Microphthalmia refers to a clinical spectrum that is characterized by a congenital reduction in the size of the optic globe(s), which may be reduced to a vestige visible only on histological analysis. This most severe form of microphthalmia is sometimes called “secondary” or “clinical” anophthalmia and occurs later in development than primary anophthalmia because of a lack of optic vesicle formation from the embryonic prosencephalon. Isolated severe microphthalmia/anophthalmia demonstrates both genetic and phenotypic heterogeneity in humans, currently implicating genes coding for transcription factors. CHX10 mutations lead to microphthalmia, coloboma, and cataracts1,2; mutations in the RAX gene have been identified in an individual with unilateral anophthalmia and sclerocornea in the other eye.3 PAX6 mutations lead to diverse congenital ocular malformations, the most common of which is aniridia, but a few genotypes have been described to date that engender primary anophthalmia4 or microphthalmia,5–7 as documented in the PAX Allelic Variant Database.
Syndromic microphthalmias (MIM 164180, 206900, 206920, 248450, 300166, 301590, 309801, 600776, 605856, 607932, 610125, 610126, and 601349) can be associated with craniofacial dysmorphic features, heart and vascular malformations, skeletal and limb anomalies, skin or gut defects, mental retardation, and hydrocephalus, or combinations thereof. Although rare, the association of severe microphthalmia and pulmonary hypoplasia (MIM 601186) is a distinct entity known as “Matthew-Wood syndrome” (MWS [MIM 601186]).8 Most authors have reported further associations of MWS with cardiac and/or diaphragmatic malformations and intrauterine growth retardation (IUGR).9–13
In two familial cases of MWS, we have excluded mutations in the FGF10 and FGFR2IIIb genes encoding fibroblast growth factor 10 and its specific receptor isoform.14 These proteins are essential for the development of all affected organs in MWS.15–17 Meanwhile, STRA6 gene mutations were recently implicated in heterogeneous postnatal associations of clinical anophthalmia, pulmonary hypoplasia, diaphragmatic hernia, and cardiac defects.18 A molecular analysis of the STRA6 gene was undertaken in the two families with MWS we had described,14 as well as in five other fetuses presenting at least one of the two major signs of clinical anophthalmia or pulmonary hypoplasia and at least one of the two associated signs of diaphragmatic closure defect or cardiopathy.
In all seven fetuses examined, the presence of severe malformations was noted on ultrasound examination, and, after genetic counseling, pregnancies were interrupted. Clinical data are summarized in table 1. Chromosome and molecular analyses and pathological examinations were performed in all cases with full parental consent. Genomic DNA was extracted from frozen tissue in fetal cases and from peripheral blood samples for parents in accordance with standard protocols.
Table 1. .
Overview of Clinical Features in Cases Undergoing STRA6 Molecular Analysis from Our Series and Pasutto et al.18[Note]
| Clinical Featuresa |
||||||||||
| Case | STRA6 Mutation(s) | Eyes | Lungs | Diaphragm | Cardiovascular | Face | Other | Growth | Age at Death | Consanguinity |
| Fetus 1b | p.D17A fsX55 | Bi AO | Bi agenesis | Bi eventr | Bi absence of PA branches | Mild dysmorphism | Duodenal stenosis, annular pancreas | IUGR | 31 wg | Yes, recurrence |
| Fetus 2b | p.G176G fsX59 | Bi AO | Bi agenesis | Bi eventr | Pulmonary trunk and PA absence, VSD | Mild dysmorphism | Duodenal stenosis, absent pancreas, polylobed spleen | IUGR | 28 wg | Yes |
| Fam2-IV:1 | p.G50A fsX22 | Bi AO | … | CDH | ASD, VSD | Mild dysmorphism | MR | SS | Alive at 14 years | Yes, recurrence |
| Fam2-IV:3 (sib) | p.G50A fsX22 | Bi AO | NA | CDH | NA | Mild dysmorphism | NA | … | 23 wg | … |
| MWS4-BE | p.T644M | Bi AO | Hypo | CDH | … | … | Bi hydronephrosis | … | Alive at 3 mo | No, recurrence |
