Abstract
The tall-cell variant (TCV) of papillary thyroid carcinoma (PTC), characterized by tall cells bearing an oxyphilic cytoplasm, is more clinically aggressive than conventional PTC. RET tyrosine kinase rearrangements, which represent the most frequent genetic alteration in PTC, lead to the recombination of RET with heterologous genes to generate chimeric RET/PTC oncogenes. RET/PTC1 and RET/PTC3 are the most prevalent variants. We have found RET rearrangements in 35.8% of TCV (14 of 39 cases). Whereas the prevalences of RET/PTC1 and RET/PTC3 were almost equal in classic and follicular PTC, all of the TCV-positive cases expressed the RET/PTC3 rearrangement. These findings prompted us to compare RET/PTC3 and RET/PTC1 in an in vitro thyroid model system. We have expressed the two oncogenes in PC Cl 3 rat thyroid epithelial cells and found that RET/PTC3 is endowed with a strikingly more potent mitogenic effect than RET/PTC1. Mechanistically, this difference correlated with an increased signaling activity of RET/PTC3. In conclusion, we postulate that the correlation between the RET/PTC rearrangement type and the aggressiveness of human PTC is related to the efficiency with which the oncogene subtype delivers mitogenic signals to thyroid cells.
Follicular-cell-derived thyroid tumors are divided into four main types: benign adenomas, well differentiated (papillary or follicular), poorly differentiated, and undifferentiated (anaplastic) carcinomas. Papillary thyroid carcinoma (PTC) is the most common thyroid carcinoma. It is defined on the basis of the architectural pattern and/or distinctive nuclear features, ie, ground glass appearance and longitudinal grooves with cytoplasm invaginations. 1,2 PTC is usually associated with a good prognosis. However, some patients develop recurrence or distant metastases or die. 1,2 Advanced age at presentation, male sex, tumor size, extrathyroidal extension, and distant metastases are associated with a more dismal prognosis. PTC is subclassified, according to morphological features, in classic, follicular, solid, diffuse/sclerosing, tall-cell, columnar-cell, and diffuse/follicular variants. The tall-cell, solid, diffuse/sclerosing, and diffuse/follicular variants are associated with a higher incidence of local and vascular invasion and of regional and distant metastases. 1,2 Solid PTC are relatively frequent in young patients living in regions (Belarus, Ukraine, and western Russia) affected by the Chernobyl nuclear disaster of 1986. 3
The tall-cell variant (TCV) of PTC has distinctive histological features: formation of papillae, a high frequency of stromal lymphoid infiltrate, and numerous tall cells (>30% of the tumor cell population). Tall cells have an abundant eosinophilic and elongated cytoplasm, their height being at least twice their width. TCV carcinomas are usually larger and, at least in older patients, more likely to extend to extrathyroidal tissues than classic PTC. They have a greater tendency to local recurrences and are associated with a higher mortality (20 to 25%) than the classic PTC variant. 4-6 Furthermore, DNA topoisomerase II 7 and p53 8 expression is higher, p27KIP1 expression lower, 9 and the frequency of trisomy of chromosome 2 higher 10 in the TCV than in classic PTC.
Somatic rearrangements of the RET proto-oncogene are the most frequent genetic lesion found in PTC (from 2.5% to 40% depending on the series). 11 Recently RET rearrangements have been identified in Hurthle cell 12 and in hyalinizing trabecular tumors of the thyroid, 13,14 which suggests that these thyroid tumor variants are genetically linked to PTC. RET encodes the tyrosine kinase (TK) receptor for ligands of the glial cell line-derived neurotrophic factor (GDNF) family. 11 In PTC, chromosomal inversions or translocations cause the TK-encoding domain of RET to fuse to heterologous genes, leading to the generation of the chimeric RET/PTC oncogenes. 11 Several RET/PTC oncogenes, which differ in the RET fusion partner, have been identified. 11 RET/PTC1 (the H4-RET fusion) 15 and RET/PTC3 (the RFG/Ele1-RET fusion) 16 are the most prevalent variants. RET/PTC oncogenes are consistently found in radiation-associated PTC 11 and the RET/PTC3 oncogene has been correlated with solid PTC in Chernobyl patients, which suggests that this variant has high oncogenic activity. 17,18 The correlation between RET/PTC rearrangements and the clinical outcome of PTC is still obscure. Some authors have proposed that RET rearrangements are associated with local invasion and distant metastases. Others have found that RET/PTC are present in early-stage small papillary thyroid carcinomas, being apparently less important in the progression to clinically evident disease. 11 A possible explanation of these discrepancies is that RET/PTC subtypes differ in oncogenicity.
Here we report that the TCV of PTC preferentially harbors the RET/PTC3 oncogene. Accordingly, we found that RET/PTC3 has higher mitogenic and signaling activity than RET/PTC1 in epithelial thyroid cells in vitro.
Materials and Methods
Tumors
Thirty-nine TCV, 39 classic PTC, and 12 PTC of the follicular variant (FV) were collected from the files of the Department of Oncology of the University of Pisa. Diagnosis of TCV was confirmed in each case according to established criteria. 1,2 Specifically, we have classified in the TCV group PTCs having at least 70% of the cells showing a height being at least twice their width; 30 of 39 samples used in this study showed more than 90% of the cells with these characteristics. The following clinicopathological data were collected: sex, age, size of tumor, location, associated thyroid lesions, and metastatic deposits (Table 1) ▶ .
