Abstract
The exact role of the mucosal immune response in the pathogenesis of human papillomavirus (HPV)-related premalignant and malignant diseases of the genital tract is poorly understood. We used immunohistochemical analysis to characterize immune cells in normal cervix (N = 21), HIV-negative high-grade dysplasia (N = 21), and HIV-positive high-grade dysplasia (N = 30). Classical germinal centers were present in 4.7% of normal cervix, 33% of high-grade lesions from HIV-negative women, and 3.3% of high-grade lesions from HIV-positive women (P = 0.003). HPV16 E7 antigen was detected in a subset of germinal centers, indicating that the secondary immune response was directed in part against HPV. Lymphoid follicles were present in 9.5% of normal cervix, 57% of HIV-negative high-grade dysplasia, and 50% of HIV-positive high-grade dysplasia (P = 0.001 normal versus high-grade). A novel type of lymphoid aggregate, consisting predominantly of CD8+ T cells, was detected in 4.8% of normal cervix, 0% of HIV-negative high-grade dysplasia, and 40% of HIV-positive high-grade dysplasia (P < 0.001). The recurrence rate of high-grade dysplasia within one year was significantly higher in women with such CD8+ T cell-dominant aggregates (P = 0.02). In summary, the types of lymphoid follicle in lesions from HIV-positive women were significantly different from those from HIV-negative women, and these differences are associated with the worse clinical outcome in HIV-positive women.
Human papillomavirus (HPV) infections of the cervix can result in a series of changes in the epithelium known as cervical dysplasia. The early changes (low-grade squamous intraepithelial lesion [SIL]) reflect active viral replication and are clinically innocuous. The more advanced forms of dysplasia (moderate and severe dysplasia and carcinoma in situ; high-grade SIL) represent potential cancer precursors; ≥ 12% of severe dysplasia will progress to cancer if left untreated. 1 HPV from high-risk viral types can be detected in >90% of high-grade SIL and cervical cancers, strongly implicating the virus as an etiological agent in cervical carcinogenesis. 2-4 However, a large percentage of HPV infections are clinically undetectable and do not result in dysplasia or cancer. Multiple epidemiological studies have reported that HPV infection is detected more frequently, and that the incidence and severity of genital neoplasia are higher among immunocompromised women, including those with HIV infection. 5-10 These data imply that the host immune response to HPV is critical in determining the outcome of HPV infection, and in 1992, invasive cervical cancer was included as an AIDS-defining illness in HIV-positive women by the Centers for Disease Control and Prevention. 11
The exact relationship between altered immune function and the development of HPV-related cervical cancers has not been elucidated. The purpose of this study was to test the hypothesis that high-grade SIL of women with HIV infection is characterized by attenuation of the numbers and function of infiltrating mucosal lymphocytes and inflammatory cells compared to lesions from uninfected women. Since low-grade SIL is unlikely to progress to cancer and has a high likelihood of spontaneous regression, we focused this study on high-grade SIL, a true cancer precursor. We performed immunohistochemical analyses to characterize the types and organization of inflammatory cells in normal cervix and samples of high-grade SIL from HIV-positive and HIV-negative women. We find that lymphoid follicles are a common feature of high-grade SIL. In addition, classical germinal centers are found in a subset of lesions from HIV-negative women but are uncommon in HIV-positive women. Finally, a novel type of aggregate is abundant in lesions from HIV-positive women and is associated with higher rate of recurrence within one year after treatment.
Materials and Methods
Specimens and data for the HIV-positive participants of this study were obtained in collaboration with the Women’s Interagency HIV Study (WIHS), a longitudinal multisite cohort study of women with or at risk for HIV infection. Study sites included consortia based at the Bronx Lebanon Hospital, State University of New York, Brooklyn, Georgetown University, Cook County Hospital, University of California, San Francisco, and the University of Southern California. Detailed methods for this study have been published previously. 12 All study procedures and consent materials were reviewed and approved by human subjects protection committees at each collaborating institution and by the WIHS executive committee. HIV infections were verified by enzyme immunoassay serology with Western blot confirmation. CD4 cell counts of peripheral blood were performed by flow cytometry in laboratories participating in the fluorescence-activated cell sorter (FACS) quality assurance program. Plasma HIV levels were determined using the Organon Teknica NASBA test in laboratories participating in the National Institutes of Health virology laboratory quality assurance program. Cervical infections with Chlamydia trachomatis (C. trachomatis) and Neisseria gonorrheae (N. gonorrheae) were identified by PACE-2 DNA probe tests (GenProbe, San Diego CA). Bacterial vaginosis was identified by gram stain as previously described. 13 Candidal and trichomonas vaginitis were identified by wet mount with saline and 10% KOH. Cervicovaginal lavage with 10 ml saline was performed at each visit, and the resulting material aliquoted and frozen within 6 hours of collection. HPV testing was performed using polymerase chain reaction (PCR) with L1 consensus primers as described previously. 14 Pap smears were obtained from each woman, and colposcopy was performed for those women with abnormal results. Loop and cone biopsies were performed for standard indications and tissues were archived as paraffin blocks after pathological evaluation at the originating institution.
Specimens for the normal cervix group were obtained as paraffin-embedded blocks from the Department of Pathology archives at UCSF from women who underwent hysterectomies for benign uterine disease with no cervical abnormalities. Specimens and data for the HIV-negative high-grade SIL group were obtained as paraffin-embedded sections of cone and loop excisions from the UCSF Department of Pathology Archives and WIHS. All samples obtained from the WIHS cohort were selected from women who had HPV 16 detected on cervicovaginal lavage specimens.
Immunohistochemistry (IHC) was performed with primary antibodies against T cells with polyclonal antibody to CD3 (clone CD3), CD4+ T cells with monoclonal antibody (mAb) to CD45R0 (clone OPD4), CD8+ T cells with mAb to CD8 (clone C8/144B), B cells with mAb to CD20 (clone L26), tissue macrophages with mAb to CD68 (clone KP1), and follicular dendritic cells with mAb to CD35 (clone Ber-MAC-DRC) purchased from DAKO (Carpenteria, CA). Antibody against perforin (clone Delta G9) was purchased from Endogen (Woburn, MA), against Ki67 (clone MIB 1) from Immunotech (Miami, FL), against BCA-1 (clone 53610.11) from R & D Systems (Minneapolis, MN), against HPV 16/18 E6 (C1P5) from Abcam (Cambridge, UK), and against HPV 16 E7 (TVG710Y) from Santa Cruz Biotechnology (Santa Cruz, CA). Each primary antibody was diluted in phosphate buffered saline (PBS) with 1% bovine serum albumin (BSA) as follows: 1:1000 (CD3), 1:500 (CD45R0), 1:200 (CD8), 1:1200 (CD20), 1:500 (CD68), 1:20 (CD35), 1:400 (perforin), 1:300 (HPV 16 E7), 1:60 (HPV16/18 E6), 1:200 (Ki67), and 1:20 (BCA-1). Isotype specific fluorescenated goat anti-mouse secondary antibodies were obtained from Molecular Probes (Eugene, OR) and used at 1:100 dilutions. We confirmed the specificity of isotype specific secondary antibodies by incubating anti-IgG1 secondary antibody with anti-CD20 primary antibody (IgG2a) and anti-IgG2a secondary antibody with anti-CD4 primary antibody (IgG1) and observed no staining, while incubations with isotype-matched secondary antibodies resulted in positive staining. Routine IHC was performed following manufacturer’s guidelines (Innogenex, San Ramon, CA). Before the inactivation of endogenous peroxidase with 0.1% hydrogen peroxide, tissue slides were digested with 0.025% trypsin for 10 minutes at 37°C. Antigen retrieval for all primary antibodies with the exception of BCA-1 was performed by heating the slides in a 1.25kW microwave for 2 minutes in 10 mmol/L Sorensen’s citric buffer (pH 6.0) and cooling in citric buffer for 30 minutes. Antigen retrieval for BCA-1 was performed by heating the slides three times in a 1.25kW microwave for 2 minutes in 100 mmol/L Tris buffer with 5% urea (pH 9.0). All antibodies with the exceptions of BCA-1 and Ki67 were incubated on tissue sections for one hour at room temperature. Antibodies against BCA-1 and Ki67 were incubated overnight at 4°C. A 1:3 dilution of hematoxylin gill 1 was used for counter staining. 3,3′-diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA) and 3-amino-9-ethylcarbozole (AEC) (BioGenex, San Ramon, CA) were used as chromogens.
