Abstract
Activation of platelets leads to cytoskeletal assembly that is responsible for platelet motility and internal contraction. We have evaluated the involvement of the cytoskeleton in platelet activation by two strong agonists, collagen and thrombin. Activation was assessed by measuring changes in cytoskeletal assembly, externalization of activation-dependent markers and expression of procoagulant activity, and tyrosine phosphorylation of proteins, in both the absence and the presence of cytochalasin B. Activation of platelets with collagen and thrombin induced morphological changes and increased the expression of CD62P, CD63, glycoprotein IV, and binding of annexin V to platelets. Moreover, both activating agents induced actin polymerization, increased the association of other contractile proteins, and promoted tyrosine phosphorylation of multiple proteins, some of which were associated with the cytoskeleton. The presence of cytochalasin B blocked the previous events when collagen was used as the activating agent, although binding of annexin V still occurred. In contrast, platelet response to thrombin was not completely prevented by the presence of cytochalasin B. Thus, activation by collagen requires a functional cytoskeleton to trigger signaling through tyrosine phosphorylation and secretion. This is not the case for thrombin, which is capable of activating signaling mechanisms in the presence of strong inhibitors of cytoskeletal assembly. Moreover, the expression of a procoagulant surface in platelets still occurs even when platelet motility has been inhibited.
Platelets are the initial elements in vascular repair after a blood vessel injury. Endothelial damage results in the exposure of the vascular subendothelium, which is a collagen-rich adhesive substrate to which platelets attach through specific receptors. 1 Moreover, the coagulation mechanisms that take place during hemostasis induce local thrombin synthesis 2 that facilitates recruitment of platelets on the exposed subendothelium. 3,4 Collagen and thrombin are the most potent platelet agonists. Activation of platelets is followed by a rapid sequence of biochemical events involving cytoskeletal reorganization, which is responsible for the morphological changes and internal contraction that platelets undergo promoting platelet response.
The platelet cytoskeleton can be considered as a multifunctional protein structure. In unstimulated platelets, it plays a major role by maintaining the discoid shape and a random organization of the granules within platelets. 5,6 The cytoskeleton seems to prevent membrane fragmentation 7 and to regulate the distribution of glycoproteins at the membrane. 8 Because GPIb is bound to the cytoskeleton, this interaction may enable platelets to resist the shear forces once attached to the subendothelium after primary adhesion. 9,10 After platelet activation a major function of the cytoskeleton is to contract, centralizing the granules and allowing the release of intraplatelet substances. 6 Platelet spreading and clot retraction are further events in which the cytoskeleton plays an active role. 11-13 Recent investigations suggest that the platelet cytoskeleton takes part in the signal transduction events that follow platelet stimulation. In this regard experimental evidence indicates that the platelet cytoskeleton localizes signaling molecules and recruits them to critical locations within platelets. 14 Therefore, in addition to its motile function, the cytoskeleton may play a major role in the signaling mechanisms occurring after platelet activation.
Platelets respond to a wide variety of stimuli in a characteristic manner. The nature and degree of the stimulus will determine the extent of platelet transformation and the amount of substances secreted. The contractile physiology dominates the platelet response before, during, and after activation. 15 The present work was designed to evaluate the role of the platelet cytoskeleton in the signaling mechanisms occurring through tyrosine phosphorylation of proteins after activation of platelets by two different agonists, thrombin and collagen. With this purpose, platelet activation with both agonists was performed in the absence and in the presence of cytochalasin B (Cyt-B), 6 an inhibitor of actin assembly and cytoskeletal reorganization. Different experimental strategies were applied, including flow cytometric evaluation of the expression of intraplatelet glycoproteins at the membrane level, electrophoretic analysis of cytoskeletal assembly, and investigation of changes in tyrosine phosphorylation of proteins. Electron microscopy was also used to visualize changes in the cytoskeletal assembly induced by both agonists and the effect of Cyt-B.
Materials and Methods
Experimental Design
The present study was designed to evaluate the role of the platelet cytoskeleton in the activation events induced by thrombin and collagen. Platelet suspensions were independently activated by purified type I collagen (Col-I) (20 μg/ml) and thrombin (0.1 U/ml), in the absence and in the presence of Cyt-B (50 μmol/L). 6 After 90 minutes, platelet suspensions were processed to 1) visualize morphological changes by electron microscopy, 2) analyze modifications in the expression of intraplatelet substances and procoagulant activity at the surface level by flow cytometry, and 3) obtain sodium dodecyl sulfate (SDS)-lysates and to extract the Triton X-100-insoluble cytoskeletal fraction to evaluate changes in cytoskeletal assembly and in tyrosine phosphorylation of proteins.
Chemical Reagents and Antibodies
Thrombin from human plasma, Cyt-B from Helminthosporium dematioideum, adenosine, benzamidine, ethylenediaminetetraacetic acid, ethylene glycol bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid, leupeptin, phenylmethyl sulfonyl fluoride, pepstatin, and orthovanadate were purchased from Sigma Chemical Co. (St. Louis, MO). Equine tendon type I collagen was obtained from Chrono-Par (Chrono-Log Co., Havertown, PA). Recombinant horseradish-conjugated anti-phosphotyrosine antibody (RC20) was from Transduction Laboratories (Lexington, KY). Phosphate-buffered saline (PBS) was from Boehringer Mannheim Corp. (Indianapolis, IN). Electrophoresis reagents and nitrocellulose membranes were from Bio-Rad Laboratories S.A. (Madrid, Spain). Enhanced chemiluminescence reagents and Hyperfilm-ECL were from Amersham Pharmacia Biotech Europe GmbH (Barcelona, Spain).
