Abstract
We recently cloned a novel gene, NPHS2, involved in autosomal recessive steroid-resistant nephrotic syndrome. This gene encodes a novel podocyte protein, podocin. Given its similarity with the stomatin family proteins, podocin is predicted to be an integral membrane protein with a single membrane domain forming a hairpin-like structure placing both N- and C-termini in the cytosol. Here, we show by in situ hybridization, that during development, the NPHS2 transcript is first expressed in mesonephric podocytes from the S-shaped body and, later, in the metanephric kidney, in the future podocytes at the late S-shaped body stage. In the mature kidney, NPHS2 is exclusively expressed in the podocytes of mature glomeruli. We generated rabbit polyclonal antibodies against fusion proteins derived from the N- and the C-terminal regions of podocin which detected a single band of 49-kd in transfected HEK293 cell lysates by immunoprecipitation and Western blotting. By immunohistology, podocin was detected in podocytes from the early capillary loop stage in the developing nephrons, and at the basal pole, along the GBM, in mature glomeruli. By electron microscopy, we demonstrate that podocin is facing the slit diaphragm with its two ends in the cytoplasm of the foot processes, in agreement with its predicted structure. Our results suggest that podocin could serve to anchor directly or indirectly components of the slit diaphragm to the cytoskeleton.
Plasma ultrafiltration during primary urine formation in the glomerulus is a central function of the kidney. The structurally complex capillary wall responsible for this function is composed of a basement membrane covered by fenestrated endothelium on the inner surface and highly specialized epithelial cells called podocytes on the outer surface. Podocytes present with interdigitating cell extensions, called foot processes, that interconnect on top of the basement membrane. These interconnections are separated by the slit diaphragm, a podocyte-specific intercellular junction with an electron-dense zipper-like structure. 1 Further information concerning the composition of the glomerular filtration barrier, and the predominant role of the podocyte for maintaining its function, has emerged through the detection of mutations in several genes encoding podocyte proteins associated with proteinuria and nephrotic syndrome, either in human diseases 2-4 or in murine models. 5,6
Nephrin, a transmembrane protein encoded by NPHS1 which is mutated in congenital nephrotic syndrome of the Finnish type, 2 was shown to be a major component of the slit diaphragm. 7-9 However, the composition of the slit diaphragm as well as the adaptor proteins linking nephrin to the cytoskeleton, is still largely unknown. CD2-associated protein (CD2AP) has been shown to interact with nephrin and could anchor nephrin to the cytoskeleton. 5 P-cadherin, FAT, a novel transmembrane protein of the P-cadherin superfamily with a unique extra-cellular domain, and ZO-1 were also shown to be associated with the slit diaphragm, which represents a unique adherens-like cell junction. 10,11
Recently, we have cloned a novel gene, NPHS2, mutated in patients presenting with autosomal recessive steroid-resistant nephrotic syndrome. 4 This gene encodes a novel podocyte protein, podocin. Podocin presents similarities with proteins of the stomatin family and is predicted to be an integral membrane protein of 383 amino acids, with a single membrane domain forming a hairpin-like structure (amino acids 105 to 121) and with both N- and C-terminal domains in the cystosol. 4 Here, we confirm the predicted structure of podocin by showing that both ends of the protein are intracellular, and we demonstrate by immunoelectron microscopy that podocin is located at the cystoplasmic face of the slit diaphragm.
Materials and Methods
Generation of Anti-Podocin Antisera
Six-histidine-tagged N- and C-terminal fragments of podocin were produced in E. coli using the QIAExpressionist kit from Qiagen (Hilden, Germany). The 5′ (nucleotides 110 to 337) and the 3′ (nucleotides 471 to 1227) podocin cDNA sequences were PCR amplified with the proofreading Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA) using primers which add BamHI and HindIII sites to the forward and reverse primers, respectively. The 5′ and 3′ podocin cDNA PCR products were then restriction enzyme digested and subcloned into the BamHI/HindIII-digested vectors pQE-32 and pQE-31, respectively. Double stranded sequencing of the constructs was performed to assure the fidelity of the polymerase and maintenance of the appropriate reading frame. The expression vectors pQE-32/5′ and pQE31/3′ were transformed in E. coli strains M15 and SG13009, respectively, and hexahistidine-tagged fusion proteins were purified on columns using a commercially prepared nickel-charged Ni-NTA agarose resin (Qiagen, Hilden, Germany), according to the manufacturer’s recommendations. The N-terminal recombinant protein was eluted under native conditions using an imidazole gradient whereas the C-terminal recombinant protein was eluted under denaturing conditions in phosphate buffer containing 8 mol/L urea at pH 4.5. Each recombinant protein was used in its elution buffer to raise polyclonal antibodies in two New Zealand White rabbits (Agrobio, La Ferti Saint-Aubin, France). The first immunization was performed with 200 μg of recombinant protein in Freund’s complete adjuvant and three booster immunizations with the same quantity of protein were performed 14, 28, and 42 days after the first immunization. Antisera were drawn at 35, 49, and 63 days after the first immunization and used without further purification.