For double-staining immunofluorescence, tissue digestion and antigen retrieval were performed as described for IHC. Alexa Fluor 488-conjugated anti-mouse IgG1 (for anti-CD35 antibody) and Alexa Fluor 546-conjugated anti-mouse IgG2a (for anti-E7 antibody) were combined and incubated with tissue slides in the dark for 3 hours at room temperature. Slides were then incubated with 4′,6-diamidino-2-phenylindole dihydrochloride: hydrate (DAPI) (Sigma, St. Louis, MO) at 1 μg/ml dilution for 5 minutes in the dark at room temperature, rinsed, and mounted with Prolong Antifade (Molecular Probes). Photographic images were captured with a digital CCD camera and superimposed using Openlab 3.0.2 (Improvision, Lexington, MA).
The E7 open reading frame was subcloned from the HPV16 E7 viral genome (from Dr. J. Palefsky, University of California San Francisco) into the pGEX plasmid (Amrad Corp.), to produce a glutathione S-transferase (GST)-E7 fusion protein. Bacterial cultures in log phase growth were induced with isopropyl β-d-1-thiogalactopyranoside (IPTG) 0.1 mmol/L, for 3 hours at 37°C. Frozen bacterial pellets were resuspended in 5 volumes of 0.025 mol/L Tris at pH 8, 0.025 mol/L NaCl, 10% sucrose containing protease inhibitors (1 mmol/L pefablock, 0.1 mg/ml aprotinin, 0.1 mg/ml leupeptin, and 5 μg/ml pepstatin), and sonicated on ice. Potassium chloride (KCl) (0.25 mol/L) and dithiothreitol (DTT) (10 mmol/L) were added and the lysates were centrifuged at 17,400 ×g for 60 minutes. Supernatants were mixed with 1 ml of a 50% slurry of glutathione-agarose beads (Sigma) and incubated for 2 hours at 4°C. The beads were washed four times with ice-cold PBS before elution with 50 mmol/L Tris (pH 8.1) containing 0.25 KCl and 5 mmol/L reduced glutathione (Sigma) for 1 hour at 4°C. The supernatant was dialyzed against 50 mmol/L Hepes (pH 7.6) and 50 mmol/L KCl at 4°C overnight. The size and purity of GST and GST-E7 proteins were verified by polyacrylamide gel elecrophoresis. The anti-E7 antibody was incubated with a 10-fold molar excess of GST-E7 or GST at 4°C for 2 hours before adding the antibody to the slides.
DNA fragmentation as a result of apoptosis was measured by end-labeling DNA with digoxigenin-labeled dUTP and terminal deoxynucleotidyl transferase, and detection with peroxidase-conjugated anti-digoxigenin secondary antibodies (Apoptag Kit, Oncor, Gaithersburg, MD). Size determinations of lymphocyte aggregates were determined using an AcCell apparatus on an Olympus microscope (Ampersand Medical Corp, Chicago, IL).
The presence of C. trachomatis infection was determined in non-WIHS samples using DNA isolated from paraffin sections in the ligase chain reaction (LCR) (Abbott, Chicago, IL), in the laboratory of Dr. Julius Schacter, San Francisco General Hospital. Briefly, each slide was treated with 0.1% trypsin at room temperature for 5 minutes. DNA was extracted by adding 4 μl of LCx urine resuspension buffer (Abbott) to each slide, which was then placed in a heating block stabilized at 97°C for 15 minutes to release the DNA into the buffer. DNA was then added to LCR reaction mixture, and the presence of C. trachomatis was determined following manufacturer’s instructions (Abbott).
The five-μm sections stained for each tissue sample in this study were selected randomly, determined by the position of the tissue at the edge of the paraffin blocks at the time of sectioning. If any section from an individual patient had a specific phenotype of aggregate present, the patient was scored as being positive for that type of aggregate. To determine whether such sampling of the paraffin blocks was representative of the entire sample, we sectioned completely through the paraffin blocks from four different surgical cases of high-grade SIL. Immunohistochemical analysis of every 15th section was then performed with antibodies against CD8 and CD20.
Univariate comparisons were carried out using a χ 2 or Fisher’s exact test for categorical data (eg, lymphoid aggregates) and analysis of variance methods for continuous variables (eg, age). For analysis, patients with any lymphoid follicles or germinal centers were combined into one category and the patients with CD8+ T cell-dominant aggregates alone were placed in another. For some of the analyses, any sample with both lymphoid follicles or germinal centers and CD8+ T cell-dominant aggregates were placed in a third group. Subsets of samples stained for E7, Ki67, BCA1, or perforin were selected based on availability of additional slides or tissue on the block as well as the presence of lymphocyte aggregates on nearby sections.
Results
The clinical characteristics of the women who contributed tissue samples to this study are summarized in Table 1 ▶ . The mean age of the normal group (50.1 years) was significantly older than the two groups who had high-grade SIL (32.4 for the HIV-negative and 32.6 for the HIV-positive cohort) (P < 0.0001). This difference in age is likely attributable to the fact that the women in the group with normal cervix were undergoing hysterectomies, and therefore were more likely to be peri- or postmenopausal. Data on smoking was not available for many cases among the normal and HIV-negative high-grade SIL group (31% missing). The reported rate of current smoking among the HIV-positive women was 16 of 28 (57%), 5 of 12 in the HIV-negative group (42%) and 5 of 10 in the normal group (50%).
Table 1.