All of the antibodies for flow cytometry were purchased from Immunotech (Marseille, France) if not otherwise stated. Platelet membrane glycoprotein IV was detected with an anti-CD36 mAb (clone FA6-152) conjugated with fluorescein-5-isothiocyanate (FITC) and with an anti-CD36 mAb (clone CB 38) conjugated with phycoerythrin (PE) (Pharmingen, San Diego, CA). P-selectin was measured with an anti-CD62P-FITC (clone CLBThromb/6) and the lysosomal integral membrane protein of 53 kd (LIMP) with an anti-CD63-FITC (clone CLBGran/12). Anionic aminophospholipid expression in the outer leaflet of platelet membrane was studied using the binding of annexin V conjugated to PE (Pharmingen). For all of the studies the negative control was an IgG1 (clone 679.1Mc7) conjugated to FITC and PE.
Blood Sampling and Platelet Activation
Human blood samples were obtained from healthy volunteers who had not taken any drug affecting platelet function in the previous 10 days. Blood was collected and anti-coagulated with citrate-phosphate-dextrose (100 mmol/L sodium citrate, 16 mmol/L citric acid, 18 mmol/L sodium hydrogen phosphate, and 130 mmol/L dextrose) (at a final citrate concentration of 19 mmol/L). Platelet-rich plasma was obtained by centrifugation at 200 × g for 10 minutes at 22°C. Platelets were isolated from platelet-rich plasma at 800 × g for 20 minutes and washed twice with equal volumes of citrate-citric acid-dextrose (93 mmol/L sodium citrate, 7 mmol/L citric acid, and 140 mmol/L dextrose), pH 6.5, containing 5 mmol/L adenosine and 3 mmol/L theophylline. 16 The final pellet was resuspended in Hanks’ balanced salt solution (136.8 mmol/L NaCl, 5.4 mmol/L KCl, 0.2 mmol/L MgSO4 × 7H2O, 0.6 mmol/L Na2HPO4, 0.4 mmol/L KH2PO4, 0.5 mmol/L MgCl2 × 6H2O, 1.3 mmol/L CaCl2) and platelet counts adjusted to 1.2 × 10 6 platelets/μl. Suspensions were kept at 37°C for 20 minutes before activation. Aliquots of platelet suspensions were kept undisturbed or were activated with Col-I (20 μg/ml) or thrombin (0.1 U/ml) for 90 seconds in the absence and in the presence of Cyt-B (50 μmol/L). For flow cytometric evaluation, platelet suspensions were activated in the presence of an antibody to GPIIb-IIIa (8 μg/ml of Fab fragments of 7E3; ReoPro, Lilly, Geneva, Switzerland) to avoid aggregate formation.
Electron Microscopy
Platelet suspensions were combined with an equal volume of 0.1% glutaraldehyde in White’s saline 17 for 5 minutes and then centrifuged. The supernatant was removed and replaced with 3% glutaraldehyde in the same buffer. The suspensions were maintained at 4°C for 30 minutes and then centrifuged. Pellets were suspended in a solution of 1% osmium tetroxide in distilled water containing 15 mg/ml of potassium ferrocyanide (pH 7.4) for 90 minutes at 2°C. After osmium fixation the samples were dehydrated in a graded series of ethanol concentrations and then treated with propylene oxide and embedded in Epon 812. The sections were stained with uranyl acetate and lead citrate to enhance contrast. Examination was performed in a Phillips (Mahwah, NJ) 301 electron microscope.
Flow Cytometric Studies
Immunolabeling of platelets with the antibodies was performed using dual-color analysis, designed to minimize artifactual activation of the sample. 18 Samples containing 1 × 10 6 resting or stimulated platelets were added to polypropylene tubes preloaded with 50 μl of PBS, pH 7.2. Next, saturating concentrations (predetermined by titration) of anti-CD36-PE were added and incubated in the dark, without stirring, for 15 minutes at room temperature, followed by the addition of saturating concentrations of FITC-conjugated antibodies and another incubation for 15 minutes. Samples were then diluted with 1 ml of PBS and analyzed immediately.
When annexin V was measured, samples were added to polypropylene tubes filled with 50 μl of Hanks’ balanced salt, pH 7.2, containing 3 mmol/L CaCl2. Incubations were performed as described before with saturating concentrations of anti-CD36-FITC and the adding annexin V-PE. Samples were diluted with 1 ml of Hanks’ balanced salt before analysis.
Samples were analyzed with a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA) at an excitation wavelength of 488 nm. Fluorescence and scatter signals were calibrated with 2-μm Calibrite beads (Becton-Dickinson). Platelets were differentiated by their characteristic forward versus side scatter and positivity for anti-CD36; histograms were composed from fluorescence data obtained in the logarithmic mode from 5000 events analyzed in each sample. When measuring GPIV the data were expressed as mean fluorescence intensity using the CellQuest conversion software (Becton Dickinson) on a Macintosh Power computer (Apple Computer Inc.). When measuring P-selectin, LIMP, and annexin V, data were expressed as the percentage of fluorescence-positive platelets. To do so, an analytical marker was set in the corresponding fluorescence channel to define 2% of the resting platelet population with the highest membrane fluorescence at the baseline level. This marker was used as a threshold to determine the proportion of platelets exhibiting immunofluorescence above this level in all subsequent samples.
Platelet Lysates and Extraction of Platelet Cytoskeletons
To obtain platelet lysates, activation was stopped by the addition of Laemmli’s buffer (125 mmol/L Tris-HCl, 2% SDS, 5% glycerol, and 0.003% bromphenol blue), containing 2 mmol/L orthovanadate and 5 mmol/L N-ethylmaleimide, and heated for 5 minutes at 90°C. Samples were kept at −20°C until electrophoretic analysis.
