Abstract
Liver repopulation could constitute a potential therapeutic alternative to liver transplantation in the future. Therefore, the development of liver repopulation strategies is of major interest. We have previously reported that Bcl-2-expressing hepatocytes are resistant to Fas-mediated apoptosis and that these hepatocytes, when transplanted into the spleen, are able to repopulate the liver of normal mice submitted to Fas-mediated apoptosis. We now show that Bcl-xL-overexpressing hepatocytes are able to repopulate up to 10% of a normal mouse liver treated with successive injections of anti-Fas antibody. We show that a twofold overexpression of Bcl-xL is sufficient to confer a selective advantage to hepatocytes submitted to anti-Fas antibody. Moreover, repopulation percentages obtained here were comparable to those obtained when Bcl-2 hepatocytes were transplanted, suggesting that both proteins are equivalent in conferring a selective advantage to hepatocytes submitted to anti-Fas antibody.
The liver is a very attractive organ for gene therapy as it is the site of many metabolic processes and also because hepatocytes can be targeted to secrete proteins into the circulation. However, until now, the use of in vivo hepatic gene transfer has been limited by the low levels of hepatocyte transduction. 1-3 An alternative approach to in vivo gene transfer consists of transplanting genetically modified hepatocytes, but this technique is hampered by the need for large numbers of transplanted hepatocytes to achieve a therapeutic effect. One way to circumvent these difficulties is to selectively amplify transduced or transplanted cells, in other words, to repopulate the liver from a small proportion of genetically modified hepatocytes.
We have previously developed a mouse model in which induction of hepatocyte apoptosis is used to create an environment in the liver for selective amplification of cells resistant to this aggression. When Bcl-2-expressing hepatocytes that are resistant to Fas-mediated apoptosis 4 are transplanted into a normal mouse, they progressively repopulate the liver after successive injections of an anti-Fas antibody, Jo2. 5,6 Furthermore, using a bicistronic retroviral vector encoding Bcl-2 and a reporter gene, we have recently demonstrated that we were able to selectively expand 1.5% of initially transduced hepatocytes to 85% of the liver after 10 weekly injections of anti-Fas antibody. 7
Transgenic mice overexpressing Bcl-xL in the liver (two- to fivefold the normal level) have also been shown to be protected against lethal injections of Jo2, even if to a lesser extent than Bcl-2 transgenic mice. 8 We therefore wondered if Bcl-xL-overexpressing hepatocytes could, as Bcl-2 transgenic hepatocytes, repopulate a normal mouse liver submitted to repeated sub-lethal Fas challenges, as an alternative strategy of liver repopulation.
Materials and Methods
Animal Procedures
A liver biopsy (the caudate lobe) was taken from four homozygous L-PK-hBcl-xL mice of B6D2 background 8 and analyzed by Western blot for the expression of human Bcl-xL. The animals with the highest and the lowest hBcl-xL expression were determined and their hepatocytes were isolated according to a standard protocol. 9 Viable hepatocytes were separated from other cells through an iso-density percoll centrifugation. 10 One million hepatocytes (>95% viability) were then injected into the spleen of 7-week-old CBA mice. Mice in the experimental groups received sub-lethal doses of Jo2, a hamster monoclonal anti-Fas antibody (Pharmingen, San Diego, CA): 0.1 mg/kg was administered intravenously once a week, beginning 48 hours after hepatocyte transplantation. At this dose, about 40% of normal hepatocytes die of apoptosis. 11 The control groups were constituted of mice not injected with Jo2. All mice were immunosuppressed with 2.5 mg/kg of FK506 injected daily by intramuscular route (kindly provided by Fujisawa GmbH, Munich, Germany).
Semiquantitative and Real-Time Polymerase Chain Reaction Analysis
Liver genomic DNA was extracted according to standard protocols. 12 Polymerase chain reaction (PCR) primers for the murine Sry gene were: 5′-GAGTACAGGTGTGCAGCT-3′ and 5′- GTGGTGAGAGGCACAAGT-3′. The conditions for amplification were as follows: 94° for 1 minute, 57° for 1 minute, and 72° for 1 minute for 30 cycles. PCR products were hybridized with an internal probe (5′-CTGTGTAGGATCTTCAATC-3′) labeled with [γ-32]P-adenosine triphosphate (ATP). Quantitation of Sry amplification was done in a PhosphorImager (Molecular Dynamic, Sunnyvale, CA). A fragment of the hBcl-xL transgene was amplified by real-time PCR in a Light Cycler (Roche, Mannheim, Germany). A SYBR Green kit (Roche, Mannheim, Germany) was used to amplify liver DNA in the Light Cycler using the following primers: 5′-CCAGGAGAAATCAAACAGAG-3′ and 5′-ACGGTGGTGGAGGAGCTCTT-3′, under the following conditions: 95° for 15 seconds, 55° for 5 seconds, and 72° for 10 seconds.