Participants’ Demographic Information
| Clinical status | Age | Dominant aggregate types | Smoking status | CD4 count** |
|---|---|---|---|---|
| Normal cervix; N = 21; mean age, 50.1 | ||||
| 1 | 44 | No | Current | |
| 2 | 44 | No | ||
| 3 | 49 | No | No | |
| 4 | 42 | No | Current | |
| 5 | 42 | No | No | |
| 6 | 49 | GC | ||
| 7 | 43 | No | ||
| 8 | 47 | No | No | |
| 9 | 42 | No | ||
| 10 | 48 | No | Current | |
| 11 | 41 | No | No | |
| 12 | 43 | No | Current | |
| 13 | 40 | No | No | |
| 14 | 81 | No | Current | |
| 15 | 49 | No | ||
| 16 | 60 | No | ||
| 17 | 62 | No | ||
| 18 | 52 | CD8 | ||
| 19 | 85 | LF-like | ||
| 20 | 51 | No | ||
| 21 | 39 | No | ||
| High grade SIL HIV-negative; N = 21; mean age, 32.4 | ||||
| 22 | 29 | No | No | |
| 23 | 39 | Both* | Current | |
| 24 | 23 | GC | No | |
| 25 | 30 | No | ||
| 26 | 84 | No | No | |
| 27 | 47 | No | ||
| 28 | 22 | GC | Current | |
| 29 | 35 | LF-like | Current | |
| 30 | 38 | No | ||
| 31 | 30 | No | No | |
| 32 | 23 | No | No | |
| 33 | 34 | No | No | |
| 34 | 26 | LF-like | ||
| 35 | 24 | GC | ||
| 36 | 24 | LF-like | Current | |
| 37 | 23 | GC | ||
| 38 | 20 | Both | ||
| 39 | 39 | No | ||
| 40 | 27 | GC | No | |
| 41 | 38 | GC | Current | |
| 42 | 26 | GC | ||
| High grade SIL HIV-positive N = 30; mean age, 32.6 | ||||
| 43 | 40 | CD8 | Current | 366 |
| 44 | 29 | LF-like | Current | 807 |
| 45 | 30 | LF-like | No | 544 |
| 46 | 40 | Both | Current | 452 |
| 47 | 43 | CD8 | Current | 687 |
| 48 | 46 | LF-like | No | 80 |
| 49 | 34 | Both | No | 883 |
| 50 | 37 | CD8 | Current | 12 |
| 51 | 27 | No | Current | 1 |
| 52 | 24 | CD8 | No | 68 |
| 53 | 46 | CD8 | Current | 108 |
| 54 | 33 | LF-like | No | 106 |
| 55 | 38 | Both | Current | 350 |
| 56 | 24 | CD8 | Current | 520 |
| 57 | 32 | CD8 | Current | 429 |
| 58 | 30 | LF-like | No | 30 |
| 59 | 28 | No | Current | 118 |
| 60 | 39 | Both | No | 1109 |
| 61 | 21 | CD8 | No | 429 |
| 62 | 28 | Both | No | 199 |
| 63 | 32 | CD8 | Current | 223 |
| 64 | 22 | LF-like | Current | 704 |
| 65 | 24 | CD8 | No | 23 |
| 66 | 27 | LF-like | Current | 1277 |
| 67 | 25 | CD8 | Current | 536 |
| 68 | 33 | CD8 | No | 280 |
| 69 | 39 | LF-like | No | 169 |
| 70 | 35 | GC | Current | 151 |
| 71 | 35 | Both | Missing | |
| 72 | 36 | No | 200 |
* Both refers to the presence of LF-like and CD8+ T cell dominant aggregates in the same sample.
** CD4 count at the time of or visit prior to loop excision or core biopys.
Organized lymphoid structures were visible in the stroma of many of the high-grade SIL samples as determined by immunostaining for CD4, CD20, CD8, and CD68 (Figure 1 a–d ▶ respectively). These dense cellular accumulations had a distinct morphology: the centers of the aggregates were characterized by a predominance of B cells with widely distributed CD4+ helper T cells and macrophages. CD8+ T cells were scattered at the periphery of the aggregates. These accumulations resemble germinal centers (GCs) that are present in secondary lymphoid organs such as tonsils and lymph nodes. Apoptotic bodies were present in the macrophages in cervical GCs as detected by morphology (tingible body macrophages, Figure 1e ▶ ) and by DNA fragmentation analysis (Figure 1f) ▶ . The mean diameter of GCs was 286.2 μm (range, 78 to 591 μm, N = 16). As shown in Table 2 ▶ , we observed well-formed GCs in 1 of 21 samples from normal cervix (4.7%), 7 of 21 samples of high-grade SIL from HIV-negative women (33.3%) and 1 of 30 samples of high-grade SIL from HIV-positive women (3.3%); differences between the 3 groups are statistically significant (P = 0.003). The difference in the frequency of GCs between high-grade SIL from HIV-negative women versus high-grade SIL from HIV-positive women is also statistically significant (P = 0.006).
Figure 1.
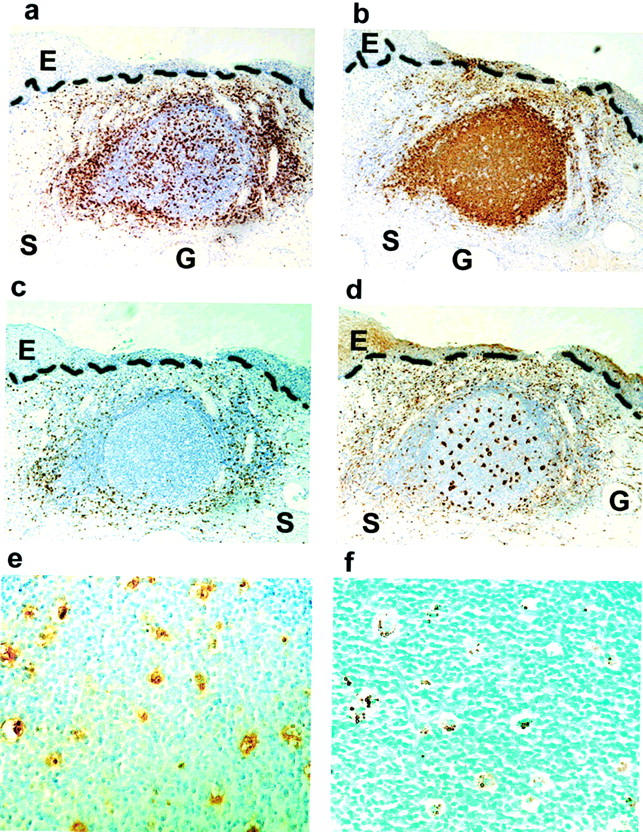
Lymphoid follicles with germinal centers are present in cervix from women with high-grade SIL. Immunohistochemistry on serial sections from a paraffin-embedded sample of high-grade SIL (HIV-negative) was performed with antibody against CD4 (a), CD20 (b), CD8 (c), CD68 (d); positively stained cells appear brown. The stromal-epithelial junction is marked with a dashed line. Serial but not adjacent sections were stained with antibodies against CD68 (e) and for DNA fragmentation (f). Photos taken with 10X (a–d) and 40X (e and f) objective. E, epithelium; S, stroma; G, endocervical gland.
Table 2.
Distribution of Lymphoid Aggregates by Clinical Status
| Status | GC | LF-like | CD8 | Both (LF-like and CD8) | None | Total |
|---|---|---|---|---|---|---|
| Normal | 1 | 1 | 1 | 0 | 18 | 21 |
| High-grade SIL; HIV-negative | 7 | 3 | 0 | 2 | 9 | 21 |
| High-grade SIL; HIV-positive | 1 | 8 | 12 | 6 | 3 | 30 |
Germinal centers have been described in the cervix in the context of chronic and follicular cervicitis. 15,16 In our study, the diagnosis of follicular cervicitis was reported on the pathology report of only 2 of the 8 samples with immunophenotypically defined GCs, and there was no mention of GCs on the other pathology reports. These data indicate that the presence of GCs is a common finding in samples of high-grade SIL from HIV-negative women, and their presence would not necessarily be appreciated by standard histological analysis.