Protein Fusion Constructs
The NPHS2 full-length cDNA coding region was PCR amplified with the Pfu Turbo DNA polymerase from Stratagene using the EcoRI-containing forward primer 5′CGGAATTCATGGAGAGGAGGGCGCGGAG3′ and the NotI-containing reverse primer 5′TTGCGGCCGCCTATAACATGGGAGAGTCTT3′, restriction enzyme digested and subcloned into the expression vector pcDNA 3.1 Zeo+ (Invitrogen, Carlsbad, CA) creating pNPHS2. Site- directed mutagenesis using the Quickchange site-directed mutagenesis kit to introduce the c-myc tag (EQKLISEEDL) was then carried out on this construct with the primer 5′GTGGTGGAATTCATGGAACAAAAACTTATTTCTGAAGAAGATCTGGAGAGGAGGGCGCGG3′ coupled with its reverse complement, creating pcMyc-NPHS2.
Cell Culture and Transient Transfections
HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin/streptomycin and 2 mmol/L L-glutamine. Confluent cells were passaged the day before transfection and 8 × 10 5 cells were distributed into 100 mm dishes. Plasmid DNA was introduced into cells by calcium phosphate-mediated transfection. 12 Five μg of each plasmid DNA were used in all transfections. Each plate was treated with 1 ml of DNA-calcium phosphate coprecipitate for a minimum of 6 hours. Transfected cells were then overlaid with supplemented DMEM and left to incubate for 48 hours.
Immunoprecipitation and Western Blotting
Non-transfected or HEK293 cells transfected with pcMyc-NPHS2 or pNPHS2 were lysed in 1 ml of ice-cold lysis buffer (1% Triton, 150 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 8.0), 5 μg/ml leupeptine, 5 μg/ml aprotinin, 5 μg/ml pepstatin, and 1 mmol/L phenymethylsulfonyl fluoride [Sigma, St Louis, MO]) and incubated on ice for 10 minutes. The cell debris and nuclei were removed by centrifugation for 10 minutes at 4°C.
For immunoprecipitation experiments, each cell lysate was incubated overnight at 4°C with 30 μl of antiserum. The immune complexes were collected after the addition of 30 μl of protein A-Sepharose CL-4B (Sigma). The pellets of sepharose beads were washed three times with 1 ml of lysis buffer.
Total cell extracts or immunoprecipitates were boiled in Laemmli sample buffer, electrophoretically separated on 12.5% SDS-PAGE and transferred to PVDF membranes Immobilon-P (Millipore, Bedford, MA). After blocking in TBS pH 7.6 (20 mmol/L Tris-HCl, 150 mmol/L NaCl) plus 5% nonfat dry milk, the membranes were incubated at 4°C overnight with a 1:3000 dilution of the 9E10 mouse monoclonal antibody directed against c-myc (Santa Cruz Biotechnology) or with a 1:3000 dilution of antipodocin antibody in TBS/Tween 0.2% (TTBS)/BSA 0.1%. After three washes in TTBS, the filters were incubated for 1 hour at room temperature with a 1:10000 dilution of the horseradish peroxidase-conjugated sheep anti-mouse or donkey anti-rabbit antibody (Amersham Pharmacia Biotech, UK). The final detection was performed using the Amersham Pharmacia ECL reagents according to the manufacturer’s recommendations.