| Brother MWS4-BE | NA | NA | Hypo unilobar | … | Fallot, PDA | … | Horseshoe kidney, undescended testes | … | 1 d | … |
| Sister MWS4-BE | NA | Bi AO | Hypo unilobar | … | PDA, CoA | … | Uterine dysplasia | … | 1 d | … |
| MWS1-EE | p.R655C | Bi AO | Hypo | Uni eventr | … | … | Hypotonia uni inguinal hernia | … | 3 mo | Yes, recurrence |
| Brother MWS1-EE | NA | Bi AO | … | … | TA, RAA, PDA, PA atresia | … | … | SS | 22 mo | … |
| MWS6-BK | p.P90L, p.T321P | Bi AO | Hypo | CDH, uni eventr | PDA | … | Hypo kidneys, bicornuate uterus | PTB (36 wg) | 1 d | Yes, recurrence? |
| Fam1-IV:2 | p.P293L | Bi AO | ACD | … | PSt, PDA | Mild dysmorphism | Ectopic kidney, DD | PTB (33 wg) | 6 mo | Yes, recurrence |
| Fam1-IV:4 (cousin) | NA | Bi AO | NA | … | Single ventricle PA atresia | NA | … | … | 2 d | … |
| CD50396b | … | Bi AO | Hypo | Uni eventr | VSD | CP hypo alae nasi | Hypo bicornuate uterus, hypo spleen | … | 1 d | No |
| Fetus 3c | … | Bi AO | Hypo | Bi CDH | Hypo L ventricle and aorta, mitral valve atresia, VSD | CP | CC agenesis, arhinencephaly, Dandy-Walker | … | 16 wg | No |
| Fetus 4 | … | Uni AO | … | … | Single ventricle tricuspid valve atresia, ASD | … | Arhinencephaly | … | 22 wg | No |
| MWS3-KH | … | Bi MO/AO | … | CDH | … | … | … | … | NA | No |
| RHP006.070 | … | Bi MO/AO | … | Bi eventr | … | … | MR | … | NA | No |
| PB-E03_053 | … | Bi MO/AO | … | CDH | … | Brachycephaly | MR, sparse hair, bi inguinal hernia | … | Alive at 10 years | No |
| GM23728 | … | Bi MO, abnormalcornea and iris | Hypo unilobar | Hypo, uni eventr | Hypo PA CoA | … | Renal dysplasia | … | Neonatal | No |
| AS20861-FF264 | … | Uni MO | … | CDH | … | … | Ocular cyst, DD | … | Alive at 13 mo | No |
| MWS2-FA | … | Bi coloboma | … | CDH | … | … | Skin patches, brittle hair | … | NA | Yes |
| MWS5-LR | … | Coloboma | … | CDH | … | … | … | … | NA | No |
| Fetus 5 | … | … | Hypo | Uni CDH | Dextroposed aorta over VSD | … | SUA | … | 32 wg | No |
| AvdW22260 | … | … | Hypo | CDH | … | … | … | PTB (28 wg) | 1 d | No |
| Twin 2 AvdW22260 | … | … | Hypo | CDH | … | CP | … | PTB (28 wg) | 1 d | … |
| PM22479d | … | … | … | CDH | … | Hypertelorism | Hypo CC omphalocoele | … | Neonatal | Yes, recurrence |
| Brotherd PM22479 | NA | … | … | CDH | ASD | Bi CLP, hypertelorism | Hypo CC | … | Neonatal | … |
| Fetus 6 | … | … | Bi hypo | L agenesis, R eventr | Hypo L heart | … | Polysplenia renal dysplasia, SUA | IUGR | 30 wg | No |
| Fetus 7 | … | … | Bi agenesis | … | L atrial isomerism, R ventricular anomaly | … | Polysplenia renal agenesis | … | 24 wg | No |
Note.— NA = not available. Fetuses 1–7 from our series are highlighted in bold.
ACD = alveolar capillary dysplasia; AO = clinical anophthalmia; ASD = atrial septal defect; bi = bilateral; CC = corpus callosum; CoA = coarctation of aorta; C(L)P = cleft (lip and) palate; DD = developmental delay; eventr = eventration; Fallot = tetralogy of Fallot; hypo = hypoplasia; L = left; MO = microphthalmia; MR = mental retardation; PA = pulmonary artery; PDA = patent ductus arteriosus; PSt = pulmonary valve stenosis; PTB = preterm birth; R = right; RAA = right aortic arch; SS = postnatal short stature; SUA = single umbilical artery; TA = truncus arteriosus; uni = unilateral; VSD = ventricular septal defect; wg = weeks gestation.
Cases given diagnosis of Matthew-Wood syndrome.
Cases given diagnosis of Fryns syndrome.
Cases with suspected Donnai-Barrow syndrome.
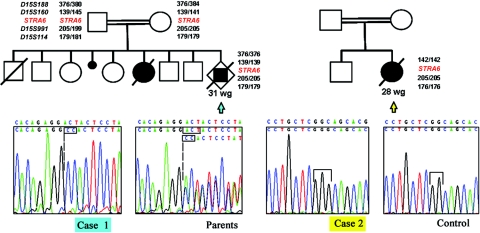
Polymorphic markers D15S188, D15S160, D15S991, and D15S114, flanking the STRA6 gene, were chosen using the UCSC Genome Browser and were examined in fetal cases 1 and 2 (fig. 1). The parents of case 1 are a consanguineous couple of Romanian origin, and the parents of case 2 are a consanguineous couple of Portuguese origin.14 Homozygous haplotypes were demonstrated in each fetus, although the clinically unaffected parents of case 1 had a heterozygous haplotype with an allele presumably inherited from a common ancestor (DNA was unavailable from the other family members of case 2).