Several studies have shown an association between GCs and chlamydia infection, 17-19 and chlamydia infection in turn is associated with the presence of SIL 18,20-22 and cervical cancer. 23 To determine whether the GCs observed in the high-grade samples were associated with the dysplastic process itself, or a reflection of a concurrent sexually transmitted disease or vaginitis, we reviewed the WIHS database of patients contributing samples to the study. Previously published work has demonstrated that chlamydial infections were uncommon (<1%) among the HIV+ women in the WIHS cohort. 13 All WIHS patients are screened for chlamydia, gonorrhea, and other vaginal infections (candida, trichomonas, and bacterial vaginosis) at enrollment to the study and at follow-up or treatment visits thereafter as necessary. None of the WIHS patients was reported to have concurrent chlamydia, gonorrhea, trichomonas, or bacterial vaginosis at the time of biopsy sampling. Two patients had concurrent yeast vaginal infection. All non-WIHS GC-positive samples with tissue remaining in the paraffin block were tested for C. trachomatis by ligase chain reaction (5 HIV-negative and 1 HIV-positive). All tested samples were C. trachomatis negative.
In many samples, lymphoid follicle-like (LF-like) structures were observed that were not as well defined structurally as the GCs described above (Figure 2) ▶ . Aggregates were scored as LF-like if they contained a dense core of CD20+ cells interspersed with CD68+, CD4+, and CD8+ cells, and lacked the distinct structure and tingible-body macrophages of GCs. Lymphoid follicles, with or without GCs, were seen in 2 of 21 samples from normal cervix (9.5%), 12 of 21 (57%) samples of high-grade SIL from HIV-negative patients, and 15 of 30 (50%) samples of high-grade SIL from HIV-positive patients (Table 2) ▶ . An increased frequency of GCs and LF-like structures was observed among high-grade SIL patients (P = 0.001). The mean diameter of LF-like structures was 243.5 μm (range, 96 to 484 μm, N = 17). There was no significant difference in the size of aggregates from HIV-negative and HIV-positive patients.
Figure 2.
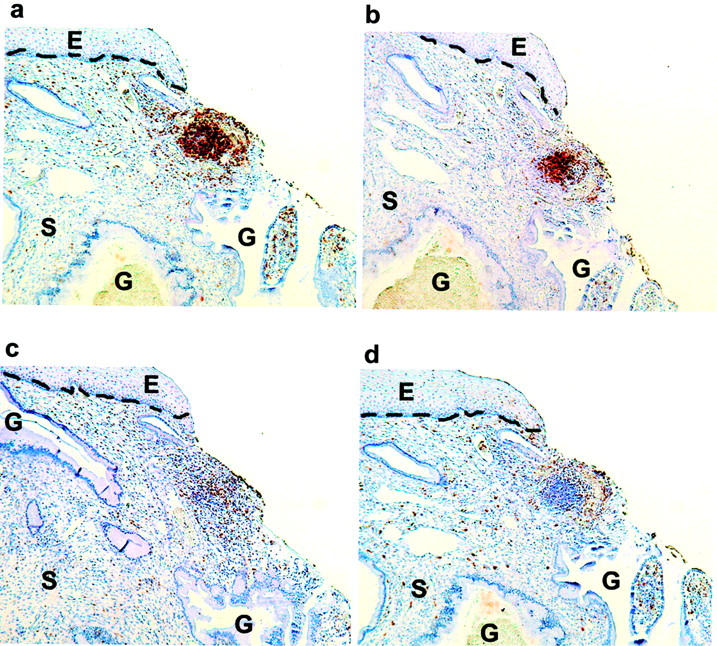
Lymphoid follicles without germinal centers are present in cervix from women with high-grade SIL. Immunohistochemistry on serial sections from a paraffin-embedded sample of high-grade SIL (HIV-negative) was performed with antibodies against CD4 (a), CD20 (b), CD8 (c), and CD68 (d); positively stained cells appear brown. The stromal-epithelial junction is marked with a dashed line. Photos taken with 10X objective. E, epithelium; S, stroma; G, endocervical gland.
A functional property of GCs is proliferation of B cells that bind to a specific antigen displayed in the follicle, allowing for clonal expansion of selective B cells. To characterize the functional status of cervical lymphoid aggregates, 10 samples known to have GC or LF-like structures from both the HIV-negative and HIV-positive patients (5 from each cohort) were selected for proliferation analysis as determined by Ki67 staining. Abundant proliferation was detected within GCs and LF-like structures independent of HIV status (Figure 3 ▶ a and b), indicating functional capacity of cells within cervical follicles to undergo clonal expansion.
Figure 3.
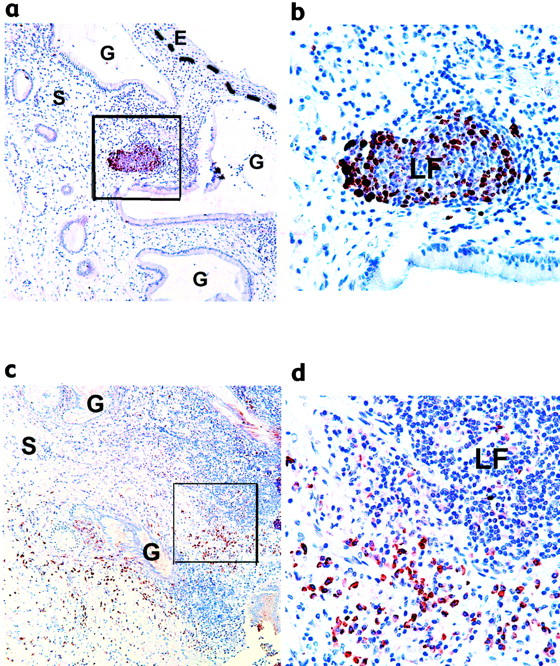
Functional characterization of cervical GCs. Immunohistochemistry on paraffin-embedded sections of high-grade SIL with GC and LF-like structures was performed with antibody against Ki67 (a and b) and BCA-1 (c and d). The stromal-epithelial junction is marked with a dashed line. Photos were taken with the 10X (a and c) or 40X objective (b and d). b and d are the areas outlined in a and c, respectively. Immunoreactivity toward mouse IgG1 (isotype control) was negligible (data not shown). E, epithelium; S, stroma; G, endocervical gland; LF, lymphoid follicle.
A complex interplay of chemokines such as B lymphocyte chemoattractant or B cell-attracting chemokine (BCA-1), secondary lymphoid tissue chemokine (SLC), lymphotoxin α1β2, and others are implicated in the recruitment, organization, and maturation of T and B cells required for the development of specific immune responses. 24,25 Recently, BCA-1 has been implicated as a critical initiating factor in the formation of lymphoid aggregates. 26 We investigated BCA-1 expression in cervical stroma of 3 samples of normal cervix and 13 samples of high-grade SIL (5 from the HIV-negative and 8 from the HIV-positive cohorts) by immunodetection. BCA-1 expression was detected in cells within and surrounding GC or LF-like structures (Figure 3, c and d) ▶ . This finding was observed in samples from both HIV-negative and HIV-positive women. Samples of normal cervix had no positively-stained cells.
To investigate the nature of the antigens displayed in the follicles found in cervix, we used IHC to determine whether HPV proteins E6 or E7, or HIV protein p24, could be detected in GCs. HIV p24 was not detected in any of the samples tested. However, HPV 16 E7 antigen was detected within the GCs and LFs of samples from both HIV-positive (2 of 4 samples tested) and HIV-negative patients (3 of 6 samples tested) (Figure 4 ▶ a, b). The specificity of the E7 antibody was tested by blocking anti-E7 antibody with synthetic GST-E7 fusion protein. Preincubation of the antibody with GST-E7 resulted in loss of immunohistochemical staining, whereas preincubation with GST alone had no effect on immunostaining (Figure 4, c–f) ▶ . Specificity for the E7 immunoreactivity was also indicated by: positive staining of dysplastic epithelium (Figure 4h) ▶ , absent or low staining of adjacent normal tissue (data not shown) and of subepithelial stroma (Figure 4, a, e, h) ▶ , and absent staining of a GC (Figure 4g) ▶ on the same section of a sample with positive staining in an adjacent GC (Figure 4a) ▶ . Furthermore, HPV protein E6 was also detected in a subset of GCs, some of which are the same GCs that showed staining for E7 (Figure 5 ▶ a, b). The staining of E6 and E7 occurred mainly in intercellular spaces and suggested that antigens were bound to interdigitating follicular dendritic cells (FDCs) in the germinal centers. Localization of E7 on the surface of FDCs was confirmed by double staining with antibodies against E7 and CD35 (Figure 5, c–f) ▶ .