Tissue Samples
Six normal fetal kidneys (12 to 28 weeks) were obtained at autopsy after spontaneous abortion or termination of pregnancy for medical reasons. Six morphologically normal intact embryos (5 to 6 weeks) were obtained after legal abortion by mifepristone (RU 486) performed at Hôpital Broussais (Paris, France). Written maternal consent was obtained after information about the research project was provided and the abortion had been performed. Inserm and the ethics committee approved the entire procedure. Six normal kidneys (1 to 51 years), unsuitable for transplantation because of vascular problems, were also used for the study. End-stage kidneys, removed at the time of renal transplantation from two patients affected with steroid-resistant nephrotic syndrome were also studied. One patient carried a homozygous stop codon mutation at amino acid 138 (R138X) previously shown to result in the absence of NPHS2 transcript, 4 and the other patient had a homozygous 855/6delAA mutation in exon 7 leading to a frameshift at amino acid 285 and to the synthesis of a putative truncated protein of 302 amino acids (O Gribouval, manuscript in preparation). Normal rat and mouse adult kidneys were also used. Specimens were immediately snap-frozen in liquid nitrogen using CRYO-H-BED (Bright, Huntingdon, England) and stored at −80°C until use, or fixed in 4% paraformaldehyde before embedding. Embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline solution (PBS), microdissected from the whole trophoblast, dehydrated, and embedded in paraplast before sectioning.
In Situ Hybridization
In situ hybridization was carried out on sections of paraffin-embedded embryos, and fetal and mature kidneys according to a standard protocol previously reported. 13 Briefly, paraffin-embedded 6 μm thick sections were deparaffinized, rehydrated, and treated by microwave heating in sodium citrate buffer (0.01 mol/L, pH 6.0). Sense and anti-sense riboprobes synthesized from a 1065-base pair PCR product (spanning bases 728 to 1792 in the NPHS2 cDNA) and labeled with either digoxigenin-11-UTP (Roche) or [35S] UTP (Amersham Pharmacia Biotech) were used for the study. 4
Immunohistology
Antibodies and Reagents
Immunostaining was performed using antibodies against the C-terminal region of podocin diluted at 1:1000, or the N-terminal region of the protein diluted at 1:50, and the labeling was detected by immunofluorescence or immunoperoxidase staining.
For double immunolabeling, the following antibodies were used: mouse anti-α3-integrin, CD49c (Beckman Coulter, Brea, CA), anti-α-3 chain of type IV collagen, MAB3 (Wieslab, Lund, Sweden), and anti-synaptopodin G1D4 (Progen Biotechnik GMBH, Heidelberg, Germany).
Cyanine2 or fluorescein (FITC)-conjugated AffiniPure goat or donkey anti-rabbit IgG and cyanine3-conjugated AffiniPure donkey anti-mouse IgG antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Vectastain Elite avidin biotin complex kit, avidin-biotin blocking kit, and diaminobenzidine (DAB) were from Vector Laboratories (Burlingame, CA).
Normal human serum adsorbed goat anti-rabbit IgG conjugated with 10 nm colloidal gold particles (GAR-gold 10 nm), and staphylococcal protein A conjugated with 10 nm colloidal gold particles (ptA-gold 10 nm) obtained from BioCell (Cardiff, UK) were used for immunoelectron microscopy.
Immunofluorescence and Immunoperoxidase Staining
Immunofluorescence labeling was performed on 3-μm-thick cryostat sections fixed in acetone for 10 minutes, and incubated for 20 minutes with 10% normal swine serum in PBST (0.01 mol/L PBS containing 0.05% Tween-20) for blocking nonspecific binding. After incubation for 1 hour at room temperature with primary antibodies diluted in the same buffer, the sections were rinsed three times in PBS and incubated for 30 minutes with FITC-conjugated anti-rabbit antibodies diluted 1:40 to 1:80 in PBS. A mounting media for immunofluorescence (Fluoprep, BioMerieux, Lyon, France) was used to delay fluorescence quenching. Labeling was examined with a orthoplan microscope equipped for light, fluorescence, and phase contrast microscopy (Leica Microscopic Systems, Heezbrugg, Switzerland).
Immunoperoxidase staining was carried out using the Vectastain Elite ABC kit and DAB substrate as previously described. 14 After washing in fresh buffer (0.01 mol/L PBS, pH 7.4), endogenous biotin was blocked by the Biotin Blocking Agent according to the instructions of the manufacturer. Sections were then incubated for 1 hour at room temperature in a moist chamber with the appropriate dilution of primary antibodies in PBST, washed in PBS and incubated with the biotinylated secondary antibody for 30 minutes. For quenching the endogenous peroxidase, sections were treated with 3% hydrogen peroxide in methanol for 5 minutes and then washed in PBS for 20 minutes. They were then incubated for 30 minutes with Vectastain Elite ABC reagent. After washing, the final staining of the sections by DAB was monitored under the microscope. Tissue sections incubated with the preimmune rabbit sera or directly with the secondary antibodies served as controls.