Table 1.
RET/PTC Expression in Thyroid Papillary Carcinoma Subtypes
| Sample | Type | Age* (years) | Sex | Nodal metastasis (no/yes) | RET/PTC1 | RET/PTC3 |
|---|---|---|---|---|---|---|
| 1 | Classic | 45 | F | N | ||
| 2 | Classic | 34 | F | N | ||
| 3 | Classic | 24 | F | N | ||
| 4 | Classic | 38 | F | N | + | |
| 5 | Classic | 33 | F | Y | ||
| 6 | Classic | 24 | F | N | + | |
| 7 | Classic | 53 | F | N | ||
| 8 | Classic | 42 | F | Y | + | |
| 9 | Classic | 22 | F | N | + | |
| 10 | Classic | 24 | M | Y | ||
| 11 | Classic | 48 | F | N | ||
| 12 | Classic | F | + | |||
| 13 | Classic | F | ||||
| 14 | Classic | 59 | F | N | ||
| 15 | Classic | 35 | F | N | ||
| 16 | Classic | F | ||||
| 17 | Classic | 34 | F | N | ||
| 18 | Classic | 34 | F | N | ||
| 19 | Classic | 53 | F | Y | + | |
| 20 | Classic | 12 | F | Y | ||
| 21 | Classic | 16 | F | N | ||
| 22 | Classic | 30 | F | N | ||
| 23 | Classic | 63 | M | Y | + | |
| 24 | Classic | 51 | F | N | ||
| 25 | Classic | M | N | |||
| 26 | Classic | F | ||||
| 27 | Classic | 75 | F | N | ||
| 28 | Classic | 9 | F | Y | + | |
| 29 | Classic | 23 | M | Y | ||
| 30 | Classic | 30 | M | N | ||
| 31 | Classic | 51 | F | + | ||
| 32 | Classic | 46 | F | |||
| 33 | Classic | 69 | F | + | ||
| 34 | Classic | 54 | F | Y | ||
| 35 | Classic | 39 | F | Y | + | |
| 36 | Classic | 29 | F | |||
| 37 | Classic | 45 | F | |||
| 38 | Classic | 53 | F | |||
| 39 | Classic | 37 | F | |||
| 40 | FV | 10 | M | N | ||
| 41 | FV | M | ||||
| 42 | FV | F | ||||
| 43 | FV | F | ||||
| 44 | FV | 40 | F | Y | + | |
| 45 | FV | 37 | M | N | ||
| 45 | FV | 30 | M | N | + | |
| 47 | FV | F | ||||
| 48 | FV | 49 | F | N | + | |
| 49 | FV | F | ||||
| 50 | FV | M | ||||
| 51 | FV | F | ||||
| 52 | FV | F | ||||
| 53 | TCV | M | + | |||
| 54 | TCV | F | ||||
| 55 | TCV | M | ||||
| 56 | TCV | 27 | F | N | ||
| 57 | TCV | 58 | F | N | ||
| 58 | TCV | M | + | |||
| 59 | TCV | 36 | F | Y | ||
| 60 | TCV | F | N | |||
| 61 | TCV | 49 | F | N | + | |
| 62 | TCV | 59 | F | N | ||
| 63 | TCV | 38 | F | N | ||
| 64 | TCV | 59 | F | N | + | |
| 65 | TCV | M | ||||
| 66 | TCV | 32 | F | Y | + | |
| 67 | TCV | 41 | F | N | + | |
| 68 | TCV | F | ||||
| 69 | TCV | F | Y | |||
| 70 | TCV | 23 | F | N | ||
| Sample | Type | Age* (years) | Sex | Nodal metastasis (no/yes) | RET/PTC1 | RET/PTC3 |
| 71 | TCV | 52 | M | N | + | |
| 72 | TCV | M | ||||
| 73 | TCV | 69 | F | N | ||
| 74 | TCV | 20 | F | + | ||
| 75 | TCV | 47 | F | N | ||
| 76 | TCV | 35 | M | |||
| 77 | TCV | 35 | F | N | + | |
| 78 | TCV | 21 | M | Y | + | |
| 79 | TCV | 45 | F | N | + | |
| 80 | TCV | 50 | F | + | ||
| 81 | TCV | 78 | F | Y | ||
| 82 | TCV | 67 | F | N | ||
| 83 | TCV | F | N | |||
| 84 | TCV | 65 | M | N | + | |
| 85 | TCV | 24 | M | N | ||
| 86 | TCV | 66 | F | N | ||
| 87 | TCV | 19 | M | Y | + | |
| 88 | TCV | F | N | |||
| 89 | TCV | 72 | F | |||
| 90 | TCV | 36 | F | N | ||
| 91 | TCV | F | N |
*Cases in which the information was not available are left blank.
Statistical Analysis
The non-parametric Fisher exact 2×2 table test was used to measure the association between the RET/PTC3 rearrangement and the TCV variant. The analysis was carried out using the STATISTICA 5.0 software (STATSOFT, Tulsa, OK).
