Abstract
Angiogenesis is a complex process, involving functional cooperativity between cytokines and endothelial cell (EC) surface integrins. In this study, we investigated the mechanisms through which the α1β1 and α2β1 integrins support angiogenesis driven by vascular endothelial growth factor (VEGF). Dermal microvascular EC attachment through either α1β1 or α2β1 supported robust VEGF activation of the Erk1/Erk2 (p44/42) mitogen-activated protein kinase signal transduction pathway that drives EC proliferation. Haptotactic EC migration toward collagen I was dependent on α1β1 and α2β1 as was VEGF-stimulated chemotaxis of ECs in a uniform collagen matrix. Consistent with the functions of α1β1 and α2β1 in supporting signal transduction and EC migration, antibody antagonism of either integrin resulted in potent inhibition of VEGF-driven angiogenesis in mouse skin. Moreover, combined antagonism of α1β1 and α2β1 substantially reduced tumor growth and angiogenesis of human squamous cell carcinoma xenografts. Collectively, these studies identify critical collaborative functions for the α1β1 and α2β1 integrins in supporting VEGF signal transduction, EC migration, and tumor angiogenesis.
Vascular endothelial growth factor (VEGF) is a cytokine essential for the vasculogenesis associated with normal embryonic development and for the angiogenesis associated with wound healing, cancers, and a variety of other important pathologies. 1-3 Through its receptors, which include two distinct receptor tyrosine kinases, 4 VEGF exerts multiple effects on vascular endothelium including stimulation of endothelial cell (EC) proliferation, 5 rapid induction of microvascular permeability, 6,7 promotion of EC survival, 8-10 stimulation of EC adhesion and migration, 11 and induction of EC gene expression. 12 Thus, the mechanisms by which VEGF promotes angiogenesis are highly complex and involve the regulation of multiple EC functions.
Adhesion to extracellular matrix through cell surface integrins is generally required for cell proliferation, survival, and migration, and for cytokine-stimulation of these processes. 13-15 The complex integrin family of transmembrane proteins consists of heterodimers, each consisting of one α and one β chain. 16,17 Previously, we reported that VEGF potently induces dermal microvascular ECs to express the α1β1 and α2β1 integrins, two important members of the β1 integrin subfamily. 18 Depending on cell type, α1β1 and α2β1 generally bind collagens and laminins. 16,17 On dermal microvascular ECs, α1β1 and α2β1 are the principal receptors for interstitial collagen type I, a major component of the extracellular matrix; and α1β1 also is a receptor for collagen IV and laminin-1. 18
Our previous findings that VEGF induces α1β1 and α2β1 expression by microvascular ECs suggested that these integrins are important to the mechanism by which VEGF promotes angiogenesis. Consistent with this hypothesis, we found that a combination of α1-blocking and α2-blocking antibodies (Abs) inhibited VEGF-driven angiogenesis in the skin of adult mice. 18 However, the individual functional contributions of these integrins remained undefined. In this study we investigated specific functions of the α1β1 and α2β1 integrins in supporting VEGF-stimulated signal transduction and EC migration. Furthermore, we used a mouse model of VEGF-driven skin neovascularization to test the importance of the α1β1 and α2β1 integrins individually for angiogenesis in vivo. To assess the involvement of α1β1 and α2β1 in tumor angiogenesis, we examined the consequences of combined α1β1 and α2β1 antagonism in a xenograft model of human squamous cell carcinoma. Collectively, findings reported here indicate that the α1β1 and α2β1 integrins each serve important functions in supporting VEGF signaling, EC migration, and tumor angiogenesis within the collagen-rich matrix of skin.
Materials and Methods
VEGF, ECs, and Cell Culture
Purified recombinant human VEGF165, expressed in Sf21 cells, was obtained from the National Cancer Institute Preclinical Repository, Biological Resources Branch, Frederick, MD. Human dermal microvascular ECs were isolated from neonatal foreskins and cultured as previously described. 19 All experiments were performed with cells at the fourth to seventh passage.