Platelet cytoskeletons were obtained according to the procedure described by Jennings and colleagues 19 with minor modifications. 20 Platelet suspensions, before and after activation with Col-I and thrombin, in the absence and in the presence of Cyt-B, were treated for 30 minutes (at 4°C) with an equal volume of lysis buffer (100 mmol/L Tris-HCl, pH 7.4, 2% Triton X-100) containing 10 mmol/L ethylene glycol bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid, 4 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L benzamidine, 2 μg/ml leupeptin, 2 μg/ml pepstatin, and 2 mmol/L sodium orthovanadate, as protease and phosphatase inhibitors. Triton-insoluble residues, corresponding to the polymerized cytoskeletal fraction, were recovered at 12,000 × g for 5 minutes at 4°C in a microfuge. Residues were washed twice with washing buffer, without Triton X-100, at 4°C, solubilized with washing buffer containing 2% SDS, and heated at 100°C for 5 minutes. Samples were frozen at −20°C until electrophoretical evaluation was performed.
Analysis of Cytoskeletal Proteins
Cytoskeletal proteins were separated by 8% SDS-polyacrylamide gel electrophoresis. 21 To evaluate the contractile proteins associated with the cytoskeleton, gels were stained with Coomassie brilliant blue R250 and densitometrically quantified as previously described. 22 Stained protein bands were densitometrically analyzed using digital-video technology provided by a computerized image analyzer running specific software (SigmaGel; Jandel GmbH, Erkrath, Germany). After selection of the bands on the monitor screen, the software automatically analyzed the color density of each protein band and integrated areas beneath densitometric peaks. Values of protein peak areas in the lanes containing Triton-insoluble residues from nonactivated platelets were considered as 100%. The association of each protein with the thrombin-activated cytoskeleton was expressed as the percentage of increase over the amount of the same protein found in the respective lane corresponding to nonactivated platelets.
Evaluation of Tyrosine-Phosphorylated Proteins
Phosphotyrosine proteins in whole platelet lysates or associated with the polymerized cytoskeletal fraction were resolved by 8% SDS-polyacrylamide gels. 21 Proteins in the gels were transferred to nitrocellulose membranes. 23 After blocking nonspecific binding, Western blots were probed with a horseradish peroxidase-conjugated anti-phosphotyrosine recombinant antibody (RC20; 1:2500). The excess of antibody was removed by extensive washing and blots were developed by the enhanced chemiluminescence method. 24
Statistics
Statistical analysis was performed using Student’s t-test and a P < 0.05 was considered statistically significant.
Results
Electron Microscopy Evaluation of Platelets Activated by Collagen and Thrombin: Effect of Cyt-B
Electron microscopy showed that most of the washed control platelets retained their discoid shape with microtubules located in the periphery of platelet cross-sections and intraplatelet granules randomly dispersed in the cytoplasm and always disconnected from membranes of the open canalicular system (Figures 1A and 2A) ▶ ▶ . Platelets exposed to Cyt-B tended to appear with more spherical shapes and dilated open canalicular system.
Figure 1.
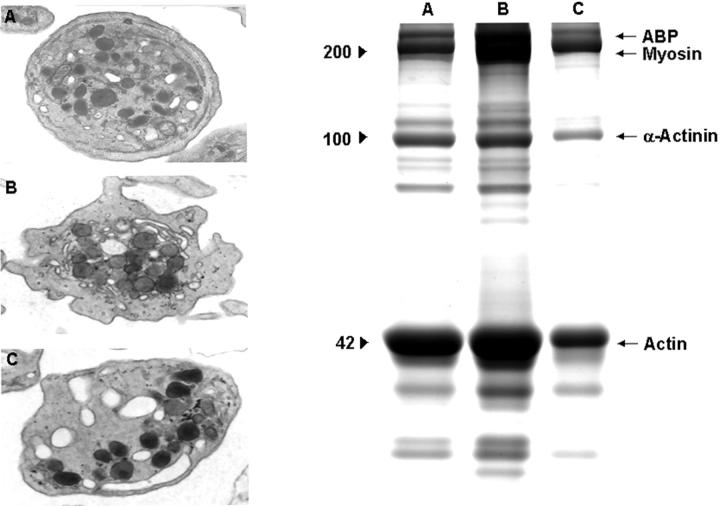
Effect of Cyt-B on platelet activation by collagen. Electron microscopy images (original magnifications, ×23,000) and electrophoretic profiles (8% SDS-PAGE) corresponding to resting platelets (A) and platelets activated with collagen (20 μg/ml) in the absence (B) and in the presence (C) of Cyt-B (50 μmol/L), respectively. Profiles are representative of eight different experiments.
Figure 2.
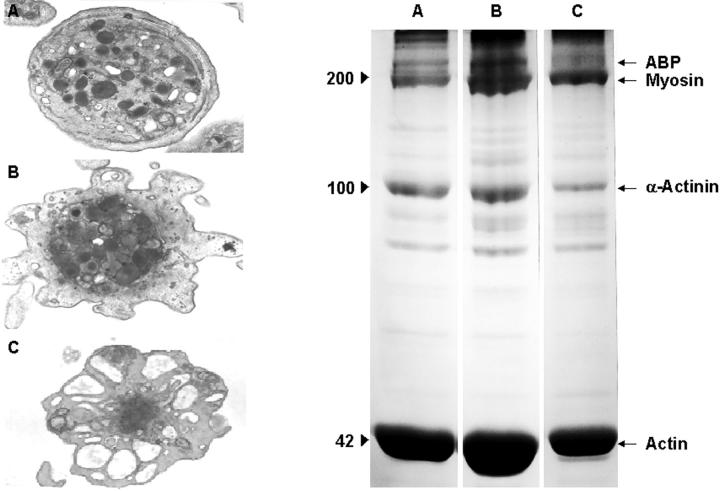
Effect of Cyt-B on platelet activation by thrombin. Electron microscopy images (original magnifications, ×23,000) and electrophoretic profiles (8% SDS-PAGE) corresponding to resting platelets (A) and platelets activated with thrombin (0.1 U/ml) in the absence (B) and in the presence (C) of Cyt-B (50 μmol/L), respectively. Profiles are representative of eight different experiments.
