Abstract
Apoptotic cell death plays an important role in limiting testicular germ cell population during spermatogenesis and its dysregulation has been shown to be associated with male infertility. The growing evidence on the role of the transcription factor nuclear factor (NF)-κB in controlling apoptosis prompted us to investigate NF-κB activity in the normal human testis and its role in testis tissue undergoing excessive apoptosis in vitro. In electrophoretic mobility shift assays, low-level constitutive NF-κB DNA-binding activity was found and, by immunostaining of the RelA and p50 NF-κB subunits, was localized to Sertoli cell nuclei. During in vitro-induced testicular apoptosis, the Sertoli cell nuclear NF-κB levels and whole seminiferous tubule NF-κB DNA-binding activity increased previous detection of germ cells undergoing apoptosis. The anti-inflammatory drug sulfasalazine effectively suppressed stress-induced NF-κB DNA binding and NF-κB-mediated IκBα gene expression. Importantly, concomitantly with inhibiting NF-κB, sulfasalazine blocked germ cell apoptosis. These results suggest that during testicular stress Sertoli cell NF-κB proteins exert proapoptotic effects on germ cells, which raises the possibility that pharmacological inhibition of NF-κB could be a therapeutic target in transient stress situations involving excessive germ cell death.
Spermatogenesis, the complex process of male germ cell proliferation and maturation from diploid spermatogonia to mature haploid spermatozoa, takes place in the seminiferous epithelium of the testis. During this process, the number of germ cells has to match with the capacity of the somatic Sertoli cells, which provide the structural support and biochemical factors essential for germ cell development. 1 In this regard, apoptotic cell death plays an important role in limiting the testicular germ cell population both in physiological conditions and under stress caused by external disturbances. 2 This programmed form of cell death is regulated by strictly controlled transcription of a number of genes. However, the transcription factors controlling the expression of the genes critical for testicular apoptosis have remained primarily unknown. Nuclear factor-κB (NF-κB), a transcription factor considered to be a major regulator of the immune and stress responses, 3,4 is an interesting candidate for the regulation of male germ cell apoptosis because there is growing evidence that this transcription factor has a function in cell proliferation and apoptosis, 4,5 and as recent studies suggest a role for NF-κB in mammalian spermatogenesis. 6
NF-κB is a dimeric DNA sequence-specific transcription factor that is assembled from two of the five known mammalian Rel/NF-κB subunits (RelA/p65, RelB, c-Rel, p50, and p52). 3,7 In most cells, the major Rel complex is the p50-RelA heterodimer. In unstimulated cells, NF-κB dimers remain sequestered in the cytoplasm by inhibitory IκB proteins, which cover the nuclear localization sequence of NF-κB and interfere with sequences important for DNA binding. Stimulation by a variety of extracellular signals leads to degradation of the IκB. 8,9 The liberated NF-κB then rapidly translocates to the nucleus, where it regulates transcription by binding to consensus κB sites in the promoters of the target genes. 4 One of the target genes activated by NF-κB is that encoding IκBα, the best known IκB protein. Newly synthesized IκBα can enter the nucleus, remove NF-κB from the DNA, and export the complex back into the cytoplasm. 10-12 In this way, NF-κB limits its own activation.
In the rat testis, the NF-κB complex of RelA and p50 proteins was found to be constitutively expressed in the nuclei of Sertoli cells at all stages of spermatogenesis. 13 In addition, nuclear NF-κB expression was elevated in Sertoli cells at stages XIV to VII, which correlates with the presence of round spermatids, and was also transiently and stage-specifically found in pachytene spermatocytes and round spermatids. 13 Moreover, in cultured rat Sertoli cells, an increase in nuclear NF-κB DNA-binding activity and κB-dependent transcription has been shown to be induced by the cytokine tumor necrosis factor-α (TNF-α). 13 As TNF-α is known to be secreted by round spermatids, 14 a paracrine mechanism has been suggested, in which this TNF-α activates NF-κB in Sertoli cells, leading to changes in the expression of Sertoli cell proteins that are able to modulate spermatogenesis. 6 In accord, TNF-α-induced activation of NF-κB in rat Sertoli cells in vitro leads to up-regulation of the cAMP-response element-binding protein, which is an important regulator of a number of cAMP-induced genes and consequently is suggested to be a regulator of spermatogenesis. 15 However, the physiological role of NF-κB in the testis still remains unclear.
Regarding apoptosis, the NF-κB transcription factors may have both anti-apoptotic and proapoptotic effects. 5 The anti-apoptotic activities of NF-κB have been observed in certain nontesticular cells after some external stimuli, such as TNF-α, ionizing radiation, and chemotherapeutic compounds. 16-19 The inhibitory effect of NF-κB on TNF-α- or chemotherapy-induced apoptosis has also been shown in chemotherapy-resistant tumors. 20 On the other hand, there is growing evidence for apoptosis-promoting functions of NF-κB. In human embryonic kidney cells, serum withdrawal induces NF-κB activation and apoptosis, which can be prevented by the overexpression of a dominant-negative form of RelA. 21 Double-positive (CD4+CD8+) T cells from mice overexpressing a dominant-negative form of IκBα are resistant to activation-induced cell death. 22 Furthermore, NF-κB stimulates the expression of the death-promoting Fas ligand (FasL) in T cells after T-cell receptor engagement or exposure to DNA-damaging agents, thus suggesting a proapoptotic role of NF-κB. 23,24 Interestingly, recent evidence indicates that NF-κB may have either proapoptotic or anti-apoptotic effects in the same cell type, depending on the death-inducing stimulus. 25 Thus, whether NF-κB promotes or inhibits apoptosis seems to depend on the specific cell type and the type of the inducer. Therefore, to understand the role of NF-κB in different physiological situations, the behavior of this transcription factor in different apoptosis models needs further characterization. In the testis tissue, studies on NF-κB have been limited to characterization of its expression. Our preliminary experiments suggested an association between NF-κB activation and apoptosis in the human testis, 26 but the role of this transcription factor in testicular apoptosis remains unresolved.
In the present study, we aimed at characterizing the role of NF-κB in human testicular apoptosis. As no studies on NF-κB expression in the human testis were available, we first studied the constitutive expression and DNA-binding activity of the NF-κB proteins in normal adult human testis. We then explored the induction of NF-κB DNA-binding activity and nuclear translocation during human testicular apoptosis, using our established in vitro tissue culture model. 27 Finally, we tested whether this activation of NF-κB can be pharmacologically modulated and evaluated the effects of NF-κB inhibition on testicular germ cell survival.
Materials and Methods
Patients
Testis tissue was obtained from 12 men aged 59 to 88 years undergoing orchidectomy as treatment for prostate cancer. They had not received hormonal, chemotherapeutic, or radiotherapeutic treatment for the cancer before the operation. They had no endocrinological disease and none of them had suffered from cryptorchidism. The operations were performed between March 2000 and January 2001 at the Department of Urology, Helsinki University Central Hospital (Helsinki, Finland). The Ethics Committees of the Hospital for Children and Adolescents and the Department of Urology, University of Helsinki, approved the study protocol.
Tissue Culture and Treatments
Apoptosis of the human testicular germ cells was induced in vitro by incubating segments of seminiferous tubules under serum-free culture conditions. We cultured segments of seminiferous tubules, rather than isolated germ cells, to maintain the physiological contact between the Sertoli cells and the germ cells. The testis tissue was microdissected on a Petri dish containing tissue culture medium (Nutrient mixture Ham’s F10; Gibco Europe, Paisley, UK) supplemented with 0.1% human albumin (Sigma Chemical Co., St. Louis, MO) and 10 μg/ml gentamicin (Gibco). Segments of seminiferous tubules (∼2 mm in length) were isolated and transferred to culture plates containing the same tissue culture medium and cultured at 34°C in a humidified atmosphere containing 5% CO2.
Sulfasalazine (SS) (Fluka Chemie Ag, Buchs, Switzerland) was dissolved in culture medium at 5 mmol/L immediately before use. Acetyl salicylic acid (ASA) (Sigma) was dissolved in 0.05 mol/L of Tris-HCl, pH 7.5, to prepare 1 mol/L of stock solution and used at a concentration of 5 mmol/L. n-Acetyl-l-cysteine (NAC) (Sigma) was prepared as 1 mol/L of stock solution in distilled water, with the pH adjusted to 7.5 with NaOH, and used at 100 mmol/L. The NF-κB SN50 peptide (Biomol Research Laboratories, Plymouth Meeting, PA) was used at 10 μg/ml.
Cytoplasmic and Nuclear Protein Extractions
Seminiferous tubules were gently homogenized with a tight-fitting Potter-Elvehjem homogenizer into ice-cold hypotonic buffer A (50 mmol/L HEPES, pH 7.4, 10 mmol/L KCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L dithiothreitol, 0.2 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 0.5% Nonidet P-40), and cytoplasmic and nuclear protein extracts were prepared as previously described. 28 Protein concentrations were determined by the Bradford method, using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA). The protein extracts were stored in aliquots at −80°C until used for electrophoretic mobility shift assays (EMSAs) or Western blotting.
