Abstract
Immune responses against cardiac myosin and group A streptococcal M protein have been implicated in the pathogenesis of rheumatic heart disease. Although cardiac myosin is known to produce myocarditis in susceptible animals, it has never been investigated for its role in production of valvular heart disease, the most serious sequelae of group A streptococcal infection in acute rheumatic fever. In our study, cardiac myosin induced valvulitis in the Lewis rat, and epitopes responsible for production of valvulitis were located in the rod region. Human and rat cardiac myosins induced severe myocarditis in the Lewis rats as expected. A purified S2 fragment (amino acid sequences 842 to 1295) produced the most severe myocarditis as well as valvulitis. Different regions of light meromyosin produced valvulitis (residues 1685 to 1936) or myocarditis (residues 1529 to 1611). Because streptococcal M proteins produced valvular heart disease in Lewis rats and have been linked to anti-cardiac myosin responses, we reacted myosin-sensitized lymphocytes isolated from the hearts of Lewis rats with peptides of streptococcal M5 protein in tritiated thymidine assays. Infiltrating lymphocytes responded most strongly to peptides within the B repeat region of streptococcal M protein. These data show direct evidence that immune responses against cardiac myosin lead to valvular heart disease and the infiltration of the heart by streptococcal M protein reactive T lymphocytes.
Infectious pathogens, including viruses, 1 group A streptococci, 2 or chlamydia 3 are important etiological agents of inflammatory heart disease. However, immune and specifically autoimmune mechanisms are the major effectors of pathogenic injury. 4 The autoantigen most often associated with both myocarditis and rheumatic carditis is cardiac myosin. 2,5-13 In 1985, myosin was identified as an autoantigen involved in cross-reactivity between the group A streptococcus and heart. 14 Since this time, evidence has supported the molecular mimicry hypothesis that streptococcal M protein and the group A carbohydrate both induce anti-myosin responses that attack the heart. 7,15-19 Rheumatic carditis and myocarditis may have similar pathogenic mechanisms because immunological mimicry was demonstrated between streptococcal M protein and myocarditic coxsackieviruses and both were associated with cytotoxic antibody against heart cells as well as T-lymphocyte responses. 18,20-22 Peptides of streptococcal M protein were shown to produce myocarditis in mice 7 and intact recombinant streptococcal M protein produced valvular heart disease in Lewis rats. 17 Studies also suggested that a myocarditic peptide of streptococcal M protein, which mimics cardiac myosin, tolerated and protected MRL/++ mice against autoimmune myocarditis after coxsackieviral infection. 23 However, it is debated as to whether or not mimicry between coxsackieviruses and cardiac myosin plays a role in the pathogenesis of myocarditis. 24,25
There is strong evidence that cardiac myosin is a dominant autoantigen in autoimmune myocarditis 8 and viral-induced myocarditis. 10 Investigators have demonstrated that myosin-induced myocarditis can be adoptively transferred by CD4+ T lymphocytes. 12 In addition to T cells, passive administration of anti-myosin monoclonal antibody was found to induce myocarditis in DBA/2 but not BALB/c mice because of the presence of myosin or a myosin-like protein in the extracellular matrix of DBA/2 mice. 26 Gauntt and colleagues 27-29 investigated the relationship between coxsackievirus and myosin and suggested that molecular mimicry between myosin and coxsackieviruses may play a role in myocarditis. Anti-coxsackieviral-neutralizing antibody produced myocardial inflammation in mice. 27 In rheumatic carditis, group A streptococcal infection plays a major role and anti-myosin antibody is associated with disease. 30,31 Monoclonal anti-myosin antibody has been shown to be cytotoxic for heart cells in culture and was found to recognize laminin on the valve and myocyte cell surface. 15,16 Antibodies in rheumatic carditis deposit in myocardium as well as valvular endothelium, 16,32,33 and streptococcal M protein reactive T cells were found in human rheumatic valves. 34 Thus, in inflammatory heart diseases, myocarditis, and rheumatic carditis, both antibody and T cells are implicated in the disease.
Although valvulitis is the most serious complication of rheumatic fever and leads to the development of rheumatic heart disease, 35 animal models of valvulitis have not been previously investigated. In addition, the role of cardiac myosin in valvular heart disease has not been previously addressed. In our study, we identified epitopes in human cardiac myosin (HCM) that induced valvulitis in Lewis rats. In the light meromyosin (LMM) region, myocarditis- and valvulitis-inducing epitopes could be separated, but the S2 rod fragment produced both myocarditis and valvulitis. Our data show that valvulitis was induced by immunization with cardiac myosin, cardiac myosin subfragments, heavy meromyosin (HMM), or S2, and with one region in LMM that was different from a site in LMM that produced myocarditis. Furthermore, lymphocytes isolated from the hearts of Lewis rats with myocarditis and valvulitis proliferated in the presence of specific streptococcal M5 protein peptides. These data show direct evidence that immune responses against cardiac myosin can induce valvular heart disease and the infiltration of the heart by streptococcal M protein-reactive T lymphocytes.
Materials and Methods
Antigens
Bovine serum albumin, rabbit skeletal tropomyosin, rabbit skeletal HMM, and rabbit skeletal LMM were purchased from Sigma Chemical Co., St. Louis, MO. Peptides from the LMM region of HCM and the streptococcal M5 protein were synthesized on a Dupont RAMPS manual peptide synthesizer using the f-moc strategy by the Molecular Biology Resource Center at the University of Oklahoma Health Sciences Center, Oklahoma City, OK (Dr. Ken Jackson, Director). Peptides were synthesized as 18-mers with a five-amino acid overlap. The streptococcal M5 peptides represented the sequence of the type 5 streptococcal M protein of the Streptococcus pyogenes (Manfraedo strain). The amino acid sequences of the human cardiac LMM peptides are shown in Table 1 ▶ and the streptococcal M5 peptides are shown in Table 2 ▶ .
Table 1.