Activation of platelets by Col-I or thrombin was followed by pseudopod formation and loss of the discoid shape, which are indirect signs of actin polymerization and cytoskeletal assembly (Figures 1B and 2B ▶ ▶ , respectively). Alpha granules decreased in frequency and appeared concentrated in the center of platelets establishing contacts with the open canalicular system. During activation, microtubules were displaced from their peripheral location toward cell centers as if they were constricting platelet organelles into a central ring. Although both Col-I and thrombin can induce these series of morphological changes, in our studies thrombin seemed to cause more frequent and drastic changes than Col-I.
Exposure of platelets to Cyt-B prevented several of the morphological changes induced by Col-I and thrombin (Figures 1C and 2C ▶ ▶ , respectively). Activation with Col-I of platelets exposed to Cyt-B did not result in pseudopod formation and/or centralization of intraplatelet granules that appeared irregularly dispersed in the cytoplasm of presumably activated platelets. Microtubules remained in polar locations and were not seen displaced toward platelet centers. Thrombin was also unable to induce either pseudopod formation or microtubule centralization, although actomyosin was observed in the platelet centers. In contrast with Col-I, thrombin was still capable of inducing a partial degranulation of intraplatelet granules.
Effect of Cyt-B on the Expression of Proteins at the Platelet Membrane after Activation by Collagen and Thrombin
Figure 3A ▶ summarizes the data obtained when platelets were stimulated with collagen. Platelet activation was measured as degranulation (expression of P-selectin and LIMP) or by increases in the procoagulant activity (measured as increases in the binding of annexin V). Activation of platelets by Col-I resulted in increases in the percentage of positive platelets from 2.0 ± 0.1% (mean ± SD, n = 5) to 27.7 ± 9.9% (P < 0.05) and from 2.4 ± 0.4% to 15.5 ± 2.2% (P < 0.05) for P-selectin and LIMP, respectively. Preincubation of platelets with Cyt-B almost blocked collagen-induced activation because the percentage of positive platelets for P-selectin and LIMP decreases to 5.7 ± 3.2% and 2.8 ± 0.3%. However, binding of annexin V was found to be increased after Col-I activation (percent positive platelets of 17.8 ± 7.5% versus 2.3 ± 0.3%, P < 0.05) and decreased, although slightly, by the presence of Cyt-B (to 8.9 ± 4.0%, P < 0.05). The mean fluorescence intensity for GPIV increased from 111.6 ± 18.9% (mean ± SD, n = 5) to 132.8 ± 21.3% after Col-I activation and decreased to 108.7 ± 22.4% when activation was performed in platelets treated with Cyt-B.
Figure 3.
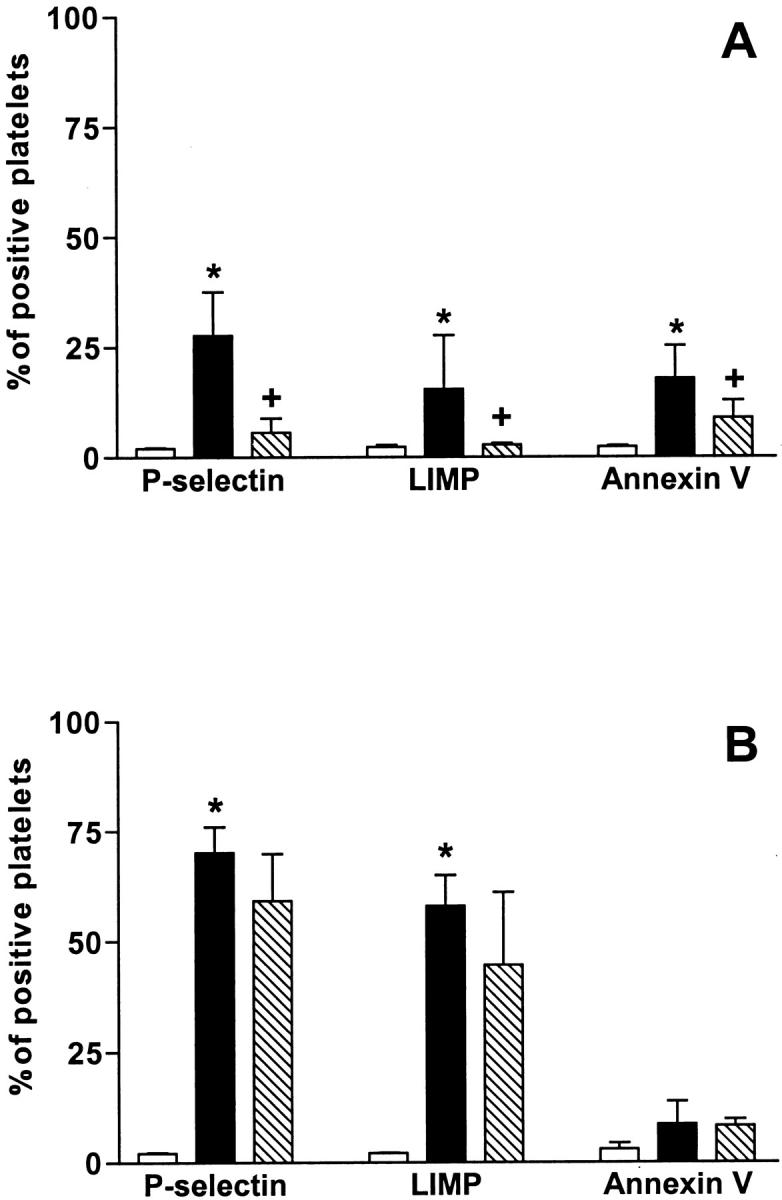
Changes in the expression of antigens after activation of platelets with collagen (A) and thrombin (B): effect of Cyt-B. Bar graphs showing the changes observed in the activation markers measured using flow cytometry. Results correspond to platelets before activation (open bars), after activation in the absence of Cyt-B (filled bars), and after activation in the presence of Cyt-B (50 μmol/L) (slashed bars). Activation was performed with collagen (20 μg/ml) (A) and with thrombin (0.1 U/ml) (B). Results are expressed as percent of positive platelets (mean ± SEM, n = 5). Asterisk indicates statistically significant differences (P < 0.05) after activation, and plus indicates statistically significant differences (P < 0.05) when Cyt-B was present.