Reverse Transcriptase-Polymerase Chain Reaction
RNA extraction, reverse transcription, and PCR amplification were performed as previously reported. 18 Positive controls were tumor samples harboring RET/PTC rearrangements. The common reverse primer (on the RET TK) was: 5′-TGCTTCAGGACGTTGAAC-3′. Forward primers, designed on the coiled-coil domains of the RET fusion partners, were as follows: RET/PTC1: 5′-ATTGTCATCTCGCCGTTC-3′; RET/PTC2: 5′-TATCGCAGGAGAGACTGTGAT-3′; RET/PTC3: 5′-AAGCAAACCTGCCAGTGG-3′; RET/PTC5: 5′-TACTAGAATACTGCAATC-3′; RET/PTC6: 5′-GCTCTACTGCATCAGTTAGAG-3′; RET/PTC7: 5′-CATTTTGCAGCTACTCAGGTG-3′; RET/PTC8: 5′-AC-AGGGAAGTGGTTACAGGA −3′.
Five hundred nanograms of RNA were reverse transcribed and subjected to 40 cycles of PCR (Perkin-Elmer, Norwalk, CT) (94°C for 30 seconds, 55°C for 2 minutes, and 72°C for 2 minutes). The product was analyzed on a 2% agarose gel and hybridized with a RET probe covering the TK domain. The human hypoxanthine phosphoribosyltransferase (HPRT) specific primers, used to assess RNA quality, were as follows: 5′-CCTGCTGGATTACATCAAAGCACTG-3′ (nucleotides 316 to 340) and 5′-CCTGAAGTATTCATTATAGTCTCAAGG-3′ (nucleotides 685 to 661). The amplified products were sequenced to confirm the rearrangement (Sequenase, USB, Cleveland, OH).
Immunohistochemistry
Anti-RET polyclonal rabbit antibodies were raised against the RET TK domain expressed in bacteria as a glutathione S-transferase (GST) fusion protein. They were affinity-purified by sequential chromatography on RET and GST-coupled agarose columns. The characterization and specificity of these antibodies are described elsewhere. 19 Formalin-fixed and paraffin-embedded 5-μm-thick tumor sections were deparaffinized, placed in a solution of absolute methanol and 0.3% hydrogen peroxide for 30 minutes, and treated with blocking serum for 20 minutes. The slides were incubated overnight with anti-RET antibody (1:100), with biotinylated anti-IgG and, finally, with premixed avidin-biotin complex (Vectostain ABC kits, Vector Laboratories, Burlingame, CA). The immune reaction was revealed with 0.06 mmol/L diaminobenzidine (DAB-DAKO, Carpinteria, CA) and 2 mmol/L hydrogen peroxide. The slides were counterstained with hematoxylin. As a control, anti-RET was preincubated with a fivefold molar excess of the antigen (GST-RET).
Cell Culture and Molecular Biology Techniques
LTR-based RET/PTC1 and RET/PTC3 expression vectors are described elsewhere. 20 PC Cl 3 cells were grown in Coon’s modified F12 medium (GIBCO-BRL, Paisley, PA) supplemented with 5% calf serum (GIBCO-BRL) and six hormones (6H; TSH, insulin, hydrocortisone, somatostatin, transferrin, and glycylhistidyl lysine) (Sigma Chemical, St. Louis, MO) and transfected as described elsewhere. 20 After selection with neomycin (G418), mass populations of several hundred clones of transfected cells were pooled and used for further analyses. For flow cytometry, cells were harvested 48 hours after reaching confluence or when subconfluent either in complete medium or in medium deprived of the 6H for 96 hours. Cells were fixed in methanol for 1 hour at −20°C, rehydrated in phosphate-buffered saline (PBS) for 1 hour at 4°C, and then treated with RNase A (50 μg/ml) for 30 minutes. Propidium iodide (25 μg/ml) was added to the cells and samples were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA) interfaced with a Hewlett Packard computer (Palo Alto, CA). The percentages of cells in the G0/G1, S, and G2/M compartments in 3 independent experiments were averaged.
TPC (RET/PTC1-positive thyroid papillary carcinoma cells) and ARO (RET/PTC-negative thyroid anaplastic carcinoma cells) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (GIBCO-BRL).
Protein Analysis
Protein extractions and immunoblotting were performed according to standard procedures by using Protran, nitrocellulose transfer membranes (Schleicher & Schuell, Dassel). Immune complexes were detected with the enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Little Chalfont, UK). The immune-complex kinase was assayed as described 21 by using 500 μg of protein lysates and 200 μmol/L of poly(l-glutamic acid-l-tyrosine) (polyGT) (Sigma) as substrate. Incorporation of labeled phosphate in the RET/PTC protein was measured by PhosphorImager analysis (GS525, Biorad, Hercules, CA) of polyacrylamide gels; phosphate incorporation in the polyGT was measured by scintillation counting of Whatman 3-mm filters spotted with the reaction. Phosphorylation-specific anti-phosphoRET(Y1062) antibodies were raised against a phosphorylated peptide spanning RET tyrosine 1062 and affinity purified as described. 22 Anti-phosphotyrosine antibodies (4G10) were from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-MAPK (mitogen-activated protein kinase) (9101) and anti-phospho-MAPK (9102) were from New England Biolabs (Beverly, MA). Secondary antibodies coupled to horseradish peroxidase were from Santa Cruz Biotechnology (Santa Cruz, CA).