Figure 4.
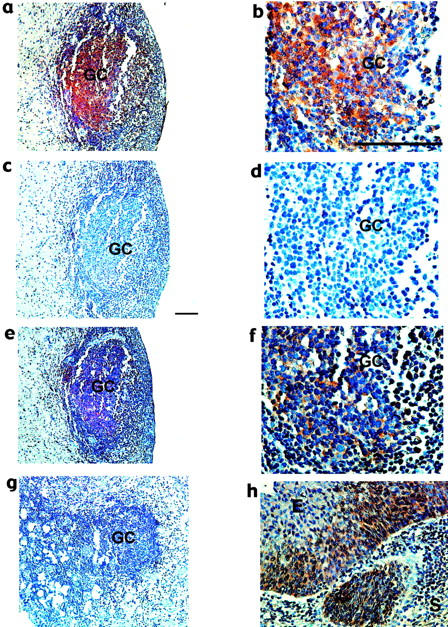
HPV16 E7 antigen in cervical GCs. Immunohistochemistry on paraffin-embedded sections of high-grade SIL with GCs was performed with antibody against HPV16 E7 antigen (a and b), antibody pre-incubated with GST-E7 (c and d), and pre-incubated with GST (e and f). g: another GC on the same slide as panel a, but negative for E7 immunostaining. h: E7 immunostaining of high-grade SIL but not of cervical stroma. The bar in b and c represent 50 μm. E, epithelium; S, stroma; GC, germinal center.
Figure 5.
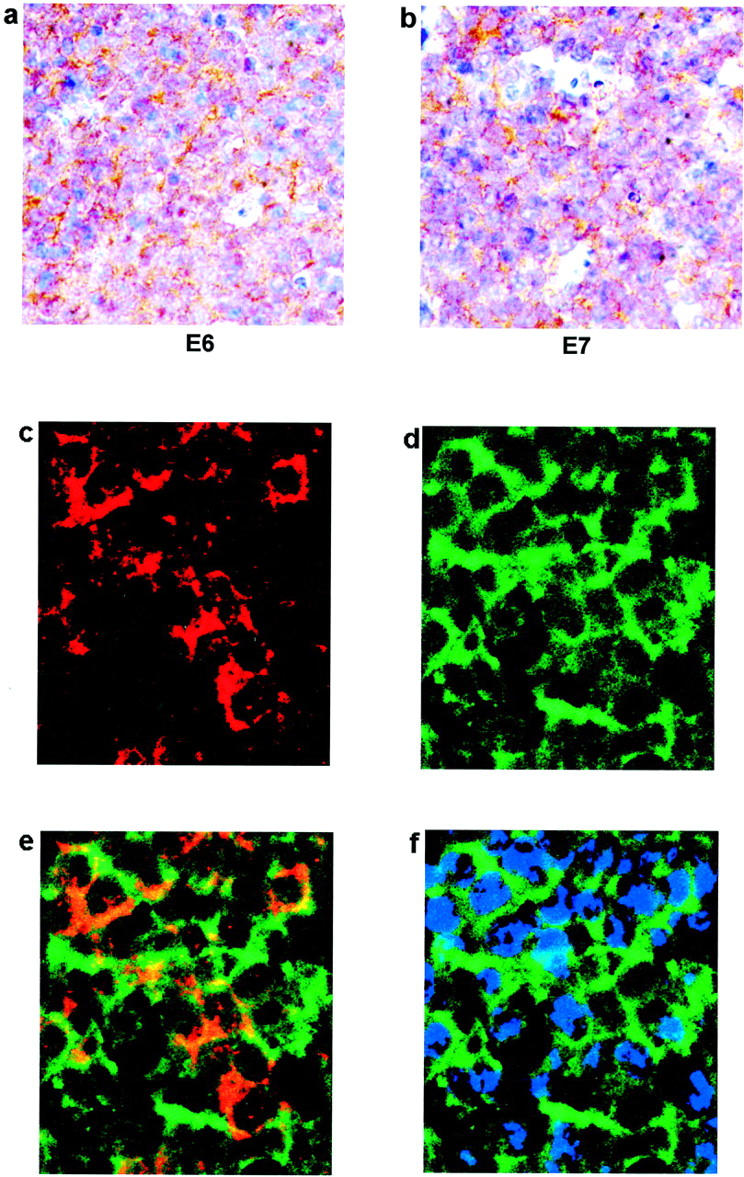
Localization of E6 and E7 antigens in the germinal center. a, b: Immunohistochemistry on sections from high-grade SIL was performed with antibodies against HPV16/18 E6 (a) and HPV16 E7 (b). Photos show areas within GCs at 40X objective magnification. c–f: Double-staining immunofluorescence on paraffin-embedded section of high-grade SIL with GCs was performed with antibodies against HPV 16 E7 (red) and CD35 (green). E7 and CD35 stained images are superimposed in panel e. Panel f shows CD35 with nuclear staining (blue). Photos were taken with the 63X objective using the DAPI, FITC, and TRITC filters.
Another type of lymphoid aggregate was commonly observed in cervical stroma of samples from HIV-positive women with high-grade SIL. These aggregates contained primarily CD8+ T cells, were interspersed with CD4+ T cells, and had few, if any, CD20+ or CD68+ cells (Figure 6) ▶ . These aggregates detected with antibody against CD8 were also positively stained with antibody against CD3 indicating that the predominant cells in the aggregates were CD3+ and CD8+ T cell (data not shown). In addition, lack of staining with antibody against Ki67 in CD8+ T cell dominant aggregates indicated that T cells within this type of aggregates were not proliferating (data not shown). The majority of CD8+ T cell-dominant aggregates were present in high-grade SIL samples from HIV-positive women who did not have co-existing LFs or GCs (Table 2) ▶ . CD8+ T cell-dominant aggregates without co-existing LFs were seen in 1 of 21 normal samples (4.8%), 0 of 21 high-grade SILs from HIV-negative patients and 12 of 30 (40%) high-grade SILs from HIV-positive patients. Thus, the occurrence of CD8+ T cell-dominant aggregates was associated with HIV infection (P ≤ 0.001). Among HIV-positive patients, there was no association between the presence of CD8+ T cell-dominant aggregates and CD4 count depletion below 400 cells/mm3. Given the high frequency (90%) of either CD8+ T cell-dominant or LF-like aggregates in HIV-positive patients, there was a highly statistically significant association between HIV infection and the presence of lymphoid aggregates in the cervix (P < 0.001).
Figure 6.