Figure 1. .
Pedigrees of cases 1 and 2, with markers flanking the STRA6 gene, and electropherograms. Case 1 (blue arrow) had a homozygous insertion/deletion in exon 2 of STRA6 (c.50_52delACTinsCC p.AspD17Ala fsX55). Case 2 (yellow arrow) had a homozygous insertion in exon 7 (c.527_528insG p.Gly176Gly fsX59). Markers D15S160, D15S991, and D15S114 were also homozygous; relatives’ DNA was unavailable for further analysis. wg = Weeks gestation.
Primers were subsequently designed to cover the 20 exons and exon-intron junctions of the STRA6 gene (UCSC Genome Browser reference sequence NM_022369), including exons 1A and 1B (the first noncoding exon may be alternatively spliced), with the use of Primer3 software,19 (table 2). PCRs were treated with the ExoSAP enzyme mix as per the manufacturer’s instructions (GE-Amersham). Sequencing was performed for all seven fetal DNA samples with the use of Big Dye v3.1 Terminator Cycle Sequencing Reactions on an ABI 3130 (Applied Biosystems). Both the sense and antisense strands of the PCR-amplified fragments were analyzed with Sequence Analysis software (Applied Biosystems).
Table 2. .
STRA6 Oligonucleotides Used for Sequencing[Note]
| Oligonucleotide Sequences (5′→3′) |
||
| Exon(s) | Forward | Reverse |
| 1a | GGGGTGGGTTCCTCTGAT | CACCCCAGGTCTCCAAACT |
| 1b | GCTGAAGGCAGGTATGTGTG | CCTCTCGTGTCCCCTCCT |
| 2 | AAGCCTCTTTTCACATCTGTAGTG | CAGTTGCAACCTCTGCCATC |
| 3 | TGGGTAAAGCCTCAGTGTGA | GTTGGACTTGCATCCTGGTT |
| 4 | CAAGCCCTCAAACTCAGACC | TGGGGGTCCTGACTAAACCT |
| 5 | CCACCTCCTTGATTTATGGAA | GCATCGTTGTAAAGACTGGATG |
| 6 and 7 | ACCTTCTCATTTTGCCCTTG | CTCAAAGGAGGCACTGTGGT |
| 8 | GCAACGGATTCTGGTTCTTG | GGAGTAGGGCTGTCTTGGG |
| 9 and 10 | ACGAATGGGTCGAGGCAG | TCTGTGCAAGGGAGGGTAAC |
| 11 | CTTGGGAGGGAGGAGGG | GGTTGAGGGCAGGGCTC |
| 12 | CCAGCGTCTCCCCTGTTAG | CATAGACCTTGGGTCTCCCC |
| 13 | TGGCAGGGGTTCTGAGG | CACAGGACTCCCACTCCTTC |
| 14 | TGGCCCAGAGGAGGATTTAG | CCAACTGAGGCCAGTGTCTG |
| 15 and 16 | AAAGCCCTTGGTTCTGGG | ACACCGAAGAAGAGGCGAG |
| 17 | AGGTCTGACACTGACCCTGG | GATGCCTTCCTCACTGCTTG |
| 18 | TGGATGCCTCCAGTGTGG | AGGGGCACACATCCTTCC |
| 19 | GATCAGGTCTGAGGGCCAG | GAGGAGGATGGTAGGCAGG |
Note.— The annealing temperature for PCR was 60°C for all primers. For QMPSF, fluorescent primers corresponding to STRA6 exon 13 were used, and MLH1 was chosen as a reference (GTAGTCTGTGATCTCCGTTT, 5′; ATGTATGAGGTCCTGTCCT, 3′). Coamplification was performed for 21 cycles, and the peaks were integrated and proportional DNA copy numbers were estimated with the use of Genotyper 3.7 software (Applied Biosystems).
Cases 1 and 2 both presented homozygous mutations in the coding sequence of STRA6 (fig. 1). A homozygous insertion/deletion in exon 2 (c.50_52delACTinsCC) for fetus 1 causes a frameshift and the appearance of a premature stop codon (p.Asp17Ala fsX55). An older brother with isolated bilateral coloboma of the retina and iris was heterozygous for this mutation, as were the clinically unaffected parents. Case 2 presented a homozygous single-base insertion in exon 7 (c.527_528insG) that also predicts a premature stop codon (p.Gly176Gly fsX59).