EMSA
DNA probe containing a consensus κB enhancer element (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was purchased from Santa Cruz (sc-2505; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The probe was 5′-end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (Promega Corp., Madison, WI). Testicular nuclear protein extracts (10 μg) or control nuclear protein extracts [Jurkat T cells, sc-2132 (Santa Cruz); KNRK cells, sc-2141 (Santa Cruz); K562 cells, sc-2130 (Santa Cruz); and LPS-stimulated human monocyte-derived macrophages (5 to 10 μg)] were incubated on ice for 10 minutes with 2 μg poly(dI-dC)(dI-dC) (Amersham Pharmacia Biotech, Piscataway, NJ) in 50 mmol/L HEPES, pH 7.6, 10% glycerol (v/v), 225 mmol/L KCl, 1 mmol/L ethylenediaminetetraacetic acid, 2.5 mmol/L dithiothreitol, 1 mmol/L MgCl2, 0.75 mmol/L phenylmethyl sulfonyl fluoride, and 1.5 μmol/L leupeptin. A 5′-end-labeled probe (15,000 to 30,000 cpm) was then added, and incubation was continued at room temperature for 30 minutes. In the competition experiments, a 100-fold molar excess of unlabeled probe or unlabeled mutated probe (sc-2511, Santa Cruz) was added before the labeled probe. Reaction products were separated on 4% polyacrylamide gels run in 22.5 mmol/L of Tris-borate and 0.5 mmol/L of ethylenediaminetetraacetic acid at 200 V at room temperature. After electrophoresis, the gels were dried and visualized by autoradiography. In the supershift assays, 2 μg of affinity-purified polyclonal antibodies were added after binding reactions and incubation was further continued for 1 hour at room temperature. The antibodies for the supershift assays were purchased from Santa Cruz (RelA/p65, sc-109X; p50, sc-7178X; c-Rel, sc-272X; p52, sc-298X; RelB, sc-226X).
Immunohistochemistry
Immunostainings of the RelA (p65) and p50 NF-κB subunits were performed on paraffin-embedded sections of formalin-fixed adult human testis tissue or isolated seminiferous tubules, or on squash preparations of human seminiferous tubules. For the squash preparations, small segments of human seminiferous tubules (∼1 mm in length) were squashed under coverslips to produce a monolayer of cells, and the preparations were fixed as previously described. 29 Paraffin sections were incubated at 60°C for 30 minutes and deparaffinized in xylene. Both paraffin sections and squash preparations were then rehydrated, permeabilized by microwaving at high power for 5 minutes in citrate buffer (10 mmol/L citrate, pH 6.0), washed, and blocked with blocking solution [phosphate-buffered saline (PBS) containing 5% goat normal serum, 3% bovine serum albumin, and 0.1% Tween 20] for at least 30 minutes at room temperature. Our preliminary experiments revealed unspecific staining for endogenous peroxidases only in the erythrocytes of the testicular capillaries found in paraffin sections of testis tissue, and therefore, endogenous peroxidases were not blocked. RelA and p50 proteins were detected with affinity-purified polyclonal antibodies to human RelA (sc-109, Santa Cruz) or p50 (sc-7178X, Santa Cruz). Both antibodies were used at 0.02 to 0.04 μg/ml for the paraffin sections and at 0.4 μg/ml for the squash preparations. The primary antibodies were added to the samples in blocking solution and incubation was performed overnight at 4°C. After incubation, the slides were washed in PBS. The primary antibodies were detected using biotin-conjugated goat anti-rabbit IgG from the ABC-Elite kit (Vector Laboratories, Inc., Burlingame, CA) followed by incubation with ABC solution. For location of the secondary antibody, 0.05% diaminobenzidine substrate (Sigma) was added. For the negative controls, the primary antibodies were replaced with nonspecific rabbit IgG (Sigma). After the staining protocols, light counterstaining was performed with hematoxylin, and the samples were dehydrated and mounted.
Southern Blot Analysis of Apoptotic DNA Fragmentation
Genomic DNA was extracted from frozen segments of human seminiferous tubules, using the Apoptotic DNA Ladder Kit (Roche Molecular Biochemicals, Mannheim, Germany), as described. 30 DNA was quantified spectrophotometrically (absorbance at 260 nm), and 1 μg of the total DNA from each sample was subjected to 3′-end-labeling with digoxigenin-dideoxy-UTP (Dig-dd-UTP; Roche) by the terminal transferase (Roche) reaction. The DNA samples were then electrophoresed on 2% agarose gels, blotted onto nylon membranes, and crosslinked to the membranes by UV irradiation. The membranes were washed and blocked with 1% Blocking reagent (Roche) in maleic buffer (100 mmol/L maleic acid, 150 mmol/L NaCl, pH 7.5) for 30 minutes at room temperature. The 3′-end-labeled DNA on the membranes was localized with alkaline phosphatase-conjugated anti-digoxigenin antibody (Anti-Digoxigenin-AP; Roche), and the bound antibody was detected by the chemiluminescence reaction (CSPD; Roche) as described. 27
In Situ End Labeling (ISEL) of Apoptotic DNA
Squash preparations of human seminiferous tubules were rehydrated, washed in distilled water, and permeabilized by microwaving at high power for 5 minutes in citrate buffer (10 mmol/L citrate, pH 6.0). After incubation for 10 minutes with terminal transferase reaction buffer (1 mol/L potassium cacodylate, 125 mmol/L Tris-HCl, and 1.25 mg/ml bovine serum albumin, pH 6.6), the apoptotic DNA was 3′-end-labeled with Dig-dd-UTP (Roche) for 1 hour at 37°C by the terminal transferase reaction. For the negative controls, the terminal transferase enzyme was replaced with the same volume of distilled water. The preparations were then blocked with blocking solution [2% Blocking reagent (Roche), in 150 mmol/L NaCl, 100 mmol/L Tris-HCl, pH 7.5], followed by location of the Dig-dd-UTP with the peroxidase-conjugated anti-digoxigenin antibody (Anti-Digoxigenin-POD, Roche). For detection of the antibody, 0.05% diaminobenzidine substrate (Sigma) was added. Light counterstaining was performed with hematoxylin, after which the samples were dehydrated and mounted.
Western Blotting
Western blotting of the IκBα was performed on cytoplasmic protein extracts of seminiferous tubules. Proteins (15 μg) were loaded into 10% sodium dodecyl sulfate-polyacrylamide gels and electrophoresis was performed at 180 V. The proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore Corp., Bedford, MA) by electrophoresis for 2 hours at 4°C in transfer buffer (26 mmol/L Tris, 192 mmol/L glycine, 10% methanol) at 100 V. The transfer was checked by staining with 0.2% Ponceau S in 3% trichloroacetic acid. IκBα protein on the membranes was detected using affinity-purified polyclonal antibodies to human IκBα (sc-371 and sc-847, Santa Cruz) at 0.2 μg/ml. The specificity of the band detected with either of the two antibodies was confirmed by preabsorption experiments with a blocking peptide (sc-371P, Santa Cruz). FasL was detected with an anti-human FasL monoclonal antibody (Transduction Laboratories, Lexington, KY). The primary antibodies were followed with peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or peroxidase-conjugated goat anti-mouse IgG (DAKO Corp. A/S, Glostrup, Denmark). The bound secondary antibody was located with the ECL detection kit (Amersham, Arlington Heights, IL). After detection of the proteins under investigation, the membranes were washed and, as a loading control, probed with an antibody to α-tubulin (Sigma).
Quantitative Analysis of X-Ray Films
The X-ray films exposed to chemiluminescence (Southern blots) or autoradiography (EMSA; see Figure 2C ▶ ) were scanned with a tabletop scanner (Hewlett Packard ScanJet 6300C) and the digital image was analyzed with the Gel plot 2 macro for Scion Image β 4.0.2 (Scion Corp., Frederick, MD) analysis software. For the Southern blots, the digitized quantification of the low molecular weight DNA fragments (<1.3 kB) in the sample cultured for 5 hours (or 10 hours in time course analysis of nuclear apoptosis; see Figure 2C ▶ ) without treatments was taken as 1.0 (100% apoptosis), and the amounts of low molecular weight DNA fragments in the other samples were expressed in relation to this. For time course analysis of NF-κB activation (EMSA; see Figure 2C ▶ ), the digitized quantification of the specific NF-κB bands in the sample cultured for 5 hours was set as 1.0, and the intensities of the bands in other samples were expressed in relation to this.
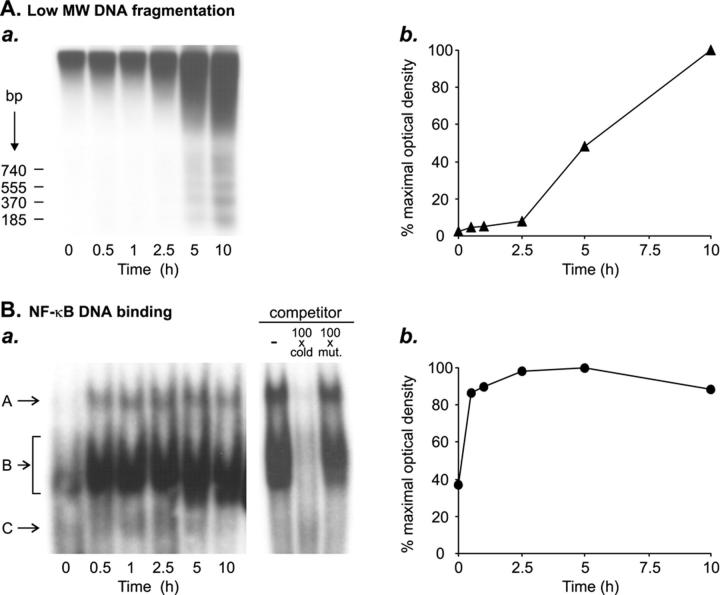
Figure 2.
Time course of activation of the transcription factor NF-κB during human testicular apoptosis. Segments of human seminiferous tubules were cultured under serum-free conditions for 0 to 10 hours to induce apoptotic cell death, after which apoptotic DNA fragmentation and activation of NF-κB were studied at different time points. A: Southern blot analysis of apoptotic low molecular weight DNA fragmentation (185 bp multiples). a: DNA was extracted from the seminiferous tubules cultured for various periods of time, after which 1 μg of the total DNA in each sample was 3′-end labeled with Dig-dd-UTP and subjected to electrophoresis. The labeled DNA was detected with chemiluminescence as described in Materials and Methods. b: Low molecular weight DNA (<1.3 kb) fragmentation was quantified from the radiograph presented in a, as described in Materials and Methods, and plotted as a function of time. The digitized quantification of the low molecular weight DNA fragments in the sample cultured for 10 hours was taken as 100% apoptosis, and the amounts of low molecular weight DNA fragments in the other samples were expressed in relation to this. B: EMSA demonstrating the increase in NF-κB DNA-binding activity during in vitro induced testicular apoptosis. a: Nuclear protein extracts (10 μg) from the seminiferous tubules were incubated with a 32P-labeled κB oligonucleotide, and the DNA-protein complexes formed were resolved on polyacrylamide gel electrophoresis. Competition experiments using an unlabeled κβ oligonucleotide (cold) or unlabeled mutated κB oligonucleotide (mut.) confirmed the specificity of binding to the κB oligonucleotide. b: NF-κB DNA binding was quantified from the radiograph presented in a, as described in Materials and Methods, and plotted as a function of time. The digitized quantification of the specific NF-κB bands in the sample cultured for 5 hours was set as 100%, and the intensities of the bands in other samples were expressed in relation to this. All of the results shown are representative of two independent experiments.