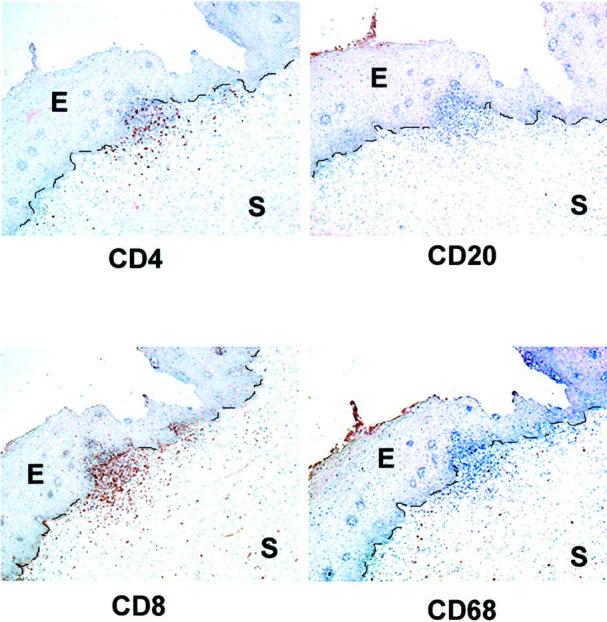
Aggregates of CD8+ T cells are present in high-grade SIL from HIV+ women. Immunohistochemistry on serial sections from high-grade SIL (HIV-positive patient) was performed with antibodies against CD4, CD20, CD8, and CD68. The stromal-epithelial junction is marked with a dashed line. Photos taken at 10X objective magnification. E, epithelium; S, stroma.
To assess a possible relationship between disease outcome and the types of aggregates, the recurrence of high-grade SIL between 3 and 12 months following treatment was compared between the groups with and without CD8+ T cell-dominant aggregates. Data on follow-up within this period were available in 34 women. Eight of 18 (44%) women who had CD8+ T cell-dominant aggregates, and 1 of 16 (6%) women lacking CD8+ T cell-dominant aggregates, developed cervical high-grade SIL in the follow-up period (P = 0.02), indicating an association between the presence of CD8+ T cell-dominant aggregates and worse disease outcome.
To assess the possibility that the small fraction of the paraffin block examined in this study produced sampling bias, we performed complete analyses of blocks from four surgical cases as described in Material and Methods. Each series contained several more examples of the same type of lymphoid aggregate scored on the initial slides. In one sample of high-grade SIL from an HIV-positive patient initially scored as having CD8+ T cell-dominant aggregates, there were 3 additional distinct CD8+ T cell-dominant aggregates and no LFs or GCs in 340 μm of tissue. In a sample of high-grade SIL from an HIV-negative patient that was scored as having both types of aggregates (LF-like and CD8+ T cell-dominant) on the original sections, there were 11 CD8+ T cell-dominant aggregates and 8 LF-like structures in 235 μm of tissue. Therefore, sections from a fraction of the tissue block appeared to accurately represent the aggregates present in the cervix.
The functional status of CD8+ T cells in the cervical tissues was assessed using a primary antibody against perforin. Perforin staining was performed on 7 random samples of normal cervix, 8 samples from HIV-negative patients with high-grade SIL, and 8 samples of HIV-positive patients with high-grade SIL (selected for samples with CD8+ T cell-dominant aggregates). Regardless of HIV status, or presence or absence of CD8+ T cell-dominant aggregates, it was unusual to see any cells that contained perforin. In the few exceptions, 1 to 2 cells per field (40X objective) did stain with perforin (data not shown). Tonsillar tissue that was processed in parallel with the cervical samples was used as a positive control for perforin staining; abundant perforin-staining cells were present in tonsils. These results indicate that the presence of perforin is rare in cervical mucosa, and presumably reflects the transient nature of perforin expression in cytotoxic T lymphocytes (CTLs) when analyzed on fixed tissue sections. Thus, we are unable to make meaningful comparisons of functional status of CTLs with respect to HIV status based on perforin staining.
Several possible confounding variables may affect the presence and types of lymphoid aggregates in these specimens. It would be unlikely for age to result in the increased number of CD8+ T cell-dominant aggregates seen in the HIV-positive group, since the mean ages of the HIV-positive and HIV-negative high-grade SIL groups were almost identical. However, the ages of women who contributed the normal samples are significantly higher than those of either of the high-grade SIL groups and only 14% of the normal group contained any aggregates. Aggregates were observed more often among younger than older women (P = 0.0001). GC or LF-like aggregates were observed in 25 of 45 women (55.6%) < 40 years of age and in 4 of 27 (14.8%) ≥ 40 (P = 0.001). CD8+ T cell-dominant aggregates were seen in 9 of 45 of women (20%) <40 and in 4 of 27 (14.8%) ≥40 years. When a similar comparison was made among women with high-grade SIL, no difference was seen, with 34 of 44 (77.3%) of samples from women <40 and 5 of 7 (71%) of samples from women ≥ 40 having aggregates.
Another possible confounding variable that may have affected the presence and types of lymphoid aggregate present is cigarette smoking. There was no statistically significant association between smoking and the type of lymphoid aggregates in the cohorts studied. However, data about smoking were available in only a subset of the patients in the normal and HIV-negative high-grade SIL cohorts (Table 1) ▶ , which constrained this analysis.
Due to reports that levels of secretory antibodies and cytokine profiles in the female lower reproductive tract are influenced by the hormonal fluctuations of the female menstrual cycle, 27-29 we compared presence and types of lymphoid follicles to phase of the menstrual cycle. Data on the last menstrual period (LMP) was available for 69% of women in the high-grade SIL cohorts (HIV-positive and HIV-negative combined). We assigned patients to follicular phase if they were 0 to 15 days since their LMP at the time that surgery was performed, and luteal phase if they were ≥ 16 days from their LMP. There was no apparent association between type of aggregate and phase of the menstrual cycle from our data (Table 3) ▶ .
Table 3.
Distribution of Lymphoid Aggregates by Phase of the Menstrual Cycle
| Phase | GC and LF-like N (%) | CD8 N (%) | None N (%) | Total number |
|---|---|---|---|---|
| Follicular | 7 (50%) | 4 (28.6%) | 3 (21.4%) | 14 |
| Luteal | 11 (52.4%) | 7 (33.3%) | 3 (14.3%) | 21 |
Discussion
This study is the first report describing classical germinal centers and lymphoid follicles as a common feature in high-grade SIL in cervical tissue. In addition, we have characterized a unique CD8+ T cell-dominant aggregate in HIV-positive women and find a statistically significant relationship between the presence of these aggregates and worse disease outcome.