Case 4 had six intronic variations and one conservative amino acid substitution (table 3), all of which were homozygous and documented SNPs in the general population (dbSNP). Parental samples for fetus 4 were not available for analysis. Since the fetus was not known to come from a consanguineous background and had a normal karyotype, the hypothesis of a small, heterozygous deletion was considered. Quantitative multiplex PCR of small fluorescent fragments (QMPSF)20 was undertaken to measure the number of genomic STRA6 copies for case 4. The results indicated that this fetus did not present a deletion of the STRA6 gene that would explain the observed homozygosity of the SNPs (data not shown).
Table 3. .
Sequence Variations in STRA6[Note]
| Fetal Case and Nucleotide Change versus NM_022369 |
Predicted Effect on ORF |
dbSNP Reference Number | Status |
| 1: | |||
| c.50_52delACTinsCC | p.Asp17Ala fsX55 | … | Homozygous |
| 2: | |||
| c.527_528insG | p.Gly176Gly fsX59 | … | Homozygous |
| 4: | |||
| c.331C→T | p.Leu111Leu | rs11857410 | Homozygous |
| c.406+97A→G | … | rs34147822 | Homozygous |
| c.406+111A→G | … | rs35255788 | Homozygous |
| c.430+24T→A | … | rs971756 | Homozygous |
| c.431−37C→T | … | rs971757 | Homozygous |
| c.1685−24T→C | … | rs12913041 | Homozygous |
| c.1840+50T→C | … | rs12912578 | Homozygous |
| 5: | |||
| c.596+9T→G | … | rs28541560 | Heterozygous |
| c.1301−43A→C | p.Ser472Ser | rs351240 | Heterozygous |
| c.1416G→A | … | rs351241 | Heterozygous |
| 6: | |||
| c.1166+32G→A | … | … | Heterozygous |
| 7: | |||
| c.1167−10C→G | … | rs2277608 | Heterozygous |
Note.— Case 3 had no sequence variations.
A single heterozygous variation located in intron 13 (c.1407+32G→A) that was observed in case 5 has not been identified to date in dbSNP (table 3). We screened 260 control chromosomes without observing the c.1407+32G→A variation. The only tissue available from fetus 5 for expression analysis was a frozen lung sample. STRA6 transcripts were not observed in either total lung RNA extracted from an age-matched fetus affected with an unrelated disorder or from the case 5 tissue sample (data not shown). Therefore, the consequence of this variation on STRA6 transcription remains to be determined.
We report homozygous mutations in the STRA6 gene in two fetuses presenting the principal features of MWS, including bilateral severe microphthalmia and pulmonary agenesis. Both also had bilateral diaphragmatic eventration, and one had a cardiac malformation. The observation that both fetuses came from consanguineous families—and, moreover, that one family demonstrated sibling recurrence—had already evoked a recessive model of inheritance for MWS.14 Since the molecular anomaly has been found, it is now possible to affirm that MWS is indeed an autosomal recessive disorder that can be ascribed to mutations in the STRA6 gene.
These two fetuses with the STRA6 mutation would not have survived postnatally. In both cases, the mutations would have led to a truncated protein if translated. Homozygous STRA6 mutations have also been observed in peri- and postnatal patients from two other families, as well as in three sporadic cases with a similar phenotypic spectrum.18 However, four missense mutations were found to be associated with a severe clinical phenotype, whereas two cases with a truncating mutation had milder clinical signs with no growth retardation nor apparent pulmonary anomalies. Indeed, one of those patients has survived into his teens. Comparison of all reported patients with STRA6 mutations (table 1) thus demonstrates that there is no correlation to date between the nature of a coding mutation and the severity of the phenotype.
The recent functional study of 50 random missense mutations introduced into bovine Stra6 has shown that a few of these are sufficient to prevent cell surface expression and that one, although allowing protein insertion into the membrane, abrogates vitamin A entry into the cell.21 Similar studies will now need to be conducted with documented human mutations to draw conclusions, but it is probable that phenotypic severity is a result of the reduction in perceived retinoic acid (RA) dose within sensitive target tissues, rather than a simple distinction between missense and nonsense mutations.
We also undertook molecular analysis of STRA6 in five other fetuses with pulmonary and ocular or cardiac malformations, but no other patent mutations were identified, despite some intriguing variations (table 3). The clinical diversity of patients with STRA6 mutations, and the large phenotypic overlap with those who do not have the mutations, strongly suggests that MWS and related syndromes are not only clinically but genetically heterogeneous.