Statistics
The experiments for Southern blot analysis of the effects of SS, ASA, NAC, or SN-50 on apoptotic DNA fragmentation were repeated on at least three independent occasions. Quantitative data represent low molecular weight DNA (integrated optical density from scanned X-ray films). Data obtained from 3 to 10 replicate experiments (mean ± SEM) were analyzed by one-way analysis of variance, and when significant differences were found, this was followed by comparison of the groups with two-tailed unpaired Student’s t-test. P < 0.05 was considered statistically significant. Data demonstrating the time courses of IκBα degradation, NF-κB DNA-binding activity, or nuclear apoptosis are representative of two independent experiments. Three independent experiments were conducted in which the effect of SS on the expressions of IκBα or FasL were studied by Western blotting, and at least three independent experiments were conducted in which the effects of SS, ASA, NAC, or SN-50 on NF-κB DNA-binding activity were studied by EMSA.
Results
Constitutive NF-κB Activity in the Adult Human Testis
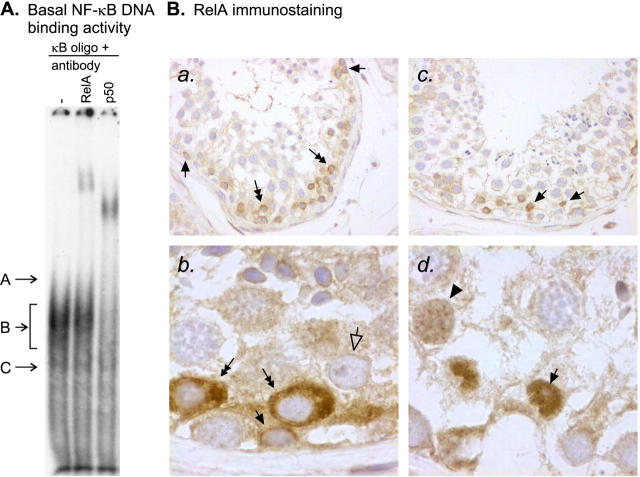
To explore the constitutive NF-κB activity in the adult human testis, the DNA-binding activity of the κB-binding proteins was first studied by EMSAs, using nuclear protein extracts from freshly isolated human seminiferous tubules and a DNA probe containing a consensus κB-binding sequence. Consistent with our previous results, 26 three DNA-protein complexes designated A, B, and C (Figure 1A) ▶ , were found. Band B was clearly seen, whereas bands A and C were poorly visible in the basal situation before exposure of the seminiferous tubules to apoptosis-inducing conditions. Competition experiments confirming the specificity of binding to the κB oligonucleotide are presented in Figure 2 ▶ . At the bottom of the gel we observed a band that seemed to represent an unspecific complex that was not eliminated by the unlabeled competitor and that was associated with the κB oligonucleotide because it was present not only in nuclear extracts of seminiferous tubules but also in control samples tested including nuclear extracts of LPS-stimulated human macrophages, Jurkat T cells, KNRK cells, or K562 cells. As demonstrated by the supershift assay, the NF-κB subunits RelA (p65) and p50 participated in the formation of the observed DNA-protein complexes (Figure 1A) ▶ . We also tested antibodies against c-Rel, p52, and RelB, but we did not find supershifts of the basal complexes with any of these antibodies. Under similar conditions these antibodies were found to bind to the corresponding DNA-protein complexes in EMSAs of control nuclear extracts (K562 for c-Rel, Jurkat for p52, and LPS-stimulated human monocyte-derived macrophage and KNRK for RelB) (data not shown).
Figure 1.
Constitutive NF-κB in the human testis. A: EMSA demonstrating basal NF-κB DNA-binding activity in human seminiferous tubules. Nuclear protein extracts (10 μg) from freshly isolated human seminiferous tubules were incubated with a 32P-labeled κB oligonucleotide. DNA-protein complexes were resolved on nondenaturing polyacrylamide gel electrophoresis and detected by autoradiography. Three NF-κB-specific bands, designated A, B, and C, were observed from which band A was only hardly detectable or absent in the majority of experiments. Competition experiments confirming the specificity of binding to the κB oligonucleotide are presented in Figure 2 ▶ . The faster migrating complex at the bottom of the gel seemed to be an unspecific complex that was not eliminated by the competitor. A supershift assay with antibodies against human RelA and p50 revealed that the RelA and p50 proteins participated in the formation of the constitutively active testicular NF-κB complexes. Antibodies against c-Rel, p52, and RelB did not affect the basal NF-κB complexes although under similar conditions they bound to the corresponding DNA-protein complexes in control nuclear extracts described in Materials and Methods (data not shown). The basal NF-κB complexes were observed in 11 independent experiments and the result of the supershift assay is representative of three independent experiments. B: RelA immunostaining showing the cellular localization of the NF-κB in normal human testis. Paraffin-embedded sections of formalin-fixed human testis tissue were immunostained for RelA as described in Materials and Methods. a: In many of the cross-sections of the seminiferous tubules, intense cytoplasmic staining of RelA was observed in spermatogonia (arrows) and early meiotic germ cells (double-headed arrows). In contrast, in the majority of the cross-sections, no nuclear RelA immunoreactive complexes were detected. Identification of spermatogonia was based on their morphology and typical localization in the seminiferous epithelium, ie, these cells are attached to the basement membrane. Original magnification, ×200. b: Higher (×1000) original magnification that better demonstrates a positive spermatogonium (arrow), positive early meiotic germ cells (double-headed arrows) and a negative Sertoli cell (open-headed arrow). c: Nuclear RelA immunostaining (arrows), indicating expression of active DNA-binding protein, was observed in only few segments of the seminiferous tubules (original magnification, ×200). d: The cells expressing nuclear RelA were identified as Sertoli cells on the basis of their typical localization in the seminiferous epithelium and the presence of a characteristic nucleolus (arrow). Arrowhead points to a weakly positive nucleus that resembles that of a spermatocyte but because of the presence of a large nucleolus and chromatin more typical to Sertoli cells the cell type could not be reliably identified. Original magnification, ×1000. The results of immunohistochemistry are representative of three independent experiments.
Immunohistochemical studies using polyclonal antibodies to human RelA and p50 proteins were then conducted on paraffin-embedded sections of formalin-fixed human testis tissue to determine the cell types expressing constitutively active NF-κB. In the majority of the tubules, strong positive staining for RelA was found in the cytoplasm of some spermatogonia and early meiotic spermatocytes (Figure 1B, a and b) ▶ . These germ cells were identified on the basis of their morphology and typical localization in the seminiferous epithelium, ie, spermatogonia are attached to the basement membrane whereas the adjacent early spermatocytes are not. In contrast, nuclear localization of the RelA, indicating expression of the active DNA-binding protein, was observed in only a few scattered tubules, and even in these tubules, most of the positive RelA immunostaining was found in the cytoplasm of immature germ cells (Figure 1B, c and d) ▶ . The cells showing positive nuclear immunostaining were identified as Sertoli cells. Identification of the Sertoli cells was based on the typical localization and morphology of the nuclei and on the presence of a characteristic nucleolus (Figure 1B, d) ▶ . Because of the spiral arrangement of the spermatogenetic stages in human seminiferous tubules, identification of adjacent stages in the human testis is difficult and, therefore, stage-specific evaluation of nuclear NF-κB expression in the human testis was not attempted. It should be noted that, on account of the very large volume of the Sertoli cell cytoplasm and its partial rupture during sample preparation, direct comparison between Sertoli cell cytoplasmic and Sertoli cell nuclear or germ cell cytoplasmic staining is difficult. Thus, light immunostaining surrounding the Sertoli cell nuclei and germ cells most likely represents cytoplasmic expression of RelA in the Sertoli cells. A similar staining pattern was observed with an antibody against p50. When the primary antibody was replaced with nonspecific rabbit IgG, there was no specific staining (negative control, shown in Figure 4 ▶ ).
Figure 4.