Histology
Liver samples fixed in 10% phosphate-buffered formalin were embedded in paraffin. Sections measuring 3 μm each were stained with hematoxylin and eosin for standard microscopy.
Fluorescent in Situ Hybridization
Fresh frozen liver sections (12 μm thick) were fixed with 4% paraformaldehyde. Fluorescent in situ hybridization (FISH) was performed as described previously. 13 Briefly, the Y chromosome was detected using a 1.5 kb RNA probe, pY3531B, that was generated against a repeat sequence of the mouse Y chromosome and labeled with dioxigenin-uridine 5′-triphosphate. After several washes, the dioxigenin was developed using an antibody against dioxigenin conjugated to peroxidase. The antibody to dioxigenin was visualized with tyramide-fluorescein isothiocyanate as substrate. Representative sections were double-stained with a mouse monoclonal antibody to cytokeratins 8, 18, and 19 (Affiniti Research Products Ltd., Exeter, UK) developed with a Texas red anti-mouse secondary antibody. Cell nuclei were counterstained with 4′,6-diamindino-2-phenylindole (DAPI).
Statistical Analysis
Repopulation values were analyzed statistically using Student-t and Mann-Whitney tests. Values are expressed as mean ± SEM.
Results
Transgenic animals overexpressing a human Bcl-xL transgene under the control of a liver-specific promoter have been previously described. 8 Bcl-xL expression in these transgenic mice has been shown to be heterogenous among animals. To verify whether this difference of expression could interfere with the resistance to apoptosis, and consequently with the selective advantage of Bcl-xL hepatocytes, a liver biopsy was taken from four male Bcl-xL transgenic mice and a Western blot against Bcl-xL was performed (data not shown). The animals with the highest and lowest human Bcl-xL expression levels (five- or twofold the endogenous level, respectively) were chosen as donors of hepatocytes (animals Bcl-xL[5x] and Bcl-xL[2x], respectively). In group I, 12 normal CBA female mice received 1 million male Bcl-xL[5x] hepatocytes by injection into the spleen, while in group II, 12 other CBA female mice received male Bcl-xL[2x] hepatocytes. Nine animals from each group were submitted to eight weekly injections of anti-Fas antibody Jo2 at sub-lethal doses and to daily immunosuppressive treatment with FK506. The other three transplanted mice in each group served as controls and received only the daily immunosuppressive treatment.
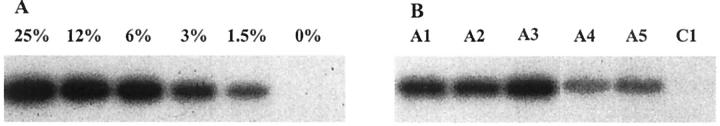
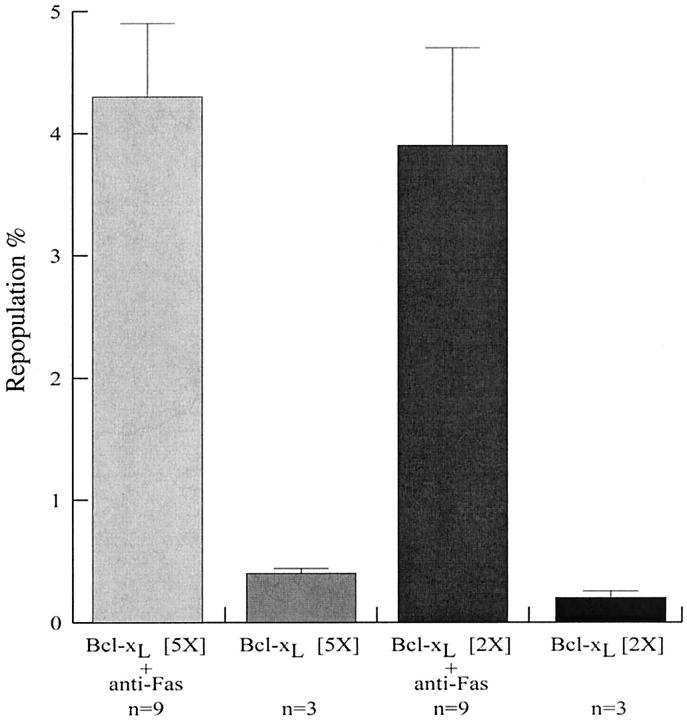