The only necessary diagnostic criterion predicting the involvement of STRA6, on the basis of the patients currently reported here and in the previous study,18 is severe microphthalmia (clinical anophthalmia). Microphthalmia with any macroscopically residual presence of the ocular globe does not correlate with STRA6 mutations in either series (table 1). Obviously, since many genes have previously been identified in both isolated and syndromic microphthalmia, this feature is not sufficient to direct molecular testing. The severe eye malformations subsequent to STRA6 mutations are always observed in association with one or more of the three following signs: pulmonary defects, congenital diaphragmatic eventration/hernia, or cardiovascular malformation involving the common aorticopulmonary trunk or pulmonary arteries. Furthermore, according to our two MWS cases and descriptions of MWS in the literature, pancreatic malformations and IUGR may also be secondary diagnostic criteria.
Pulmonary defects range from agenesis (this report) to hypoplasia or unilobar lung (among families with MWS mutations) to no obvious lung problems (in either member of family 2 examined by Pasutto et al).18 Pulmonary and diaphragmatic malformations (eventration/hernia) are not always associated and occur separately or in combination even among members of the same family.18 This observation leads us to conclude that, in the context of STRA6 mutations, the pulmonary phenotype of patients with mutations is a primary malformation and is not a consequence of diaphragmatic hernia. However, the joint presence of clinical anophthalmia and pulmonary and/or diaphragmatic anomalies is still not sufficient to guarantee STRA6 involvement, because other cases with bilateral anophthalmia and hypoplastic lungs (patients with MWS GM23728 and CD50396 from Pasutto et al.18 and our case 4) do not present coding-sequence mutations (table 1).
Cardiovascular involvement is frequent but inconstant. Case 2 had a ventricular septal defect and pulmonary trunk agenesis, whereas case 1 presented isolated agenesis of the pulmonary arteries. Furthermore, STRA6 mutations described by Pasutto et al. also give rise to conotruncal or great-artery malformations (i.e., truncus arteriosus, tetralogy of Fallot, pulmonary valve or arterial stenosis, and right aortic arch) in at least some family members.18 Other affected members with identical mutations had no cardiovascular signs (cf. MWS4-BE). Cases of MWS described elsewhere9,12 also show a preponderance of pulmonary artery absence, ductus arteriosus, or ventricular septal defects.
Fryns syndrome (MIM 229850) has a clinical spectrum that includes diaphragmatic hernia and, less frequently, microphthalmia, facial dysmorphy, and distal limb anomalies. Fetal case 3, presenting with bilateral microphthalmia, pulmonary hypoplasia, diaphragmatic hernia, cardiac involvement, and cleft palate, was given a diagnosis of Fryns syndrome. Despite the implication of the same organ systems as in MWS and absence of a digital phenotype, no mutations in the STRA6 coding sequence were found. Patients GM23728 and CD50396 from Pasutto et al.18 also had a similar phenotype (table 1); the latter was given a diagnosis of MWS, presented true clinical anophthalmia, and had a cleft palate. Palate involvement might therefore be suggestive of Fryns syndrome rather than MWS. Phenotypic overlap between these two disorders indicates that similar cases given a diagnosis of Fryns syndrome or MWS have either a noncoding mutation in STRA6 or involvement of another gene necessary for the cellular interpretation of RA levels. For some authors, animal models of retinoid deficiency also evoke the PAGOD syndrome (pulmonary tract and pulmonary artery, agonadism, omphalocele, diaphragmatic defect, and dextrocardia [MIM 202660]), which shares features with Fryns syndrome and MWS.22
RA, a small lipophilic hormone derived from retinol (vitamin A), is a ligand for nuclear receptors (RARα, -β, and -γ) that act in homodimers or in heterodimers with retinoid X receptor partners to bind DNA and regulate the expression of many genes, including the Stra (stimulated by retinoic acid) targets.23,24 The functionally identified Stra genes have different roles and structurally unrelated products. For example, Stra1 encodes ephrin B1, a bidirectional, membrane-bound signaling molecule highly expressed in the embryonic neural crest25; Stra7, later identified as the evolutionarily conserved transcription factor Gbx2,26 partners with the homeobox transcription factor Otx2 in the specification of the isthmic organizer (midbrain/hindbrain junction).27
Otx2 was also subsequently identified as a transcriptional target of RA, which leads to derepression of Pax6 transcription in the optic cup.28 Interestingly, both OTX229 and PAX64 are responsible for human anophthalmias (MIM 610125 and 607108 [allelic variant .0005], respectively), through heterozygous loss-of-function with incomplete penetrance for the former and compound heterozygous loss-of-function engendering a primary anophthalmia for the latter. Mutations in EFNB1 (encoding human ephrin B1) induce craniofrontonasal syndrome (MIM 304110), sometimes in association with congenital diaphragmatic hernia (CDH).30,31 We note that CRABP1 (cellular retinoic acid–binding protein 1), another transcriptional target and effector of cytoplasmic RA levels,32 is located close to reported CDH loci in the long arm of chromosome 15. Experimental or teratogenic reductions in RA levels also lead to CDH in both animals and humans.33,34
The murine Stra6 gene encodes an integral transmembrane protein that is expressed in the developing eye, lung, other endodermal gut derivatives, limbs, and somites.23 In addition to being stimulated by RA, Stra6 encodes a receptor for soluble retinol-binding protein, efficiently mediating retinol uptake from the circulatory system into target cells.21
Signaling by RA within the caudal pharyngeal endoderm of the vertebrate embryo is critical for the organization of the adjacent aortic arch vessels and heart. Sensitivity of only the most posterior aortic arches, which persist in direct continuity with the outflow tract of the heart, may be a result of the localized mesodermal production of retinaldehyde dehydrogenase 2 (Raldh2), a major enzyme for RA synthesis from retinol during development.35 Raldh2−/− mice demonstrate third- and fourth-arch artery malformations, with agenesis of the sixth arch36 in addition to cardiac septation defects37 and partial pancreatic agenesis.38 The variable implication of the cardiac outflow tract and vascular derivatives of the embryonic fourth (definitive aorta) and sixth (ductus arteriosus and proximal pulmonary artery) aortic arches in our patients is consistent with an underlying field defect affecting the perception of RA dose by the endoderm.