As summarized in Figure 3B ▶ , the activation of platelets by thrombin caused a significant increase in the expression of GPIV from 124.4 ± 14.7% to 154.5 ± 18.5% (P < 0.05). Thrombin activation also produced significant increases in the expression of P-selectin and LIMP, from 2.1 ± 0.2% to 70.2 ± 5.8% (P < 0.05) and 58.0 ± 7.0% (P < 0.05), respectively. As expected, a mild increase in the binding of annexin V, which did not reach statistical significance (8.6 ± 5.2%), was seen after activation when thrombin was used. These changes were not modified when platelets were incubated with Cyt-B before activation.
Effect of Cyt-B on Cytoskeletal Assembly and Tyrosine Phosphorylation of Proteins after Activation by Collagen and Thrombin
Aliquots of activated platelet suspensions, in the absence or in the presence of Cyt-B, were treated to obtain platelet lysates or to extract platelet cytoskeletons. The effect of Cyt-B was monitored by electrophoretic analysis of cytoskeletal assembly (see electrophoretic profiles in Figures 1 and 2 ▶ ▶ ). Tyrosine phosphorylation of proteins was evaluated in both platelet lysates and the polymerized cytoskeletal fraction.
Densitometric analysis of protein profiles showed that the insoluble cytoskeletal fraction in resting platelets consisted mainly of actin-binding protein, myosin, α-actinin, and actin. Platelet activation by Col-I and thrombin induced polymerization of actin (increase in actin of 80.4 ± 5.6% and 110.24 ± 3.8%, respectively, with respect to actin present in resting platelets) (mean ± SEM, n = 8) and increased the association of actin-binding protein (in 50 ± 11.4% and 60.4 ± 5.6%, respectively), myosin (in 115 ± 3.8% and 140 ± 5%), and α-actinin (in 61.4 ± 7.9% and 60.6 ± 2.1%) with the cytoskeleton (see electrophoretic profiles in Figures 1 and 2 ▶ ▶ ). The presence of Cyt-B inhibited the effect induced by Col-I and thrombin because actin polymerization and association of the contractile proteins induced by both agonists were almost absent.
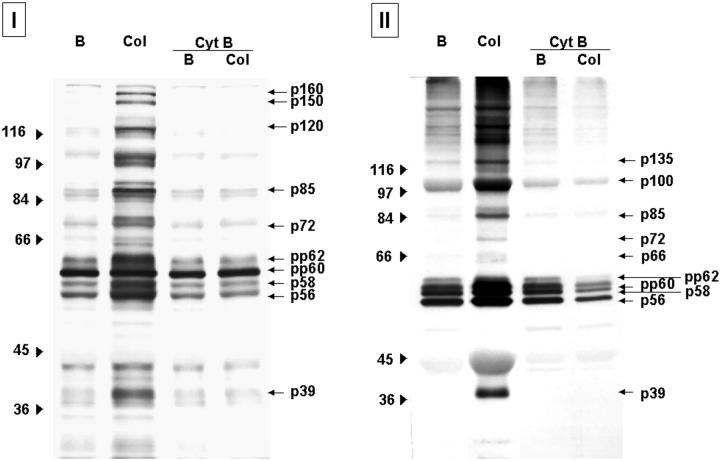
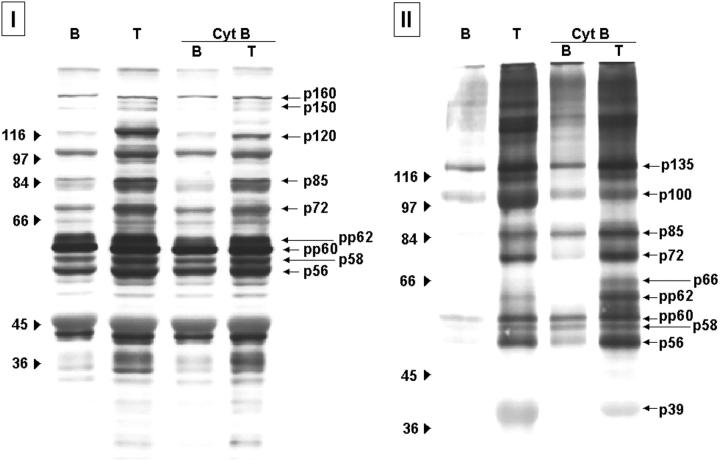
Activation of platelet suspensions by Col-I and thrombin resulted in phosphorylation at tyrosine residues of multiple proteins, including p160, p150, p125, p100, p97, p85, p72, p66, p64, pp62, pp60, p58, p56, and p39 (Figures 4I and 5I ▶ ▶ , respectively). Some of the tyrosine-phosphorylated proteins (p135, p100, p85, p72, pp62, pp60, p58, p56, and p39) appeared consistently associated with the cytoskeletal fraction after stimulation by both agonists (Figures 4 ▶ II and 5II, respectively). Presence of Cyt-B blocked tyrosine phosphorylation of proteins after stimulation with Col-I, because no detectable signal was observed when analyzing both platelet lysates and proteins associated with the polymerized cytoskeletal fraction (Figure 4 ▶ , I and II). Different results were obtained in platelets activated by thrombin in the presence of Cyt-B. The degree of tyrosine phosphorylation of some of the proteins observed in platelet lysates was decreased, although very mildly, especially for protein p120 and p110 (Figure 5I) ▶ . In contrast, no changes were detected when analyzing those phosphotyrosine proteins associated with the polymerized cytoskeletal fraction, which remained the same as in the absence of Cyt-B (Figure 5I ▶ I).