One week after the last Jo2 injection, all animals were killed and their livers analyzed. To estimate the percentage of repopulation, liver DNA was analyzed by two different PCR approaches: a semiquantitative PCR for the Sry gene and a real-time PCR for the Bcl-xL transgene. We compared the signals obtained from our animals with those from serial dilutions of liver DNA of a male Bcl-xL transgenic mouse. Actually, this method underestimates the repopulation percentage, as only ∼ 60% of total liver DNA originates from hepatocytes. 14 Therefore, to obtain the real percentage of transgenic hepatocytes present in the livers, the values corresponding to the signals obtained by PCR were multiplied by 1.6. Figure 1 ▶ illustrates results obtained for the Sry gene. In group I, repopulation percentages of the animals that received Jo2 ranged from 2 to 6% of the hepatocytes (mean of 4.3 ± 0.6%), while the control animals not submitted to Jo2 share a mean repopulation of 0.4% (±0.05%). In group II, the repopulation varied from 1.5 to 10% in the animals that received Jo2 (mean of 3.9 ± 0.8%), while the mice not submitted to it showed a mean percentage of 0.2% (±0.06%). Equivalent results were obtained using real-time PCR for the Bcl-xL transgene (Figure 2) ▶ . There was no significant difference between the animals treated with Jo2 in groups I and II, suggesting that the hepatocytes with a lower expression of Bcl-xL were as resistant to the sub-lethal doses of Jo2 used as the hepatocytes with a higher level of Bcl-xL.
Figure 1.
Semiquantitative PCR analysis of Sry DNA in transplanted mouse livers. A: Serial dilutions of male Bcl-xL transgenic mouse liver DNA in female non-transgenic DNA. B: Liver DNA of five representative animals which received the weekly Jo2 injections (A1 and A2: group I; A3 to A5: group II) and of one animal that did not receive Jo2 (C1: group 1).
Figure 2.
Real-time PCR quantification of liver repopulation. Results obtained from amplification of the Bcl-xL transgene using a Light Cycler are represented as graphs. Error bars indicate SEM. The number of mice used in each group is shown below the graph.
To verify that the liver morphology remained intact at the end of the treatment, we performed a routine histological staining. Repopulated livers appeared morphologically and histologically normal (Figure 3A) ▶ . Moreover, to visualize male Bcl-xL hepatocytes, a fluorescent in situ hybridization against the Y chromosome was performed. Y-chromosome-positive Bcl-xL hepatocytes could be identified forming repopulation clusters in the liver parenchyma of female recipients (Figure 3, B–F) ▶ .
Figure 3.