Indeed, murine Stra6 is highly expressed in the pharyngeal endoderm and mesenchyme along the embryonic gut.23 Our two severely affected patients with mutations had duodenal stenosis and pancreatic malformations in addition to lung agenesis. These organs are among the many derivatives of the embryonic endoderm produced by localized outpocketings into the mesoderm that will consolidate into the definitive structure.
RA is particularly necessary for normal growth and formation of the lung. Fgf10−/− mice demonstrate complete lung agenesis,15,16 whereas, in knockout mice for the appropriate Fgf10-binding isoform of Fgfr2, the tracheal bifurcation at the origin of the bronchi is absent.17 In Raldh2−/− mouse embryos, Fgf10 is no longer expressed in the lung bud, and complete agenesis results.39 It appears likely that Stra6 expressed, among other places, in the Raldh2+ bronchial mesenchyme of the early lung23 mediates retinol entry into the mesoderm and a subsequent effect on Fgfr2 signaling in the endoderm. Indeed, the supply of exogenous RA for short periods can partially rescue both Fgf10 expression and lung agenesis, leading to unilobar or unilateral right-sided lung development; longer rescue periods lead to better recovery and more subtle alveolar malformations.40
Stra6 is also expressed at all stages of eye development—initially, within the optic vesicle and, later, within the periocular mesenchyme, the choroid, and the optic nerve (and forebrain) meninges. Expression in the retinal pigment epithelium persists throughout adult life in both mice and humans,18,23 which is indicative of the continued need for RA for ocular function. The consistency of clinical anophthalmia in patients with STRA6 mutations argues for the need for vitamin A uptake to further all stages of eye development after initial optic specification.
Stra6 transcripts are also detected in several other sites, including the forebrain, the isthmic organizer, and the neurohypophysis. However, no patients with STRA6 mutations present CNS malformations or pituitary anomalies, although IUGR or short stature may indicate a more subtle effect (table 1). Murine expression patterns do not always suffice to explain clinical outcome.41 Despite the strong, localized brain expression of the RA target Gbx2 (Stra7), its absence in mice gives rise only to posterior branchial arch anomalies and cardiac malformations, reminiscent of those observed in patients with STRA6 mutations or in Raldh2−/− mice.42 There may also be species-specific differences in the RA sensitivity of the developing brain; the clinical spectrum of human vitamin A deficiency syndrome does not include the exencephaly observed in mouse models.43
In conclusion, STRA6 mutations are responsible for a large spectrum of congenital malformations with no current evidence of a genotype-phenotype correlation. Different transcriptional targets of RA signaling in humans appear to effect subset phenotypes of those observed in more generalized deficiencies.43 MWS is thus part of a growing family of human syndromes due to mutations in genes encoding effectors of the powerful developmental morphogen, RA.
