Abstract
Autoimmune ovarian disease (AOD) is a probable cause of human premature ovarian failure, and a potential complication of contraceptive vaccines based on ovarian antigens. The diagnosis depends on detection of noninfectious ovarian inflammation (oophoritis) and serum antibody to ovarian and placental antigens. Mechanisms underlying AOD have been investigated in mice but not in primates. Herein, we report induction of AOD in primates, and compare the immunopathology between monkey and murine AOD. Four cynomolgus macaques immunized with monkey or human zona pellucida 3 peptide (pZP3) in adjuvant, developed T-cell responses to the immunizing peptide and produced antibody that bound to native zona pellucida in vivo. Immunostaining of ovaries from pZP3-immunized macaques showed numerous clusters of T cells co-localized with major histocompatibility complex II-positive macrophages in the ovarian interstitium. Such foci were not detected in untreated or adjuvant-treated control monkeys. This finding is comparable to murine pZP3-induced AOD. However, unlike murine AOD in which numerous granulomatous lesions are detected, severe granulomatous inflammation was detected in only one of three monkeys with abnormal immunohistology. Similar to mice with pZP3-induced AOD, the immunized monkeys retained normal ovarian function. The results are discussed in the context of complications of ZP-based human immunocontraceptive vaccines and case reports of human autoimmune oophoritis.
Autoimmune ovarian disease (AOD) is considered to be a cause of human premature ovarian failure (POF). 1-3 However, the immunological basis of POF remains poorly understood. This is in part because early POF is clinically silent and advanced stages of the disease lack ovarian inflammation (oophoritis), a diagnostic feature of autoimmune etiology. 4 The strongest evidence for human AOD is the finding of oophoritis in a subset of patients with POF, 4,5 as well as the co-existence of ovarian inflammation with autoimmune adrenal disease or autoimmune polyglandular syndrome. 6 The diagnosis of immune POF is based on detection of circulating autoantibodies to ovarian or placental antigens. However, there is currently no consensus on the antigen specificity of these antibodies or the clinical assay system for their detection. Although serum autoantibodies to ovarian 17α-hydroxylase and side-chain cleavage enzyme of steroid-producing cells have been characterized, they are detected only in POF patients associated with autoimmune polyglandular syndrome type I or type II. 7,8
Studies on rodent models of AOD have added some insights into the pathology and mechanisms of immune-mediated ovarian injury. Neonatal thymectomy in susceptible mouse strains causes ovarian inflammation and autoantibodies resulting in ovarian atrophy and ovarian dysfunction. 9 This model has been used to understand regulation of autoimmune disease in general and AOD in particular. 10,11 The second rodent model of AOD is elicited by immunization with a self-peptide of murine zona pellucida (ZP)3 (330 to 342). 12 Murine pZP3 immunization causes activation of autoreactive T cells and generation of anti-ZP autoantibody. The autoreactive T cells mediate autoimmune oophoritis characterized by focal or diffuse inflammation in the ovarian interstitium and organized monocytic granulomata. Although oophoritis alone does not affect ovarian function, a high level of ZP autoantibody reduces fertility in female mice. 13 On the other hand, combined ZP antibody and T-cell-mediated inflammation are required for the induction of ovarian atrophy and infertility. 14 Chimeric peptides that contain foreign T-cell epitope and native ZP3 B-cell epitope induce ZP antibody without inducing ovarian inflammation, and they are potential candidate antigens for contraceptive vaccine development. 15
In primates, long-term immunization with zona pellucida proteins for >1 year resulted in antibodies to zona pellucida with contraceptive effect. However, the infertility was irreversible and the monkeys developed POF. 16,17 The ovaries from immunized animals show few oocytes or developing follicles but increased numbers of aberrant granulosa cell nests. Ovarian inflammation was not reported in the atrophic ovaries of animals after long-term immunization. To investigate AOD as a basis for POF and a complication of ZP vaccination, we have developed a primate model of AOD. In this study, we demonstrate that cynomolgus macaques are indeed susceptible to AOD using human and macaque homologues of murine pZP3 (330 to 342). The resulting ovarian histopathology is compared to murine AOD, and with the reported findings in human AOD.
Materials and Methods
Peptides
The ZP3 peptides from different species used in this study are as follows: human 328 to 341 [GTPSHSRRQPHVMS pZP3(hu)]; macaque 328 to 341 [GTPSHSRRQPHVVS pZP3(mac)], and mouse 330 to 342 [NSSSSQFQIHGPR pZP3 (mou)]. 18 The peptides were synthesized in an automated peptide synthesizer (Beckman Instruments, Fullerton, CA) using F-moc chemistry. Deprotection was achieved by 20% piperidine in dimethyl formamide, and the peptides were chemically cleaved from the resin with 85% trifluoroacetic acid. The peptides were purified by HPLC eluting as a single peak on PRLP-S columns (Waters, Milford, MA) and were of >90% purity.
Monkeys: Immunization, Cyclicity, Surgery, Sacrifice
All animal housing and procedures were performed in accordance with the National Institutes of Health guidelines. Adult female cynomolgus macaques (Macaca fascicularis) were anesthetized with ketamine hydrochloride (10 to 20 mg/kg) for immunization and bleeding. Experimental groups included immunization with peptides [pZP3(mac) or pZP3(hu): two animals per group] or controls (untreated or adjuvant-treated: two animals per group). Immunizations were done on days 0, 14, and 28 intramuscularly with 50 nmol/L of peptide and 0.1 mg of nor-muramylated dipeptide (a gift from Dr. Vernon Stevens, Ohio State University, Columbus, OH) in phosphate-buffered saline (PBS) emulsified in equal volume of squalene:arlacel A (4:1, v:v; Sigma Chemical Co., St. Louis, MO). Animals were given booster immunizations at times indicated. Blood collected from the femoral vein was used for serum antibody analyses, T-cell responses, and circulating steroid levels. All of the animals were monitored daily for menstrual bleeding. Ovaries were surgically removed under general anesthesia through a midline abdominal incision. To study ovarian immunopathology at multiple time points, the first ovary was removed between 1.5 to 6 months and the second between 11 to 16.5 months. Each ovary was cut into 4 to 5 pieces collected in buffered formalin for histopathology. One piece was snap-frozen in liquid nitrogen for detection of ZP-bound IgG. The animals were euthanized at the end of the study and organs collected for histopathological analysis.