Mitogen-Activated Protein Kinase (MAPK) Analyses
Experiments were performed in Costar 96-well EIA plates coated first overnight with 10 μg/ml Fc-specific goat anti-mouse IgG (Sigma Chemical Co., St. Louis, MO), followed by blocking of remaining nonspecific protein binding sites with 100 mg/ml bovine serum albumin (BSA) (fraction V, no. A9306; Sigma) for 2 hours at 37°C, followed by incubation for 1 hour with either 10 μg/ml or 0.2 μg/ml mouse monoclonal Abs (mAbs), as indicated. mAbs included the following: anti-human integrin α1 [(clone 5E8D9 (Upstate Biotechnology, Lake Placid, NY) and clone FB12 (Chemicon, Temecula, CA)], anti-human integrin α2 (clone A2-IIE10, Upstate Biotechnology), isotype IgG1 control Ab (clone G192-1; PharMingen, La Jolla, CA), and IgG2a isotype control Ab (clone G192-428, PharMingen). After incubation with mAbs, wells were washed three times with phosphate-buffered saline.
Cells were gently trypsinized, and washed twice in serum-free medium (EBM-2; Clonetics, San Diego, CA), and 8 × 10 4 cells in serum-free medium were added to Ab-coated wells. Cells were allowed to attach and spread, and after decay of MAPK activity to baseline, cells were stimulated with 20 ng/ml of VEGF. At harvest, the entire contents (cells and medium) of each well were lysed in standard Laemmli sodium dodecyl sulfate sample buffer without reducing agents but containing protease and phosphatase inhibitors: 1 mmol/L 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 1 μm aprotinin, 20 μm leupeptin, 35 μm bestatin, 15 μm pepstatin A, and 15 μm E-64 (all from Sigma), and 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L EGTA, 2.5 mmol/L sodium pyrophosphate, 5 mmol/L sodium orthovanadate, and 50 mmol/L sodium fluoride. One half the total volume of each sample was electrophoresed under reducing conditions on standard Laemmli gels containing 10% (w/v) polyacrylamide followed by electrophoretic transfer to Transblot membranes (BioRad, Richmond, CA). Blots were blocked for 1 hour with 5% (w/v) nonfat dry milk and stained with phospho-MAPK (Erk1/2) rabbit polyclonal Ab (New England Biolabs, Beverly, MA) and subsequently with total Erk1/2 rabbit polyclonal Ab (K-23; Santa Cruz Biotechnology, Santa Cruz, CA). Bound primary Ab was detected by staining with horseradish peroxidase-conjugated goat anti-rabbit IgG (New England Biolabs) followed by visualization with chemiluminescence (NEN Renaissance). All experiments were repeated at least three times with similar results.
Cell Migration Assays
Before assay, cells were induced for maximal expression of α1β1 and α2β1 by stimulating with 20 ng/ml of VEGF165 for 3 days as previously described. 19 Cell migration was assayed with 8-μm-pore size Transwell migration chambers (Costar). For haptotaxis assays, the undersides of membranes were coated at room temperature for 1 hour with 10 μg/ml rat tail collagen (BD Biosciences). For chemotaxis assays, both sides of the membranes were coated with collagen. After 60 minutes, coating solutions were removed and remaining protein-binding sites were blocked by incubation with a solution of 100 mg/ml of BSA at room temperature for 60 minutes. For chemotaxis assays only, 20 ng/ml of VEGF165 was included in the lower chambers as a chemoattractant. Cells (8 × 104) were added to the upper chambers in serum-free EBM-2 containing 10 mg/ml of BSA. Integrin-blocking or control isotype Abs in solution (10 μg/ml) were mixed with cells for 15 minutes before the addition of cells to chambers. The integrin Abs were identical to those used as immobilized ligands to support cell adhesion (see MAPK analyses, above). Cell migration was allowed to proceed for 4 hours at 37°C in a standard tissue culture incubator; cells then were removed from the upper surface of the membranes with a cotton swab, and cells that migrated to the lower surface were stained with 0.2% (w/v) crystal violet in 2% ethanol for 15 minutes and washed with water. Dried membranes were cut out and mounted on glass slides in immersion oil. At least 10 random high-power fields from each of triplicate membranes were counted for each experimental condition. No cell migration was observed when membranes were coated with BSA alone, and in no cases did we observe cells in the lower chamber that had traversed the membranes but did not remain attached. All migration assays were repeated at least twice with similar results.