Results and Discussion
High Prevalence of RET/PTC3 in TCV
To screen for RET rearrangements, 39 TCV samples were subjected to RT-PCR. As a control, 39 samples of classic PTC and 12 samples of the follicular variant were examined. The clinical and pathological data of the cases are summarized in Table 1 ▶ . We used one common primer on RET exon 12 and forward primers mapping on the different RET fusion partners. The results are summarized in Tables 1 and 2 ▶ ▶ and representative examples are shown in Figure 1 ▶ . In summary, a rearranged RET oncogene was detected in 14 of 39 TCV samples. This prevalence was not significantly different from that observed in the classic (11 of 39 samples) and follicular (3 of 12 samples) variants. However, there was a striking correlation between the TCV phenotype and the RET/PTC3 rearrangement. Whereas the prevalences of RET/PTC1 and RET/PTC3 in classic and follicular PTC were similar (5 RET/PTC1 and 6 RET/PTC3 in classic and 1 RET/PTC1 and 2 RET/PTC3 in follicular cases), RET/PTC3 was the only rearrangement found in TCV carcinomas, thus, there was a significant correlation between this rearrangement and TCV (P = 0.015). RET/PTC1 and RET/PTC3 were the only RET/PTC variants found in this series.
Table 2.
RET/PTC Expression in PTC Subtypes
| PTC subtype | RET/PTC-positive | RET/PTC1 | RET/PTC3 |
|---|---|---|---|
| Classic | 11/39 (27.5%) | 5/39 (10.3%) | 6/39 (17.2%) |
| FV | 3/12 (25%) | 1/12 (8.3%) | 2/12 (16.6%) |
| TCV | 14/39 (35.8%) | 0/39 | 14/39 (35.8%) |
Figure 1.
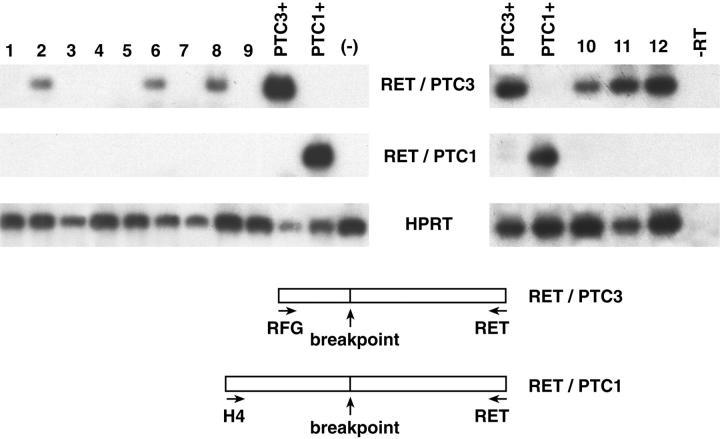
RT-PCR detection of RET/PTC rearrangements in TCV. TCV samples were analyzed for RET/PTC activation by RT-PCR. The reaction products were hybridized with a RET TK probe. The housekeeping HPRT mRNA was amplified for normalization. The results obtained with representative samples are shown. RNA extracted from PTC samples previously shown to carry a RET/PTC1 (lane PTC1+) or a RET/PTC3 (lane PTC3+) rearrangement served as positive controls. RNA from a thyroid neoplastic sample previously shown to be negative for RET/PTC1 and RET/PTC3 was used as a negative control (lane (-)). There was no amplification when samples did not undergo previous reverse transcription (lane –RT contains sample 12 amplified without previous reverse transcription). A schematic representation of the RET/PTC1 and RET/PTC3 rearrangements and of the primers used is shown.
We used immunohistochemistry to confirm RET kinase expression in RET/PTC-positive TCV. Seven TCV-positive samples and 5 TCV-negative samples, as shown by RT-PCR, were studied. All of the 7 RET/PTC3-TCV cases were intensely stained with anti-RET antibodies, the staining being confined to the cytoplasm of neoplastic cells (two representative samples are shown in Figure 2 ▶ ). The RT-PCR-negative cases and 10 samples of normal thyroid tissue (one sample is shown in Figure 2 ▶ ) were negative at immunohistochemistry. An immunoblot analysis of cells expressing RET/PTC oncogenes (NIH 3T3 fibroblasts transfected with RET/PTC1 or RET/PTC3, and the RET/PTC1-positive human thyroid papillary carcinoma cell line, TPC) or cells not expressing RET/PTC (untransfected NIH 3T3 and the ARO, human thyroid anaplastic carcinoma cell line) 15,16 was performed to assess antibody specificity (Figure 2G) ▶ .
Figure 2.