Acknowledgments
The authors warmly thank Chantal Esculpavit, Annie Ebrac, Géraldine Goudefroye, and Catherine Ozilou, for their assistance, and Jeanne Amiel and Patrick Calvas, for critical discussion and reflection. Support has been provided by National Institutes of Health grant R01 NS039818-09 (to M.V. and S.T.) and the Association Française contre les Myopathies (to H.E.), in addition to the Assistance Publique and the Institut National de la Santé et de la Recherche Médicale.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for syndromic microphthalmias, anophthalmia, anophthalmia and pulmonary hypoplasia, MWS, Fryns syndrome, PAGOD syndrome, and craniofrontonasal syndrome)
- PAX6 Allelic Variant Database, http://pax6.hgu.mrc.ac.uk/
- Primer3 software, http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi
- UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgTracks (for reference sequence NM_022369)
References
- 1.Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, et al (2000) Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet 25:397–401 10.1038/78071 [DOI] [PubMed] [Google Scholar]
- 2.Bar-Yosef U, Abuelaish I, Harel T, Hendler N, Ofir R, Birk OS (2004) CHX10 mutations cause non-syndromic microphthalmia/anophthalmia in Arab and Jewish kindreds. Hum Genet 115:302–309 10.1007/s00439-004-1154-2 [DOI] [PubMed] [Google Scholar]
- 3.Voronina VA, Kozhemyakina EA, O’Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH (2004) Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet 13:315–322 10.1093/hmg/ddh025 [DOI] [PubMed] [Google Scholar]
- 4.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL (1994) PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 7:463–471 10.1038/ng0894-463 [DOI] [PubMed] [Google Scholar]
- 5.Azuma N, Yamaguchi Y, Handa H, Hayakawa M, Kanai A, Yamada M (1999) Missense mutation in the alternative splice region of the PAX6 gene in eye anomalies. Am J Hum Genet 65:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent MC, Pujo AL, Olivier D, Calvas P (2003) Screening for PAX6 gene mutations is consistent with haploinsufficiency as the main mechanism leading to various ocular defects. Eur J Hum Genet 11:163–169 10.1038/sj.ejhg.5200940 [DOI] [PubMed] [Google Scholar]
- 7.Azuma N, Yamaguchi Y, Handa H, Tadokoro K, Asaka A, Kawase E, Yamada M (2003) Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. Am J Hum Genet 72:1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Wei J (2006) A newborn with anophthalmia and pulmonary hypoplasia (the Matthew-Wood syndrome). Am J Med Genet A 140:1564–1566 [DOI] [PubMed] [Google Scholar]
- 9.Berkenstadt M, Lev D, Achiron R, Rosner M, Barkai G (1999) Pulmonary agenesis, microphthalmia, and diaphragmatic defect (PMD): new syndrome or association? Am J Med Genet 86:6–8 [DOI] [PubMed] [Google Scholar]
- 10.Engellenner W, Kaplan C, Van de Vegte GL (1989) Pulmonary agenesis association with nonimmune hydrops. Pediatr Pathol 9:725–730 [DOI] [PubMed] [Google Scholar]
- 11.Seller MJ, Davis TB, Fear CN, Flinter FA, Ellis I, Gibson AG (1996) Two sibs with anophthalmia and pulmonary hypoplasia (the Matthew-Wood syndrome). Am J Med Genet 62:227–229 [DOI] [PubMed] [Google Scholar]
- 12.Spear GS, Yetur P, Beyerlein RA (1987) Bilateral pulmonary agenesis and microphthalmia. Am J Med Genet Suppl 3:379–382 10.1002/ajmg.1320280543 [DOI] [PubMed] [Google Scholar]
- 13.Steiner RD, Dignan P St J, Hopkin RJ, Kozielski R, Bove KE (2002) Combination of diaphragmatic eventration and microphthalmia/anophthalmia is probably nonrandom. Am J Med Genet 108:45–50 10.1002/ajmg.10167 [DOI] [PubMed] [Google Scholar]
- 14.Martinovic-Bouriel J, Bernabe-Dupont C, Golzio C, Grattagliano-Bessieres B, Malan V, Bonniere M, Esculpavit C, Fallet-Bianco C, Mirlesse V, Le Bidois J, et al (2007) Matthew-Wood syndrome: report of two new cases supporting autosomal recessive inheritance and exclusion of FGF10 and FGFR2. Am J Med Genet A 143:219–228 [DOI] [PubMed] [Google Scholar]
- 15.Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS (1998) Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 12:3156–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, et al (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21:138–141 10.1038/5096 [DOI] [PubMed] [Google Scholar]
- 17.De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C (2000) An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127:483–492 [DOI] [PubMed] [Google Scholar]
- 18.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, FitzPatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, et al (2007) Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80:550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- 20.Saugier-Veber P, Goldenberg A, Drouin-Garraud V, de La Rochebrochard C, Layet V, Drouot N, Le Meur N, Gilbert-Du-Ssardier B, Joly-Helas G, Moirot H, et al (2006) Simple detection of genomic microdeletions and microduplications using QMPSF in patients with idiopathic mental retardation. Eur J Hum Genet 14:1009–1017 10.1038/sj.ejhg.5201661 [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315:820–825 10.1126/science.1136244 [DOI] [PubMed] [Google Scholar]