Human Cardiac Light Meromyosin Peptides
| Peptide no. | Amino acid sequence | Residue no. |
|---|---|---|
| LMM-1 | KEALISSLTRGKLTYTQQ | 1295–1312 |
| LMM-2 | TYTQQLEDLKRQLEEEVK | 1308–1325 |
| LMM-3 | EEEVKAKNALAHALQSAR | 1321–1338 |
| LMM-4 | LQSARHDCDLLREQYEEE | 1334–1351 |
| LMM-5 | EQYEEETEAKAELQRVLSK | 1346–1364 |
| LMM-6 | RVLSKANSEVAQWRTKYE | 1360–1376 |
| LMM-7 | RTKYETDAIQRTEELEEA | 1373–1390 |
| LMM-8 | ELEEAKKKLAQRLQEAEE | 1386–1403 |
| LMM-9 | QEAEEAVEAVNAKCSSLE | 1399–1416 |
| LMM-10 | CSSLEKTKHRLQNEIEDL | 1412–1429 |
| LMM-11 | EIEDLMVDVERSNAAAAA | 1425–1442 |
| LMM-12 | AAAAALDKKQRNFDKILA | 1438–1455 |
| LMM-13 | DKILAEWKQKYEESQSEL | 1451–1468 |
| LMM-14 | SQSELESSQKEARSLSTE | 1464–1481 |
| LMM-15 | SLSTELFKLKNAYEESLE | 1477–1494 |
| LMM-16 | EESLEHLETFKRENKNLQ | 1490–1507 |
| LMM-17 | NKNLQEEISDLTEQLGSS | 1503–1520 |
| LMM-18 | EQLGSSGKTIHELEKVRKQ | 1516–1533 |
| LMM-19 | KVRKQLEAEKMELQSALE | 1529–1546 |
| LMM-20 | LQSALEEAEASLEHEEGKI | 1542–1559 |
| LMM-21 | EEGKILRAQLEFNQIKAE | 1555–1572 |
| LMM-22 | NQIKAEIERKLAEKDEEME | 1568–1585 |
| LMM-23 | DEEMEQAKRNHLRVVDSL | 1581–1598 |
| LMM-24 | VVDSLQTSLDAETRSRNE | 1594–1611 |
| LMM-25 | RSRNEALRVKKKMEGDLN | 1607–1624 |
| LMM-26 | EGDLNEMEIQLSHANRMA | 1620–1637 |
| LMM-27 | ANRMAAEAQKQVKSLQSL | 1633–1650 |
| LMM-28 | SLQSLLKDTQIQLDDAVR | 1646–1663 |
| LMM-29 | RANDDLKENIAIVERRNN | 1659–1676 |
| LMM-30 | IAIVERRNNLLQAELEEL | 1672–1689 |
| LMM-31 | ELEELRAVVEQTERSRKL | 1685–1702 |
| LMM-32 | RSRKLAEQELIETSERVQ | 1698–1715 |
| LMM-33 | SERVQLLHSQNTSLINQK | 1711–1728 |
| LMM-34 | LINQKKKMDADLSQLQTE | 1724–1741 |
| LMM-35 | TEVEEAVQESRNAEEKAKK | 1737–1754 |
| LMM-36 | RNAEEKAKKAITDAAMMA | 1750–1767 |
| LMM-37 | AAMMAEELKKEQDTSAHL | 1763–1780 |
| LMM-38 | TSAHLERMKKNMEQTIKDL | 1777–1794 |
| LMM-39 | TIKDLQHRLDEAEQIALK | 1790–1807 |
| LMM-40 | EQIALKGGKKQLQKLEARV | 1802–1820 |
| LMM-41 | LEARVRELENELEAEQKR | 1816–1833 |
| LMM-42 | AEQKRNAESVKGMRKSER | 1829–1846 |
| LMM-43 | RKSERRIKELTYQTEEDR | 1842–1859 |
| LMM-44 | TEEDRKNLLRLQDLVDKL | 1855–1872 |
| LMM-45 | LVDKLQLKVKAYKRQAEE | 1868–1885 |
| LMM-46 | RQAEEAEEQANTNLSKFR | 1881–1898 |
| LMM-47 | LSKFRKVQHELDEAEERA | 1894–1910 |
| LMM-48 | AEERADIAESQVNKLRAK | 1907–1923 |
| LMM-49 | KLRAKSRDIGTKGLNEE | 1920–1936 |
Table 2.
Amino Acid Sequence of Type 5 M Protein Peptides*
| NT-1† | AVTRGTINDPQRAKEALD |
| NT-2 | KEALDKYELENHDLKTKN |
| NT-3 | LKTKNEGLKTENEGLKTE |
| NT-4 | GLKTENEGLKTENEGLKTE |
| NT-5 | KKEHEAENDKLKQQRDTL |
| NT-6 | QRDTLSTQKETLEREVQN |
| NT-7 | REVQNTQYNNETLKIKNG |
| NT-8 | KIKNGDLTKELNKTRQEL |
| B1-A | TRQELANKQQESKENEKAL |
| B1-B | ENEKALNELLEKTVKDKI |
| B1-B2 | VKDKIAKEQENKETIGTL |
| B2 | TIGTLKKILDETVKDKIA |
| B2-B3A | KDKIAKEQBNKETIGTLK |
| B3A | ITGLKKILDETVKDKLAK |
| B2-B3B | DKLAKEQKSKQNIGALKQ |
| B3B | GALKQELAKKDEANKISD |
| C1A | NKISDASRKGLRRDLDAS |
| C1B | DLDASREAKKQLEAEHKQ |
| C1-C2 | AEHQKLEEQNKISEASRK |
| C2A | EASRKGLRRDLDASREAK |
| C2B | SREAKKQLEAEQQKLEEQ |
| C2-C3 | KLEEQNKISEASRKGLRR |
| C3 | KGLRRDLDASREAKKQ |
*Predicted amino acid sequence of serotype 5 M protein from S. pyogenes, strain Manfredo.
†Peptides represent the sequence found in the amino terminal region (NT), and B (B) and C (C) repeat regions of the M5 protein.
Myosin Purification
Cardiac myosin was purified from human (HCM) and rat (RCM) heart tissue, and human skeletal myosin was purified from human quadriceps tissue according to Tobacman and colleagues 36 with slight modifications. Briefly, heart tissue was homogenized in a low salt buffer [40 mmol/L KCl, 20 mmol/L imidazole (pH-7.0), 5 mmol/L EGTA, 5 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride (PMSF), and 1 μg/ml leupeptin] for 15 seconds on ice. The washed myofibrils were collected by centrifugation at 16,000 × g for 10 minutes. The myofibrils were then resuspended in high-salt buffer (0.3 mol/L KCl, 0.15 mol/L K2HPO4, 1 mmol/L EGTA, 5 mmol/L dithiothreitol, 0.5 mmol/L PMSF, and 1 μg/ml leupeptin) and homogenized for three 30-second bursts on ice. The homogenized tissue was further incubated on ice with stirring for 30 minutes to facilitate actomyosin extraction. After clarification by centrifugation, actomyosin was precipitated by adding 10 volumes of cold water and adjustment of the pH to 6.5. Dithiothreitol was added to 5 mmol/L and the precipitation allowed to proceed for 30 minutes. The actomyosin was then collected by centrifugation at 16,000 × g. The actomyosin pellet was then resuspended in high-salt buffer, ammonium sulfate was increased to 33%, and the KCl concentration was increased to 0.5 mol/L. After the actomyosin pellet and salts were dissolved, ATP was added to 10 mmol/L, MgCl2 was added to 5 mmol/L, and the solution was centrifuged at 20,000 × g for 15 minutes to remove actin filaments. The supernatant was removed and stored at 4°C in the presence of the following inhibitors: 0.5 mmol/L PMSF, 5 μg/ml TLCK, and 1 μg/ml leupeptin.
Isolation of Cardiac Myosin Fragments
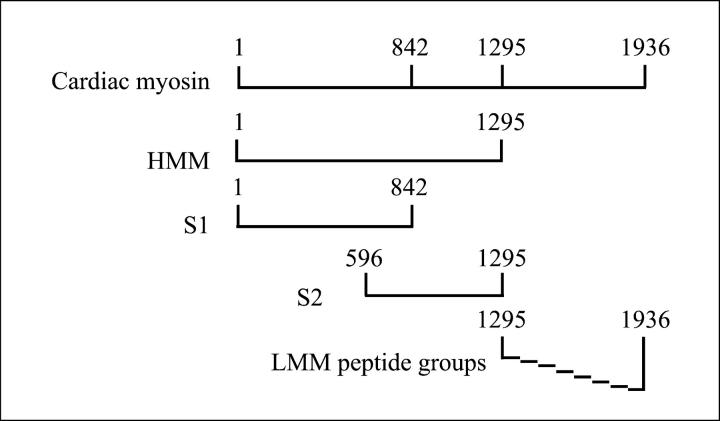
The myosin molecule can be divided into subfragments through proteolysis. At lower ionic strengths, chymotryptic cleavage yields subfragment-1 (S1) and myosin rod; whereas, at higher ionic strength, chymotryptic cleavage yields HMM and LMM. Further cleavage of HMM yields S1 and S2 subfragments. Purified subfragments and synthetic LMM peptides from HCM that were tested in Lewis rats are shown in Figure 1 ▶ . Purity of all proteins was assessed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and in some cases, fragments required further purification by ammonium sulfate precipitation and/or diethyl amino ethyl ion exchange chromatography.