Double Immunofluorescence Labeling and Confocal Microscopy
For dual fluorochrome labeling, the slides were simultaneously incubated with rabbit anti-podocin antibodies and mouse anti-α3(IV), anti-α3-integrin or anti-synaptopodin antibodies, as previously described. 15 After washing with PBS, the slides were simultaneously incubated with Cy2-conjugated goat anti-rabbit IgG and Cy3-conjugated donkey anti-mouse IgG. Sections were examined with a orthoplan microscope equipped with appropriate filters (Leica Microscopic Systems) and with a Zeiss confocal microscope (Carl Zeiss Microscopy, Jena, Germany).
Immunogold Electron Microscopy
Kidneys from anesthesized adult mice were fixed by perfusion of periodate-lysine-paraformaldehyde solution (PLP) through the left ventricle. They were removed and postfixed for 1 hour in PLP, incubated for 1 hour in glycine-PBS before embedding in LRWhite (London Resin, Besingstoke, UK). The resin was polymerized at −24°C. Ultrathin sections were cut, mounted on nickel grids coated with collodium and carbon films, and processed for immunocytochemistry.
For immunolabeling, tissue sections were first incubated for 5 minutes on a drop of 0.1 mol/L PBS, pH 7.4, containing 5% bovine serum albumin (BSA) and 10% normal goat serum (G), and then with the primary antibodies diluted in the blocking solution [1% BSA, 1% G and 0.1% Tween 20 (T)] and incubated at room temperature for 2 hours. The grids were then rinsed with the same buffer and incubated for 45 minutes on a drop of the secondary antibody (GAR-gold 10 nm) or ptA-gold 10 nm, diluted in the same buffer containing gelatin 0.1%. They were then washed with PBS, post-fixed for 15 minutes with 2% glutaraldehyde, briefly stained with 2% osmium tetroxyde, and dried. After staining with uranyl acetate and lead citrate, the sections were examined with a Zeiss EM 902 electron microscope. Direct incubation with the secondary antibody or the protein A-gold alone was performed as controls.
Results
Characterization of Polyclonal Podocin Antibodies
Polyclonal rabbit antisera were raised against fusion proteins derived from two regions of podocin: P18 and P21 against the whole N-terminal region before the membrane-associated domain (amino acids 15 to 89), P35 and P39 against the C-terminal region downstream of the membrane-associated domain (amino acids 135 to 383). The specificity of these antisera was first tested by Western blot analysis of HEK293 cells transiently transfected with a c-myc-tagged podocin expression construct, pcMyc-NPHS2. Both N- and C-terminal antibodies detected a thick band of 49-kd in transfected cells (Figure 1a) ▶ . This band was also detected by the 9E10 antibody directed against the c-myc epitope. In contrast, this band was not detected by these antibodies in the non- transfected cells (Figure 1a) ▶ nor by the preimmune antisera in the transfected and non-transfected cells (data not shown).
Figure 1.
Characterization of podocin antibodies by Western blotting and immunoprecipitation. a: Lysates from non-transfected HEK293 cells (lanes 1, 3, 5) or HEK293 cells transiently transfected with pcMyc-NPHS2 (lanes 2, 4, 6) were separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with P21 antiserum (lanes 1 and 2), P35 antiserum (lanes 3 and 4) and 9E10 antibody (lanes 5 and 6). b: Lysates from non-transfected HEK293 cells (lanes 1, 3, 5) or HEK293 cells transiently transfected with pNPHS2 (lanes 2, 4, 6) were separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with P35 antiserum. Lysates were previously immunoprecipitated with P21 antiserum (lanes 3 and 4) or with P35 antiserum (lanes 5 and 6). P21 and P35 immunoprecipitated podocin (arrow). A strong non-specific signal is observed around 55-kd in the immunoprecipated lysates, corresponding to the rabbit Ig present in the antisera.
Furthermore, the four antibodies immunoprecipitated podocin when overexpressed in HEK293 cells transfected with pNPHS2. The P35 antibody detected a single band of 49 kd in cell lysates of transfected cells as well as in the pNPHS2 transfected cell lysates immunoprecipitated with P21 or P35, whereas no band was seen in controls (Figure 1b) ▶ . The same results were obtained by immunoprecipitation with P18 and P39 (data not shown).