Induction of Angiogenesis in Mouse Skin, Administration of Integrin-Blocking Abs, and Quantitation of Angiogenesis
Assays were based on a previously described model 20 with the following modifications. Athymic NCr nude mice (females, 11 weeks old) were injected subcutaneously midway on the right and left backsides with 0.25 ml of Matrigel (BD Biosciences) at a final concentration of 9 mg/ml together with 1.5 × 10 6 SK-MEL-2 cells transfected for stable expression of human VEGF165. Soon after injection, the Matrigel implant solidified and persisted without apparent deterioration throughout the 6-day assay interval. Isotype-matched control hamster mAb (150 μg, clone Ha 4/8) or blocking hamster anti-mouse α1 antibody (Ab) (clone Ha 31/8) 21 or blocking hamster anti-mouse α2 Ab (clone Ha 1/29) 21 were administered to five animals per group by intraperitoneal injection on days 1, 3, and 5. Five additional animals were treated with α1 Ab and α2 Ab in combination (150 μg each), and five animals were treated with the corresponding dose (300 μg) of control isotype Ab. After 6 days, the animals were euthanized and dissected.
Implants together with associated skin were fixed for 3 hours in 10% buffered formalin and embedded in paraffin. Sections were cut, deparaffinized, and treated with 0.1% trypsin for 30 minutes at 37°C to enhance antigen availability before staining with 2 μg/ml rat anti-mouse CD31 mAb (clone MEC 13.3, PharMingen). Bound Ab was stained with secondary rabbit anti-rat Ab coupled to horseradish peroxidase (Vectastain Elite Kit; Vector Laboratories, Burlingame, CA) and visualized with liquid DAB-Plus substrate (Zymed, San Francisco, CA). Sections were counterstained with hematoxylin (Vector Laboratories). Cross-sectional diameters of individual new blood vessels within the overlying skin at the Matrigel implant/host interface were measured from representative digitized images (three specimens from each group) with NIH Image Program 1.61 and data were expressed as average diameter ± SE (n = 80 for each group). Combined blood vessel cross-sectional areas within the overlying skin at the Matrigel/host interface, determined as a percentage of the total tissue, were measured from representative digitized images obtained from six specimens of each group, using NIH Image (n = 30 for each group). Statistical analyses were performed with the two-sided unpaired t-test (InStat Program).
Tumor Xenograft Model and Ab Administration
A431 squamous cell carcinoma cells (2 × 106) (American Type Culture Collection, Rockville, MD) were injected intradermally into both flanks of 8-week-old female BALB/c (nu/nu) mice (two sites per mouse) as described. 22 Beginning 1 day after implantation, mice (n = 5) received intraperitoneal injections, every third day, of 250 μg of the hamster α1 mAb (clone Ha 31/8) together with 250 μg of the hamster α2 mAb (clone Ha 1/29). The control group (n = 5) received 500 μg of isotype control Ab according to the same schedule. The smallest and largest tumor diameter were measured weekly, using a digital caliper, and tumor volumes were calculated using the following formula: volume = (4/3)(π)(1/2 × smaller diameter) 2 (1/2 × larger diameter). Tumor data were analyzed by the two-sided unpaired t-test. Mice were sacrificed after 18 days.
Computer-Assisted Morphometric Analysis of Tumor Vessels
Blood vessel size and number within the viable regions of tumors were determined as follows. Six-μm cryostat sections were stained with an anti-mouse CD31 mAb (Pharmingen). Representative sections obtained from five tumors from each cell clone were analyzed using a Nikon E-600 microscope. Images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI), and morphometric analyses were performed using the IP LAB software (Scanalytics, Billerica, MA). Three different fields in each section were examined at ×10 magnification, and the number of vessels per mm2, the average vessel size, and the relative area occupied by tumor blood vessels were determined as described. 23 The two-sided unpaired t-test was used to analyze differences in microvessel density and vascular size.
Results
The α1β1 and α2β1 Integrins Each Support VEGF Signal Transduction Necessary for EC Proliferation
Angiogenesis requires EC proliferation, and activation of the Erk1/Erk2 (p44/42) MAPK signal transduction pathway is pivotal for cell cycle progression. 24,25 VEGF potently activates the MAPK pathway and VEGF-stimulated EC proliferation is blocked by inhibitors of MAPK activation. 26,27 Consequently, VEGF activation of this pathway in ECs is most probably required for VEGF stimulation of angiogenesis. Integrins have been implicated critically in supporting cytokine activation of the MAPK pathway, 15 raising the possibility that α1β1 and/or α2β1 collaborate with VEGF in promoting MAPK activation and angiogenesis.