Figure 1.
Diagram of HCM, cardiac myosin subfragments, and LMM peptide groups. HMM, subfragment-1 (S1), and subfragment-2 (S2) were enzymatically cleaved and purified as described in Materials and Methods and as shown in the diagram. Each of the eight LMM peptide groups consisted of six consecutive, overlapping, synthetic peptides. The LMM peptides correspond to the LMM region of HCM as shown.
Human Myosin Subfragment-1
In our studies, S1 was produced from purified HCM according to Tobacman and colleagues 36 with slight modification. Briefly, myosin was dialyzed against digestion buffer (0.1 mol/L NaCl, 0.01 mol/L imidazole-HCl, pH 7, and 0.001 mol/L dithiothreitol) and cleaved with a 1:100 w/w ratio of α-chymotrypsin to myosin for 15 minutes at 25°C. The reaction was terminated by the addition of PMSF to a final concentration of 0.3 mmol/L. The rod and uncleaved myosin were precipitated by dialysis in a low salt solution (10 mmol/L NaCl, 10 mmol/L imidazole, pH 7.0, 1 mmol/L dithiothreitol) and then separated from the soluble S1 by centrifugation at 20,000 × g.
Human Cardiac HMM
The HMM subfragment of HCM was prepared according to a previously described procedure with slight modifications. 37 Briefly, HMM was prepared by digesting myosin with α chymotrypsin 100:1 w/w ratio in 0.6 mol/L KCl, 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, and 0.01 mol/L Tris-HCl (pH 7.6) for 10 minutes at 25°C. The reaction was terminated by adding PMSF to a final concentration of 0.2 mmol/L. The LMM and uncleaved myosin were precipitated by dialysis in a low-salt solution (0.03 mol/L KCl, 0.01 mol/L potassium phosphate, pH 6.3, 1 mmol/L dithiothreitol, and 1 mmol/L MgCl2) and then separated from the soluble HMM by centrifugation at 20,000 × g.
S2 Fragment of Human Cardiac Myosin
Normally, S2 subfragment of cardiac myosin was prepared by controlled proteolysis of purified HMM subfragment. However, in these studies, an S2-containing fragment was produced from purified preparations of human cardiac HMM that seemed to be proteolyzed by an innate protease. The S2 fragment was purified by applying the innately proteolyzed preparation of HMM to S-400 gel filtration chromatography. Identity of S2 was confirmed by N-terminal sequencing using the Edman degradation method. Myosin subfragments are shown in a diagram in Figure 1 ▶ .
Immunization
Female Lewis rats (6- to 8-weeks old) were purchased from Harlan-Sprague-Dawley (Indianapolis, IN) and maintained in groups of three or four at the Animal Resources Facility on the campus of the University of Oklahoma Health Sciences Center. Five hundred μg of protein or peptide was emulsified in Freund’s complete adjuvant at a 1:1 ratio (v/v) and injected into one hind footpad of rats anesthetized with 10 mg of ketamine/0.2 mg of xylazine. On days 1 and 3 after immunization, the rats were intraperitoneally administered 1 × 10 10 killed Bordetella pertussis organisms as an adjuvant. Seven days after the primary immunization rats were boosted subcutaneously with 500 μg of protein or peptide emulsified in Freund’s incomplete adjuvant (1:1 ratio). Control rats received phosphate-buffered saline (PBS) or bovine serum albumin emulsified in Freund’s adjuvant and B. pertussis injections.
Histopathological Examination of Tissue
Rats were terminated 21 days after the primary immunization by cardiac puncture while under anesthesia. Heart, liver, and kidneys were excised and fixed in 10% buffered formalin. After routine tissue preparation (ie, paraffin embedding, slide mounting, and hematoxylin and eosin staining), myocardium was blindly scored for the presence of histopathological myocarditis according to the following scale: 0 = normal, 1 = mild (<5% of heart cross-section involved), 2 = moderate (5 to 10% of cross-section involved), 3 = marked (10 to 25% of cross-section involved), 4 = severe (>25% of cross-section involved). Valve tissue was observed for the presence of cellular infiltrates. Liver and kidneys were also evaluated for abnormalities, but were found to be normal.
Isolation and Proliferation Assay of Lymphocytes Infiltrating Hearts in Cardiac Myosin-Induced Myocarditis and Valvulitis
Inflamed hearts from Lewis rats with myocarditis/valvulitis were perfused with heparinized Iscove’s modified Dulbecco’s medium, minced, and treated with collagenase at 160 U/ml (Sigma Chemical Co.) for 30 minutes at 37°C. The tissue was then pressed through a screen to obtain a single cell suspension and washed with Iscove’s modified Dulbecco’s medium. The cell suspension was separated in Ficoll Hypaque (Sigma Chemical Co.) and lymphocytes recovered after centrifugation. The cells were washed in Iscove’s modified Dulbecco’s medium and were resuspended in proliferation medium and reacted with M5 peptides in the tritiated thymidine uptake assay using 5 × 10 3 cells/well. To provide additional antigen presenting cells, 5 × 10 3 mitomycin C-treated normal rat spleen cells were added to each well. Proliferation medium consisted of Iscove’s modified Dulbecco’s medium, 2% rat serum, 50 μmol/L 2-mercaptoethanol, 100 U penicillin, and 100 μg streptomycin and streptococcal M5 peptide at 20 μg/ml. Wells were pulsed with 1.0 μCi of tritiated thymidine (ICN, Irvine, CA) 18 hours before being harvested onto filters with a cell harvester. Tritiated thymidine incorporation was measured by a liquid scintillation counter. Values represent the stimulation index (stimulation index equals test counts per minute/media control counts per minute).
Results
Induction of Myocarditis with Cardiac Myosin, Subfragments, and LMM Peptides
Lewis rats immunized with HCM developed severe clinical and histological myocarditis as expected (Table 3 ▶ and Figure 2a ▶ ). This response was specific for the cardiac isoform as immunization with human skeletal myosin, rabbit skeletal LMM, and rabbit skeletal HMM did not cause myocarditis (Table 3) ▶ . Additionally, rabbit skeletal tropomyosin, and bovine serum albumin did not induce myocarditis (Table 3) ▶ . Lewis rats immunized with rat cardiac myosin (RCM) developed myocarditis that was clinically and histologically indistinguishable from myocarditis induced by HCM (Table 3 ▶ and Figure 2b ▶ ). Histological lesions displayed intense inflammatory infiltrates consisting of lymphocytes, macrophages, neutrophils, and multinucleated giant cells (Figure 2, a and b) ▶ .
Table 3.