Expression of NPHS2 Transcript in the Human Kidney
In the 5- to 6-week-old embryos, no signal was detected except in the mesonephros. No signal was detected in the primitive metanephros consisting of the uninduced mesenchyma and the branching ureteric bud or in other developing structures. In contrast, NPHS2 was specifically expressed in mesonephric podocytes from the S-shaped body stage, then in the large fully developed glomeruli (Figure 2a) ▶ . In the developing metanephric kidney, no NPHS2 transcript was detected at the initial steps of nephron formation whereas hybridization signals were seen at the late S-shaped body stage, in the future podocytes of the inferior segment of the S-shaped body (Figure 2, b and c) ▶ . Strong expression persisted in the immature and mature glomeruli (Figure 2b) ▶ . In postnatal kidneys, all glomeruli were strongly labeled with the antisense probe. At high magnification, expression of the transcript was clearly localized within podocytes, at the periphery of the glomerular tuft (Figure 2, d–f) ▶ . NPHS2 expression was seen in the patient with a frame-shift mutation (855/6delAA) in exon 7 (Figure 2f) ▶ . No specific signal was obtained with the sense probe (data not shown).
Figure 2.
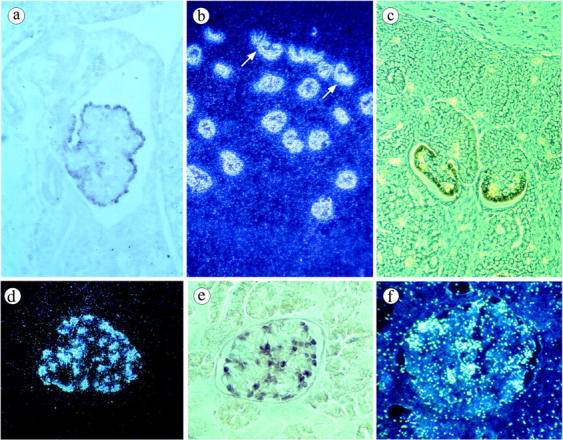
In situ hybridization with antisense NPHS2 riboprobe labeled with [35S]VTP (b, d, f) or digoxigenin (a, c, e). a: In the mesonephros of a 5 week-old human embryo, strong expression is seen in the visceral epithelial cells of a mature glomerulus. No labeling is detected in the surrounding tissue. b: Dark field micrograph: all of the glomeruli of a 15 gestational week (GW) fetus are strongly labeled with the probe. The transcript is also seen in immature podocytes of the inferior segment of the S-shaped body (arrow). c: Phase contrast micrograph. In the superficial cortex of a 12 GW fetus, strong signals are observed in the developing podocytes of the late S-shaped body and the immature glomerulus, whereas no labeling is detected in earlier structures. d–e: Normal mature kidney. d: Dark field micrograph of a glomerulus shows intense labeling of podocytes at the periphery of the tuft. e: The same distribution is observed with the digoxigenin-labeled probe. f: End-stage kidney from the patient with a frame-shift mutation of NPHS2. Strong expression of the transcript is seen in preserved podocytes. Magnifications: a, ×160; b, ×50; c, ×200; d–f, ×250.
Podocin Expression in the Kidney
Polyclonal antibodies against the C- or the N-terminal regions of podocin gave the same pattern of immunolabeling but the staining was stronger with P35 and P39, the anti-C-terminal antibodies. In the fetus, the most primitive nephronic structures, vesicles, comma-shaped and early S-shaped bodies were unlabeled. A faint signal first appeared at the base of the primitive podocytes along the vascular cleft of the S body at the beginning of the indentation of the inferior segment of the S-shaped body (Figure 3a) ▶ . The signal increased with glomerular maturation (Figure 3, a–c) ▶ . During the capillary loop and maturing glomerulus stages, immunolabeling was intense in the basal region of the podocytes, along the glomerular basement membrane, but was also detected as thin spiky projections between the developing podocytes (Figure 3, b and c) ▶ . Later, in the mature glomeruli located in the deep cortex of fetal kidneys, podocin labeling strictly followed the course of the GBM. The same distribution was maintained in mature kidneys where all podocytes were regularly labeled with the antibodies (Figure 3, d–f) ▶ . No staining was detected in other glomerular or extraglomerular cells. No labeling was seen in control experiments or in the kidney of the patient with a homozygous stop codon mutation (R138X) known to result in the absence of a transcript. 4 In contrast, a very strong podocyte labeling was observed in the patient with the 855/6delAA mutation using antibodies directed against the N-terminal part of the protein whereas no labeling was seen with the anti-C terminal domain (Figure 3, g and h) ▶ . The same pattern as in the human mature glomeruli was obtained on mouse and rat mature kidney sections labeled with P21 or P35 (Figure 3i) ▶ .