Mice: Immunization and Sacrifice
(C57BL/6 X A/J)F1 females were obtained from the National Cancer Institute, Bethesda, MD and housed as per National Institutes of Health guidelines. As previously described, for induction of AOD, mice were anesthetized by intraperitoneal injection of tribromoethanol (0.5 mg/mouse) and immunized subcutaneously with 50 nmol/L of pZP3(mou) emulsified in CFA (Mycobacterium tuberculosis H37RA strain, 0.16 mg/mouse) (Difco Laboratories, Detroit, MI) and sacrificed 14 days later. 12 Control mice were injected with CFA alone. One ovary was collected in Bouin’s fixative for histopathological grading of AOD and the other ovary was collected in 4% paraformaldehyde in PBS (w/v), pH 7.4, for immunohistochemical staining.
Detection of Antibody to Peptides by Enzyme-Linked Immunosorbent Assay
Serum IgG to pZP3(hu) or pZP3(mac) were detected by enzyme-linked immunosorbent assay. 18 Ninety-six well plates (Corning Glass Works, Corning, NY) were coated overnight at 4°C with 25 nmol/L of peptide in 0.1 mol/L bicarbonate buffer, pH 9.0. All subsequent incubations were performed for 2 hours at room temperature. The plates were washed with 0.05% Tween 20 in PBS, pH 7.4, and blocked with 3% bovine serum albumin (BSA) in PBS. The plates were incubated with serial dilutions of monkey sera. The plates were then washed and incubated with horseradish peroxidase-labeled goat anti-monkey IgG (Nordic Immunological Laboratories, Capistrano Beach, CA) (1:3000 dilution). After washing, the reaction was developed by o-phenylene diamine (0.5 mg/ml; Sigma Chemical Co.) with freshly added 0.06% H2O2 in 0.1 mol/L citrate buffer, pH 5.0. The color intensity was read at 490 nmol/L by an enzyme-linked immunosorbent assay reader (Molecular Devices, Menlo Park, CA). The end-point titer was calculated as dilution of serum giving an absorbance of 2 SD above the background reading using preimmune monkey serum.
Detection of Anti-ZP Antibody by Direct and Indirect Immunofluorescence
In vivo binding of ZP antibody was detected by direct immunofluorescence. Frozen monkey ovary sections from peptide-immunized macaques or adjuvant-treated controls were fixed in 95% ethanol for 10 minutes and rinsed with PBS. The sections were then blocked with normal goat serum (1:10 v/v) diluted in PBS with 3% BSA for 20 minutes in a humid chamber at room temperature. After rinsing with PBS, the bound antibody was detected by fluorescein isothiocyanate conjugated to anti-monkey IgG (1:100; Nordic Immunological Laboratories) for 30 minutes. The slides were rinsed and mounted in Vectashield medium (Vector Laboratories, Burlingame, CA).
Antibody to zona pellucida in serum was detected by indirect immunofluorescence. The procedure followed was same as above with some differences. Five μm sections of snap-frozen normal monkey ovary were fixed in 95% ethanol, blocked with normal goat serum in PBS with 3% BSA (1:10 v:v). The sections were then incubated with immune serum or adjuvant control serum diluted in 3% BSA in PBS for 1 hour. IgG antibody bound to ZP was detected by fluorescein isothiocyanate conjugated to anti-monkey IgG (Nordic Immunological Laboratories).
Lymphocyte Proliferative Responses in Monkeys
Estimation of T-cell proliferative responses was done as previously described. 18 Monkey peripheral blood was collected in heparinized tubes and centrifuged to separate supernatant plasma. The cell pellet was resuspended in sterile PBS, layered over Histopaque 1083 (Sigma), and centrifuged at 400 × g for 20 minutes. Mononuclear cells at the interphase were collected, washed, and resuspended at 2 × 106/ml in Dulbecco’s minimum essential medium supplemented with 1% sodium pyruvate, 1% of 200 mmol/L glutamine, 1% nonessential amino acids, 5 × 10−5 mol/L β-mercaptoethanol, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated fetal calf serum. The assay was set up in triplicates in which cells (0.1 ml/well) were cultured with an equal volume of peptide (0 to 30 μmol/L) in 96-well flat-bottom plates for 4 days at 37°C in 5% CO2. [3H]-thymidine (0.5 μCi/well; Dupont NEN Products, Boston, MA) was added to each well 16 hours before harvesting the cells (Skatron Instruments, Sterling, VA), and the cell-associated thymidine measured by scintillation counting (Beckman Instruments). Results are presented as stimulation index (experimental counts per minute with peptide/background counts per minute without peptide). The monkeys Babs and Harriette gave high background counts when cultured in media with 10% fetal calf serum. For these animals, fetal calf serum was substituted with 10% autologus heat-inactivated plasma.
Immunohistology: Monkey and Mouse Ovaries
Serial sections (5 μm) were cut through the entire formalin-fixed, paraffin-embedded, monkey ovaries. Every 10th section was stained with hematoxylin and eosin. Adjacent sections were stained with the following antibodies at indicated dilutions. Polyclonal rabbit antibody to human CD3 and mouse monoclonal antibodies to HLA (CR3/43), CD20 (L26), CD 68 (KP1), and myeloid/histiocyte antigen (Mac 387) obtained from DAKO Corporation, Carpinteria, CA. A rabbit polyclonal antibody to HLA DR (a gift from Dr. S. M. Fu, University of Virginia, Charlottesville, VA) was also used in some of the studies. Optimal dilutions of primary antibody (1:20 for rabbit anti-HLA polyclonal; 1:50 for L26, Mac387, CR3/43; and 1:100 for anti-CD3 and KP1) were determined using control monkey spleen sections.