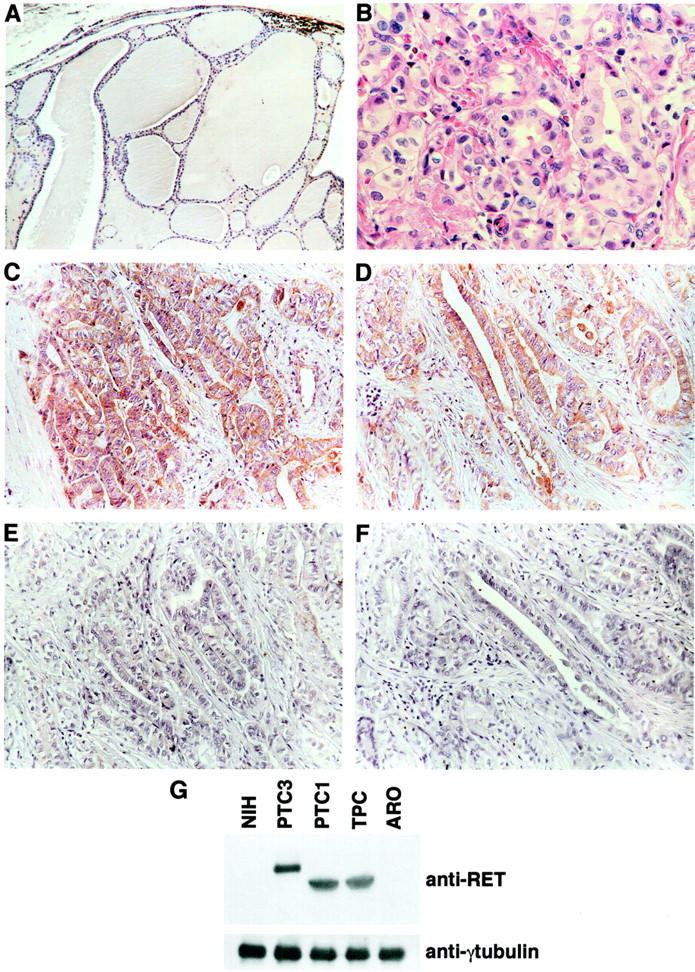
Immunohistochemical detection of RET protein products in TCV. Seven of the TCV samples that were positive for RET/PTC rearrangements were analyzed by immunohistochemistry for the expression of the RET protein. As a control, 10 non-neoplastic thyroid samples and 5 TCV samples that did not bear RET/PTC rearrangements (RT-PCR) were analyzed. Representative examples are shown. A: Normal thyroid tissue. B: One TCV sample (same as in C) stained with H/E. C and D: Two independent TCV samples showing intense RET immunoreactivity in neoplastic cells. E and F: Same samples as in C and D: anti-RET was pre-incubated with a fivefold molar excess of the antigen as a control of the specificity of the reaction. G: protein lysates (100 μg) obtained from the indicated cell lines were immunoblotted with anti-RET, to assess antibody specificity or anti-γ-tubulin to assess loading levels.
Increased Mitogenicity of RET/PTC3 with Respect to RET/PTC1
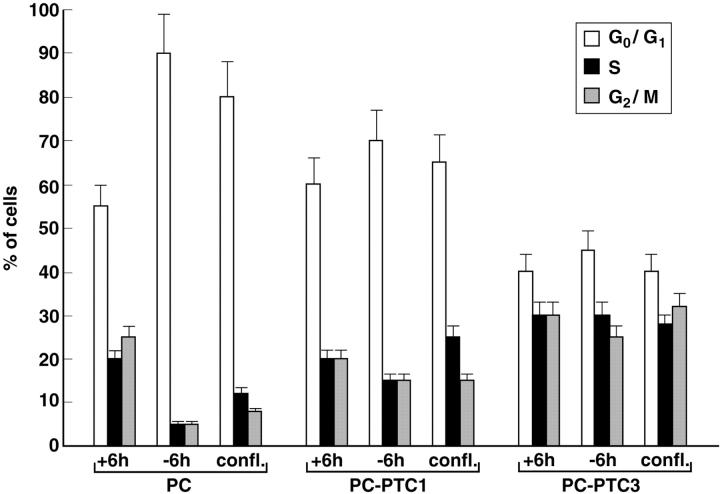
PC Cl 3, a continuous line of Fischer rat thyroid cells, is a model with which to study growth regulation in an epithelial thyroid cell setting. PC Cl 3 cells express thyroid differentiation markers and depend on a mixture of six hormones, including TSH and insulin, for proliferation. Expression of oncogenes of different categories, including RET/PTC1, results in 6H-independent proliferation. 23 We transfected PC Cl 3 cells with expression vectors for RET/PTC1 and RET/PTC3, and mass populations of transfected cells (PC-PTC1 and PC-PTC3) were marker-selected. Cell cycle kinetics was examined by flow cytometry in different growth conditions: 1) the logarithmic phase of growth, 2) on 6H-deprivation (96 hours), and 3) 48 hours after cells had reached confluence. Parental cells were arrested in G1 phase by hormone deprivation and contact-inhibition whereas a significant fraction of PC-PTC1 and PC-PTC3 cells remained in the S and G2/M compartments in both conditions (Figure 3) ▶ . Notably, PC-PTC3 cells had a significantly higher proliferative fraction than PC-PTC1 cells in all of the three growth conditions.
Figure 3.