Induction of Myocarditis in Lewis Rats by Immunization with Cardiac Myosin, Myosin Subfragments, or LMM Peptides
| Immunogen | Myocarditis (positive/total) | Average lesion score* |
|---|---|---|
| Whole molecule | ||
| Human cardiac myosin | 18 /23 | 3.0 |
| Rat cardiac myosin | 13 /13 | 3.4 |
| Subfragments of HCM | ||
| HMM subfragment of HCM | 5 /5 | 4.0 |
| S1 subfragment of HCM | 0 /7 | 0 |
| S2 subfragment of HCM† | 3 /3 | 3.8 |
| Related molecules and fragments | ||
| Human skeletal myosin | 0 /6 | 0 |
| Rabbit skeletal LMM | 0 /5 | 0 |
| Rabbit skeletal HMM | 0 /3 | 0 |
| Rabbit skeletal tropomyosin | 0 /6 | 0 |
| Light meromyosin peptides | ||
| LMM peptides 1–6‡ | 1 /9 | 2.5 |
| LMM peptides 7–12 | 1 /6 | 0.5 |
| LMM peptides 13–18 | 0 /6 | 0 |
| LMM peptides 19–24 | 5 /11 | 2.0 |
| LMM peptides 25–30 | 0 /6 | 0 |
| LMM peptides 31–36 | 0 /6 | 0 |
| LMM peptides 37–42 | 0 /4 | 0 |
| LMM peptides 43–49 | 0 /6 | 0 |
| Controls | ||
| Bovine serum albumin | 0 /6 | 0 |
| PBS + adjuvants | 0 /34 | 0 |
*Lesions were scored histologically based on the following scale: 0 = normal, 1 = mild (less than 5% of cross section involved), 2 = moderate (5 to 10% of cross section involved), 3 = marked (10–25% of cross section involved), 4 = severe (greater than 25% of cross section involved).
†Ninety five kd subfragment of human cardiac myosin containing the S2 region as determined by Edman degradation.
‡LMM peptide sequences are listed in Table 1 ▶ .
Figure 2.
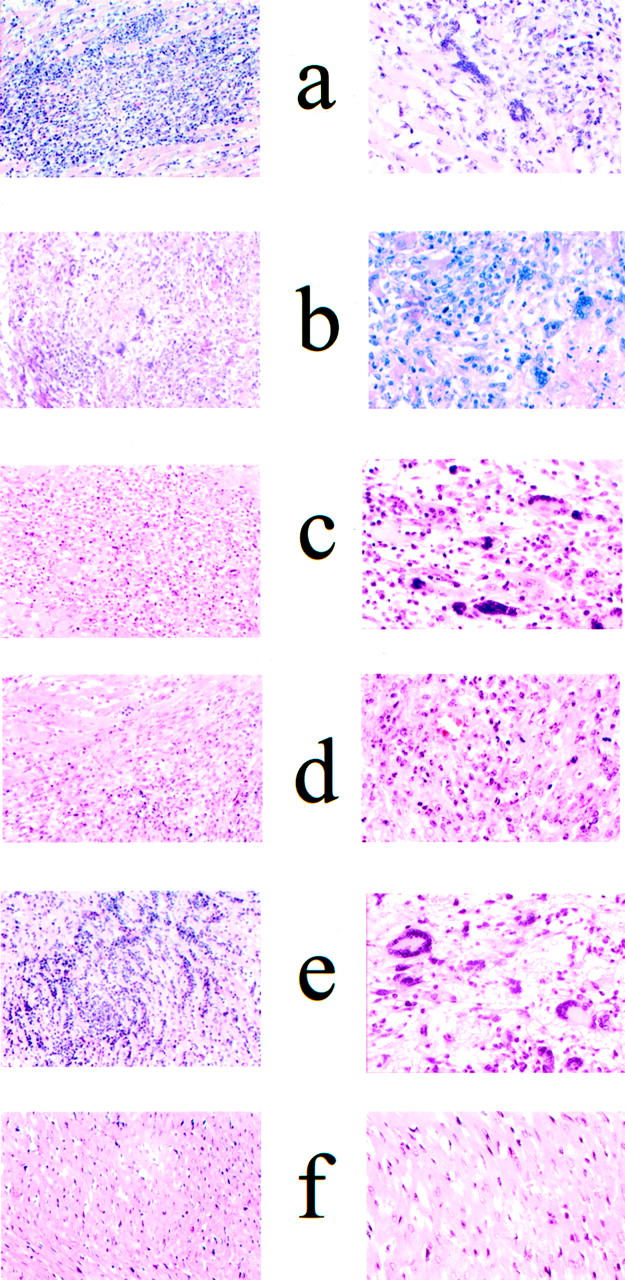
Histopathological features of autoimmune myocarditis in Lewis rats. Animals were immunized with HCM (a), RCM (b), HMM fragment of HCM (c), S2 fragment of HCM (d), HCM LMM peptides 19 to 24 (e), and PBS plus adjuvants (f) as described in Material and Methods. Lewis rats immunized with human or RCM, LMM peptide group 19 to 24, HMM fragment, or S2 fragment of HCM exhibit myocyte necrosis in the myocardium with intense inflammatory infiltrates (a–e). Control rats immunized with PBS plus adjuvants (f) or with bovine serum albumin plus adjuvants (not shown) have no detectable histopathological lesions. Original magnifications: ×200 (left panels); ×400 (right panels).
To confirm and extend knowledge about epitopes that produce myocarditis in the Lewis rat, purified HCM subfragments HMM, S1, and S2, were administered to Lewis rats. HMM is a large subfragment of myosin comprised of the S1 and S2 segments of the molecule (see diagram in Figure 1 ▶ ). When administered to Lewis rats, HMM induced severe disease in 100% (five of five) of rats (Table 3 ▶ , Figure 2c ▶ ). All five animals had severe clinical signs of disease, and the histopathological lesions were throughout a large portion of the heart and scored as severe (Table 3) ▶ . Immunization with the globular head or S1 portion of the cardiac myosin molecule did not induce myocarditis (Table 3) ▶ , suggesting that disease-producing epitopes in the Lewis rat were confined to the rod region.
To further analyze the rod region, an S2 fragment, whose molecular mass was estimated at 95 kd by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was purified from human cardiac HMM. Edman degradation sequence analysis of the first five amino acids of this fragment revealed that the N terminus was at amino acid residue 596 (sequence: KNKDP). Therefore, this HCM fragment extended from amino acid residue 596 to residue 1295 (the C-terminal end of the HMM molecule) and contained the entire S2 portion of the HCM molecule. When administered to Lewis rats, the extended S2 subfragment of HCM induced severe myocarditis, comparable to that induced by the intact myosin molecule and HMM (Table 3) ▶ . The histopathological lesions from Lewis rats immunized with the S2 fragment are shown in Figure 2d ▶ . Severe myocyte necrosis of the myocardium was observed and the inflammatory cell infiltrate consisted of mononuclear cells and neutrophils. Control animals immunized with bovine serum albumin or PBS plus adjuvants had no detectable myocardial inflammation (Table 3 ▶ and Figure 2f ▶ ). These results confirmed that the S1 fragment did not induce disease and that the S2 region strongly induced myocarditis in Lewis rats.