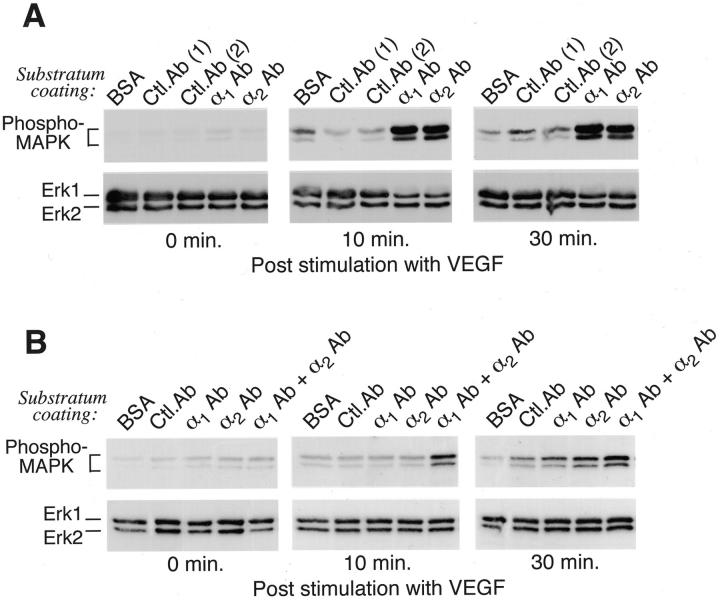
To test directly α1β1 and α2β1 integrin function in regulating VEGF activation of the Erk1/Erk2 (p44/42) MAP kinase pathway, dermal microvascular ECs in suspension were added to plastic wells coated with isotype control Abs or functional integrin mAbs directed against either the α1 integrin subunit or the α2 integrin subunit. Because these two α subunits pair exclusively with the β1 integrin subunit, the chosen α1 and α2 Abs selectively probe α1β1 and α2β1 function, respectively. Although these Abs in solution function as integrin antagonists by sterically blocking attachment of α1β1 and α2β1 to collagen I, these same Abs, when immobilized to plastic substratum, serve as α1β1-specific and α2β1-specific ligands that support cell attachment and spreading similar to collagen I. ECs did not attach and spread on plastic coated with control Abs, and VEGF only marginally activated the Erk1/Erk2 MAP kinases in these cells (Figure 1A) ▶ . In contrast, dermal microvascular ECs adhered and spread efficiently on plastic coated with either α1 Ab or α2 Ab, and attachment of ECs through either α1 Ab or α2 Ab supported marked activation of Erk1/Erk2 by VEGF, as determined with phospho-specific Abs (Figure 1A) ▶ . Phosphorylation of Erk1/Erk2 after VEGF stimulation in cells attached to either α1 Ab or α2 Ab was rapid (within 10 minutes) and sustained through 30 minutes. Thus, these data demonstrate that dermal microvascular EC adhesion through either the α1β1 integrin or the α2β1 integrin is sufficient to support VEGF activation of the Erk1/Erk2 MAPK pathway. Furthermore, activation was comparable to that observed in cells attached to natural ligands including type I collagen and vitronectin (not shown). At the concentrations of Abs used in Figure 1A ▶ (10 μg/ml), we did not observe additive effects of coating substratum with α1 Ab in combination with α2 Ab. However, in related experiments in which substratum was coated with reduced concentrations of Abs (0.2 μg/ml), we observed that both Abs in combination supported MAPK activation by VEGF more potently than either Ab alone, suggesting cooperation between α1β1 and α2β1 (Figure 1B) ▶ . Finally, we have observed that microvascular EC adhesion through integrins α5β1 and αvβ3 also fulfills the adhesion requirement for MAPK activation by VEGF (data not shown). Regardless, as the most prominent receptors for collagen on dermal microvascular ECs, 18 integrins α1β1 and α2β1 likely serve prominent roles in supporting VEGF activation of MAPK in the collagen-rich matrix of skin.
Figure 1.