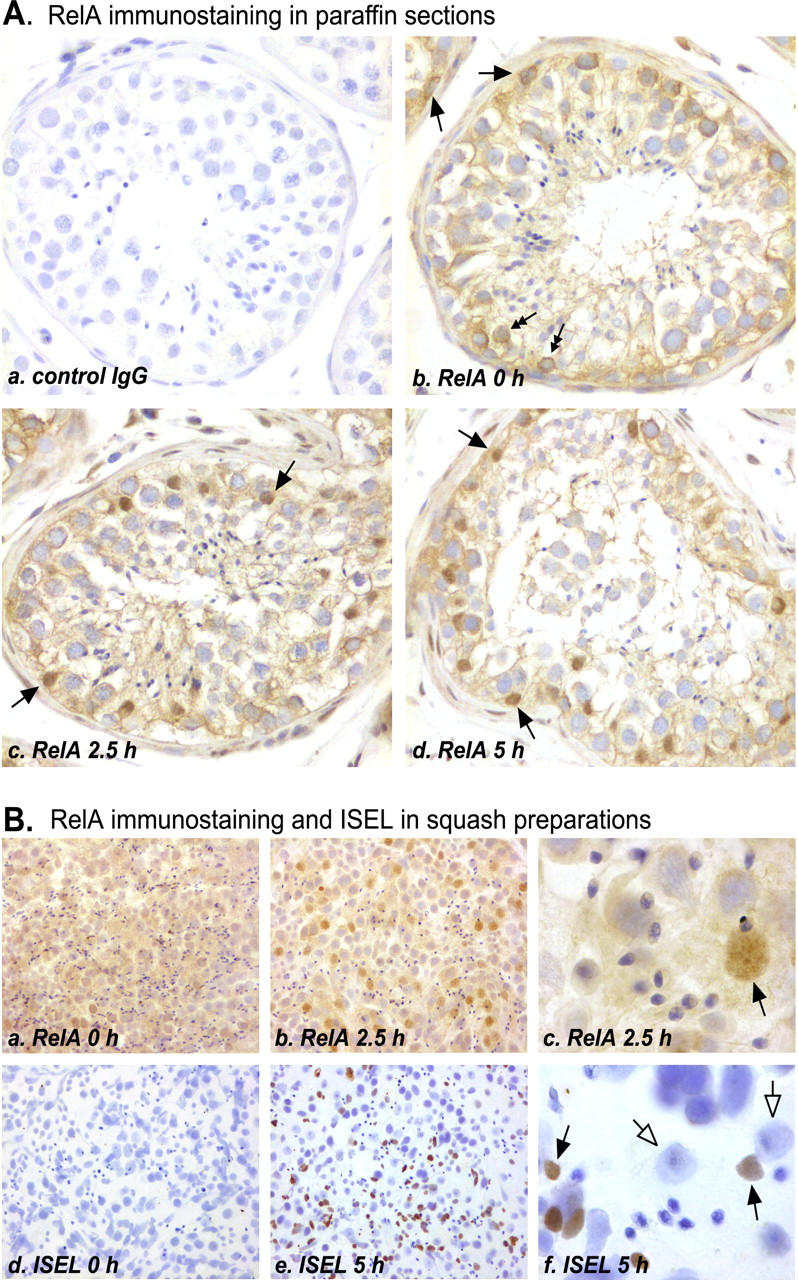
Cellular localization of NF-κB nuclear translocation and apoptotic DNA fragmentation during in vitro-induced human testicular apoptosis. A: RelA immunostaining in paraffin-embedded sections of formalin-fixed human seminiferous tubules cultured for 0 to 5 hours in serum-free conditions. a: Nonimmune control in which nonspecific rabbit IgG was used as the primary antibody (original magnification, ×200). b: No nuclear staining for RelA was observed in the majority of the segments of the seminiferous tubules that were not exposed to apoptosis-inducing conditions. Cytoplasmic immunopositivity was found in some spermatogonia (arrows) and early meiotic germ cells (double-headed arrows). In addition, light immunostaining extending from the basal membrane to the tubule lumen is likely to represent RelA in the Sertoli cell cytoplasm. c–d: After culture for 2.5 or 5 hours, all of the segments of the tubules showed intense staining for the RelA in the Sertoli cell nuclei (arrows). B: Comparison of RelA immunostaining and in situ 3′-end labeling (ISEL) of apoptotic DNA. Segments of human seminiferous tubules were cultured in serum-free conditions for 0 to 5 hours, after which the samples were squashed and fixed, and immunostaining and ISEL protocols were performed as described in Materials and Methods. a and b: RelA nuclear translocation was observed in Sertoli cells (original magnifications, ×200). c: Original magnification of ×1000 showing the typical size and shape of a positively stained Sertoli cell nucleus and the characteristic nucleolus (arrow). d: Very rare ISEL-positive apoptotic cells were observed in seminiferous tubules squashed immediately after the orchidectomy (original magnification, ×200). e: In samples cultured for 5 hours in apoptosis-inducing conditions, the number of apoptotic cells was greatly increased. f: Original magnification of ×1000 demonstrating that apoptotic DNA fragmentation occurred in germ cells (arrows; late phase of germ cell apoptosis) whereas the Sertoli cell nuclei remained ISEL-negative (open-headed arrows). Immunostaining of RelA in uncultured and cultured seminiferous tubules was repeated three independent times and ISEL of uncultured and cultured seminiferous tubules is representative of at least 20 independent experiments.
Early Activation of NF-κB during in Vitro-Induced Testicular Apoptosis
The activation of NF-κB during testicular apoptosis was studied in our established in vitro model. 27 As shown in Figure 2A ▶ , incubation of segments of human seminiferous tubules under serum-free culture conditions resulted in induction of apoptotic DNA fragmentation within 5 hours. Apoptosis had further increased at 10 hours of culture. In the same tubules, NF-κB DNA-binding activity had strongly and rapidly (within 30 minutes) increased from the basal level and it remained at this elevated level throughout the culture (Figure 2B) ▶ . Especially band A in EMSA was markedly intensified during induction of testicular apoptosis (Figure 2B) ▶ . We therefore concluded that NF-κB activation occurs very early as compared with nuclear apoptosis (Figure 2) ▶ .
NF-κB Activation Is Induced in Sertoli Cells whereas Apoptotic Cell Death Occurs in Germ Cells
To study the site of NF-κB activation in the human seminiferous epithelium, we first tested the subunit composition of the inducible DNA-protein complexes by supershift assays using nuclear protein extracts from seminiferous tubules cultured for 2.5 hours in serum-free conditions. As demonstrated in Figure 3 ▶ , complexes A and B, representing nuclear proteins specifically binding to the κB consensus oligonucleotide, were retarded by the anti-p50 and anti-RelA antibodies, but not by the other antibodies tested (c-Rel, p52, RelB, and an unrelated control antibody), indicating that the inducible NF-κB in the human testis is composed of the p50 and RelA subunits. The ability of the antibodies against c-Rel, p52, and RelB to bind to the corresponding DNA-protein complexes was confirmed in EMSAs of control nuclear extracts (K562 for c-Rel, Jurkat for p52, and LPS-stimulated human monocyte-derived macrophage and KNRK for RelB) (data not shown).
Figure 3.
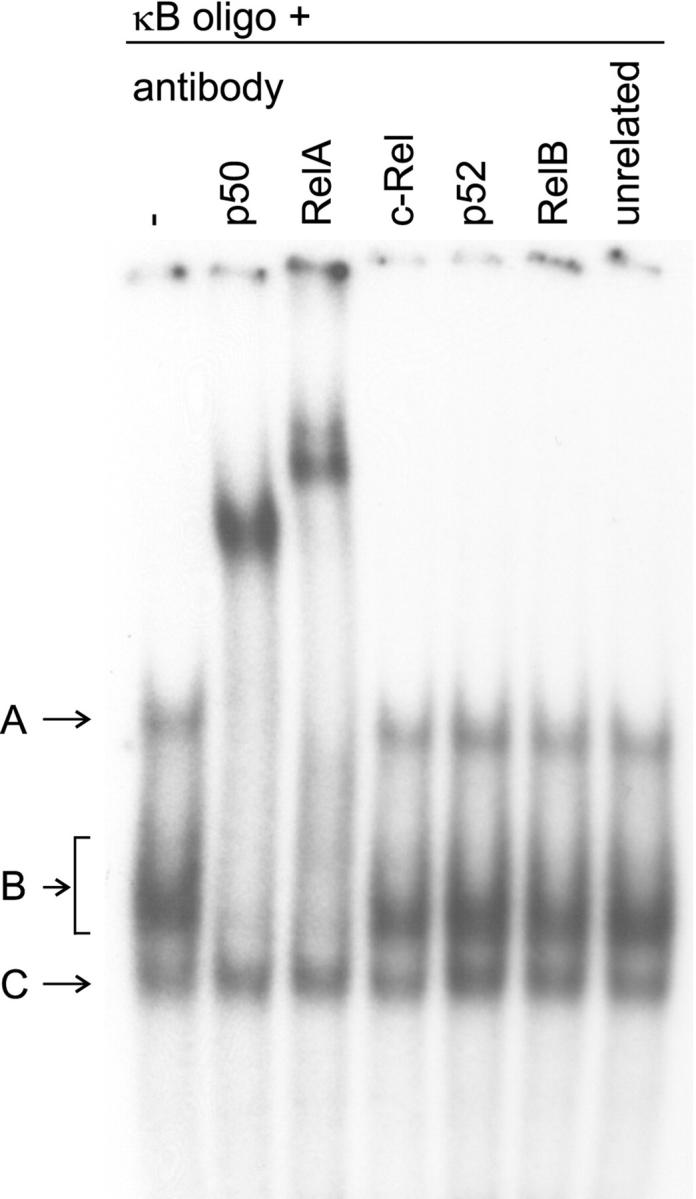
Supershift assay identifying the subunit composition of the inducible NF-κB complexes in the human testis. Nuclear protein extracts (10 μg) of human seminiferous tubules cultured for 2.5 hours in serum-free conditions were incubated with a 32P-labeled κB oligonucleotide, after which various antibodies were added to the binding reactions as indicated. The NF-κB complexes A and B were retarded by the anti-p50 and anti-RelA antibodies, indicating that the inducible NF-κB in the human testis is composed of the corresponding proteins. The other antibodies, which did not affect the electrophoretic mobility of the testicular NF-κB complexes, bound to the corresponding DNA-protein complexes in EMSAs of control nuclear extracts described in Materials and Methods (data not shown). The three NF-κB complexes in cultured seminiferous tubules were observed in 11 independent experiments and the result of the supershift assay is representative of two independent experiments.