The tissues were deparaffinized in xylene, transferred to absolute alcohol, rehydrated with decreasing grades of alcohol, and rinsed in PBS. Endogenous peroxidase was inactivated with 0.6% hydrogen peroxide in methanol PBS (4:1, v:v). Because some antibodies (CD3, CD68, and Mac 387) did not recognize their cognate antigens, unmasking of epitopes was performed by digestion with pepsin (4 mg/ml) in 0.1% HCl at 37°C for 7 minutes. For HLA monoclonal and polyclonal antibodies, antigen retrieval was done by heating slides in 10 mmol/L of citrate buffer, pH 6.0, for 20 minutes in a microwave. Evaporated fluid was replaced with water every 4 minutes. The slides were allowed to cool to room temperature before use. Sections were rinsed in PBS after antigen retrieval and blocked with normal goat or rabbit serum followed by primary antibody diluted in 3% BSA PBS. After washing, the slides were incubated with biotinylated goat anti-mouse or anti-rabbit Ig (Vector Laboratories) (1:100 dilution). Detection of bound antibody was done by avidin-biotinylated enzyme complex (Vectastain ABC kit; Vector Laboratories) and visualized by incubation with diaminobenzidine substrate (Biogenex Corp., San Ramon, CA). The sections were counterstained with methylene blue, rinsed with water, and dehydrated with ascending grades of alcohol. The slides were then rinsed with xylene and mounted with Cytoseal60 (Stephens Scientific, Kalamazoo, MI). Monkey spleen sections concomitantly processed were positive controls and ovaries without primary antibody served as negative controls.
Immunochemical staining of mouse ovaries was done as previously described. 13 Briefly, 5-μm sections from mouse ovaries fixed in 4% paraformaldehyde were incubated with primary antibody to CD5 (Lyt1 53-7.313), major histocompatibility complex (MHC) class II (M5/114.15.2), B220 (RA3-3A1/61), or macrophage (F4/80). After washing in PBS, the sections were incubated with biotinylated rabbit anti-rat antibody. Bound antibody was visualized by the ABC detection system followed by diaminobenzidine and counterstained with methylene blue as described above.
Steroid Assays
For analysis of ovarian function, monkeys were bled every 3 days for 1 to 3 consecutive months at two phases of the study, with the first early phase starting 1 to 2 months before immunization until 2 months after immunization and the second late phase between 3 to 11 months when the antibody titers were high. Endocrine function was monitored by estimation of circulating steroid hormone levels. One hundred μl of serum was used in competitive radioimmunoassays for progesterone and estradiol estimations, as per the manufacturer’s instructions (ICN Pharmaceuticals, Costa Mesa, CA, for progesterone and Diagnostic Products Corporation, Los Angeles, CA, for estradiol). The detection limit for progesterone was 0.27 ng/ml with an interassay coefficient of variation (CV) of 11.7% and intra-assay CV of 8.9%. For estradiol, the sensitivity limit was 27 pg/ml with an interassay and intra-assay CV being 7.4% and 4.5%, respectively.
Results
ZP3 Peptide from Macaque or Human Elicits an Autoimmune Response in Female Cynomolgus Macaques
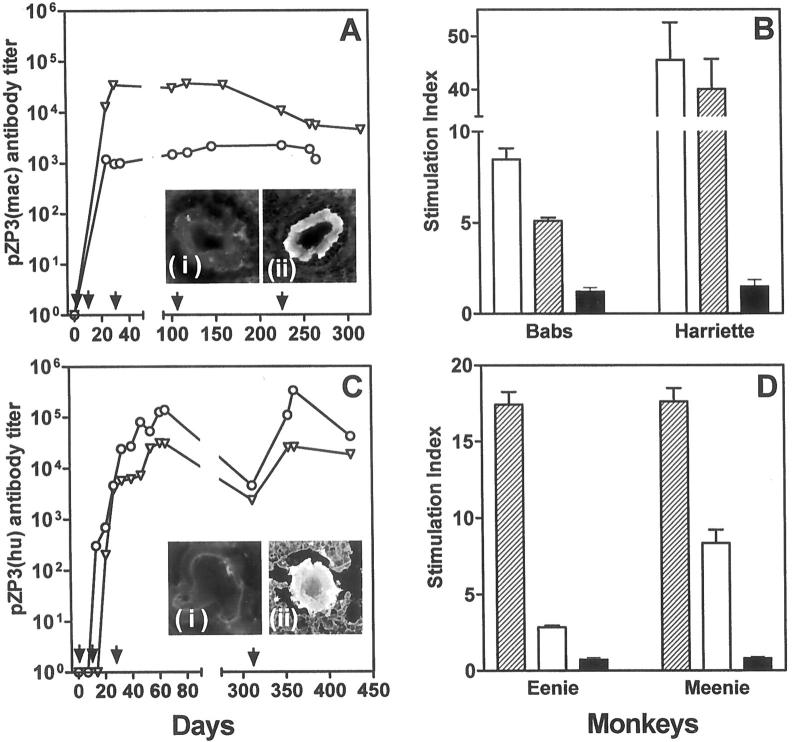
Two normal, cycling, adult female cynomolgus macaques were immunized with 50 nmol/L of pZP3(mac) in a squalene arlacel A emulsion. Both animals (Babs and Harriette) had detectable serum anti-peptide antibody on day 24 after immunization (Figure 1A) ▶ . The titers were maintained at 10 3 to 10 5 by subsequent booster immunizations until sacrifice. Their serum IgG reacted with macaque ZP by indirect immunofluorescence (Figure 1A ▶ , inset). Antibody bound to ovarian ZP was also detected by direct immunofluorescence at time of sacrifice (data not shown). Thus pZP3(mac) includes a native B-cell epitope. The antibody detected was of the IgG isotype suggesting a helper T-cell response and the presence of a T-cell epitope in pZP3(mac). T-cell proliferative response was detected in vitro by days 28 to 32 in both animals (data not shown). The T-cell proliferative response could also be recalled 10 days after the final booster immunization (Figure 1B) ▶ .
Figure 1.