Figure 4.
Tyrosine-phosphorylated proteins present in platelet lysates (I) and associated with the polymerized cytoskeletal fraction (II) after collagen activation: effect of Cyt-B. Protein profiles corresponding to resting platelets (B), platelets activated with 20 μg/ml of collagen (Col) in the absence and in the presence of Cyt-B (50 μmol/L). After activation, platelets were lysated to analyze whole platelet lysates (I) or processed to extract the polymerized cytoskeletal fraction (II). Samples were resolved by 8% SDS-PAGE and proteins transferred to nitrocellulose membranes. Phosphotyrosine proteins were detected by a specific antibody and the enhanced chemiluminescence technique. Images are representative of eight different experiments.
Figure 5.
Tyrosine-phosphorylated proteins present in platelet lysates (I) and associated with the polymerized cytoskeletal fraction (II) after thrombin activation: effect of Cyt-B. Protein profiles corresponding to resting platelets (B), platelets activated with 0.1 U/ml of thrombin (T) in the absence and in the presence of Cyt-B (50 μmol/L). After activation, platelets were lysated to analyze whole platelet lysates (I) or processed to extract the polymerized cytoskeletal fraction (II). Samples were resolved by 8% SDS-PAGE and proteins transferred to nitrocellulose membranes. Phosphotyrosine proteins were detected by a specific antibody and the enhanced chemiluminescence technique. Images are representative of eight different experiments.
Discussion
Activation of platelets triggers a sequence of intracellular mechanisms that amplify platelet response. Platelets are essentially motile cells that respond to activating stimuli with cytoskeletal reorganization characterized by shape change and internal contraction. The extent of platelet transformation and the amount of substances secreted during platelet response depend on the nature and degree of the stimulus. In the present study we used Cyt-B, an agent that prevents formation of new actin filaments, to evaluate the relevance of cytoskeletal assembly in the signaling mechanisms occurring in platelets as a response to stimulation by collagen or thrombin. Our data indicate that activation by Col-I requires a functional cytoskeleton to trigger signaling through tyrosine phosphorylation. This is not the case for thrombin, which can activate signaling mechanisms and release intraplatelet substances in the presence of strong inhibitors of actin polymerization. These results provide further evidence that collagen and thrombin, the two most potent physiological platelet agonists, elicit platelet activation through different signaling pathways.
Ultrastructural studies have confirmed that activation of platelets with the agonists used in our study results in a sequence of events including pseudopod formation, constriction of microtubule coils, and degranulation. The morphological changes observed in response to collagen were abolished in the presence of Cyt-B, because intraplatelet granules appeared randomly dispersed in the cytoplasm and the microtubule coils remained located in the periphery. In contrast with collagen, thrombin was still able to induce association and centralization of actomyosin, and degranulation in the presence of Cyt-B, as visualized by electron microscopy, 6,25 although pseudopod formation was not observed. These observations were confirmed by electrophoresis and flow cytometric analysis because in the presence of Cyt-B the expression of activation-dependent markers occurred after thrombin but not after collagen activation. However, binding of annexin V promoted by collagen activation was only moderately inhibited by Cyt-B, suggesting that exposure of negatively charged phospholipids was not affected by inhibition of cytoskeletal assembly.
In the present study, collagen activation of platelets induced signaling through tyrosine phosphorylation of proteins, which was completely blocked when actin polymerization was inhibited by the presence of Cyt-B. Collagen exhibits at least three receptors in platelets: GPIa-IIa, 26 GPIV, 27 and GPVI. 28 Activation of platelets after binding of collagen to the corresponding receptors seems to occur in a first phase with activation of phospholipase (PL) A2 and thromboxane A2 (TXA2) production, and a second phase that seems to be TXA2-dependent. 29 Recent reports have shown that activation of both GPIa-IIa 30 and GPVI 31 also elicits PLCγ2 activation, which induces Ca++ mobilization via the inositol phospholipid-dependent pathway. Both mechanisms have been shown to trigger tyrosine phosphorylation of proteins. It has been reported that GPIa-IIa is linked to the platelet cytoskeleton 32 and that the first phase of collagen activation can be inhibited by the presence of cytochalasins, although phospholipases are not affected. 29 On the other hand, GPIV and GPVI are directly related to tyrosine kinases. Signaling through GPVI occurs independently of the cytoskeleton because the collagen-related peptide, which is a direct agonist of this glycoprotein, elicits signal transduction 33 even in the presence of cytochalasin. 30 Despite this fact, as demonstrated in the present study, once platelet cytoskeletal assembly was blocked, collagen was unable to induce signaling through tyrosine phosphorylation, suggesting that GPIa-IIa may be the collagen receptor initiating the signaling cascade in association with platelet cytoskeleton. Moreover, all these observations together suggest that cytoskeletal assembly and exocytosis are two linked processes when collagen is used as activating agent.