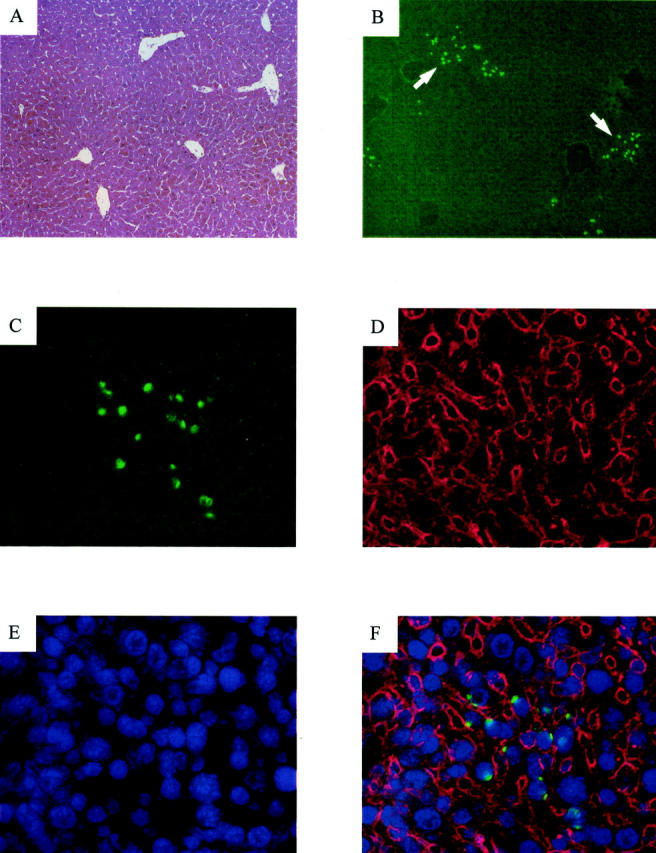
Histology and fluorescent in situ hybridization of a Bcl-xL-repopulated liver. A: Liver section examined by routine hematoxylin and eosin staining. Original magnification, ×100. B: Y chromosome staining in a liver section of the same animal. Arrows indicate repopulating-hepatocyte clusters. Original magnification, ×100. C–F: Identical fields showing a detailed view of a hepatocyte cluster. Original magnification, ×400. C: Staining for the Y chromosome FISH (green). D: Immunostaining with an antibody to cytokeratins 8, 18, and 19 (red). E: DAPI nuclei staining (blue). F: Overlays of the Y chromosome, cytokeratins and DAPI fluorescence.
Discussion
We have previously shown that Bcl-2-expressing hepatocytes were able to repopulate a normal mouse liver 5 and that these repopulating hepatocytes could restore a physiological function of the liver. 6 However, our strategy seems hardly transposable to a clinical setting since Bcl-2 has been associated with various oncogenic processes; although never in the liver 8,15 and since it does not seem to play a role in hepatocyte physiology. 16 Indeed, the expression of Bcl-2 in the liver seems restricted to bile duct cells. 15
In the present paper we showed that Bcl-xL, another anti-apoptotic member of the Bcl-2 family, is also able to confer a selective advantage to hepatocytes submitted to anti-Fas antibody. Bcl-xL is normally expressed in hepatocytes and its expression stimulated during liver regeneration after partial hepatectomy. 16 This upregulation of Bcl-xL 4 hours after partial hepatectomy could be involved in the greater resistance of hepatocytes to Fas-mediated apoptosis detected after partial hepatectomy. 17 The fact that hepatocytes overexpressing Bcl-xL are resistant to sub-lethal doses of anti-Fas antibody corroborates this hypothesis.
We show here that hepatocytes expressing high or low levels of a human Bcl-xL transgene were able to be selectively expanded and repopulate a normal mouse liver up to 10%. Moreover, the repopulation percentages were not significantly different between the two experimental groups. We are aware that our strategy is not directly applicable in a clinical setting, mainly because of the involvement of Fas pathway in fulminant hepatitis in humans. 18 Nevertheless, our present results indicate that a slight overexpression (about twofold) of a physiologically expressed protein is sufficient to confer a selective advantage to hepatocytes. When compared with the results obtained with the allogenic transplantation of Bcl-2 transgenic hepatocytes, 5 our present results are not statistically different, suggesting that both proteins are equivalent in conferring a selective advantage to hepatocytes submitted to sub-lethal doses of anti-Fas antibody. The divergence between this result and the one showing a slightly differential protective effect of Bcl-2 and Bcl-xL in Fas-mediated apoptosis induced by lethal injections of Jo2 8 may be due to a difference in the strength of the death stimulus, since here sub-lethal doses of the anti-Fas antibody were used to allow the repopulation to occur. In our prior studies on liver repopulation by allogenic Bcl-2 hepatocytes, we have discussed the reasons why repopulation extent was limited to about 10%. This is most likely due to the persistent immune rejection of allogenic hepatocytes despite the use of an immunosuppressive regimen. Indeed, endogenous hepatocytes transduced with a Bcl-2 retrovirus can almost totally repopulate the liver. 7
In conclusion, our results show that Bcl-xL-overexpressing hepatocytes are able to repopulate a normal mouse liver to a similar extent to Bcl-2 hepatocytes. This approach could represent an alternative model of liver repopulation, in which a low overexpression of a physiological protein confers a selective advantage to hepatocytes.