Immune response in female macaques immunized with pZP3(mac) (A and B) or pZP3(hu) (C and D). Serum antibody titers to the immunizing peptide were detected by enzyme-linked immunosorbent assay (A and C). Arrows indicate time of primary and booster immunizations. A: Antibody titers to pZP3(mac) [Babs (circles) and Harriette (triangles)]. Serum antibody from peptide-immunized Harriette recognized macaque zona pellucida (inset Aii) whereas sera from adjuvant-treated controls did not (inset Ai). In lymphocyte proliferation assays (B and D) animals immunized with pZP3(mac) (B) respond to the immunizing macaque peptide (open bars) and its human homologue (hatched bars) but not to the mouse peptide (solid bars). C: Antibody titers to pZP3(hu) [Meenie (circles) and Eenie (triangles)]. Direct immunofluorescence showed antibody bound to ZP (inset Cii) in Meenie but not in an adjuvant-treated macaque (inset Ci). In lymphocyte proliferative assays animals immunized with pZP3(hu) (D) respond to the immunizing peptide (hatched bars) and its homologous macaque peptide (open bars) but not to the dissimilar mouse peptide (solid bars). Data show stimulation index (±SEM) at 30 μmol/L concentration for each peptide. C is reprinted with permission from the Society of Reproduction, Inc. (Biol Reprod 1997, 56:767).
Two macaques immunized with a homologous human ZP3 peptide [pZP3(hu)] were also studied for AOD. Therefore, we evaluated the immunological cross-reactivity between the two peptides. The human ZP3 protein has 94% homology with the monkey ZP3, and the peptides used in this study differ at only a single amino acid residue (340V to M). 19 As previously reported, macaques (Eenie and Meenie) were immunized with pZP3(hu) in adjuvant. 18 Antibody to pZP3(hu) of the IgG isotype was detected in the serum and titers sustained at 10 4 to 10 5 for >400 days by repeated boosting (Figure 1C) ▶ . Antibody bound to ovarian ZP was detected by direct immunofluorescence at the time of sacrifice (Figure 1C ▶ , inset). Circulating antibody to ZP was also detectable by indirect immunofluorescence (data not shown). T cells from pZP3(hu)-immunized animals proliferated strongly in response to the immunogen and cross-reacted to a lesser extent with the homologous pZP3(mac) but not at all with pZP3(mou) (Figure 1D) ▶ . These experiments demonstrate that the macaque and human peptides cross-react at the B-cell and T-cell levels even in outbred macaques. Our subsequent investigations showed that immunization with these immunologically cross-reactive peptides have similar effects on the ovary and on ovarian function.
pZP3(mac) and pZP3(hu) Immunization Is Associated with Ovarian Infiltration of T Cells and Activated Macrophages without Detectable Oophoritis
The ovary has many leukocytes that play a critical role in normal ovarian function including follicular atresia, ovulation, and involution of the corpus luteum. 20-22 Thus we first examined the distribution of T cells and macrophages in the ovaries from two untreated and two adjuvant-treated cycling adult macaques. As described in Materials and Methods, serial sections were cut through the entire ovary for each animal, and every 10th section was studied histologically. Adjacent sections were immunostained with antibodies to CD3, MHC II, macrophages (CD68, Mac 387) and B cells. As reported in humans, 23 T cells and macrophages expressing MHC II were detected in normal or adjuvant-treated monkey ovaries. In addition, atretic follicles and corpora lutea were infiltrated by small numbers of T cells and MHC II-positive macrophages (Figure 2, A and B) ▶ . Occasional clusters of T cells were also located in the ovarian cortex and medulla (Figure 2C) ▶ . However, these T-cell clusters did not contain any detectable MHC II-positive macrophages (Figure 2D) ▶ .
Figure 2.
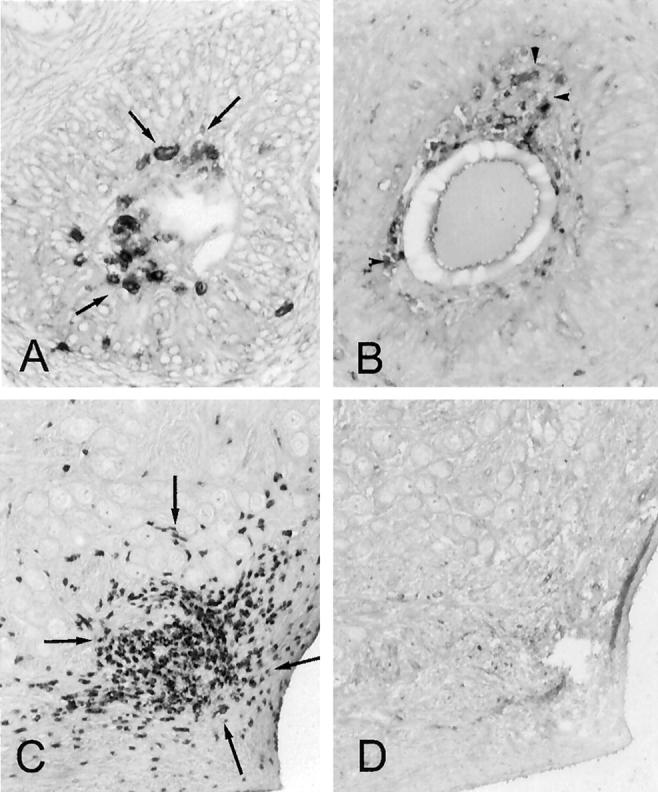
Immunoperoxidase staining for CD3+ T cells (arrows) and MHC II+ macrophages (arrowheads) in control macaque ovaries. A and B: Atretic follicles in adjacent sections have invading CD3+ T cells (A) along with MHC II-expressing cells (B). C and D: Adjacent sections of ovarian cortex reveal a cluster of CD3+ T cells (C) but absence of MHC II+ cells (D). Original magnifications, ×400.
In contrast, a unique pattern of cellular infiltration was detected in three of four pZP3-immunized monkeys. In these lesions, the T cells were found to co-localize with MHC II-expressing cells (Figure 3 ▶ ; A to D). These clusters of inflammatory cells were found in the interstitial or perifollicular locations. Because these typical leukocyte infiltrates were detected in pZP3-immunized monkeys but not in monkeys injected with adjuvant alone, they were considered to be pathologically relevant. Although macrophages (Mac 387 or CD68 positives) were detected in these T-cell- and MHC II-positive cellular infiltrates, they did not account for all of the MHC II staining in the inflammatory foci (data not shown). The additional MHC II-expressing cells may represent ovarian dendritic cells or granulosa cells of adjacent follicles, as described in human AOD. 24
Figure 3.