Increased mitogenicity of RET/PTC3 with respect to RET/PTC1. Parental, PC-PTC1, and PC-PTC3 cells were harvested when subconfluent in complete medium (+6H), after 96 hours of hormones deprivation (−6H) or 48 hours after they had reached confluence (confl.) and analyzed by flow cytometry. The percentage of cells in each phase of the cell cycle is depicted. The results are representative of three independent experiments. Bars indicate SD.
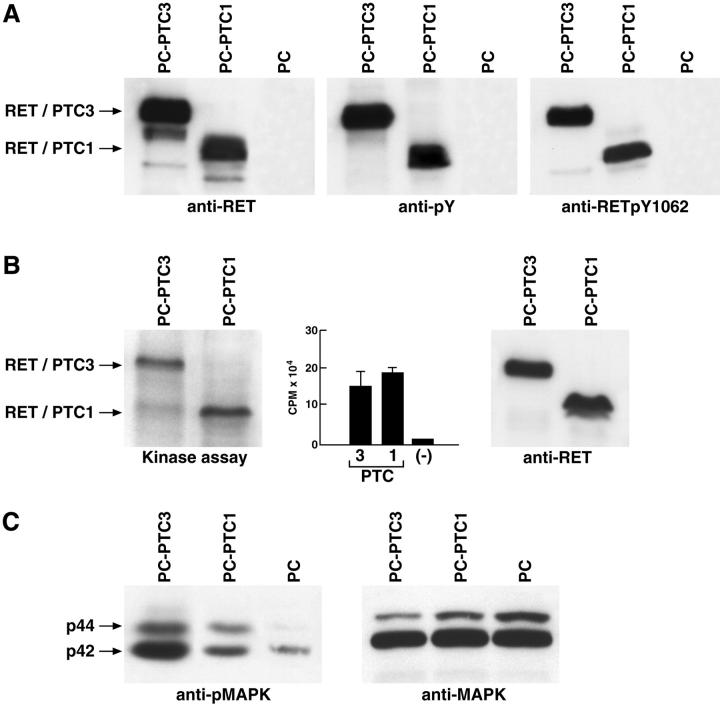
We next investigated the molecular mechanism of the powerful mitogenic effect of RET/PTC3. RET/PTC rearrangements lead to the fusion of the RET TK domain to protein motifs capable of oligomerization. 24 This results in constitutive activation of the kinase; autophosphorylation of RET/PTC tyrosine residues; and activation of downstream signaling events culminating in the mitogenic response. We used immunoblot analysis to examine these three biochemical steps in RET/PTC-expressing cells, and found that the RET/PTC1 and RET/PTC3 proteins were expressed to the same extent (Figure 4A) ▶ . The two proteins had also comparable levels of in vivo tyrosine phosphorylation as assessed with a monoclonal antibody that recognizes phosphotyrosine (Figure 4A) ▶ . RET/PTC-mediated signaling depends on a specific RET tyrosine residue, Y1062 (numbering is referred to the full-length RET protein). On phosphorylation, Y1062 binds to multiple docking proteins (Shc and FRS2) which recruit Sos, the exchange factor for Ras, to the plasma membrane thereby leading to Ras and MAPK activation. 20 We analyzed the extent of Y1062 phosphorylation in the RET/PTC1 and RET/PTC3 proteins with an antibody against phosphoY1062 of RET. Figure 4A ▶ shows that two proteins had also comparable levels of Y1062 phosphorylation. An in vitro immunocomplex kinase assay, which measures both autophosphorylation of the kinase and phosphorylation of a synthetic peptide, confirmed that the two RET/PTC variants had comparable intrinsic catalytic activity (Figure 4B) ▶ . Triggering of the Ras/MAPK cascade is a common endpoint of receptor tyrosine kinase signaling, and can so be used as a signaling “read-out.” The MAPK cascade elicits a mitogenic response in most cell types, including thyroid cells. 25,26 Thus, we determined MAPK phosphorylation, a measure of their activity, in PC-PTC cells by immunoblot with an antibody against the phosphorylated active p42 and p44 MAPK. Surprisingly, we found that MAPK phosphorylation was significantly higher (3- ± 0.5-fold) in PC-PTC3 than in PC-PTC1 cells (Figure 4C) ▶ . Therefore, the two oncogenes have the same enzymatic activity and autophosphorylation levels, but activation of MAPK is greater in RET/PTC3.
Figure 4.
Increased signaling ability of RET/PTC3 with respect to RET/PTC1. A: 50 μg of protein lysates obtained from indicated cells were immunoblotted with anti-RET, anti-phosphotyrosine (pY), or anti-phosphoRET (pY1062). Filters were stripped and stained with anti-γ-tubulin to verify loading (not shown). B:RET/PTC autophosphorylation (autokinase assay) and the phosphorylation of an exogenous substrate (polyGT) was evaluated in an immunocomplex kinase assay. One representative autoradiography is shown for the autokinase and the bar graph of the average scintillation counts of three independent experiments is reported for the polyGT assay; bars indicate SD. 1 of 10 of the immunocomplexes were immunoblotted with anti-RET for normalization. C: 50 μg of protein lysates were immunoblotted with anti-phosphoMAPK or anti-MAPK antibodies.