Figure 3.
Immunostaining of podocin in fetal and mature kidneys using P35 (a–e, h–i) and P21 antibodies (f–g) directed against the C- and N-terminal regions of podocin, respectively. a: In the developing human kidney, podocin is initially detected in the inferior segment of the S-shaped body when the future glomerulus becomes indented (arrow). b and c: At the glomerular capillary loop stage, podocin expression in podocytes is basolateral. d–f: In glomeruli from human adult kidneys, podocin labeling strictly follows the course of the GBM. g: In the patient with the NPHS2 homozygous 855/6delAA mutation, podocyte expression of the N-terminal domain of the protein is very strong. h: It contrasts with the absence of labeling with the antibodies against the C-terminal domain. i: In normal mature mouse kidney, podocin distribution is similar to that in human kidney. Magnifications: a, × 50; b and c, ×350; d, f, g, and h, ×250; e and i, ×300.
Double Immunofluorescence Labeling and Confocal Microscopy
To better delineate the subcellular localization of podocin, double immunofluorescence labeling was performed on human mature glomeruli, with anti-podocin antibodies (in green) and antibodies directed against the α3 chain of type IV collagen (Figure 4a ▶ -c), synaptopodin (Figure 4, d–f) ▶ and the α3 chain of α3β1 integrin (Figure 4, g–i) ▶ (in red).
Figure 4.

Dual immunofluorescence and confocal microsopy with P35 polyclonal anti-podocin antibodies (green) (a, d, g) and monoclonal antibodies (red) against type IV collagen α-3 chain (b and c), synaptopodin (e and f) or α3 integrin (h and i) in human mature kidney. The green podocin labeling of podocytes follows the external aspect of the red GBM (c), is co-localized and extends focally beyond synaptopodin labeling (f) and is tightly contiguous or colocalized with the α3 integrin (i). Magnification: a–i, ×650.
The anti-α3 chain of type IV collagen gave a strong linear labeling of the glomerular basement membrane (GBM). Dual stained sections seen at low magnification showed a nearly complete superposition of the red and green labeling. However, high magnification and confocal microscopy examination clearly disclosed the red linear labeling of the GBM with the anti-α3(IV) antibodies from the green, somewhat granular, labeling of the base of the podocytes with the anti-podocin antibodies (Figure 4, a–c) ▶ . Synaptopodin is an actin-associated protein in the podocyte foot processes. 16 Dual labeling with podocin showed a tight superposition of both markers evidenced by the yellow color of the labeling outlining the GBM (Figure 4, d–f) ▶ . In addition, the green podocin labeling could be focally seen at the periphery of the synaptopodin staining (Figure 4f) ▶ . CD49c recognizes the α3 chain of the α3β1 integrin, an adhesion molecule highly expressed in the glomerular epithelial cells along the GBM. 17 It gave a strong labeling of the podocytes at their site of adhesion to the GBM. Dual labeling showed that podocin and α3 integrin are co-distributed along the GBM with focally a tight alternance of red and green spots well discriminated by confocal microscopy (Figure 4, g–i) ▶ .
Immunoelectron Microscopy
The localization of podocin molecules was precisely determined by immunoelectron microscopy in mature mouse glomeruli. Both anti-C- and N-terminal antibodies gave the same labeling pattern. Gold particles were distributed at the base of the podocyte foot processes, near the GBM (Figure 5) ▶ . They were primarily located at the cytoplasmic face of the plasma membrane adjacent to the filtration slits which were globally unlabeled. Small clusters of particles were occasionally found along the surface of the foot processes at a distance from the GBM. No significant labeling was observed on the GBM or the podocyte cell bodies. No labeling was detected on Bowman’s capsule epithelial cells, endothelial, or mesangial cells. Control stainings were negative.
Figure 5.

Localization of podocin in the glomerular capillary wall of normal mice using immunogold labeling and P35 (a and c) or P21 (b and d) antibodies. With both antibodies, podocin is seen at the base of podocyte foot processes, along the GBM, on either part of the slit diaphragm (arrow), predominantly at the inner face of the plasma membrane. Focally some gold particles are seen at distance of the GBM (arrowhead). Magnifications: a–b, ×24,000; c–d, ×40,000.