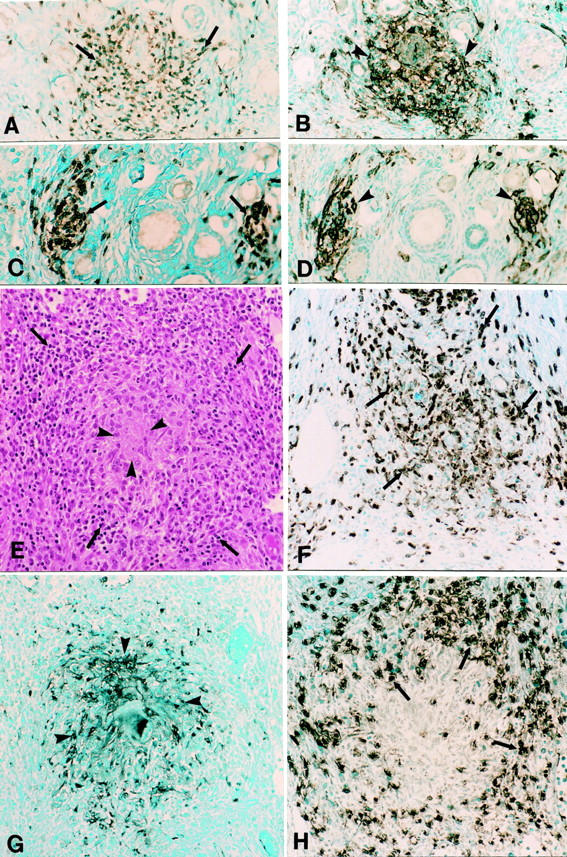
Ovarian inflammation in pZP3(mac)- and pZP3(hu)-immunized macaques. A–D: Adjacent sections of a typical primate AOD lesion consisted of clusters of CD3+ T cells (A, arrows) and MHC II + cells (B, arrowheads). C and D: Adjacent sections of another typical primate AOD lesion stained for CD3 (C) and MHC II (D). Adjacent sections of a large granuloma in the ovary of Eenie that was immunized with pZP3(hu) in adjuvant. E: Typical granulomatous inflammation with eosinophilic center (arrowheads) surrounded by cuff of lymphocytes (arrows) (H&E). The granuloma contains many macrophage (Mac 387+) (F), a central core of MHC II-positive cells (G), and a rim of CD3+ T cells (H). Original magnifications, ×400.
Interestingly, despite the impressive cellular infiltrates detected immunohistologically, inflammatory leukocytes were not visualized histologically on adjacent sections of the same ovaries. This was true for formalin-fixed or the Bouin’s-fixed tissues. An exception was the ovary of Eenie, which was studied at 3 months after immunization with pZP3(hu) and adjuvant. This ovary contained several foci of granulomatous inflammation characterized by a central eosinophilic area surrounded by a cuff of lymphocytes (Figure 3E) ▶ . Immunostaining of adjacent sections showed invasion of macrophages in the region (Figure 3F) ▶ . The granuloma showed characteristic features of central MHC II-expressing cells surrounded by a ring of CD3-positive T lymphocytes (Figure 3, G and H) ▶ . Histopathological evaluation of other organs collected at sacrifice failed to show any detectable inflammation.
Ovarian Function Is Retained in the Presence of Oophoritis
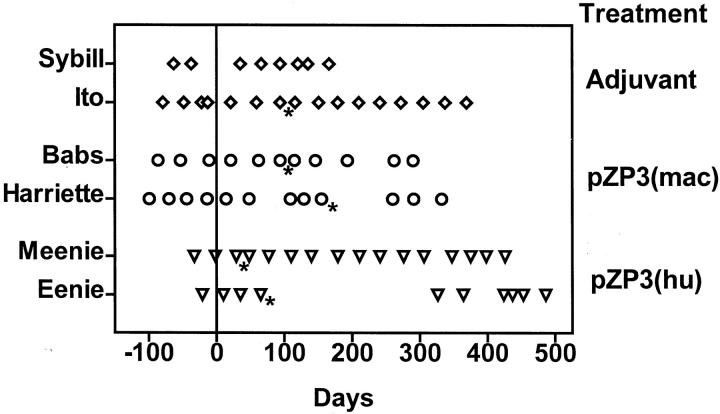
Menstrual bleeding in the pZP3-immunized and adjuvant-treated animals was monitored. The cycles in all of the animals except Eenie (the animal with granulomatous oophoritis) were found to be regular and comparable to adjuvant-treated controls (Figure 4) ▶ . Eenie stopped menstruating for 7 months after ovariectomy after which menstrual bleeding resumed spontaneously. Sera were collected at two phases of the study to monitor endocrine function as described in Materials and Methods. The mean peak estradiol and progesterone levels studied early (when antibody titers were low) and late (when ZP antibody titers remained high) were comparable in all of the animals except Eenie (Table 1) ▶ . Although the data collected is during a limited period of study, the pattern showed functioning ovaries with an estradiol peak preceding the progesterone peak (data not shown). Steroid analysis after ovariectomy in Eenie showed loss of cyclicity with estradiol <20 pg/ml and peak progesterone reaching only 2.3 ng/ml.
Figure 4.
Ovarian cyclicity in adjuvant-immunized (Sybill, Ito), pZP3(mac)-immunized (Harriette, Babs), and pZP3(hu)-immunized (Meenie, Eenie) macaques monitored until sacrifice. Each point indicates time of menstrual bleeding. Time of first immunization is indicated as day 0. Asterisk denotes surgical removal of one ovary.
Table 1.