Acknowledgments
We thank Éva Mezey for her help with FISH. We also thank M. Lambert for technical assistance and Fujisawa GmbH, Germany, for kindly providing us with FK506. C. Mitchell is recipient of a fellowship from National Council for Scientific and Technological Development (CNPq)-Brazil.
Footnotes
Address reprint requests to Hélène Gilgenkrantz, U129 INSERM, 24, rue du Faubourg Saint Jacques, 75014 Paris, France. E-mail: gilgenkrantz@cochin.inserm.fr.
Supported by Association Française contre la Myopathie.
References
- 1.Miao CH, Snyder RO, Schowalter DB, Patijn GA, Donahue B, Winther B, Kay MA: The kinetics of rAAV integration in the liver. Nat Genet 1998, 19:13-15 [DOI] [PubMed] [Google Scholar]
- 2.Yant SR, Meuse L, Chiu W, Ivics Z, Izsvak Z, Kay MA: Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet 2000, 25:35-41 [DOI] [PubMed] [Google Scholar]
- 3.Park F, Ohashi K, Chiu W, Naldini L, Kay MA: Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet 2000, 24:49-52 [DOI] [PubMed] [Google Scholar]
- 4.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A: Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 1996, 2:80-86 [DOI] [PubMed] [Google Scholar]
- 5.Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A, Soubrane O, Gilgenkrantz H, Kahn A: Selective repopulation of normal mouse liver by Fas/CD95-resistant hepatocytes. Nat Med 1998, 4:1185-1188 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell C, Mignon A, Guidotti JE, Besnard S, Fabre M, Duverger N, Parlier D, Tedgui A, Kahn A, Gilgenkrantz H: Therapeutic liver repopulation in a mouse model of hypercholesterolemia. Hum Mol Genet 2000, 9:1597-1602 [DOI] [PubMed] [Google Scholar]
- 7.Guidotti JE, Mallet VO, Mitchell C, Fabre M, Schoevaert D, Opolon P, Parlier D, Lambert M, Kahn A, Gilgenkrantz H: Selection of in vivo retrovirally transduced hepatocytes leads to efficient and predictable mouse liver repopulation. FASEB J 2001, 15:1849-1851 [DOI] [PubMed] [Google Scholar]
- 8.de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C, Kahn A, Mignon A: Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol 1999, 277:G702-G708 [DOI] [PubMed] [Google Scholar]
- 9.Berry MN, Friend DS: High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 1969, 43:506-520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC: Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol 1986, 22:201-211 [DOI] [PubMed] [Google Scholar]
- 11.Guidotti JE, Mallet VO, Parlier D, Mitchell C, Fabre M, Jaffray P, Lambert M, Kahn A, Gilgenkrantz H: Fas/CD95 pathway induces mouse liver regeneration and allows for highly efficient retrovirus-mediated gene transfer. Hepatology 2001, 33:10-15 [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989. New York, Cold Spring Harbor Laboratory Press, Cold Spring Harbor
- 13.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR: Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290:1779-1782 [DOI] [PubMed] [Google Scholar]
- 14.Daoust R, Cantero A: The numerical proportion of cell types in rat liver during carcinogenesis by 4-dimethylaminoazobenzene (DAB). Cancer Res 1959, 19:757-762 [PubMed] [Google Scholar]
- 15.Vail ME, Pierce RH, Fausto N: Bcl-2 delays and alters hepatic carcinogenesis induced by transforming growth factor alpha. Cancer Res 2001, 61:594-601 [PubMed] [Google Scholar]
- 16.Tzung SP, Fausto N, Hockenbery DM: Expression of Bcl-2 family during liver regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol 1997, 150:1985-1995 [PMC free article] [PubMed] [Google Scholar]
- 17.Takehara T, Hayashi N, Mita E, Kanto T, Tatsumi T, Sasaki Y, Kasahara A, Hori M: Delayed Fas-mediated hepatocyte apoptosis during liver regeneration in mice: hepatoprotective role of TNF alpha. Hepatology 1998, 27:1643-1651 [DOI] [PubMed] [Google Scholar]
- 18.Kanzler S, Galle PR: Apoptosis and the liver. Semin Cancer Biol 2000, 10:173-184 [DOI] [PubMed] [Google Scholar]