Germinal centers are a feature of the secondary immune response commonly found in lymph nodes and mucosal-associated lymphoid tissue (MALT). In GCs, antigen is displayed on FDCs; those B cells that bind antigen proliferate and undergo genetic rearrangement of the immunoglobulin genes to produce cellular clones with higher affinity for antigen binding (somatic hypermutation). B cells that do not have high affinity to specific antigens undergo programmed cell death and are ingested by macrophages. The cervical GCs described here are presumably recruited by the production of BCA-1 in the stroma, indicating that similar mechanisms govern GC formation in the cervix as found in secondary lymphoid tissues. In addition, our data demonstrate that cervical GCs have functional properties of mature GCs found in other secondary lymphoid tissue, including cellular proliferation, apoptosis, and tingible body macrophages in the B-cell-rich centers. Cervical GC formation has been previously described in association with cervicitis. In one study by Crum et al, 17 GCs were found in 9 of 102 (8.8%) of cervical biopsies selected due to the presence of a chronic inflammatory infiltrate; in another study by Roberts et al, 15 lymphoid follicles were present in 2.4% of 450 consecutive hysterectomy specimens. These rates of GC detection are similar to those found in our samples of benign cervix (4.7%), and indicate that GC formation can occur in cervix under a variety of situations other than the presence of SIL. Several reports have documented a relationship between cervical GCs and C. trachomatis infection. 17-19 However, it is unlikely that the GCs and LFs described here can be attributed entirely to the presence of C. trachomatis because LCR assays performed on 6 of the 8 study samples with well-formed GCs were negative for C. trachomatis. In addition, women in the WIHS cohort were well-screened clinically, had C. trachomatis and gonorrhea cultures, wet mounts and gram stains performed at entry into the study, and had these tests repeated if obvious cervicitis or vaginitis was detected at follow-up visits. The overall rate of STDs was low in this cohort, 13 and none of the patients had known C. trachomatis or gonorrhea at the time of the procedure that generated the study sample. The high incidence of GCs in samples of high-grade SIL from HIV-negative women (33%) are likely to reflect a mucosal response to the dysplastic process itself, and specifically to HPV antigens present in the lesions. This proposition is supported by the presence of HPV 16 E7 and E6 antigens in a subset of GCs. CD35 is a receptor for a breakdown product of complement, C3b, and is abundantly expressed on the surface of FDCs. It is also known as a key immune complex-trapping molecule in the follicle. 30,31 The double staining of E7 and CD35 on FDCs and the interdigitating staining pattern of both E6 and E7 antigens in the GCs indicated that these antigens are indeed localized on the cell surface of FDCs in cervical GCs.
Our data demonstrate differences in the properties of lymphoid aggregates in high-grade SIL from HIV-positive women. Specifically, women with HIV infection and high-grade SIL have a significantly lower frequency of well-formed GCs than women without HIV infection. Given the known correlation between HIV infection and higher rates of SIL and of recurrence after treatment, 5,6,8-10 our data suggest that failure to generate GCs may explain in part this difference in outcomes in HIV-infected individuals. A second profound difference in the mucosal immune response in SIL samples from HIV-positive women is the finding of a distinct type of accumulation, consisting primary of densely clustered CD8+ T cells, almost entirely restricted to samples from HIV-positive women. There are several recent observations of increased numbers or clusters of CD8+ T cells in dysplastic or cancerous cervix, 32-34 including samples from HIV-positive women. 35,36 Our data are the first to demonstrate an association between the presence of CD8+ T cell-dominant aggregates and recurrence of cervical high-grade SIL within one year after therapy. One explanation for this apparently counterintuitive association is that the CD8+ T cells in the aggregates are not functioning effectively as CTLs, consistent with published data about CD8+ T cells from HIV-positive individuals. 37-40 Our data suggest that CD8+ T cell-dominant aggregates might be playing a permissive role in the persistence and recurrence of HPV-induced disease. Recent data from model systems of progressive carcinogenesis have raised questions about the appropriateness of increased immune responses during cancer development. In a transgenic mouse model of HPV-induced squamous carcinogenesis, production of a matrix metalloproteinase (MMP9) is necessary for development of SIL and cancer. 41 The same finding has been reported in a transgenic animal model of pancreatic cancer of the β-cell islets. 42 Interestingly, MMP9 was not produced by the neoplastic cells in either model, but was instead contributed by infiltrating cells adjacent to the neoplastic lesion in one case 42 and from bone-marrow derived mast cells and neutrophils in another. 41 The pivotal role of MMP9 in carcinogenesis was attributed in part to its role in releasing active growth factors otherwise sequestered in the extracellular matrix. 42 Therefore, by analogy, proteases and other factors secreted by cells in cervical CD8+ T cell-dominant aggregates may contribute to the maintenance, persistence, or progression of HPV infection in women. If so, these findings would have significant implications for therapeutic and protective vaccine trials currently underway. Further investigation of the functional properties of lymphocytes and inflammatory cells in high-grade SIL will contribute to our understanding of both the protective and the potentially permissive effects of the immune response in HPV-induced cervical neoplasia.
Acknowledgments
We would like to acknowledge J.M. McCune for scientific input; J. Schacter and J. Morcada for performing C. trachomatis LCR assays; M. Weinberg, M. Takeda and N. Sharkey for expert technical assistance; L. Lamarcq for graphical assistance; and C. Miaskowski and L. Coussens for critical review of the manuscript. In addition, the authors acknowledge Ampersand Medical Corp for providing the AcCell Cytopathology System.
Footnotes
Address reprint requests to Karen Smith-McCune, Cancer Research Institute and Department of Obstetrics and Gynecology, 2340 Sutter Street, Room S331, UCSF, San Francisco, CA 94143-0128 (Zip for courier 94115). E-mail: kmccune@cc.ucsf.edu.
Supported by The National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute, the National Institute of Child Health & Human Development, the National Institute on Drug Abuse, the National Institute of Dental Research, the Agency for Health Care, Policy and Research, and the Centers for Disease Control and Prevention, U01-AI-35004, U01-AI-31834, U01-AI-34994, AI-34989, U01-HD-32632 (NICHD), U01-AI-34993, U01-AI-42590 (for WIHS). Additional funding from the UCSF Cancer Center Support Grant CA82103 (to V.W.), P30 MH59037 from CFAR UCSF Gladstone Institute of Virology and Immunology (to B.H.), and the General Clinical Research Centers at UCSF and the San Francisco General Hospital through the National Center for Research Resources grants M01-RR-00079 and M01-RR-0083 (for the GCRCs) and NIAID grant UO1-AI-34989 (for WIHS).