- 22.Macayran JF, Doroshow RW, Phillips J, Sinow RM, Furst BA, Smith LM, Lin HJ (2002) PAGOD syndrome: eighth case and comparison to animal models of congenital vitamin A deficiency. Am J Med Genet 108:229–234 10.1002/ajmg.10262 [DOI] [PubMed] [Google Scholar]
- 23.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P (1997) Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 63:173–186 10.1016/S0925-4773(97)00039-7 [DOI] [PubMed] [Google Scholar]
- 24.Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dolle P, Chambon P (1995) Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol 170:420–433 10.1006/dbio.1995.1226 [DOI] [PubMed] [Google Scholar]
- 25.Davy A, Aubin J, Soriano P (2004) Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18:572–583 10.1101/gad.1171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouillet P, Chazaud C, Oulad-Abdelghani M, Dolle P, Chambon P (1995) Sequence and expression pattern of the Stra7 (Gbx-2) homeobox-containing gene induced by retinoic acid in P19 embryonal carcinoma cells. Dev Dyn 204:372–382 [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo-Sanchez M, Millet S, Bloch-Gallego E, Alvarado-Mallart RM (2005) Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary. Brain Res Brain Res Rev 49:134–149 10.1016/j.brainresrev.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 28.Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, Studer M (2007) Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol 303:362–375 10.1016/j.ydbio.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 29.Ragge NK, Brown AG, Poloschek CM, Lorenz B, Henderson RA, Clarke MP, Russell-Eggitt I, Fielder A, Gerrelli D, Martinez-Barbera JP, et al (2005) Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet 76:1008–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaughran J, Rees M, Battin M (2002) Craniofrontonasal syndrome and diaphragmatic hernia. Am J Med Genet 110:391–392 10.1002/ajmg.10176 [DOI] [PubMed] [Google Scholar]
- 31.Vasudevan PC, Twigg SR, Mulliken JB, Cook JA, Quarrell OW, Wilkie AO (2006) Expanding the phenotype of craniofrontonasal syndrome: two unrelated boys with EFNB1 mutations and congenital diaphragmatic hernia. Eur J Hum Genet 14:884–887 10.1038/sj.ejhg.5201633 [DOI] [PubMed] [Google Scholar]
- 32.Means AL, Thompson JR, Gudas LJ (2000) Transcriptional regulation of the cellular retinoic acid binding protein I gene in F9 teratocarcinoma cells. Cell Growth Differ 11:71–82 [PubMed] [Google Scholar]
- 33.Clugston RD, Klattig J, Englert C, Clagett-Dame M, Martinovic J, Benachi A, Greer JJ (2006) Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am J Pathol 169:1541–1549 10.2353/ajpath.2006.060445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holder AM, Klaassens M, Tibboel D, de Klein A, Lee B, Scott DA. Genetic factors in congenital diaphragmatic hernia. Am J Hum Genet (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berggren K, McCaffery P, Drager U, Forehand CJ (1999) Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol 210:288–304 10.1006/dbio.1999.9286 [DOI] [PubMed] [Google Scholar]
- 36.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P (2003) The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130:2525–2534 10.1242/dev.00463 [DOI] [PubMed] [Google Scholar]
- 37.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P (2001) Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128:1019–1031 [DOI] [PubMed] [Google Scholar]
- 38.Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G (2005) Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol 284:399–411 [DOI] [PubMed] [Google Scholar]
- 39.Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV (2004) Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol 273:402–415 10.1016/j.ydbio.2004.04.039 [DOI] [PubMed] [Google Scholar]
- 40.Vermot J, Messaddeq N, Niederreither K, Dierich A, Dolle P (2006) Rescue of morphogenetic defects and of retinoic acid signaling in retinaldehyde dehydrogenase 2 (Raldh2) mouse mutants by chimerism with wild-type cells. Differentiation 74:661–668 10.1111/j.1432-0436.2006.00094.x [DOI] [PubMed] [Google Scholar]
- 41.Fougerousse F, Bullen P, Herasse M, Lindsay S, Richard I, Wilson D, Suel L, Durand M, Robson S, Abitbol M, et al (2000) Human-mouse differences in the embryonic expression patterns of developmental control genes and disease genes. Hum Mol Genet 9:165–173 10.1093/hmg/9.2.165 [DOI] [PubMed] [Google Scholar]
- 42.Byrd NA, Meyers EN (2005) Loss of Gbx2 results in neural crest cell patterning and pharyngeal arch artery defects in the mouse embryo. Dev Biol 284:233–245 10.1016/j.ydbio.2005.05.023 [DOI] [PubMed] [Google Scholar]
- 43.Kastner P, Mark M, Chambon P (1995) Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83:859–869 10.1016/0092-8674(95)90202-3 [DOI] [PubMed] [Google Scholar]