Thrombin activation of platelets resulted in tyrosine phosphorylation of proteins that was not blocked in the presence of Cyt-B, despite the fact that Cyt-B inhibited actin polymerization and association of other contractile proteins induced by thrombin. Interestingly, some of the phosphotyrosine proteins were associated to the remaining polymerized cytoskeletal fraction. Platelets exhibit a high-affinity thrombin receptor, the GPIb-IX-V complex, 34 and moderate affinity thrombin receptors called protease-activated thrombin receptors (PARs), with a seven transmembrane domain structure. 35,36 GPIb is in tight connection with the cytoskeleton, a fact that may be involved in its function. Previous unpublished work by our group indicates that efficient tyrosine phosphorylation of proteins after activation of platelets with thrombin depends on intact GPIb. When GPIb is blocked, higher concentrations of thrombin are required suggesting the involvement of the moderate affinity receptor. The PAR1 receptor is a seven-transmembrane G protein-coupled receptor that activates phospholipase C, generating IP3, that increases intraplatelet calcium, and diacylglycerol. Both second messengers induce activation of protein kinases. Phosphorylated proteins and increases in cytosolic calcium regulate events such as cytoskeletal changes, expression of adhesive receptors, and exocytosis. If cytoskeletal changes are inhibited by the presence of cytochalasin, release of intraplatelet substances may only be explained on the basis that the PAR1-dependent pathway remains functional and may result in activation of specific mechanisms of exocytosis that would be independent of cytoskeletal assembly. Therefore, cytoskeletal assembly and exocytosis are two dissociated events during thrombin activation.
Secretion of intraplatelet substances occurs by fusion of the granule membranes with the open canalicular system in human platelets whereas platelets from other species, such as bovine platelets, 37 show differential mechanisms. Several studies point out to the existence of molecular mechanisms of platelet exocytosis, apart from internal contraction. These mechanisms seem to be dependent on a group of proteins, including NSF (N-ethylmaleimide sensitive factor), SNAP-23, and syntaxins, that are located in the open canalicular system, α-granules, and lysosomes. 38,39 These proteins would be responsible for membrane fusion and seem to be dependent on calcium increases and independent of cytoskeletal assembly.
Collagen and thrombin are probably the more physiological platelet agonists. Collagen is exposed at sites of vascular damage. Thrombin is rapidly generated through tissue factor exposure and facilitated by anionic phospholipids exposed on platelets attaching to the damaged surface. Most of the effort in anti-platelet therapy has been focused on preventing platelet-platelet interactions. According to our data, platelets can still express negatively charged phospholipids, after collagen activation, and release the contents of their granules, after exposure to thrombin, even in the presence of a strong inhibitor of basic contractile functions such as Cyt-B. We cannot ignore that expression of a procoagulant surface on platelets ensures assembly of coagulation complexes and perpetuation of coagulation mechanisms implied in the extension of local thrombotic events. In the same manner, we should not neglect the possibility that released selectins initiate cross-talk with other cells, and may be responsible for promoting atherothrombotic mechanisms. These basic mechanisms of exocytosis and procoagulant activity should be considered when developing new anti-thrombotic strategies.
Acknowledgments
We thank Marcy Krumwiede, University of Minnesota, for technical assistance in the electron microscopy and Byron Buckley, University of North Carolina–Chapel Hill, for assistance in the preparation of the manuscript.
Footnotes
Address reprint requests to Maribel Diaz-Ricart, Servicio de Hemoterapia y Hemostasia, Hospital Clinic, Villarroel 170, 08036 Barcelona, Spain. E-mail: mdiaz@clinic.ub.es.
Supported by the Ministerio de Ciencia y Tecnología (SAF2000-0041 and HF1999-0059), the Fondo de Investigaciones de la Seguridad Social (FIS 99/0110, 99/0106, and 99/0108), and the Generalitat de Catalunya (CIRIT SGR 99–227, 2001 TDOC 00002).
References
- 1.Clemetson KJ: Platelet collagen receptors: a new target for inhibition? Haemostasis 1999, 29:16-26 [DOI] [PubMed] [Google Scholar]
- 2.Weiss HJ, Lages B: Evidence for tissue factor-dependent activation of the classic extrinsic coagulation mechanism in blood obtained from bleeding time wounds. Blood 1988, 71:629-635 [PubMed] [Google Scholar]
- 3.Wagner WR, Hubbell JA: Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J Lab Clin Med 1990, 116:636-650 [PubMed] [Google Scholar]
- 4.Diaz-Ricart M, Estebanell E, Lozano M, Aznar-Salatti J, White JG, Ordinas A, Escolar G: Thrombin facilitates primary platelet adhesion onto vascular surfaces in the absence of plasma adhesive proteins: studies under flow conditions. Haematologica 2000, 85:280-288 [PubMed] [Google Scholar]
- 5.White JG: Platelet membrane ultrastructure and its changes during platelet activation. Prog Clin Biol Res 1988, 283:1-32 [PubMed] [Google Scholar]
- 6.Escolar G, Krumwiede M, White JG: Organization of the actin cytoskeleton of resting and activated platelets in suspension. Am J Pathol 1986, 123:86-94 [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JE, Austin CD, Boyles JK, Steffen PK: Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J Cell Biol 1990, 111:483-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer SC, Sanan DA, Fox JEB: Role of actin-binding protein in insertion of adhesion receptors into the membrane. J Biol Chem 1998, 273:3013-3020 [DOI] [PubMed] [Google Scholar]