Discussion
Recently, several genes expressed by the glomerular podocytes have been identified. Among them, NPHS1 and NPHS2, encoding nephrin and podocin respectively, were shown to play a major role in the glomerular permeability as mutations in these genes result in early onset nephrotic syndrome in human. 2,4 Nephrin is a transmembrane protein that contains an extracellular portion including eight Ig-like motifs and a type III fibronectin domain and a short intracellular domain. 2 Nephrin is specifically located in the slit diaphragm, 7-9 and is expressed as soon as podocytes differentiate in the fetal kidney. 8,9,18,19 Podocin is a new member of the stomatin protein family. Given its primary structure similarities with stomatin and MEC-2, a protein involved in mechanosensation in Caenorhabditis elegans, 20-22 podocin was predicted to be an integral membrane protein with a short membrane domain forming a hairpin-like structure and with N- and C-terminal cytosolic domains. 4
Here, we describe the expression pattern of podocin during human embryogenesis and kidney development and the subcellular localization of podocin in the podocytes, using in situ hybridization and immunohistochemistry. For this purpose, we raised antibodies against both the N- and C-terminal regions of podocin. The specificity of these antibodies was demonstrated by Western blotting and immunoprecipitation and confirmed by the absence of staining of the kidney of a patient with a homozygous NPHS2 mutation (R138X) resulting in the absence of NPHS2 transcript. 4 Furthermore, no labeling was observed with the anti-C-terminal antibodies on kidney sections from a patient carrying a NPHS2 homozygous 855/6delAA mutation, whereas a strong labeling was observed on kidney sections of this patient with antibodies directed against the N-terminal domain of podocin. As the 855/6delAA mutation is predicted to introduce a frame-shift at amino acid 285 and to lead to the production of a truncated podocin lacking the last 98 amino acids, this suggests that the epitopes recognized by the C-terminal antibodies, P35 and P39, are localized in this 98-amino-acid sequence.
NPHS2 expression was detected in 5-week human embryos, the earliest developmental stage studied. The gene was specifically expressed in the mesonephros, and more precisely in the mesonephric future podocytes from the late S-shaped body. No other embryonic structure was found to express NPHS2. In metanephric kidneys, the NPHS2 transcript was initially detected in the lower limb of the late S-shaped body, in the presumptive podocytes but not in future parietal epithelial cells. This expression persisted throughout glomerular differentiation and in mature glomeruli. Expression of the protein was detected somewhat later than the transcript, at a time when the inferior limb of the S-shaped body becomes indented. At this stage, as well as at the early capillary loop stage, podocin was distributed at the basal pole and along the lateral surface of immature podocytes that had not yet developed foot processes. Podocin expression increased with glomerular maturation and persisted at a high level in mature kidneys at the basal pole of podocytes. Double immunolabelings and confocal microscopy examination confirmed this localization. Podocin labeling followed the external aspect of the GBM stained for type IV collagen α3 chain. By light microscopy, podocin was tightly contiguous to α-3 integrin expressed at the site of foot process attachment to the GBM. It co-localized with synaptopodin, a prolin-rich cytoplasmic protein intimately associated with actin filaments present in the foot processes. 16 An interesting observation was the strong expression of the N-terminal domain of podocin in the patient with the NPHS2 homozygous 855/6delAA mutation. This shows that the increased synthesis of the truncated podocin is not able to compensate for its defective function and emphasizes the crucial role of the C-terminal region of podocin, which contains proline residues that might be the site of interaction with Src homology 3 (SH3) domain-encoding proteins. 23
Using immunogold labeling and electron microsopy, we showed the podocin distribution at the base of the foot processes and precisely determined its localization on either side of the slit diaphragm, the slit membrane itself being unlabeled. Interestingly, both the C- and N-terminal domains of the protein identified by specific antibodies are co-localized at the cytoplasmic face of the plasma membrane, a finding in agreement with the hairpin-like predicted structure of podocin. This structure is also reminiscent of that of other proteins such as caveolins which incorporate in specialized membrane microdomains. 24
More generally, podocin shows the same pattern of distribution during kidney development as that described for nephrin. 8,18,19,25 Indeed, it is expressed in the mesonephros, in future podocyte from the late S-shaped body. Later, the expression is restricted to the base of the cells as soon as they differentiate with the appearance of foot processes. However, the nephrin extracellular domain is localized within the slit diaphragm, 9,25 whereas podocin, as ZO-1, 26 is present on the cytoplasmic face, at the point of attachment of the slit diaphragm. This suggests that podocin, as a membrane protein anchored to the plasma membrane, could interact with the intracellular domains of the transmembrane proteins localized in the slit diaphragm such as nephrin, P-cadherin, or FAT. Given the structure and the subcellular localization of the protein, it is tempting to speculate that podocin might assemble in lipid microdomains in the podocyte membrane and recruit other proteins such as CD2AP and ZO-1. More generally, podocin might serve to connect directly or indirectly the components of the slit diaphragm to the actin cytoskeleton.