References
- 1.Ostor AG: Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993, 12:186-192 [PubMed] [Google Scholar]
- 2.Lorincz AT, Reid R, Jensen AB, Greenberg MD, Lancaster W, Kurman RJ: Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol 1992, 79:328-337 [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Meijer CJ: Do HPV-negative cervical carcinomas exist? J Pathol 1997, 181:253-254 [DOI] [PubMed] [Google Scholar]
- 4.Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, Scott DR, Sherman ME, Kurman RJ, Wacholder S, Stanton CK, Manos MM: Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst 1993, 85:958-964 [DOI] [PubMed] [Google Scholar]
- 5.Ahdieh L, Munoz A, Viahov D, Trimble CL, Timpson LA, Shah K: Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol 2000, 151:148-157 [DOI] [PubMed] [Google Scholar]
- 6.Maiman M: Management of cervical neoplasia in human immunodeficiency virus-infected women. J Natl Cancer Inst Monogr 1998, 23:43-49 [DOI] [PubMed] [Google Scholar]
- 7.Penn I: Cancers of the anogenital region in renal transplant recipients: analysis of 65 cases. Cancer 1986, 58:611-616 [DOI] [PubMed] [Google Scholar]
- 8.Vernon SD, Holmes KK, Reeves WC: Human papillomavirus infection and associated disease in persons infected with human immunodeficiency virus. Clin Infect Dis 1995, 21(Suppl 1):S121-S124 [DOI] [PubMed] [Google Scholar]
- 9.Fowler MG, Melnick SL, Mathieson BJ: Women and HIV: epidemiology and global overview. Obstet Gynecol Clin North Am 1997, 24:705-729 [DOI] [PubMed] [Google Scholar]
- 10.Chopra KF, Tyring SK: The impact of the human immunodeficiency virus on the human papillomavirus epidemic. Arch Dermatol 1997, 133:629-633 [PubMed] [Google Scholar]
- 11.: Centers for Disease Control and Prevention: 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993, 269:729-730 [PubMed] [Google Scholar]
- 12.Barkan S, Melnick S, Preston-Martin S, Weber K, Kalish L, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J: The Women’s Interagency HIV Study: WIHS collaborative study group. Epidemiology 1998, 9:117-125 [PubMed] [Google Scholar]
- 13.Greenblatt RM, Bacchetti P, Barkan S, Augenbraun M, Silver S, Delapenha R, Garcia P, Mathur U, Miotti P, Burns D: Lower genital tract infections among HIV-infected and high-risk uninfected women: findings of the Women’s Interagency HIV Study (WIHS). Sex Transm Dis 1999, 26:143-151 [DOI] [PubMed] [Google Scholar]
- 14.Palefsky J, Minnkoff H, Kalish L, Levine A, Sacks H, Garcia P, Young M, Mioti P, Burk R: Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV-1)-positive and high-risk HIV-negative women. J Natl Cancer Inst 1999, 91:226-236 [DOI] [PubMed] [Google Scholar]
- 15.Roberts T, Ng A: Chronic lymphocytic cervicitis: cytologic and histopathologic manifestations. Acta Cytol 1975, 19:235-243 [PubMed] [Google Scholar]
- 16.Wright T, Ferenczy A: Benign Diseases of the Cervix. Kurman RJ eds. Blaustein’s Pathology of the Female Genital Tract. 1994, :pp 203-227 Springer-Verlag, New York [Google Scholar]
- 17.Crum CP, Mitao M, Winkler B, Reumann W, Boon ME, Richart RM: Localizing chlamydial infection in cervical biopsies with the immunoperoxidase technique. Int J Gynecol Pathol 1984, 3:191-197 [DOI] [PubMed] [Google Scholar]
- 18.Paavonen J, Vesterinen E, Meyer B, Saksela E: Colposcopic and histologic findings in cervical chlamydial infection. Obstet Gynecol 1982, 59:712-715 [PubMed] [Google Scholar]
- 19.Hare MJ, Toone E, Taylor-Robinson D, Evans RT, Furr PM, Cooper P, Oates JK: Follicular cervicitis: colposcopic appearances and association with Chlamydia trachomatis. Br J Obstet Gynaecol 1981, 88:174-180 [DOI] [PubMed] [Google Scholar]
- 20.Schachter J, Hill EC, King EB, Coleman VR, Jones P, Meyer KF: Chlamydial infection in women with cervical dysplasia. Am J Obstet Gynecol 1975, 123:753-757 [DOI] [PubMed] [Google Scholar]
- 21.Carr MC, Hanna L, Jawetz E: Chlamydiae, cervicitis, and abnormal Papanicolaou smears. Obstet Gynecol 1979, 53:27-30 [PubMed] [Google Scholar]
- 22.Paavonen J, Vesterinen E, Meyer B, Saikku P, Suni J, Purola E, Saksela E: Genital Chlamydia trachomatis infections in patients with cervical atypia. Obstet Gynecol 1979, 54:289-291 [PubMed] [Google Scholar]
- 23.Anttila T, Saikku P, Koskela P, Bloigu A, Dillner J, Ikaheimo I, Jellum E, Lehtinen M, Lenner P, Hakulinen T, Narvanen A, Pukkala E, Thoresen S, Youngman L, Paavonen J: Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 2001, 285:47-51 [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG: Chemokines and cell migration in secondary lymphoid organs. Science 1999, 286:2098-2102 [DOI] [PubMed] [Google Scholar]
- 25.Fu Y, Chaplin DD: Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999, 17:399-433 [DOI] [PubMed] [Google Scholar]
- 26.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG: BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 2000, 12:471-481 [DOI] [PubMed] [Google Scholar]
- 27.Lu FX, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller CJ: Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun 1999, 67:6321-6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin RD, Kutteh WH: Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J Reprod Immunol 1999, 42:93-106 [DOI] [PubMed] [Google Scholar]
- 29.Kutteh WH, Moldoveanu Z, Mestecky J: Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses 1998, 14(Suppl 1):S51-S55 [PubMed] [Google Scholar]
- 30.Fang Y, Xu C, Fu YX, Holers VM, Molina H: Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol 1998, 160:5273-5279 [PubMed] [Google Scholar]
- 31.Yoshida K, van den Berg TK, Dijkstra CD: Two functionally different follicular dendritic cells in secondary lymphoid follicles of mouse spleen, as revealed by CR1/2 and FcR gamma II-mediated immune-complex trapping. Immunology 1993, 80:34-39 [PMC free article] [PubMed] [Google Scholar]
- 32.Cromme F, Walboomers M, Van Oostveen J, Stukart M, De Gruijl T, Kummer J, Leonhart A, Helmerhorst T, Meijer C: Lack of granzyme expression in T lymphocytes indicates poor cytotoxic T lymphocyte activation in human papillomavirus-associated cervical carcinomas. Int J Gynecol Cancer 1995, 5:366-373 [DOI] [PubMed] [Google Scholar]
- 33.Edwards RP, Kuykendall K, Crowley-Nowick P, Partridge EE, Shingleton HM, Mestecky J: T lymphocytes infiltrating advanced grades of cervical neoplasia. CD8-positive cells are recruited to invasion. Cancer 1995, 76:1411-1415 [DOI] [PubMed] [Google Scholar]
- 34.Bethwaite PB, Holloway LJ, Thornton A, Delahunt B: Infiltration by immunocompetent cells in early stage invasive carcinoma of the uterine cervix: a prognostic study. Pathology 1996, 28:321-327 [DOI] [PubMed] [Google Scholar]
- 35.Bell MC, Schmidt-Grimminger D, Turbat-Herrera E, Tucker A, Harkins L, Prentice N, Crowley-Nowick PA: HIV+ patients have increased lymphocyte infiltrates in CIN lesions. Gynecol Oncol 2000, 76:315-319 [DOI] [PubMed] [Google Scholar]
- 36.D’Amico M, Cannone M, Vago L, Martini I, Cecchini G, Costanzi G, Barberis M: Human immunodeficiency virus localization in human papillomavirus-related, high-grade squamous intraepithelial lesions of the cervix in women with HIV infection: microdissection and molecular analysis on formalin-fixed and paraffin-embedded specimens. J Lower Genital Tract Disease 1999, 3:254-259 [DOI] [PubMed] [Google Scholar]
- 37.Andersson J, Behbahani H, Lieberman J, Connick E, Landay A, Patterson B, Sonnerborg A, Lore K, Uccini S, Fehniger TE: Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS 1999, 13:1295-1303 [DOI] [PubMed] [Google Scholar]
- 38.Brinchmann JE, Rosok BI, Spurkland A: Activation and proliferation of CD8+ T cells in lymphoid tissues of HIV-1-infected individuals in the absence of the high-affinity IL-2 receptor. J Acquir Immune Defic Syndr 1998, 19:332-338 [DOI] [PubMed] [Google Scholar]
- 39.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA: CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 1995, 95:2061-2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BN, Follen M, Tortolero-Luna G, Eriksen N, Helfgott A, Hammill H, Shearer WT, Reuben JM: Synthesis of IFN-gamma by CD8(+) T cells is preserved in HIV-infected women with HPV-related cervical squamous intraepithelial lesions. Gynecol Oncol 1999, 75:379-386 [DOI] [PubMed] [Google Scholar]
- 41.Coussens L, Tinkle C, Hanahan D, Werb Z: MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103:481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergers G, Brekken R, McMahon G, Vu T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan G: Matrix metalloproteinase-9 triggers the angiongenic switch during carcionogenesis. Nature Cell Biol 2000, 2:737-744 [DOI] [PMC free article] [PubMed] [Google Scholar]