Conclusions
The phenotype of a tumor is dictated by its specific complement of genetic lesions. In this framework, allelic variants of a single gene, if endowed with different oncogenic potential, may condition tumor aggressiveness. Post-Chernobyl solid PTC are significantly associated with RET/PTC3, suggesting that this RET/PTC variant has high oncogenic potential. Here we show that the TCV is also significantly associated with RET/PTC3. The linkage of RET/PTC3 with aggressive (solid and TCV) histological PTC subtypes may be explained by a high oncogenicity of this RET/PTC variant or by preferential association with other, as yet unknown, genetic lesions that, in turn, condition the tumor phenotype. To discriminate between the two possibilities, we have examined the mitogenic ability of RET/PTC1 and RET/PTC3 in vitro and found that RET/PTC3 is clearly more efficient in promoting proliferation of cultured thyroid cells. Neither enzymatic activity nor autophosphorylation was higher in RET/PTC3 than in RET/PTC1. However, we found that the extent of MAPK activation, a common endpoint of receptor mitogenic signaling, was significantly higher in RET/PTC3- than in RET/PTC1-expressing cells. Kinase signaling does not solely depend on the intrinsic stoichiometry of phosphorylation. A number of other factors can influence signaling; for instance, parallel triggering of negative signal transducers, like phosphatases, or the intracellular localization of the kinase which can affect the engagement of specific intracellular effectors. Signals leading to activation of Ras/MAPK and other pathways are initiated at the plasma membrane level where Ras is positioned. We have recently demonstrated that the RET/PTC3 protein is recruited to the plasma membrane by heterodimerizing with the membrane-linked RFG protein 24 and preliminary biochemical fractionation data (RM Melillo, F Carlo-magno, M Santoro, unpublished data) reveal at least threefold more RET/PTC3 than RET/PTC1 protein at membrane level. Therefore, we hypothesize that the differential intracellular localization of the two RET/PTC (1 and 3) proteins might account for their different signaling ability. Whatever the mechanism, we propose a model whereby the ability of RET/PTC3 to transduce mitogenic signals contributes to the higher aggressiveness of the PTC subtypes harboring this variant. We speculate that genetic alterations other than RET/PTC3 may occur in aggressive PTC to determine the specific subtype (solid or tall-cell).
In conclusion, the results reported herein indicate that RET/PTC3 is a marker for aggressive PTC variants. Furthermore, they prompt the novel concept that specificity of the fusion partner determines the signaling and the oncogenic ability of the rearranged kinase, a concept that can be extended to other oncogenic kinases activated in human tumors.
Acknowledgments
We are grateful to Jean Ann Gilder for editing the text. We thank A.M. Cirafici and D. Salvatore for the anti-RET antibodies.
Footnotes
Address reprint requests to Fulvio Basolo, M.D., Department of Oncology, Division of Pathology, Via Roma 57, 56126 Pisa, Italy. E-mail: fbasolo@do.med.unipi.it.
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro, European Community (grant EC FIGH-CT1999-CHIPS), Ministero dell’ Universita ’e della Ricerca Scientifica e Tecnologica, Progetto Biotecnologie 5% of the Consiglio Nazionale delle Ricerche.
References
- 1.Hedinger C, Williams ED, Sobin LH: Histological Typing of Thyroid Tumours, ed 2 1988, Germany, Springer-Verlag, Number 11 of International Classification of Tumours. Berlin
- 2.Rosai J, Carcangiu ML, DeLellis RA: Atlas of Tumor Pathology. Tumors of the Thyroid Gland, 3rd series. 1992, DC, Armed Forces Institute of Pathology, Fascicle 5. Washington
- 3.Nikiforov YE, Gnepp DR: Pathomorphology of thyroid gland lesions associated with radiation exposure: the Chernobyl experience and review of the literature. Adv Anat Pathol 1999, 6:78-91 [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski ML, Merino MJ: Tall-cell variant of papillary thyroid carcinoma: a reassessment and immunohistochemical study with comparison to the usual type of papillary carcinoma of the thyroid. Am J Surg Pathol 1996, 20:964-974 [DOI] [PubMed] [Google Scholar]
- 5.Ruter A, Nishiyama R, Lennquist S: Tall-cell variant of papillary thyroid cancer: disregarded entity? World J Surg 1997, 21:15-20 [DOI] [PubMed] [Google Scholar]
- 6.Filie AC, Chiesa A, Bryant BR, Merino MJ, Sobel ME, Abati A: The Tall-cell variant of papillary carcinoma of the thyroid: cytologic features and loss of heterozygosity of metastatic and/or recurrent neoplasms and primary neoplasms. Cancer 1999, 87:238-242 [DOI] [PubMed] [Google Scholar]