The antipodocin antibodies provided here will be useful tools for testing the potential interaction of these proteins with podocin, for identifying unknown podocin partners, and for studying the podocin expression pattern in various glomerular nephropathies.
Acknowledgments
We thank L. Heidet and V. Kalatzis for critical reading of the manuscript, G. Mollet for invaluable help and advice, Y. Goureau for technical assistance with the confocal microscope, and Y. Deris for assistance with figure preparation.
Footnotes
Address reprint requests to Corinne Antignac, M.D., Ph.D., Inserm U423, Tour Lavoisier 6ème étage, 149, rue de Sèvres, 75015 Paris, France. E-mail: antignac@necker.fr.
Supported in part by grants from Association pour l’Utilisation du Rein Artificiel, Fondation pour la Recherche Médicale, and Association Claude Bernard. S.R. is a fellow of the Ministère de l’Education Nationale, de la Recherche, et de la Technologie.
References
- 1.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974, 60:423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein, nephrin, is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 2000, 24:251-256 [DOI] [PubMed] [Google Scholar]
- 4.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 2000, 24:349-354 [DOI] [PubMed] [Google Scholar]
- 5.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 6.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking neph1, a novel protein with homology to nephrin. Mol Cell Biol 2001, 21:4829-4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holthofer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzman LB, St. John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 9.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000, 11:1-8 [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Kihara I, Yamamoto T: FAT is a component of glomerular slit diaphragms. Kidney Int 2001, 59:1003-1012 [DOI] [PubMed] [Google Scholar]
- 12.Pear WS, Nolan GP, Scott ML, Baltimore D: Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA 1993, 90:8392-8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidet L, Cai Y, Guicharnaud L, Antignac C, Gubler MC: Glomerular expression of type IV collagen chains in normal and X-linked Alport syndrome kidneys. Am J Pathol 2000, 156:1901-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Jeanpierre C, Dressler GR, Lacoste M, Niaudet P, Gubler MC: WT1 and PAX-2 podocyte expression in Denys-Drash syndrome and isolated diffuse mesangial sclerosis. Am J Pathol 1999, 154:181-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Y, Beziau A, Sich M, Kleppel MM, Gubler MC: Collagen distribution in human membranous glomerulonephritis. Pediatr Nephrol 1996, 10:14-21 [DOI] [PubMed] [Google Scholar]
- 16.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korhonen M, Ylänne J, Laitinen L, Virtanen I: The α1-α6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol 1990, 111:1245-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawachi H, Abrahamson DR, St. John PL, Goldstein DJ, Shia MA, Matsui K, Shimizu F, Salant DJ: Developmental expression of the nephritogenic antigen of monoclonal antibody 5–1-6. Am J Pathol 1995, 147:823-833 [PMC free article] [PubMed] [Google Scholar]
- 19.Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain, and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001, 10:1-8 [DOI] [PubMed] [Google Scholar]
- 20.Stewart GW, Hepworth-Jones BE, Keen JN, Dash BC, Argent AC, Casimir CM: Isolation of cDNA coding for an ubiquitous membrane protein deficient in high Na+, low K+ stomatocytic erythrocytes. Blood 1992, 79:1593-1601 [PubMed] [Google Scholar]
- 21.Huang M, Gu G, Ferguson EL, Chalfie M: A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 1995, 378:292-295 [DOI] [PubMed] [Google Scholar]
- 22.Salzer U, Ahorn H, Prohaska R: Identification of the phosphorylation site on human erythrocyte band 7 integral membrane protein: implications for a monotopic protein structure. Biochim Biophys Acta 1993, 1151:149-152 [DOI] [PubMed] [Google Scholar]
- 23.Cohen GB, Ren R, Baltimore D: Modular binding domains in signal transduction proteins. Cell 1995, 80:237-248 [DOI] [PubMed] [Google Scholar]
- 24.Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP: p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J Biol Chem 1999, 274:32333-32341 [DOI] [PubMed] [Google Scholar]
- 25.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestila M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H: Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 2000, 157:1905-1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurihara H, Anderson JM, Farquhar MG: Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA 1992, 89:7075-7079 [DOI] [PMC free article] [PubMed] [Google Scholar]