Nuclear translocation of the p50 and RelA proteins was next studied immunohistochemically in paraffin-embedded sections of formalin-fixed human seminiferous tubules, using antibodies to human p50 and RelA. Because the presence and the nuclear translocation of these two proteins were found in the same cell type, only the RelA immunostaining is shown in Figure 4 ▶ . When the primary antibodies were replaced with a similar concentration of nonspecific rabbit IgG, there was no specific staining (Figure 4A, a) ▶ . In the majority of the freshly isolated segments of seminiferous tubules that were not exposed to apoptosis-inducing conditions, strong cytoplasmic immunostaining was seen in the spermatogonia and the early meiotic germ cells as well as light cytoplasmic immunostaining in the Sertoli cells, but there was no nuclear NF-κB expression (Figure 4A, b) ▶ . In contrast, in all of the segments of tubules cultured for 2.5 or 5 hours in serum-free conditions, positively staining Sertoli cell nuclei were observed (Figure 4A, c and d) ▶ . Of note, nuclear translocation of the NF-κB proteins was not observed in the spermatogonia or early meiotic spermatocytes, in which cytoplasmic staining for the RelA and p50 was intense in the 0-hour samples. Thus, the site of NF-κB activation, as indicated by nuclear translocation of the RelA and p50 proteins, was the Sertoli cells. The significance of the positive cytoplasmic NF-κB staining in the early meiotic germ cells remains unclear.
When considering the potential role of NF-κB in the regulation of testicular apoptosis, it was of interest to determine whether NF-κB activation occurred in the same cell types where apoptosis took place. Therefore, we performed RelA immunostaining and ISEL of apoptotic DNA fragments using squash preparations of human seminiferous tubules that had been exposed to apoptosis-inducing conditions or that had been cultured in serum-free conditions to induce apoptotic cell death (Figure 4B) ▶ . We used squash preparations rather than paraffin sections 1) because of the lack of false-positive labeling, which may be caused after accidental formation of free DNA 3′-ends during permeabilization of paraffin sections, we considered the results of ISEL more reliable, and 2) because in squash preparations, the nuclei of the cells maintain their characteristic morphology better, allowing more accurate identification of the individual cell types. In agreement with the results in the paraffin sections, no nuclear RelA immunostaining was observed in the majority of the segments of tubules squashed immediately after the orchidectomy (Figure 4B, a) ▶ , whereas, in the samples cultured for 2.5 hours, a great number of positively staining Sertoli cell nuclei were found (Figure 4B, b and c) ▶ . In the squash preparations, the identification of Sertoli cell nuclei was based on their typical size and shape and on the presence of a characteristic nucleolus. In contrast, in the ISEL analysis, apoptotic DNA fragmentation was predominantly observed in late meiotic and postmeiotic germ cells, whereas, the Sertoli cell nuclei remained negative (Figure 4B ▶ ; d, e, and f). This result is in agreement with the results of our previous studies 31,32 in which we have shown with ISEL and electron microscopy that the cells undergoing apoptosis in the present in vitro model are mainly spermatocytes and spermatids.
Inhibition of Testicular NF-κB Induction and Apoptosis by SS
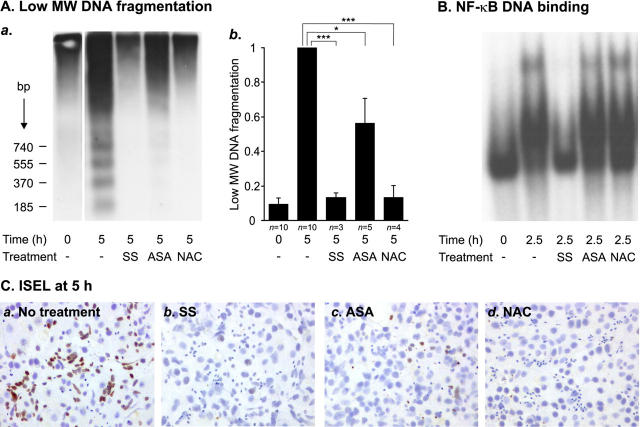
To evaluate the effects of NF-κB activation on testicular germ cell survival, we next tested the ability of previously reported NF-κB inhibitors to modulate NF-κB activation and apoptotic germ cell death in our in vitro model. Both testicular apoptosis and NF-κB induction were effectively blocked by the anti-inflammatory drug SS (Figure 5) ▶ . A 5 mmol/L concentration of SS inhibited apoptotic DNA fragmentation during 5 hours of culture by 87% (P < 0.001) (Figure 5A) ▶ and concomitantly retained NF-κB DNA-binding activity at the basal (0 hour) level after 2.5 hours (Figure 5B) ▶ and 5 hours (data not shown) in three independent experiments. Interestingly, effective inhibition of apoptotic DNA fragmentation by SS was still observed after 48 hours of culture in two independent experiments (data not shown). In one experiment, culture of seminiferous tubules was continued for 4 days, after which SS still prevented apoptotic DNA laddering but some smearing typical for necrosis was observed in SS-treated tubules (data not shown). A NF-κB inhibitory peptide SN-50 (10 μg/ml) also slightly inhibited testicular apoptosis (24%, P = 0.01), but an inhibitory effect of this peptide on NF-κB activation could not be reliably detected with EMSAs (data not shown). Other previously reported NF-κB inhibitors, ASA and NAC, were also found to be effective inhibitors of testicular apoptosis (Figure 5A) ▶ . Apoptotic DNA fragmentation was reduced by 44% (P < 0.05) with ASA at 5 mmol/L and by 87% (P = 0.001) with NAC at 100 mmol/L. However, although capable of inhibiting apoptosis, ASA and NAC had no effect on NF-κB DNA binding (Figure 5B) ▶ . Thus, the anti-apoptotic effects of ASA and NAC seem to be mediated by mechanisms other than NF-κB inhibition. In contrast, the strong apoptosis-blocking effect of SS may, at least partly, be mediated via NF-κB inhibition.
Figure 5.
Effects of NF-κB inhibitors on human testicular apoptosis and NF-κB activation. Segments of human seminiferous tubules were cultured in serum-free conditions in the absence or presence of 5 mmol/L SS, 5 mmol/L ASA, or 100 mmol/L NAC. After culture for 2.5 hours, samples of the tubules were snap-frozen and nuclear protein extracts were prepared for analysis of NF-κB activity. The rest of the tubules were cultured for 5 hours and analyzed for apoptotic DNA fragmentation by Southern blot and ISEL analyses. A: Southern blot analysis of apoptotic DNA fragmentation. Equal amount (1 μg) of the total DNA from each sample was 3′-end labeled with Dig-dd-UTP, after which the DNA samples were electrophoresed and blotted onto nylon membranes and the labeled apoptotic DNA fragments were detected with chemiluminescence as described in Materials and Methods. a: Radiograph from a representative experiment in which 5 mmol/L SS, 5 mmol/L ASA, or 100 mmol/L NAC was added to the culture medium. b: Quantification of SS-, ASA-, and NAC-mediated inhibition of low molecular weight DNA (<1.3 kb) fragmentation. Each value represents a mean of the indicated number of independent experiments ± SEM. In all experiments, SS and NAC blocked apoptosis extremely effectively. The anti-apoptotic effect of ASA was strong in three experiments but only moderate in two experiments, which led to the variation indicated in the figure. *, P < 0.05; ***, P = 0.001. B: EMSA showing the effects of 5 mmol/L SS, 5 mmol/L ASA, or 100 mmol/L NAC on testicular NF-κB DNA-binding activity. Nuclear protein extracts (10 μg) were analyzed for NF-κB DNA-binding activity, as described in the legends to Figures 1 and 2 ▶ ▶ and in Materials and Methods. The result is representative of three independent experiments. C: ISEL analysis of the inhibition of germ cell apoptosis by SS, ASA, or NAC. Apoptotic DNA fragments in squash preparations of seminiferous tubules cultured for 5 hours in serum-free conditions in the absence or presence of indicated compounds were detected by 3′-end labeling with Dig-dd-UTP as described in Materials and Methods. a: A great number of ISEL-positive germ cells were observed in the seminiferous tubules after 5 hours of exposure to apoptosis-inducing conditions. Germ cell death was effectively suppressed by 5 mmol/L SS (b), 5 mmol/L ASA (c), or 100 mmol/L NAC (d). The overall morphology of the testicular cells was not disturbed by the treatments.
To confirm the Southern blot results indicating inhibition of apoptosis by SS, ASA, and NAC, and to be sure that the overall morphology of the seminiferous epithelial cells had remained normal during the treatments, we further performed ISEL analysis of squash preparations of seminiferous tubules cultured for 5 hours in the absence or presence of these compounds. In agreement with the results obtained in the Southern blot analyses, germ cell death was effectively suppressed by 5 mmol/L of SS, 5 mmol/L of ASA, or 100 mmol/L of NAC (Figure 5C) ▶ . No severe abnormalities in the morphology of the testicular cells were observed. Because of the presence of different populations of germ cells in the adjacent spirally oriented stages of the human seminiferous tubules, the varying amounts of ISEL positivity in individual squash preparations could be because of varying amounts of apoptotic activity in distinct types of germ cells presented in the preparation. Therefore, a large number of squash preparations were examined, and the result presented in Figure 5C ▶ represents the most typical finding. Of note, in all of the squash preparations made from the tubules treated with SS or NAC, disappearance of the apoptotic cells was complete. Negative controls, in which the terminal transferase enzyme was replaced with distilled water, showed no staining (data not shown).
SS-Mediated Inhibition of Sertoli Cell NF-κB Activation at the Level of Nuclear DNA Binding
Because SS in certain cell types has previously been reported to inhibit NF-κB activity by inhibiting IκB degradation, 33,34 we wished to know whether the same inhibitory mechanism functions in the seminiferous epithelium. Western blot analysis of IκBα protein in the seminiferous tubules showed that, in agreement with the rapid increase in the NF-κB DNA-binding activity, IκBα was readily degraded after the onset of serum withdrawal (Figure 6A, a) ▶ . Even during 10 hours of culture, however, the IκBα protein did not completely disappear; the lowest level was observed at 2.5 hours, after which the expression of IκBα gradually increased slightly. This is likely to be explained by the known ability of activated NF-κB to stimulate IκBα gene transcription. IκBα degradation was not prevented in the samples treated with 5 mmol/L of SS (Figure 6A, b) ▶ , thus suggesting that SS inhibits NF-κB activation at a level distal to IκB degradation. Interestingly, Western blotting revealed the complete disappearance of the IκBα protein in the SS-treated tubules after 5 hours of culture (Figure 6A, b) ▶ , suggesting that SS prevented the NF-κB-induced synthesis of new IκBα protein. Similar results were obtained in three independent experiments and with two different antibodies to human IκBα. Equal loading of the samples was confirmed by reprobing the blots with an antibody against α-tubulin. In agreement with the results of the IκBα Western blots, RelA immunostaining of SS-treated seminiferous tubules showed that nuclear translocation of the RelA protein in the Sertoli cells was not prevented by SS (Figure 6B) ▶ . Thus, in the present apoptosis model, SS seems to inhibit NF-κB at the level of DNA binding in the nucleus.