- 7.Lee A, LiVolsi VA, Baloch ZW: Expression of DNA topoisomerase IIα in thyroid neoplasia. Mod Pathol 2000, 13:396-400 [DOI] [PubMed] [Google Scholar]
- 8.Ruter A, Dreifus J, Jones M, Nishiyama R, Lennquist S: Overexpression of p53 in tall-cell variants of papillary thyroid carcinoma. Surgery 1996, 120:1046-1050 [DOI] [PubMed] [Google Scholar]
- 9.Tallini G, Garcia-Rostan G, Herrero A, Zelterman D, Viale G, Bosari S, Carcangiu ML: Down-regulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol 1999, 23:678-685 [DOI] [PubMed] [Google Scholar]
- 10.Roque L, Clode AL, Gomes P, Rosa-Santos J, Soares J, Castedo S: Cytogenetic findings in 31 papillary thyroid carcinomas. Genes Chromosomes Cancer 1995, 13:157-162 [DOI] [PubMed] [Google Scholar]
- 11.Jhiang SM: The RET proto-oncogene in human cancers. Oncogene 2000, 19:5590-5597 [DOI] [PubMed] [Google Scholar]
- 12.Cheung CC, Ezzat S, Ramvar L, Freeman JL, Asa SL: Molecular basis of Hurthle cell papillary thyroid carcinoma. J Clin Endocrinol Metab 2000, 85:878-882 [DOI] [PubMed] [Google Scholar]
- 13.Papotti M, Volante M, Giuliano A, Fassina A, Fusco A, Bussolati G, Santoro M, Chiappetta G: RET/PTC activation in hyalinizing trabecular tumors of the thyroid. Am J Surg Pathol 2000, 24:1615-1621 [DOI] [PubMed] [Google Scholar]
- 14.Cheung CC, Boerner SL, MacMillan CM, Ramyar L, Asa SL: Hyalinizing trabecular tumor of the thyroid: a variant of papillary carcinoma proved by molecular genetics. Am J Surg Pathol. 2000, 24:1622-1626 [DOI] [PubMed] [Google Scholar]
- 15.Grieco M, Santoro M, Berlinghieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G: PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990, 60:557-563 [DOI] [PubMed] [Google Scholar]
- 16.Santoro M, Dathan NA, Berlingieri MT, Bongarzone I, Paulin C, Grieco M, Pierotti MA, Vecchio G, Fusco A: Molecular characterization of RET/PTC3: a novel rearranged version of the RET proto-oncogene in a human thyroid papillary carcinoma. Oncogene 1994, 9:509-516 [PubMed] [Google Scholar]
- 17.Nikiforov Y, Rowland JM, Bove KE, Monfore Munoz H, Fagin JA: Distinct patterns of ret rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997, 57:1690-1694 [PubMed] [Google Scholar]
- 18.Thomas GA, Bunnell H, Cook HA, Cook HA, Williams ED, Neroynya A, Cherstvoy ED, Tronko ND, Bogdanova TI, Chiappetta G, Viglietto G, Pettimalli F, Salvatore G, Fusco A, Santoro M, Vecchio G: High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab 1999, 84:4232-4238 [DOI] [PubMed] [Google Scholar]
- 19.Viglietto G, Chiappetta G, Martinez-Tello FJ, Fukunaga FH, Tallini G, Rigopoulu D, Visconti R, Mastro A, Santoro M, Fusco A: RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene 1995, 11:1207-1210 [PubMed] [Google Scholar]
- 20.Melillo RM, Santoro M, Ong SH, Billaud M, Fusco A, Schlessinger J, Lax I: The docking protein FRS2 links RET and its oncogenic forms with the RAS/MAPK cascade. Mol Cell Biol 2001, 21:4177-4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlomagno F, De Vita G, Berlingieri MT, de Franciscis V, Melillo RM, Colantuoni V, Kraus MH, Di Fiore PP, Fusco A, Santoro M: Molecular heterogeneity of RET loss of function in Hirschsprung’s disease. EMBO J 1996, 15:2717-2725 [PMC free article] [PubMed] [Google Scholar]
- 22.Salvatore D, Melillo RM, Monaco C, Visconti R, Fenzi G, Vecchio G, Fusco A, Santoro M: Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res 2001, 61:1426-1431 [PubMed] [Google Scholar]
- 23.Santoro M, Melillo RM, Grieco M, Berlingieri MT, Vecchio G, Fusco A: The TRK and RET tyrosine kinase oncogenes cooperate with ras in the neoplastic transformation of a rat thyroid epithelial cell line. Cell Growth Differ 1993, 4:77-84 [PubMed] [Google Scholar]
- 24.Monaco C, Visconti R, Barone MV, Pierantoni GM, Berlingieri MT, De Lorenzo C, Mineo A, Vecchio G, Fusco A, Santoro M: The RFG oligomerization domain mediates kinase activation and re-localization of the RET/PTC3 oncoprotein to the plasma membrane. Oncogene 2001, 20:599-608 [DOI] [PubMed] [Google Scholar]
- 25.Cobellis G, Missero C, Di Lauro R: Concomitant activation of MEK-1 and Rac-1 increases the proliferative potential of thyroid epithelial cells, without affecting their differentiation. Oncogene 1998, 17:2047-2057 [DOI] [PubMed] [Google Scholar]
- 26.Gire V, Marshall CJ, Wynford-Thomas D: Activation of mitogen-activated protein kinase is necessary but not sufficient for proliferation of human thyroid epithelial cells induced by mutant Ras. Oncogene 1999, 18:4819-4832 [DOI] [PubMed] [Google Scholar]