For localization of myocarditic epitope(s) in the LMM tail fragment of the myosin rod, 49 overlapping 18-mer peptides that span the LMM region of the cardiac myosin molecule were synthesized (Table 1) ▶ based on published sequences. 38 Because no single LMM peptide could induce disease, the LMM peptides were tested for myocarditis as eight peptide groups with six consecutive peptides comprising each LMM peptide group (Table 3) ▶ . The LMM peptide group containing peptides 1 to 6 induced moderate inflammation in only one of nine animals indicating a very minor pathogenic epitope (Table 3) ▶ . However, the peptide group containing LMM peptides 19 to 24 induced moderate myocarditis in 45% (5 of 11) of the animals tested (Table 1 ▶ , Figure 2e ▶ ). LMM peptides 19 to 24 corresponded to amino acid residues 1529 to 1611 in HCM. Although, myocarditis was induced by the LMM 19- to 24-peptide group in ∼50% of the rats, severity was less than that induced by intact cardiac myosin, HMM, or S2 subfragments, suggesting that the site in LMM may not be a dominant myocarditic region for Lewis rats. By comparing the different regions of the cardiac myosin molecule in a single study, it allowed us to identify the most and the least myocarditic regions in the Lewis rat model and to put these epitopes in perspective with disease.
Cardiac Myosin-Induced Valvulitis in Lewis Rats
Valve tissue from Lewis rats immunized with HCM and its subfragments was obtained and examined histopathologically for the presence of inflammatory cells (ie, valvulitis). Forty-three percent of Lewis rats immunized with HCM and 46% of rats given RCM developed valvulitis (Table 4) ▶ . Although not every rat that developed myocarditis developed valvulitis, at least half of the rats that developed myocarditis developed valvulitis. The valvular inflammation was characterized histopathologically as the presence of cellular infiltrates composed primarily of mononuclear cells within valve tissue (Figure 3, A and B) ▶ . Skeletal myosin did not induce valvular inflammation (Table 4) ▶ .
Table 4.
Induction of Valvulitis in Lewis Rats by Immunization with Cardiac Myosin, Myosin Subfragments, or LMM Peptides
| Immunogen | Valvulitis* (positive/total) |
|---|---|
| Whole molecule | |
| Human cardiac myosin | 10 /23 |
| Rat cardiac myosin | 6 /13 |
| Subfragments of HCM | |
| HMM | 4 /5 |
| S1 | 0 /4 |
| S2 | 2 /3 |
| Related molecules | |
| Human skeletal myosin | 0 /6 |
| Light meromyosin peptides | |
| LMM peptides 1–6 | 0 /6 |
| LMM peptides 7–12 | 0 /6 |
| LMM peptides 13–18 | 0 /6 |
| LMM peptides 19–24 | 0 /11 |
| LMM peptides 25–30 | 0 /6 |
| LMM peptides 31–36 | 2 /6 |
| LMM peptides 37–42 | 2 /4 |
| LMM peptides 43–49 | 2 /6 |
| Controls | |
| Bovine serum albumin | 0 /6 |
| PBS+ adjuvants | 0 /34 |
*Presence of inflammatory cells in valve tissue were indicated as shown in Figure 3 ▶ . The level of valvulitis was similar in peptide groups 31 to 36, 37 to 42, and 43 to 49. These peptide groups produced a similar valvulitis that was seen as cellular infiltration at the valvular surface endothelium. In some instances, the valvulitis was more severe for the whole cardiac myosin molecule.
Figure 3.
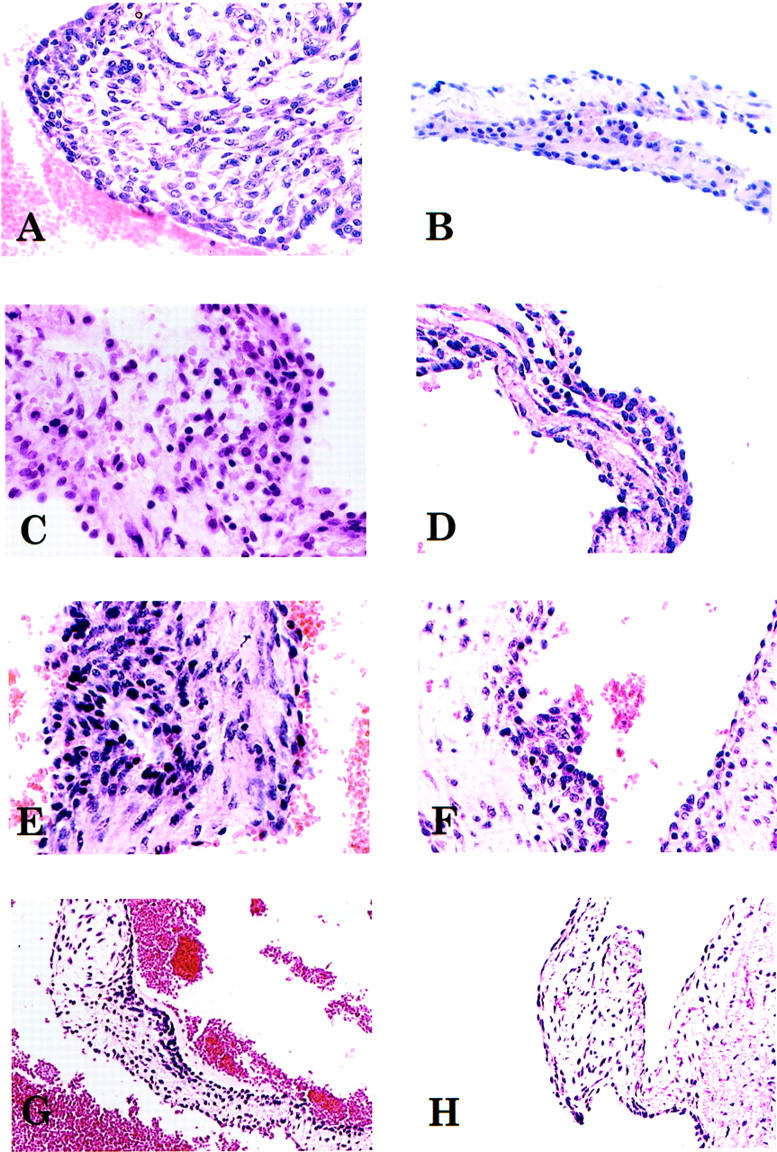
Histopathological features of autoimmune valvulitis in Lewis rats. Animals were immunized with HCM (A), RCM (B), HMM fragment of HCM (C), S2 fragment of HCM (D), LMM peptide group 31 to 36 (E), LMM peptide group 37 to 42 (F), LMM peptide group 43 to 49 (G), or PBS plus adjuvants (H). Mononuclear cell infiltrates can be distinguished in the valves of Lewis rats immunized with human or RCM, HMM fragment, S2 fragment of HCM, or LMM peptide groups (A–G). No mononuclear cell infiltrates could be detected in valves from control animals (H). Original magnifications: ×400 (A–F); ×200 (G and H).