Figure 6.
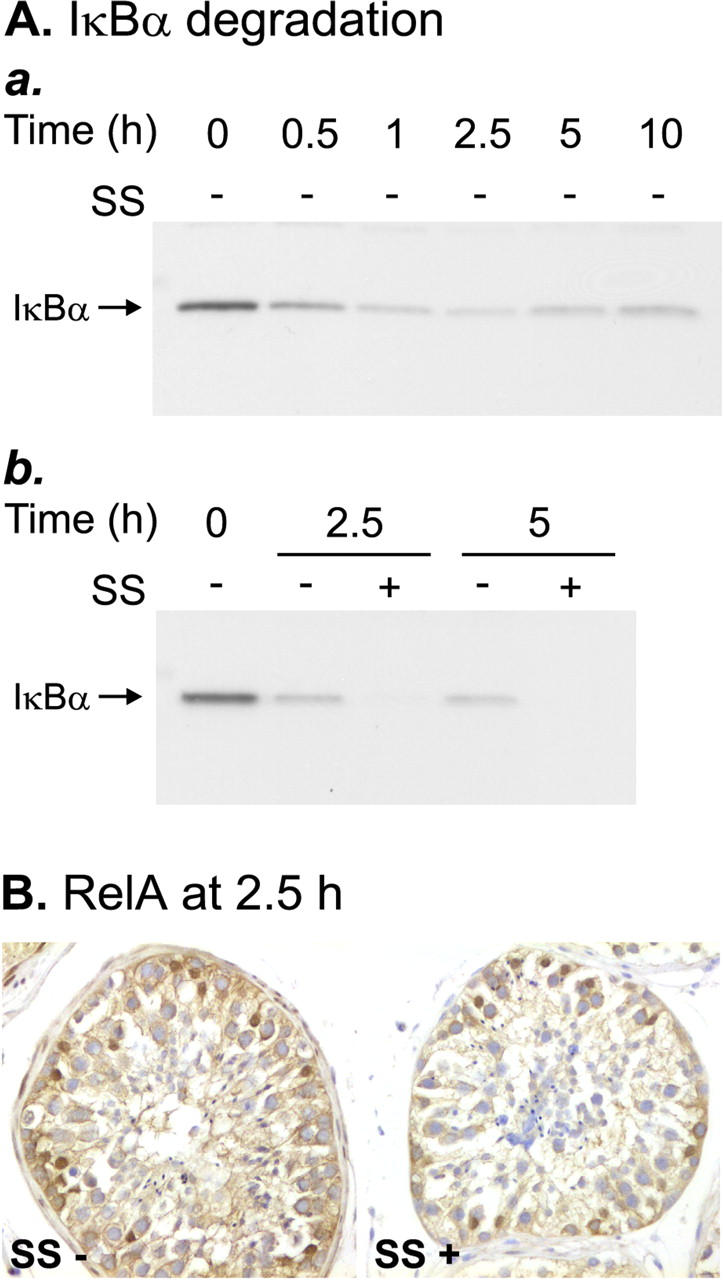
Effects of SS on IκBα protein expression and RelA nuclear translocation. A: Western blots demonstrating the effect of SS (SS) on the testicular IκBα. Cytosolic protein extracts were prepared from human seminiferous tubules cultured in serum-free conditions for increasing lengths of time in the absence or presence of 5 mmol/L of SS. Equal amounts of protein (15 μg) were subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted on polyvinylidene difluoride membranes, which were then probed with a rabbit polyclonal antibody to human IκBα, as described in Materials and Methods. a: Time course of IκBα degradation during testicular apoptosis. b: SS did not block IκBα degradation, but prevented formation of new IκBα protein after 2.5 hours and 5 hours. The Western blots shown are representative of three independent experiments. B: RelA immunostaining of paraffin sections of seminiferous tubules cultured for 2.5 hours in the absence (SS−) or presence (SS+) of 5 mmol/L of SS. Immunopositive Sertoli cell nuclei were observed in both SS-treated and untreated tubules, indicating that nuclear translocation of the RelA was not affected by SS. The results shown are representative of three independent experiments.
Testicular FasL Expression Seems Not to Be Regulated by the Inducible NF-κB
Finally, because the promoter of the gene encoding the Fas ligand (FasL) is known to contain NF-κB-binding sites, 24,35 and because the regulation of this gene has been suggested to be a potential target for NF-κB in the testis, we tested the effect of the SS-mediated NF-κB blockade on the expression of the FasL protein in Western blot analysis. Despite the effective inhibition of NF-κB activation, SS had no effect on the protein expression of the FasL after 2.5 hours and 5 hours culture (data not shown), suggesting that NF-κB does not regulate testicular FasL expression during stress-induced apoptosis.
Discussion
Recent data have suggested a potential role for the transcription factor NF-κB in regulating rodent spermatogenesis, 6 but the physiological significance of NF-κB in the testis tissue has remained unclear. Moreover, studies on NF-κB expression and activity in the human testis have been limited to our preliminary findings on NF-κB DNA-binding activity in isolated human seminiferous tubules. 26 In view of the growing evidence on the role of NF-κB in controlling apoptosis, 5 and the importance of apoptotic cell death during spermatogenesis, 2 we set out to elucidate NF-κB activation during testicular apoptosis and its consequences. In the present study we have shown that constitutively active nuclear NF-κB exists at low levels in the Sertoli cells of the human testis. Furthermore, using cultured human seminiferous tubules as a model, 27 we demonstrated that nuclear NF-κB expression and DNA binding in Sertoli cells increases rapidly during in vitro-induced apoptosis of male germ cells. Finally, we found that pharmacological inhibition of testicular NF-κB activation was associated with effective suppression of germ cell death.
Our first aim was to describe the constitutive expression and DNA-binding activity of NF-κB transcription factors in normal adult human testis. Consistently with the results for the rat testis, 13 we found low basal NF-κB DNA-binding activity, which, by immunostaining of the RelA and p50 subunits, was localized to Sertoli cells. However, in contrast to the rat testis, in which Sertoli cells were found to express nuclear NF-κB at all stages of spermatogenesis, 13 only a few segments of the seminiferous tubules contained positively staining Sertoli cell nuclei in the human testis. Moreover, no germ cells showing nuclear NF-κB were found, which contrasts with the previously observed stage-dependent expression of nuclear NF-κB in the late meiotic and postmeiotic germ cells of the rat testis. 13 However, intense cytoplasmic staining for NF-κB was found in the spermatogonia and early meiotic germ cells of the human testis. As no nuclear translocation of this cytoplasmic NF-κB was observed during in vitro-induced testicular stress, the physiological significance of this finding remains unclear. In light of the importance of the stable pool of immature germ cells for continuous spermatogenesis, 36 it can be hypothesized that the extensive deposition of NF-κB in the cytoplasm of the spermatogonia and immature spermatocytes can be used for rapid nuclear translocation and transcriptional activation of protective genes on certain stimuli.
A strong and rapid increase in nuclear NF-κB DNA-binding activity was observed in human seminiferous tubules cultured in serum-free conditions to induce testicular apoptosis. NF-κB induction was already evident after 30 minutes of culture, but clear apoptotic DNA fragmentation only appeared in Southern blot analysis after 5 hours of culture. The very early activation of NF-κB, as compared with nuclear apoptosis, led us to suggest a regulatory role for NF-κB in testicular apoptosis. In this regard, it was of interest to know whether NF-κB activation and apoptosis occur in the same cell types. Increased positive nuclear immunostaining for RelA and p50 was observed in the Sertoli cells of all of the tubules cultured under serum-free conditions, indicating that the site of NF-κB activation was in the Sertoli cells. However, ISEL analysis of apoptotic DNA fragmentation revealed that apoptosis was induced in late meiotic and postmeiotic germ cells whereas the Sertoli cells survived. Thus, it could be hypothesized that during severe stress NF-κB activation in Sertoli cells functions to protect the Sertoli cells themselves and, to keep the germ cell population small enough for the limited nursing capacity of the Sertoli cells, simultaneously induces transcription of Sertoli cell gene(s) that are able to mediate germ cell death. This would be a reasonable way for the Sertoli cells to act, because they are essential for functional spermatogenesis 1 and they are terminally differentiated cells with no capacity for renewal. Interestingly, induction of testicular NF-κB DNA-binding activity was completely blocked by the anti-inflammatory aminosalicylate drug SS, which has also been shown to specifically inhibit NF-κB activation in colon epithelial cells and in Jurkat T cells. 33,34 Concomitantly, germ cell apoptosis was effectively suppressed, supporting the hypothesized role of NF-κB as one of the factors controlling stress-induced germ cell death. However, Sertoli cells survived despite SS treatment, which does not support the view that Sertoli cell survival depends on their NF-κB activation. It also suggests that the apoptotic death of meiotic and postmeiotic male germ cells can be pharmacologically modulated by NF-κB inhibitory drugs with no hazardous effects on Sertoli cells.