Peak Serum Estradiol and Progesterone in Macaques Immunized with ZP3 Peptides
| Animal | Early phase | Late phase | ||
|---|---|---|---|---|
| Peak estradiol (pg/ml) | Peak progesterone (ng/ml) | Peak estradiol (pg/ml) | Peak progesterone (ng/ml) | |
| Babs | 127.3 ± 79.5* (3)† | 37.1 ± 13.5 (3) | 56.9 ± 14.9 (2) | 40.0 ± 31.82 (2) |
| Harriette | 61.8 ± 37.5 (3) | 24.6 ± 6.2 (3) | 64.9 ± 44.2 (3) | 21.9 ± 3.18 (2) |
| Meenie | 145.7 ± 26.5 (3) | 21.1 ± 5.5 (3) | 84.3 ± 50.5 (2) | 27.7 ± 7.4 (2) |
| Eenie‡ | 31.4 ± 11.7 (2) | 26.9 (1) | 119.6 (1) | 15.7 (1) |
*Data are mean ± SD of peak values in sera.
†No of peaks studied.
‡Includes only those cycles where menstrual bleeding was detected.
Comparative Histology and Immunopathology of Murine ZP3 Peptide-Induced AOD
AOD is induced by injection of pZP3(mou) in adult B6AF1 female mice, 12 but a detailed analysis of the immunopathology of the oophoritic lesion has not been described. To compare murine and macaque AOD, we evaluated mouse ovaries for the distribution of T cells (CD5), macrophages (F4/80), MHC II, and B cells (B220).
Immunostaining of the ovarian inflammatory infiltrates in murine AOD and the primate AOD are similar. In murine AOD, the infiltrating lymphocytes were predominantly T cells (Figure 5A) ▶ , with only occasional B cells (data not shown). Like the lesion in macaque AOD, T cells were found to form clusters with F4/80-positive macrophages that expressed MHC II (Figures 5, A and C) ▶ . Macrophages were normally present in large numbers in the normal mouse ovary. Adjuvant treatment resulted in the increase in ovarian macrophages that expressed MHC class II (Figure 5D) ▶ , but there was no increase in T-cell infiltration (Figure 5B) ▶ .
Figure 5.
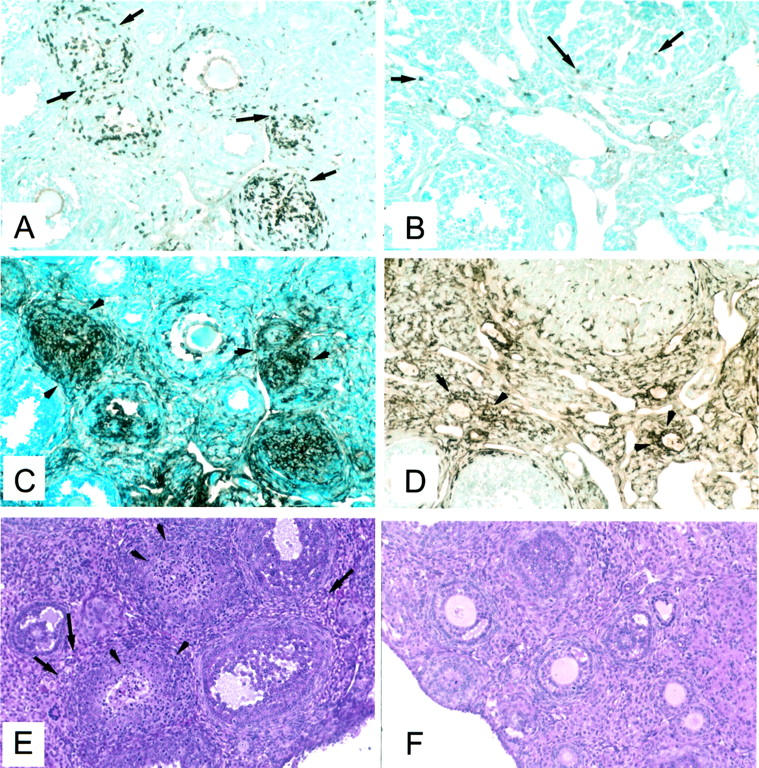
Immunohistopathology of murine AOD. A, C, and E: Ovary of a mouse immunized with pZP3(mou) in CFA. Adjacent sections showing clusters of cells T cells (CD5+) (A, arrows) that co-localize with intensely MHC II+ cells (C, arrowheads). The opposite ovary stained with H&E (E) shows lymphocytic infiltration in the interstitial regions (arrows) and the ovarian follicles (arrowheads). B, D, and F: Ovary of an adjuvant-treated mouse showing a few T (CD5+) cells (B) and an adjacent section (D) with many MHC II+ macrophages in the interstitium and the corpus luteum. The opposite ovary (F) stained with H&E appears completely normal.
In contrast to macaque ovaries, histological evidence of oophoritis was readily detected in murine AOD. The lesions consisted of inflammation in the interstitium and some involvement of developing follicles (Figure 5E) ▶ . The inflammation was focal or diffuse, frequently appearing as monocytic granulomata and in some cases, multinucleated giant cells. No inflammatory lesions were seen in animals immunized with adjuvant alone (Figure 5F) ▶ .