Localization of Valvulitis-Inducing Fragments of Cardiac Myosin
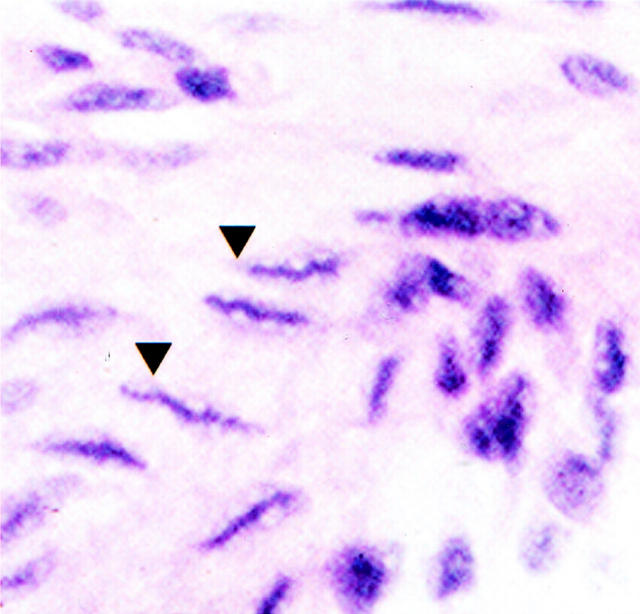
Immunization with the cardiac HMM subfragment produced valvulitis in four of five animals (Table 4 ▶ , Figure 3C ▶ ) and immunization with S2 produced valvulitis in two of three animals (Table 4 ▶ , Figure 3D ▶ ). Using groups of synthetic peptides (Table 1) ▶ , three valvulitis-inducing epitopes were discovered in the LMM region of HCM (Table 4) ▶ . One-third of Lewis rats immunized with LMM peptides 31 to 36 developed valvulitis (Table 4 ▶ , Figure 3E ▶ ). LMM groups containing peptides 37 to 42 (Table 4 ▶ , Figure 3F ▶ ) and 43 to 49 (Table 4 ▶ , Figure 3G ▶ ) also induced valvulitis in two of four and two of six animals, respectively. Control rats immunized with bovine serum albumin plus adjuvants or PBS plus adjuvants had no detectable valvular inflammation (Table 4 ▶ , Figure 3H ▶ ). Cells with caterpillar nuclei characteristic of Anitschkow cells, present in the valves of patients with rheumatic fever, 35 could be found scattered throughout the valves of Lewis rats immunized with cardiac myosin, HMM, or S2 subfragments, and LMM peptides 31 to 36, 37 to 42, and 43 to 49 (Figure 4) ▶ .
Figure 4.
Presence of Anitschkow-like cells in valves from Lewis rats immunized with HMM from HCM. In addition to inflammatory cell infiltrates, cells with caterpillar nuclei morphology were observed (arrowheads). The Anitschkow-like cells shown in the figure are cut longitudinally and the condensed chromatin gives the appearance of a caterpillar. Cells with caterpillar nuclei were observed in valves from rats immunized with intact HCM, RCM, and HCM subfragments, and LMM peptides.
In summary, valvulitis-producing epitopes in cardiac myosin were located in the rod region. However, the epitopes in LMM that produced myocarditis (residues 1529 to 1611) were located in a different region from those producing valvulitis (residues 1685 to 1936). However, both myocarditis and valvulitis were produced by the HMM and S2 subfragments, but not by the S1 subfragment, suggesting that the most pathogenic epitope is in the S2 subfragment.
Lymphocytes Infiltrating Hearts in Myocarditis and Valvulitis Proliferate to Group A Streptococcal M5 Peptides
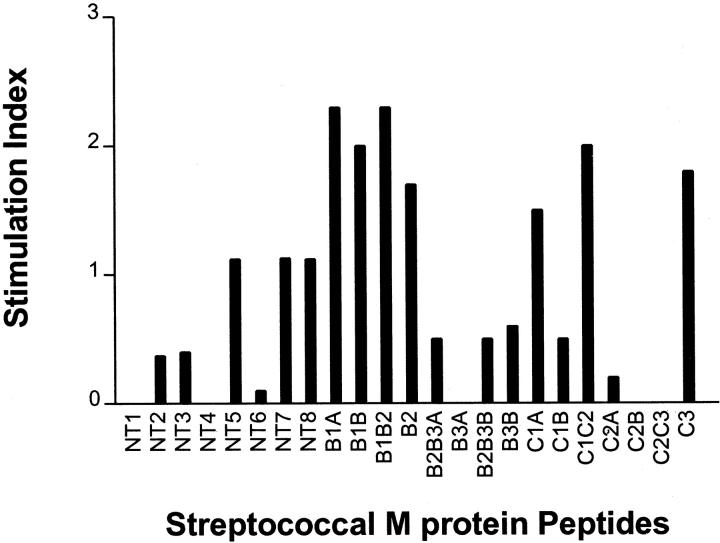
Lymphocytes isolated from hearts after induction of cardiac myosin-induced inflammatory heart disease were reacted with streptococcal M5 protein peptides to determine the responsiveness of myosin-sensitized lymphocytes to streptococcal M protein. Lymphocytes tested were isolated from inflamed hearts from four rats showing clinical signs of myocarditis. Lymphocytes from the myocardial infiltrate proliferated to four M5 peptides including a group of overlapping peptides in the B repeat region of the streptococcal M protein including M5 peptides B1A, B1B, B1B2 that indicated stimulation indices of >2.0 (Figure 5) ▶ . Also elevated were two peptides in the C repeat region including C1C2 and C3. These data provide evidence that streptococcal M protein cross-reacts with myosin-sensitized T cells in hearts developing inflammatory heart disease such as myocarditis and valvulitis.
Figure 5.
Reaction of lymphocytes infiltrating hearts in cardiac myosin-induced myocarditis and valvulitis proliferated to group A streptococcal M5 peptides. Lymphocytes from the myocardial infiltrate proliferated to four M5 peptides including M5 peptides B1A, B1B, and B1B2 with stimulation indices of >2.0. Also with elevated stimulation indices were two peptides in the C repeat region, C1C2 and C3. These data provide evidence that streptococcal M protein cross-reacts with myosin sensitized T cells in hearts developing inflammatory heart disease.
Discussion
Herein we report an animal model of valvular heart disease that to our knowledge has not been described. In addition, our novel observations in the Lewis rat show that cardiac myosin-induced valvular heart disease resembled valve disease in rheumatic fever. Recently, we have shown that streptococcal recombinant M6 protein produced a striking cellular infiltration of the valve with focal lesions in myocardium. 17 In the M protein-induced valvulitis, the cellular infiltrates entered through the valve surface suggesting that the valve endothelium is an important location for entry of inflammatory cells rather than from myocardium. However, in the valvulitis associated with cardiac myosin-induced myocarditis, cellular infiltrates were often observed in the myocardium adjacent to the valve. The evidence also suggests that sensitivity of Lewis rats to valvular heart disease is novel and will be useful to investigate the pathogenesis of rheumatic and immune-mediated valvular heart disease.
Using rodent models, the role of cardiac myosin as an autoantigen in the pathogenesis of autoimmune myocarditis has been well established 8,9,11-13,39,40 . Myocarditis can be induced by cardiac myosin in A/J mice, 8,12 BALB/c mice, 39,41 and in Lewis rats. 9,13 However, C57BL/6 mice are resistant to myosin-induced myocarditis. Exposure of cardiac myosin in the heart may be an important event leading to the onset of disease in the susceptible host. 42 Evidence has shown that in normal myocardium myosin-class II major histocompatibility antigen complexes are present before the induction of autoimmune myocarditis. 43 Induction of myocarditis is seen only with cardiac myosin and not skeletal myosin. 8 Unique epitopes within cardiac myosin have been described to produce myocarditis. Myocarditis was induced by amino acid residues 334 to 352, located in the S1 region of A/J mouse cardiac myosin, 11 residues 736 to 1032 in BALB/c cardiac myosin, 41 acetylated residues 614 to 643 of RCM-produced disease in BALB/c mice, 35 residues 1070 to 1165 of porcine cardiac myosin-induced disease in Lewis rats, 38 residues 1107 to 1186 in the Lewis rat, 44 and acetylated Lewis rat LMM region residues 1539 to 1555. 13 Our study in Lewis rats suggests that epitopes within the LMM region produce valvulitis whereas the most severe myocarditis was produced by the S2 region of cardiac myosin.