SS has been previously reported to inhibit NF-κB activation by directly inhibiting the IκB kinases α and β, which leads to inhibition of the IκBα degradation. 34 Interestingly, in the present study, SS inhibited neither IκBα degradation nor nuclear translocation of the RelA subunit, but prevented the formation of new IκBα protein, which may indicate a decrease in NF-κB-mediated transcriptional activation of the IκBα gene. That the cytoplasmic NF-κB in some immature germ cells did not translocate into the nucleus despite disappearance of the IκBα may indicate that the type of IκB in these cells is other than IκBα. Indeed, high levels of IκBβ and IκBε mRNA have been found in the testis, 37,38 which suggests that these forms of IκB participate in the regulation of testicular NF-κB. Thus, the mechanism by which SS appears to inhibit NF-κB DNA binding in the present apoptosis model differs from the previously documented SS-mediated inhibition of the IκB kinases, and remains to be clarified. Interestingly, the DNA-binding and transactivating capacities of RelA and p50 are also positively regulated by selective phosphorylation of these proteins. 39 That the phosphorylation of the NF-κB proteins can be pharmacologically modulated without affecting IκB phosphorylation and degradation is exemplified by the finding that mesalamine, an analogue of SS that lacks an azo-group present in SS, stimulates interleukin-1-induced NF-κB-dependent transcription in colon epithelial cells by selectively inhibiting RelA phosphorylation and has no effect on IκB degradation, NF-κB nuclear translocation, or NF-κB DNA binding. 40 As the DNA binding of RelA and p50 may also be disturbed by their hypophosphorylation 39 and as different NF-κB-inducing stimuli may cause phosphorylation of NF-κB proteins at unique sites, 41,42 which possibly differentially affects their DNA binding, selective inhibition of RelA or p50 phosphorylation could be the mechanism by which SS blocks NF-κB DNA binding in human testicular cells under the conditions induced in the present model. Because of the general anti-inflammatory action of SS, other simultaneous NF-κB-independent pathways affecting either Sertoli cells or germ cells directly may also contribute to the SS-mediated germ cell survival. In this context, apoptosis inhibition because of the anti-oxidative properties of SS 43,44 seems unlikely, because IκBα degradation, which in many cell types is known to be blocked by anti-oxidative compounds, 45 was not prevented. However, this possibility cannot be completely ruled out, because a delayed reactive oxygen species-dependent signaling pathway, which is required for NF-κB transcriptional activation but is separable from that required for its nuclear translocation and DNA binding, has also been described. 46
Because of the complex cell-specific network of interacting proteins in the NF-κB signaling pathway, diverse compounds may act at multiple levels to inhibit NF-κB activation and, importantly, the ability of a given compound to modulate NF-κB activity depends on the cell type and the NF-κB-inducing signal. 45 Therefore, in parallel to SS, we tested the ability of other compounds, previously reported to inhibit NF-κB in other cellular systems, to suppress testicular NF-κB activity and apoptosis. Of these compounds, a NF-κB-inhibitory peptide, SN-50, which competes for the nuclear transport system with NF-κB, 47 also slightly inhibited testicular apoptosis. A concomitant weak inhibition of NF-κB DNA binding in SN-50-treated seminiferous tubules was observed in some experiments, but perhaps because of the large amount of inhibition needed for clear results in the EMSA experiments, this inhibitory effect of the SN-50 peptide on NF-κB activation was not reliably detected in all experiments. In contrast, ASA 48 and NAC 49 effectively suppressed testicular apoptosis, but had no effect on NF-κB activation. Thus, in the present study, the anti-apoptotic effects of ASA and NAC seem to be mediated by mechanisms that do not involve Sertoli cell NF-κB inhibition and may affect germ cells directly. Although this result could be interpreted as showing that NF-κB activation does not play an obligatory role in the induction of testicular apoptosis, it more likely reflects the presence of many compensatory or parallel apoptotic pathways in the testis. The importance of apoptotic cell death for functional spermatogenesis would support the idea that apoptosis induction in the testis does not rely on a single pathway.
Finally, we wished to find out whether the testicular expression of the death-promoting cytokine FasL is regulated by NF-κB, because this cytokine is known to play an important role in the control of testicular apoptosis, 50-52 and because its expression has been shown to be directly regulated by NF-κB in certain forms of apoptosis. 23,24 We have previously shown that the expression of the FasL is up-regulated during in vitro-induced testicular apoptosis. 26 If this was because of NF-κB-dependent transcription of the gene encoding the FasL, then SS, which effectively blocks NF-κB DNA-binding activity, should affect the expression of the FasL. However, we found no effect of SS on the expression of the FasL suggesting that, at least in the present in vitro model, NF-κB does not regulate testicular FasL gene expression during stress-induced apoptosis. This result agrees with our previous results showing that the cytokine TNF-α inhibited testicular apoptosis and concomitantly down-regulated the expression of the FasL, but had no effect on the inducible NF-κB activity. 26 Thus, in a testicular stress situation caused by external disturbances, the NF-κB- and FasL-mediated pathways seem to function in parallel to regulate testicular germ cell death.
In the present study, we used an in vitro tissue culture for studying NF-κB activation in human testicular apoptosis. Our culture model, like all in vitro models, has limitations. NF-κB activation depends on the type of stressor and the culture of seminiferous tubules under serum-free conditions involves not only serum deprivation but also relative hyperoxia because of high partial oxygen pressure in normal atmosphere as compared with that found in peripheral organs such as the testis. Thus, the apoptotic pathways induced in the present model are potentially multiple and, accordingly, there may also be multiple inducers of NF-κB. Moreover, various stimuli may differentially activate NF-κB in different types of cells. It would be interesting to study NF-κB activity after either specific Sertoli cell toxicants, such as mono-(2-ethylhexyl)phthalate or 2,5-hexanedione, 53,54 or disturbers of germ cells such as radiation. 55,56 However, the culture conditions are difficult to optimize so that no spontaneous germ cell apoptosis occurs, which is a prerequisite for conduction of such experiments. Furthermore, using those kinds of treatments in vivo is virtually impossible in humans. Moreover, because of the potential species specificity of cellular responses, results obtained from animal studies do not necessarily apply to humans. Finally, because the contacts between Sertoli cells and germ cells play an important role in testicular physiology and pathology, culturing isolated cells is inappropriate. Therefore, we feel that despite its limitations, the present in vitro model, which maintains the physiological contacts between the cells of the seminiferous epithelium, gives the best available way to detect human testicular apoptotic mechanisms involving interaction between different cell types, such as is presented here, or has been shown for FasL-mediated testicular germ cell apoptosis. 50 The present culture system models a situation in which human testicular homeostasis is threatened, demonstrates how different types of cells in the seminiferous epithelium may act during severe stress, and gives the opportunity to study the effects of pharmacological modulation of stress-induced apoptosis in the human testis. Here we have found that the in vitro-induced death of male germ cells can be effectively suppressed by SS treatment. Our finding is supported by reports of reversible infertility and sperm abnormalities, including abnormally large numbers of immature germ cells in the sperm, in men treated with SS for inflammatory bowel diseases. 57-61 This finding suggests that SS affects germ cell maturation and apoptosis also in vivo. Moreover, the reversibility of the infertility supports our observation that only the apoptotic death of the germ cells in later phases of maturation is affected by the SS treatment, leaving the Sertoli cells and the immature germ cells undisturbed and potentially capable of functional spermatogenesis after cessation of the treatment.
In conclusion, we have shown that constitutive NF-κB DNA-binding activity in the Sertoli cells of the human testis was strongly and rapidly increased during in vitro-induced testicular apoptosis. Interestingly, apoptotic death occurred in maturing germ cells, whereas the Sertoli cells containing the inducible NF-κB survived. Thus, the stress-induced NF-κB nuclear translocation in Sertoli cells could be hypothesized to induce the transcription of genes encoding factors involved in the regulation of germ cell death. Such a mechanism would serve to maintain the sufficiency of the testicular environment for functional spermatogenesis. The role of NF-κB as one of the important factors regulating male germ cell apoptosis was supported by the finding that both testicular NF-κB activation and germ cell death were effectively suppressed by a previously reported NF-κB inhibitor and established clinical drug SS. Although the tissue culture model used in the present study induces an extreme stress situation in the seminiferous tubules, it may represent a model showing how different cell types in the seminiferous epithelium react during the more subtle stress situations occurring in vivo. Moreover, the results obtained with SS raise the possibility that pharmacological inhibition of NF-κB could be a therapeutic target in transient stress situations involving excessive apoptosis of male germ cells.
Acknowledgments
We thank Ms. Virpi Aaltonen, Ms. Kaisa Alasalmi, and Ms. Sinikka Heikkilä for their skillful technical assistance; and the staff of the Department of Surgery, Helsinki University Central Hospital, for providing the orchidectomy samples.
Footnotes
Address reprint requests to Virve Pentikäinen, M.D., Hospital for Children and Adolescents, University of Helsinki Biomedicum Helsinki, P.O. Box 700, FIN-00029 HUS, Finland. E-mail: vpentika@mappi.helsinki.fi.
Supported by the Helsinki Biomedical Graduate School, University of Helsinki; the Foundation for Pediatric Research, Finland; and the Sigrid Juselius Foundation, Finland.