Discussion
Human AOD is characterized by a wide spectrum of histopathological changes. 3-6 Lonsdale and colleagues 25 have summarized a comprehensive list of histologically confirmed reports of human autoimmune oophoritis. The inflammation may involve ovarian follicular structures or interstitial regions. The interstitial inflammation may be restricted to the hilar region, perifollicular regions, or may extend diffusely throughout the ovary. The wide range of inflammatory lesions is associated with varying clinical presentation from asymptomatic oophoritis, menstrual disturbances, to POF and infertility. Lymphocytes and plasma cells are the most common infiltrating cells. The distributions of inflammatory cells in normal human ovary defined by immunohistology serve as a baseline for identification of pathological infiltrates. 23 However, immunohistological studies on the ovarian disease are limited to three published reports on four patients. 25-27 They have identified T cells as the dominant infiltrating cells. Some macrophages were also detected in the infiltrates. Local production of proinflammatory cytokines such as interferon-γ by infiltrating T cells has been implicated in the aberrant expression of MHC II on granulosa cells at the site of inflammation. 24 These features are similar to our findings in the macaque and mouse ZP-induced AOD. Although typical granulomatous oophoritis resembling the inflammation seen in Eenie, has been reported in patients their immunohistological findings were not studied. 5 B cells and plasma cells are identified in the human AOD as well as murine AOD induced by neonatal thymectomy (preliminary data) but are rarely found in monkey or murine AOD induced by pZP3. Another type of human oophoritis contains mainly eosinophils, 28 and similar pathology has been elicited in mice by adoptive transfer of Th2 (interleukin-4 and interleukin-5 producing), pZP3-specific T cells (P Alard and KSK Tung, unpublished), and in pinworm-infected neonatal mice injected with pZP3(mou) without adjuvant. 29
Immunization with zona pellucida proteins and peptides has also been investigated as immunocontraceptive vaccine. Vaccines based on the whole porcine zona pellucida proteins have been applied effectively to control feral horse and elephant populations. 30,31 As a potential vaccine for human population control, primates (marmoset, baboons, squirrel monkeys, and macaques) have been immunized with heterologous zona pellucida proteins. Although this approach resulted in infertility, 16,17,32 varying extents of irreversible ovarian dysfunction were also observed. Antibody to ZP was postulated to interfere with junctional complexes between granulosa cells and oocytes leading to the loss of developing follicles, and accelerated recruitment of the primordial follicles. However, other studies on squirrel monkeys and bonnet monkeys immunized with porcine ZP proteins with alum and muramyl di-peptide (MDP) adjuvants were found to develop high levels of circulating ZP antibodies. These studies showed a reversible contraceptive effect without any loss of ovarian function. 33,34 These contradictory data suggest that the high titers of ZP antibody alone are not sufficient to explain irreversible loss of ovarian function after ZP protein immunization in primates. Because no obvious inflammation was detected, autoimmune oophoritis was not considered a basis for ovarian failure in ZP-immunized primates.
The ZP3 peptide with well-defined T- and B-cell epitopes capable of eliciting a restricted immune response has been used to dissect the mechanism of AOD 12 in the mouse model. Transfer of ZP3 peptide-specific T cells into naïve recipient mice resulted in granulomatous oophoritis and enhanced ovarian expression of interleukin-1, tumor necrosis factor-α, and interferon-γ. However, the ovarian function of cell recipients was normal and the mice remained fertile. 13 Antibody to ZP3 also does not cause any ovarian pathology. These results indicate that T-cell-mediated ovarian inflammation alone or ZP antibody alone does not affect ovarian function. However, co-transfer of pathogenic T cells and ZP antibody targets the inflammation into developing follicles leading to their destruction and the development of ovarian atrophy. 14 Thus combined action of both antibody and proinflammatory T cells is required for severe ovarian injury leading to POF. In this study we show that ZP3 peptide immunization in primates can elicit a T-cell response and cause ovarian immunopathology that is similar to murine AOD. These data suggest a role for T cells in macaque AOD. It will be important to determine in the future whether the combined effects of antibody and proinflammatory T cells are responsible for the long-term ovarian changes in monkeys injected with ZP3.
Acknowledgments
We thank Melissa Bevard and Joyce Nash, Cell Science Core, for expert technical assistance; Valerie Long, Ligand Preparation and Assay Core, Center for Molecular Studies in Reproduction, University of Virginia for steroid analysis; and the Tissue Specificity Core, Center of Reproductive Gamete Contraceptive Vaccinogens, University of Virginia for normal ovarian tissue for the frozen sections and immunohistochemical staining.
Footnotes
Address reprint requests to Harini Bagavant, Department of Pathology, Box 800214, Health Sciences Center, University of Virginia, Charlottesville, VA 22908. E-mail: hb5u@virginia.edu.
Supported by the National Institutes of Health (grant U54 HD 29099) and a Fogarty Foundation fellowship (to H. B.).
References
- 1.Wheatcroft N, Weetman : Is premature ovarian failure an autoimmune disease? Autoimmunity 1997, 25:157–165 [DOI] [PubMed]
- 2.Hoek A, Schoemaker J, Drexhage HA: Premature ovarian failure and ovarian autoimmunity. Endocr Rev 1997, 18:107-134 [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Kempers RD, Randall RV: Premature ovarian failure: evidence for the autoimmune mechanism. Fertil Steril 1981, 36:238-240 [DOI] [PubMed] [Google Scholar]
- 4.Bannatyne P, Russel P, Shearman RP: Autoimmune oophoritis: a clinicopathological assessment of 12 cases. Int J Gynecol Pathol 1990, 9:191-207 [DOI] [PubMed] [Google Scholar]
- 5.Russel P, Bannatyne P, Shearman RP: Premature hypergonadotropic ovarian failure: clinicopathologic study of 19 cases. Int J Gynecol Pathol 1982, 1:186-201 [DOI] [PubMed] [Google Scholar]