We have found both CD4+ and CD8+ T cells present in valves and myocardium of cardiac myosin-induced myocarditis and valvulitis (data not shown). This is similar to what we have found in human rheumatic heart disease. 45 The data also link epitopes of streptococcal M protein and cardiac myosin together because myosin-sensitized lymphocytes from inflamed hearts proliferated to several streptococcal M protein peptides (Figure 5) ▶ . This further supports the hypothesis that the Lewis rat is similar to the human, because T cells isolated from valves of humans with rheumatic heart disease proliferate to a similar group of peptides of streptococcal M5 protein. 34 The homology and α-helical coiled-coil structure shared between M protein and the cardiac myosin rod is significant enough that both produce inflammatory heart disease in Lewis rats. Although many M protein epitopes have been shown to be cross-reactive with myosin, only streptococcal M protein peptides sharing sequence homology with cardiac myosins produced myocardial inflammation in mice. 7,23 Repeated regions of M proteins that share homology with only cardiac myosins may break tolerance to cardiac myosin and induce myocardial disease. 23
Because cardiac myosin is not known to be present in the valve or expressed extracellularly in normal heart, how mimicry of cardiac myosin produces myocarditis and valvular heart disease is an important question. Our studies suggest that laminin may link myosin with the valve through cross-reactivity between the α-helical structures. Recently, a cytotoxic anti-myosin/anti-streptococcal monoclonal antibody from rheumatic carditis was shown to recognize laminin, an extracellular matrix α-helical coiled-coil protein that is an integral part of the valve structure as well as present in the extracellular matrix surrounding the cardiomyocyte. 16,20,46 Evidence presented in previous work supports the hypothesis that laminin, present in the basement membrane of the valve and secreted by endothelial cells, is a target of cross-reactive anti-myosin/anti-streptococcal antibody. Laminin present in valves and myocardium may cross-react with anti-myosin/anti-laminin T cells and antibody that recognize M protein, myosin, and laminin. Because cardiac myosin is an intracellular molecule, peptides of it may be presented to T cells during turnover in cardiac tissues. 43 Exposure of cardiac myosin in damaged regions of the heart may also lead to inflammatory heart disease.
A systematic approach was used to identify cardiac myosin epitopes responsible for myocarditis and valvulitis. Valvulitis and myocarditis were observed in Lewis rats given the S2 fragment and HMM. In LMM, different groups of peptides produced valvulitis (residues 1685 to 1936) or myocarditis (residues 1529 to 1611). Three different LMM regions (amino acids 1685 to 1767, 1763 to 1846, and 1842 to 1936) were involved in production of valvulitis in the rats. The incidence of valvulitis induced by the cardiac myosin subfragments and LMM peptide groups was decreased when compared to intact cardiac myosin. This suggests that the valvulitis-inducing epitopes in cardiac myosin may act in concert to produce valvular inflammation. The present studies provide a direct link between cardiac myosin and valvular inflammation suggesting that exposure of cardiac myosin may actively contribute to valvulitis during development of rheumatic heart disease. Collectively, our results demonstrate that myocarditic epitopes for Lewis rats are located within the rod region of the cardiac myosin molecule in S2 and LMM with the most pathogenic epitopes in S2. The myocarditis- and valvulitis-producing epitopes in the LMM region could be separated and were distinctly different and in two separate regions of the molecule.
Our results demonstrated that myocarditis induced by self antigen RCM was indistinguishable from that induced by HCM. Rat cardiac α-myosin (dominant isoform expressed in adult rat heart) and human cardiac β-myosin (dominant isoform expressed in human ventricle tissue) are 93% identical and 98% homologous at the amino acid sequence level. Mimicry of epitope(s) between HCM and RCM was sufficient to produce severe disease. Regardless of the source of cardiac myosin, myocarditis and valvulitis were induced in Lewis rats and multiple epitopes seemed to contribute to both diseases. The Lewis rat model of inflammatory heart disease will be a powerful tool for further investigation of the immunopathogenesis of human myocarditis and rheumatic valvulitis.
Acknowledgments
We thank Ms. Janet Heuser and Ms. Michelle Smart for expert technical assistance; and Dr. Ken Jackson and the W. K. Warren of the Molecular Biology Resource Facility at the University of Oklahoma Health Sciences Center for synthesis of the human cardiac light meromyosin and group A streptococcal M5 peptides.
Footnotes
Address reprint requests to Dr. Madeleine Cunningham, Department of Microbiology and Immunology, University of Oklahoma Health Sciences Center, Biomedical Research Center, 975 NE 10th Street, Oklahoma City, OK 73104. E-mail: madeleine-cunningham@ouhsc.edu.
Supported by grants HL35280 and HL56267 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (to M. W. C.).
Current address for A. Quinn: La Jolla Institute of Allergy and Immunology, La Jolla, CA.