References
- 1.Griswold MD: The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 1998, 9:411-416 [DOI] [PubMed] [Google Scholar]
- 2.Dunkel L, Hirvonen V, Erkkilä K: Clinical aspects of male germ cell apoptosis during testis development and spermatogenesis. Cell Death Differ 1997, 4:171-179 [DOI] [PubMed] [Google Scholar]
- 3.Baldwin Jr AS: The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 1996, 14:649–683 [DOI] [PubMed]
- 4.Pahl HL: Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18:6853-6866 [DOI] [PubMed] [Google Scholar]
- 5.Barkett M, Gilmore TD: Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999, 18:6910-6924 [DOI] [PubMed] [Google Scholar]
- 6.Delfino F, Walker WH: Hormonal regulation of the NF-κB signaling pathway. Mol Cell Endocrinol 1999, 157:1-9 [DOI] [PubMed] [Google Scholar]
- 7.Gilmore TD: The Rel/NF-κB signal transduction pathway: introduction. Oncogene 1999, 18:6842-6844 [DOI] [PubMed] [Google Scholar]
- 8.Whiteside ST, Israel A: I κB proteins: structure, function and regulation. Semin Cancer Biol 1997, 8:75-82 [DOI] [PubMed] [Google Scholar]
- 9.Karin M: How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 1999, 18:6867-6874 [DOI] [PubMed] [Google Scholar]
- 10.Sun SC, Ganchi PA, Ballard DW, Greene WC: NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science 1993, 259:1912-1915 [DOI] [PubMed] [Google Scholar]
- 11.Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay RT, Virelizier JL, Dargemont C: Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J Cell Sci 1997, 110:369-378 [DOI] [PubMed] [Google Scholar]
- 12.Sachdev S, Hoffmann A, Hannink M: Nuclear localization of IκBα is mediated by the second ankyrin repeat: the IκBα ankyrin repeats define a novel class of cis-acting nuclear import sequences. Mol Cell Biol 1998, 18:2524-2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delfino F, Walker WH: Stage-specific nuclear expression of NF-κB in mammalian testis. Mol Endocrinol 1998, 12:1696-1707 [DOI] [PubMed] [Google Scholar]
- 14.De SK, Chen HL, Pace JL, Hunt JS, Terranova PF, Enders GC: Expression of tumor necrosis factor-α in mouse spermatogenic cells. Endocrinology 1993, 133:389-396 [DOI] [PubMed] [Google Scholar]
- 15.Delfino FJ, Walker WH: NF-κB induces cAMP-response element-binding protein gene transcription in Sertoli cells. J Biol Chem 1999, 274:35607-35613 [DOI] [PubMed] [Google Scholar]
- 16.Beg AA, Baltimore D: An essential role for NF-κB in preventing TNF-α-induced cell death. Science 1996, 274:782-784 [DOI] [PubMed] [Google Scholar]
- 17.Liu ZG, Hsu H, Goeddel DV, Karin M: Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 1996, 87:565-576 [DOI] [PubMed] [Google Scholar]
- 18.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM: Suppression of TNFα-induced apoptosis by NF-κB. Science 1996, 274:787-789 [DOI] [PubMed] [Google Scholar]
- 19.Wang CY, Mayo MW, Baldwin AS: TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 1996, 274:784-787 [DOI] [PubMed] [Google Scholar]
- 20.Wang CY, Cusack JC, Liu R, Baldwin AS: Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat Med 1999, 5:412-417 [DOI] [PubMed] [Google Scholar]
- 21.Grimm S, Bauer MK, Baeuerle PA, Schulze-Osthoff K: Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol 1996, 134:13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hettmann T, DiDonato J, Karin M, Leiden JM: An essential role for nuclear factor κB in promoting double positive thymocyte apoptosis. J Exp Med 1999, 189:145-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR: DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell 1998, 1:543-551 [DOI] [PubMed] [Google Scholar]
- 24.Kasibhatla S, Genestier L, Green DR: Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J Biol Chem 1999, 274:987-992 [DOI] [PubMed] [Google Scholar]
- 25.Kaltschmidt B, Kaltschmidt C, Hofmann TG, Hehner SP, Droge W, Schmitz ML: The pro- or anti-apoptotic function of NFκB is determined by the nature of the apoptotic stimulus. Eur J Biochem 2000, 267:3828-3835 [DOI] [PubMed] [Google Scholar]
- 26.Pentikäinen V, Erkkilä K, Suomalainen L, Otala M, Pentikäinen MO, Parvinen M, Dunkel L: Tumor necrosis factor α downregulates the Fas ligand and inhibits germ cell apoptosis in the human testis. J Clin Endocrinol Metab 2001, 86:4480-4484 [DOI] [PubMed] [Google Scholar]
- 27.Erkkilä K, Henriksen K, Hirvonen V, Rannikko S, Salo J, Parvinen M, Dunkel L: Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab 1997, 82:2314-2321 [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Brasier A: Mechanism for biphasic RelA NF-κB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J Biol Chem 1997, 272:9825-9832 [DOI] [PubMed] [Google Scholar]
- 29.Parvinen M, Hecht NB: Identification of living spermatogenic cells of the mouse by transillumination-phase contrast microscopic technique for ‘in situ’ analyses of DNA polymerase activities. Histochemistry 1981, 71:567-579 [DOI] [PubMed] [Google Scholar]
- 30.Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L: Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab 2000, 85:2057-2067 [DOI] [PubMed] [Google Scholar]
- 31.Erkkilä K, Hirvonen V, Wuokko E, Parvinen M, Dunkel L: N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro. J Clin Endocrinol Metab 1998, 83:2523-2531 [DOI] [PubMed] [Google Scholar]
- 32.Erkkilä K, Pentikäinen V, Wikström M, Parvinen M, Dunkel L: Partial oxygen pressure and mitochondrial permeability transition affect germ cell apoptosis in the human testis. J Clin Endocrinol Metab 1999, 84:4253-4259 [DOI] [PubMed] [Google Scholar]
- 33.Wahl C, Liptay S, Adler G, Schmid RM: Sulfasalazine: a potent and specific inhibitor of nuclear factor κB. J Clin Invest 1998, 101:1163-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM: Suppression of NF-κB activity by sulfasalazine is mediated by direct inhibition of IκB kinases alpha and beta. Gastroenterology 2000, 119:1209-1218 [DOI] [PubMed] [Google Scholar]
- 35.Matsui K, Fine A, Zhu B, Marshak-Rothstein A, Ju ST: Identification of two NF-kappa B sites in mouse CD95 ligand (Fas ligand) promoter: functional analysis in T cell hybridoma. J Immunol 1998, 161:3469-3473 [PubMed] [Google Scholar]
- 36.de Rooij DG: Stem cells in the testis. Int J Exp Pathol 1998, 79:67-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S: IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 1995, 80:573-582 [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Nabel GJ: A new member of the IκB protein family, IκB epsilon, inhibits RelA (p65)-mediated NF-κB transcription. Mol Cell Biol 1997, 17:6184-6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen FE, Ghosh G: Regulation of DNA binding by Rel/NF-κB transcription factors: structural views. Oncogene 1999, 18:6845-6852 [DOI] [PubMed] [Google Scholar]
- 40.Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, Lipsky JJ, McKean DJ: Inhibition of interleukin-1-stimulated NF-κB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem 1999, 274:26448-26453 [DOI] [PubMed] [Google Scholar]
- 41.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S: The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 1997, 89:413-424 [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Baldwin Jr AS: Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem 1998, 273:29411–29416 [DOI] [PubMed]
- 43.Aruoma OI, Wasil M, Halliwell B, Hoey BM, Butler J: The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol 1987, 36:3739-3742 [DOI] [PubMed] [Google Scholar]
- 44.Gionchetti P, Guarnieri C, Campieri M, Belluzzi A, Brignola C, Iannone P, Miglioli M, Barbara L: Scavenger effect of sulfasalazine, 5-aminosalicylic acid, and olsalazine on superoxide radical generation. Dig Dis Sci 1991, 36:174-178 [DOI] [PubMed] [Google Scholar]
- 45.Epinat JC, Gilmore TD: Diverse agents act at multiple levels to inhibit the Rel/NF-κB signal transduction pathway. Oncogene 1999, 18:6896-6909 [DOI] [PubMed] [Google Scholar]
- 46.Vlahopoulos S, Boldogh I, Casola A, Brasier AR: Nuclear factor-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor α: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 1999, 94:1878-1889 [PubMed] [Google Scholar]
- 47.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J: Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem 1995, 270:14255-14258 [DOI] [PubMed] [Google Scholar]
- 48.Yin MJ, Yamamoto Y, Gaynor RB: The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396:77-80 [DOI] [PubMed] [Google Scholar]
- 49.Schreck R, Rieber P, Baeuerle PA: Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J 1991, 10:2247-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pentikäinen V, Erkkilä K, Dunkel L: Fas regulates germ cell apoptosis in the human testis in vitro. Am J Physiol 1999, 276:E310-E316 [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Richburg JH, Younkin SC, Boekelheide K: The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997, 138:2081-2088 [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K: The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology 1999, 140:852-858 [DOI] [PubMed] [Google Scholar]
- 53.Richburg JH, Boekelheide K: Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol 1996, 137:42-50 [DOI] [PubMed] [Google Scholar]
- 54.Blanchard KT, Allard EK, Boekelheide K: Fate of germ cells in 2,5-hexanedione-induced testicular injury. I. Apoptosis is the mechanism of germ cell death. Toxicol Appl Pharmacol 1996, 137:141-148 [DOI] [PubMed] [Google Scholar]
- 55.Hasegawa M, Wilson G, Russell LD, Meistrich ML: Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat Res 1997, 147:457-467 [PubMed] [Google Scholar]
- 56.Hasegawa M, Zhang Y, Niibe H, Terry NH, Meistrich ML: Resistance of differentiating spermatogonia to radiation-induced apoptosis and loss in p53-deficient mice. Radiat Res 1998, 149:263-270 [PubMed] [Google Scholar]
- 57.Levi AJ, Fisher AM, Hughes L, Hendry WF: Male infertility due to sulphasalazine. Lancet 1979, 2:276-278 [DOI] [PubMed] [Google Scholar]
- 58.Traub AI, Thompson W, Carville J: Male infertility due to sulphasalazine. Lancet 1979, 2:639-640 [DOI] [PubMed] [Google Scholar]
- 59.Toth A: Reversible toxic effect of salicylazosulfapyridine on semen quality. Fertil Steril 1979, 31:538-540 [DOI] [PubMed] [Google Scholar]
- 60.Toovey S, Hudson E, Hendry WF, Levi AJ: Sulphasalazine and male infertility: reversibility and possible mechanism. Gut 1981, 22:445-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giwercman A, Skakkebaek NE: The effect of salicylazosulphapyridine (sulphasalazine) on male fertility. A review. Int J Androl 1986, 9:38-52 [DOI] [PubMed] [Google Scholar]