- 6.Irvine WJ, Chan MMW, Scath L, Kolb FO, Hartog M, Bayliss RIS, Drury MI: Immunological aspects of premature ovarian failure associated with idiopathic Addison’s disease. Lancet 1968, 2:883-887 [DOI] [PubMed] [Google Scholar]
- 7.Arif S, Vallian S, Farzaneh F, Zanone MM, James SL, Pietropaolo M, Hettirachchi S, Vergani D, Conway GS, Peakman M: Identification of 3 beta hydroxysteroid dehydrogenase as a novel target of steroid cell autoantibodies with endocrine autoimmune disease. J Clin Endocrinol Metab 1996, 81:4439-4445 [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Sawika J, Betterle C, Powell M, Prentice L, Volapto M, Rees Smith B, Furmaniak J: Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison’s disease, and premature ovarian failure. J Clin Endocrinol Metab 1996, 81:1871-1876 [DOI] [PubMed] [Google Scholar]
- 9.Miyake T, Taguchi O, Ikeda H, Sato Y, Takeuchi S, Nishizuka Y: Acute oocyte loss in experimental autoimmune oophoritis is a possible model of premature ovarian failure. Am J Obstet Gynecol 1988, 156:186-192 [DOI] [PubMed] [Google Scholar]
- 10.Asano M, Toda M, Sakaguchi N, Sakaguchi S: Autoimmune disease as a consequence of developmental abnormality of a T cell population. J Exp Med 1996, 184:387-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KSK: Endogenous oocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day 3 thymectomy. J Immunol 2001, 166:4363-4369 [DOI] [PubMed] [Google Scholar]
- 12.Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T, Allen P, Dean J, Tung KSK: Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest 1992, 89:28-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagavant H, Adams SE, Terranova P, Chang A, Kramer FW, Lou YH, Kasai K, Tung KSK: Autoimmune ovarian inflammation triggered by proinflammatory (Th1) T cells is compatible with normal ovarian function. Biol Reprod 1999, 61:635-642 [DOI] [PubMed] [Google Scholar]
- 14.Lou YH, Park KK, Agersborg SS, Alard P, Tung KSK: Retargetting of T cell mediated inflammation: a new perspective on autoantibody. J Immunol 2000, 164:5251-5257 [DOI] [PubMed] [Google Scholar]
- 15.Lou YH, Ang J, Thai H, McElveen F, Tung KSK: A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J Immunol 1995, 155:2715-2720 [PubMed] [Google Scholar]
- 16.Paterson M, Thillai Koothan P, Morris KD, O’Byrne KT, Braude P, Williams A, Aitken RJ: Analysis of contraceptive potential of antibodies against native and deglycosylated porcine ZP3 in vivo and in vitro. Biol Reprod 1992, 46:523-534 [DOI] [PubMed] [Google Scholar]
- 17.VandeVoort CA, Schwoebel ED, Dunbar BS: Immunization of monkeys with recombinant complimentary deoxyribonucleic acid expressed zona pellucida proteins. Fertil Steril 1995, 64:838-847 [DOI] [PubMed] [Google Scholar]
- 18.Bagavant H, Fusi FM, Baisch J, Kurth BK, David CS, Tung KSK: Immunogenicity and contraceptive potential of a human zona pellucida 3 peptide vaccine. Biol Reprod 1997, 56:764-770 [DOI] [PubMed] [Google Scholar]
- 19.Kolluri SK, Kaul R, Banerjee K, Gupta SK: Nucleotide sequence of cDNA encoding bonnet monkey (Macaca radiata) zona pellucida glycoprotein 3. Reprod Fertil Dev 1995, 7:1209-1212 [DOI] [PubMed] [Google Scholar]
- 20.Gaytan F, Morales C, Bellido C, Aguilar E, Sanchez-Criado JE: Ovarian follicle macrophages: is follicular atresia in the immature rat a macrophage mediated event? Biol Reprod 1998, 58:52-59 [DOI] [PubMed] [Google Scholar]
- 21.Presl J, Bukovsky A: Role of Thy-1+ and Ia+ cells in ovarian function. Biol Reprod 1986, 34:159-169 [DOI] [PubMed] [Google Scholar]
- 22.Gaytan F, Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE: Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod 1998, 59:417-425 [DOI] [PubMed] [Google Scholar]
- 23.Best CL, Pudney J, Welch WR, Burger N, Hill JA: Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod 1996, 11:790-797 [DOI] [PubMed] [Google Scholar]
- 24.Hill JA, Welch WR, Faris HMP, Anderson DJ: Induction of class II histocompatibility complex antigen expression on human granulosa cells by interferon gamma: a potential mechanism contributing to autoimmune failure. Am J Obstet Gynecol 1990, 162:534-540 [DOI] [PubMed] [Google Scholar]
- 25.Lonsdale RN, Roberts PF, Trowell JE: Autoimmune oophoritis associated with polycystic ovaries. Histopathology 1991, 19:77-81 [DOI] [PubMed] [Google Scholar]
- 26.Sommerville JE, Iftikhar M, O’Sullivan JF, Hayes D: Autoimmune oophoritis an incidental finding. Pathol Res Pract 1991, 189:475-477 [DOI] [PubMed] [Google Scholar]
- 27.Sedmak DD, Hart WR, Tubbs RR: Autoimmune oophoritis: a histopathologic study of involved ovaries with immunologic characterization of mononuclear cell infiltrate. Int J Gynecol Pathol 1987, 6:73-81 [PubMed] [Google Scholar]
- 28.Lewis J: Eosinophilic perifolliculitis: a variant of autoimmune oophoritis? Int J Gynecol Pathol 1993, 12:360-364 [PubMed] [Google Scholar]
- 29.Agersborg SS, Garza KM, Tung KSK: Intestinal parasitism terminates self tolerance and enhances neonatal induction of autoimmune disease and memory. Eur J Immunol 2001, 31:851-859 [DOI] [PubMed] [Google Scholar]
- 30.Kirkpatrick JF, Liu IM, Turner Jr JW, Naugle R, Keiper R: Long term effect of procine zonae pellucidae immunocontraception on ovarian function in feral horses (equus cabalus). J Reprod Fertil 1992, 94:437–444 [DOI] [PubMed]
- 31.Frayer Hoskin RA, Grobler D, Van Altena JJ, Bertschinger HJ, Kirkpatrick JF: Immunocontraception of African elephants. Nature 2000, 407:149. [DOI] [PubMed] [Google Scholar]
- 32.Gulyas BJ, Gwatkin RBL, Yuan LC: Active immunization of cynomolgus monkeys (Macaca fascicularis) with porcine zonae pellucidae. Gamete Res 1983, 4:299-307 [Google Scholar]
- 33.Sacco AG, Subramanian MG, Yurewicz EC, DeMayo FJ, Dukelow WR: Heteroimmunization of squirrel monkeys (Saimiri sciureus) with a purified porcine zona antigen (PPZA): immune response and biologic activity of antiserum. Fertil Steril 1983, 39:350-358 [DOI] [PubMed] [Google Scholar]
- 34.Bagavant H, Thillaikoothan P, Sharma MG, Talwar GP, Gupta SK: Antifertility effects of porcine zona pellucida-3 immunization using permissible adjuvants in female bonnet monkeys (Macaca radiata): reversibility, effect on follicular development and hormone profiles. J Reprod Fertil 1994, 102:17-25 [DOI] [PubMed] [Google Scholar]