- 9.Mistry N, Cranmer SL, Yuan Y, Mangin P, Dopheide SM, Harper I, Giuliano S, Dunstan DE, Lanza F, Salem HH, Jackson SP: Cytoskeletal regulation of the platelet glycoprotein Ib/V/IX-von Willebrand factor interaction. Blood 2000, 96:3480-3489 [PubMed] [Google Scholar]
- 10.Cunningham JG, Meyer SC, Fox JE: The cytoplasmic domain of the alpha-subunit of glycoprotein (GP) Ib mediates attachment of the entire GP Ib-IX complex to the cytoskeleton and regulates von Willebrand factor-induced changes in cell morphology. J Biol Chem 1996, 271:11581-11587 [DOI] [PubMed] [Google Scholar]
- 11.Nachmias VT: Cytoskeleton of human platelets at rest and after spreading. J Cell Biol 1980, 86:795-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White JG: Ultrastructural analysis of platelet contractile apparatus. Methods Enzymol 1992, 215:109-127 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Onji T, Okamoto K, Matsusaka T, Taniguchi H, Shibata N: Reorganization of contractile elements in the platelet during clot retraction. J Ultrastruct Res 1984, 89:98-109 [DOI] [PubMed] [Google Scholar]
- 14.Fox JEB: The platelet cytoskeleton. Thromb Haemostasis 1993, 70:884-893 [PubMed] [Google Scholar]
- 15.White JG: Ultrastructural modifications in platelet membranes and cytoskeleton following activation. Blood Cells 1983, 9:237-261 [PubMed] [Google Scholar]
- 16.Rao GH, Escolar G, White JG: Epinephrine reverses the inhibitory influence of aspirin on platelet-vessel wall interactions. Thromb Res 1986, 44:65-74 [DOI] [PubMed] [Google Scholar]
- 17.White JG, Krumwiede M: Influence of cytochalasin B on the shape change induced in platelets by cold. Blood 1973, 41:823-832 [PubMed] [Google Scholar]
- 18.Lozano M, Estebanell E, Cid J, Diaz-Ricart M, Mazzara R, Ordinas A, Escolar G: Platelet concentrates prepared and stored under currently optimal conditions: minor impact on platelet adhesive and cohesive functions after storage. Transfusion 1999, 39:951-959 [DOI] [PubMed] [Google Scholar]
- 19.Jennings LK, Fox JEB, Edwards HH, Phillips DR: Changes in the cytoskeletal structure of human platelets following thrombin activation. J Biol Chem 1981, 256:6927-6932 [PubMed] [Google Scholar]
- 20.Kometani M, Sato T, Fujii T: Platelet cytoskeletal components involved in shape change and secretion. Thromb Res 1986, 41:801-809 [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 22.Escolar G, Diaz-Ricart M, Cases A, Castillo R, Ordinas A, White JG: Abnormal cytoskeletal assembly in platelets from uremic patients. Am J Pathol 1993, 143:823-831 [PMC free article] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979, 76:4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cichowski K, McCormick F, Brugge JS: p21rasGAP association with Fyn, Lyn, and Yes in thrombin-activated platelets. J Biol Chem 1992, 267:5025-5028 [PubMed] [Google Scholar]
- 25.Lefebvre P, White JG, Krumwiede MD, Cohen I: Role of actin in platelet function. Eur J Cell Biol 1993, 62:194-204 [PubMed] [Google Scholar]
- 26.Nieuwenhuis HK, Sakariassen KS, Houdijk WP, Nievelstein PF, Sixma JJ: Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood 1986, 68:692-695 [PubMed] [Google Scholar]
- 27.Tandon NN, Kralisz U, Jamieson GA: Identification of GPIV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem 1989, 264:7576-7583 [PubMed] [Google Scholar]
- 28.Moroi M, Jung SM, Okuma M, Shinmyozu K: A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest 1989, 84:1440-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano T, Hanasaki K, Arita H: Possible involvement of cytoskeleton in collagen-stimulated activation of phospholipases in human platelets. J Biol Chem 1989, 264:5400-5406 [PubMed] [Google Scholar]
- 30.Inoue K, Ozaki Y, Satoh K, Wu Y, Yatomi Y, Shin Y, Morita T: Signal transduction pathways mediated by glycoprotein Ia/IIa in human platelets: comparison with those of glycoprotein VI. Biochem Biophys Res Commun 1999, 256:114-120 [DOI] [PubMed] [Google Scholar]
- 31.Pasquet JM, Bobe R, Gross B, Gratacap MP, Tomlinson MG, Payrastre B, Watson SP: A collagen-related peptide regulates phospholipase Cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem J 1999, 342:171-177 [PMC free article] [PubMed] [Google Scholar]
- 32.Fox JEB: Identification of actin-binding protein as the protein linking the membrane cytoskeleton to glycoproteins on platelet plasma membranes. J Biol Chem 1985, 260:11970-11977 [PubMed] [Google Scholar]
- 33.Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, Barnes MJ, Watson SP: A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1. Blood 1997, 89:1235-1242 [PubMed] [Google Scholar]
- 34.Greco NJ, Jamieson GA: High and moderate affinity pathways for alpha-thrombin-induced platelet activation. Proc Soc Exp Biol Med 1991, 198:792-799 [DOI] [PubMed] [Google Scholar]
- 35.Vu TK, Hung DT, Wheaton VI, Coughlin SR: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 [DOI] [PubMed] [Google Scholar]
- 36.Coughlin SR, Scarborough RM, Vu TK, Hung DT: Thrombin receptor structure and function. Cold Spring Harb Symp Quant Biol 1992, 57:149-154 [DOI] [PubMed] [Google Scholar]
- 37.White JG: The secretory pathway of bovine platelets. Blood 1987, 69:878-885 [PubMed] [Google Scholar]
- 38.Chen D, Lemons PP, Schraw T, Whiteheart SW: Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood 2000, 96:1782-1788 [PubMed] [Google Scholar]
- 39.Lemons PP, Chen D, Whiteheart SW: Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem Biophys Res Commun 2000, 267:875-880 [DOI] [PubMed] [Google Scholar]