References
- 1.Huber SA, Gauntt CJ, Sakkinen P: Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res 1998, 51:35-80 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham M: Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000, 13:470-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmaier K, Neu N, de la Maza L, Pal S, Hessel A, Penninger JM: Chlamydia infections and heart disease linked through antigenic mimicry. Science 1999, 283:1335-1339 [DOI] [PubMed] [Google Scholar]
- 4.Brown CA, O’Connell JB: Myocarditis and idiopathic dilated cardiomyopathy. Am J Med 1995, 99:309-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham MW, Hall NK, Krisher KK, Spanier AM: A study of monoclonal antibodies against streptococci and myosin. J Immunol 1985, 136:293-298 [PubMed] [Google Scholar]
- 6.Cunningham MW, Swerlick RA: Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med 1986, 164:998-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham MW, Antone SM, Smart M, Liu R, Kosanke S: Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun 1997, 65:3913-3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW: Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol 1987, 139:3630-3636 [PubMed] [Google Scholar]
- 9.Kodama M, Matsumoto Y, Fujiwara M, Massani M, Izumi T, Shibota A: A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol 1991, 57:250-262 [DOI] [PubMed] [Google Scholar]
- 10.Neu N, Beisel KW, Traystman MD, Rose NR, Craig SW: Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3 induced myocarditis. J Immunol 1987, 138:2488-2492 [PubMed] [Google Scholar]
- 11.Donermeyer DL, Beisel KW, Allen PM, Smith SC: Myocarditis-inducing epitope of myosin binds constitutively and stably to I-A K on antigen presenting cells in the heart. J Exp Med 1995, 182:1291-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SC, Allen PM: Myosin-induced acute myocarditis is a T cell mediated disease. J Immunol 1991, 147:2141-2147 [PubMed] [Google Scholar]
- 13.Wegmann KW, Zhao W, Griffin AC, Hickey WF: Identification of myocarditogenic peptides derived from cardiac myosin capable of inducing experimental allergic myocarditis in the Lewis rat. J Immunol 1994, 153:892-900 [PubMed] [Google Scholar]
- 14.Krisher K, Cunningham MW: Myosin: a link between streptococci and heart. Science 1985, 227:413-415 [DOI] [PubMed] [Google Scholar]
- 15.Adderson EE, Shikhman AR, Ward KE, Cunningham MW: Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetyl-glucosamine/anti-myosin antibody V region genes. J Immunol 1998, 161:2020-2031 [PubMed] [Google Scholar]
- 16.Galvin JE, Hemric ME, Ward K, Cunningham M: Cytotoxic monoclonal antibody from rheumatic carditis reacts with human endothelium: implications in rheumatic heart disease. J Clin Invest 2000, 106:217-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn A, Kosanke S, Fischetti VA, Factor SM, Cunningham M: Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect Immun 2001, 69:4072-4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shikhman AR, Greenspan NS, Cunningham MW: A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J Immunol 1993, 151:3902-3913 [PubMed] [Google Scholar]
- 19.Malkiel S, Liao L, Cunningham MW, Diamond B: T-cell-dependent antibody response to the dominant epitope of streptococcal polysaccharide, N-acetyl-glucosamine, is cross-reactive with cardiac myosin. Infect Immun 2000, 68:5803-5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ: Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA 1992, 89:1320-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber SA, Moraska A, Cunningham M: Alterations in major histocompatibility complex association of myocarditis induced by coxsackievirus B3 mutants selected with monoclonal antibodies to group A streptococci. Proc Natl Acad Sci USA 1994, 91:5543-5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber S, Polgar J, Moraska A, Cunningham M, Schwimmbeck P, Schultheiss P: T lymphocyte responses in CVB3-induced murine myocarditis. Scand J Infect Dis 1993, 88(Suppl):S67-S78 [PubMed] [Google Scholar]
- 23.Huber SA, Cunningham MW: Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J Immunol 1996, 156:3528-3534 [PubMed] [Google Scholar]
- 24.Rose N: Viral damage or ‘molecular mimicry’—placing the blame in myocarditis. Nat Med 2000, 6:631-632 [DOI] [PubMed] [Google Scholar]
- 25.Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N: Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med 2000, 6:693-697 [DOI] [PubMed] [Google Scholar]
- 26.Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B: Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med 1995, 187:1123-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauntt C, Arizpe H, Higdon A, Bowers D, Rozek M, Crawley R: Molecular mimicry, anti-coxsackievirus B2 neutralizing monoclonal antibodies and myocarditis. J Immunol 1995, 154:2983-2995 [PubMed] [Google Scholar]
- 28.Gauntt CJ, Arizpe HM, Higdon AL, Rozek MM, Crawley R, Cunningham MW: Anti-Coxsackievirus B3 neutralizing antibodies with pathological potential. Eur Heart J 1991, 12:124-129 [DOI] [PubMed] [Google Scholar]
- 29.Gauntt CJ, Higdon AL, Arizpe HM, Tamayo MR, Crawley R, Henkel RD, Pereira ME, Tracy SM, Cunningham MW: Epitopes shared between coxsackievirus B3 (CVB3) and normal heart tissue contribute to CVB3-induced murine myocarditis. Clin Immunol Immunopathol 1993, 68:129-134 [DOI] [PubMed] [Google Scholar]
- 30.Cunningham MW, McCormack JM, Talaber LR, Harley JB, Ayoub EM, Muneer RS, Chun LT, Reddy DV: Human monoclonal antibodies reactive with antigens of the group A streptococcus and human heart. J Immunol 1988, 141:2760-2766 [PubMed] [Google Scholar]
- 31.Cunningham MW, McCormack JM, Fenderson PG, Ho MK, Beachey EH, Dale JB: Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol 1989, 143:2677-2683 [PubMed] [Google Scholar]
- 32.Kaplan MH, Bolande R, Ratika L, Blair J: Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. N Engl J Med 1964, 271:637-645 [DOI] [PubMed] [Google Scholar]
- 33.Gulizia JM, Cunningham MW, McManus BM: Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves. Evidence for multiple cross-reactive epitopes. Am J Pathol 1991, 138:285-301 [PMC free article] [PubMed] [Google Scholar]
- 34.Guilherme L, Cunha-Neto E, Coelho V, Snitcowsky R, Pomerantzeff PMA, Assis RV, Pedra F, Neumann J, Goldberg A, Patarroyo ME, Pileggi F, Kalil J: Human heart-filtrating T cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation 1995, 92:415-420 [DOI] [PubMed] [Google Scholar]
- 35.Liao L, Sindhwani R, Leinwand L, Diamond B, Factor S: Cardiac α-myosin heavy chains differ in their induction of myocarditis: identification of pathogenic epitopes. J Clin Invest 1993, 92:2877-2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McManus BM, Chow LH, Wilson JE, Anderson DR, Gulizia JM, Gauntt CJ, Klingel KE, Beisel KW, Kandolf R: Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol 1993, 68:159-169 [DOI] [PubMed] [Google Scholar]
- 37.Smith SC, Allen PM: Expression of myosin-class II major histocompatibility complexes in the normal myocardium occurs before induction of autoimmune myocarditis. Proc Natl Acad Sci USA 1992, 89:9131-9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohno K, Takagaki Y, Nakajima Y, Izumi T: Advantage of recombinant technology for the identification of cardiac myosin epitope of severe autoimmune myocarditis in Lewis rats. Jpn Heart J 2000, 41:67-77 [DOI] [PubMed] [Google Scholar]
- 39.Stollerman GH: Rheumatic Fever and Streptococcal Infection. 1975. Grune and Stratton, New York
- 40.Tobacman LS, Adelstein RS: Enzymatic comparisons between light chain isozymes of human cardiac myosin subfragment-1. J Biol Chem 1984, 259:11226-11230 [PubMed] [Google Scholar]
- 41.Margossian SS, Lowey S: Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 1982, 85:55-71 [DOI] [PubMed] [Google Scholar]
- 42.Jaenicke T, Diederich KW, Haas W, Scheich J, Lichter P, Pfordt M, Bach A, Vosberg HP: Complete sequence of human β myosin. Genomics 1990, 8:194-207 [DOI] [PubMed] [Google Scholar]
- 43.Pummarer CL, Luze K, Grassl G, Bachmaier K, Offner F, Lenz DM, Zamborelli TJ, Penninger JM, Neu N: Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest 1996, 97:2057-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inomata T, Hanawa T, Mihanishi E, Yajima S, Nakayama T, Maita M, Kodama T, Izumi A, Shibata A, Abo T: Localization of porcine cardiac myosin epitopes that induce experimental autoimmune myocarditis. Circ Res 1995, 76:726-733 [PubMed] [Google Scholar]
- 45.Roberts S, Kosanke S, Dunn ST, Jankelow D, Duran CMG, Cunningham M: Immune mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis 2001, 183:507-511 [DOI] [PubMed] [Google Scholar]
- 46.Antone SM, Adderson EE, Mertens NMJ, Cunningham MW: Molecular analysis of V gene sequences encoding cytotoxic anti-streptococcal/anti-myosin monoclonal antibody 36.2.2 that recognizes the heart cell surface protein laminin. J Immunol 1997, 159:5422-5430 [PubMed] [Google Scholar]