Adhesion of dermal microvascular ECs through either the α1β1 integrin or the α2β1 integrin supports VEGF activation of Erk1/Erk2 MAP kinases. Functional integrin Abs or control Abs were immobilized on plastic and remaining protein-binding sites were blocked with BSA, as described in Materials and Methods. After plating, cells attached and spread on substratum coated with α1 Ab or α2 Ab, but remained unattached to wells coated with isotype control Abs or BSA alone. After plating, MAPK phosphorylation was allowed to decay for 3 hours before stimulation with VEGF. A: Wells coated with 10 μg/ml Ab. As indicated by staining with phospho-specific MAPK Abs, VEGF induced marked activation in cells plated on α1 Ab or α2 Ab but poorly induced MAPK activation in cells plated on control Abs or BSA. Staining with Ab that recognizes both phosphorylated and nonphosphorylated forms of Erk1 and Erk2 established that they were present equally in all samples. B: VEGF more efficiently induced activation of MAPK in cells plated on substratum coated with a combination of α1 Ab and α2 Ab (0.2 μg/ml of each) than in cells plated on substratum coated with each Ab alone (0.2 μg/ml). For all experiments (A and B), failure of VEGF to activate MAPK in cells plated on BSA or control (Ctl) Abs was not attributable to anoikis because cell viability remained >90%, as determined by replating of cells on collagen-coated plastic.
The α1β1 and α2β1 Integrins Support Migration of Dermal Microvascular ECs
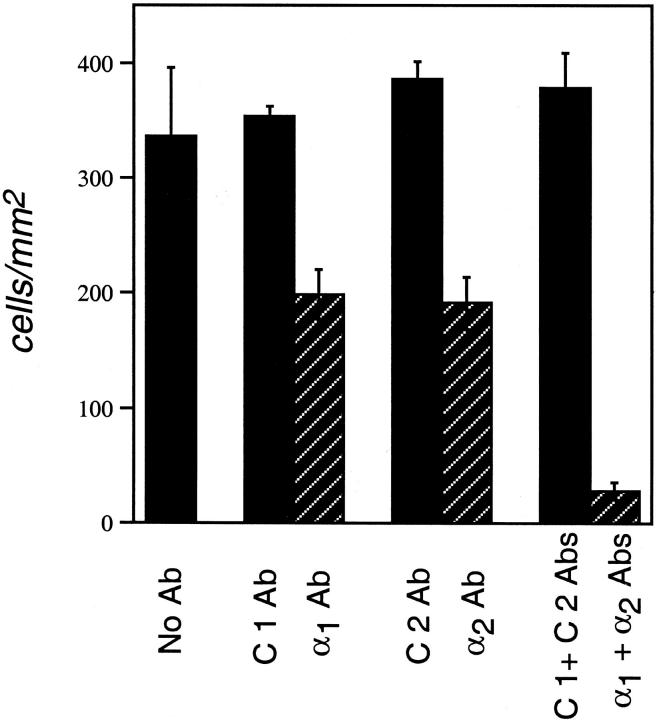
We first examined the functions of the α1β1 and α2β1 integrins in supporting haptotactic migration in a gradient of immobilized collagen I. The activities of the α1β1 and α2β1 integrins in supporting haptotaxis were tested by including soluble α1 Ab and α2 Ab at concentrations sufficient to provide the maximum inhibition of cell attachment to collagen I as determined with cell adhesion assays. 18 As shown in Figure 2 ▶ , antagonism of each integrin individually resulted in ∼40% inhibition of migration toward collagen type I, in comparison with isotype control Abs. Thus, these data indicate that the α1β1 and α2β1 integrins each function in directed migration toward collagen I. Importantly, both α1 Ab and α2 Ab in combination blocked haptotaxis toward collagen I by nearly 90% (Figure 2) ▶ .
Figure 2.
The α1β1 and α2β1 integrins each support dermal microvascular EC-directed migration toward collagen I (haptotaxis). Cells were incubated with integrin-blocking or control Abs, and then placed in Transwell migration chambers containing filters coated on the undersides with collagen I. α1 Ab and α2 Ab were each inhibitory, but isotype control Abs (C1 Ab, C2 Ab) were without effect, indicating that both α1β1 and α2β1 function in migration toward collagen I. Error bars indicate standard deviations.
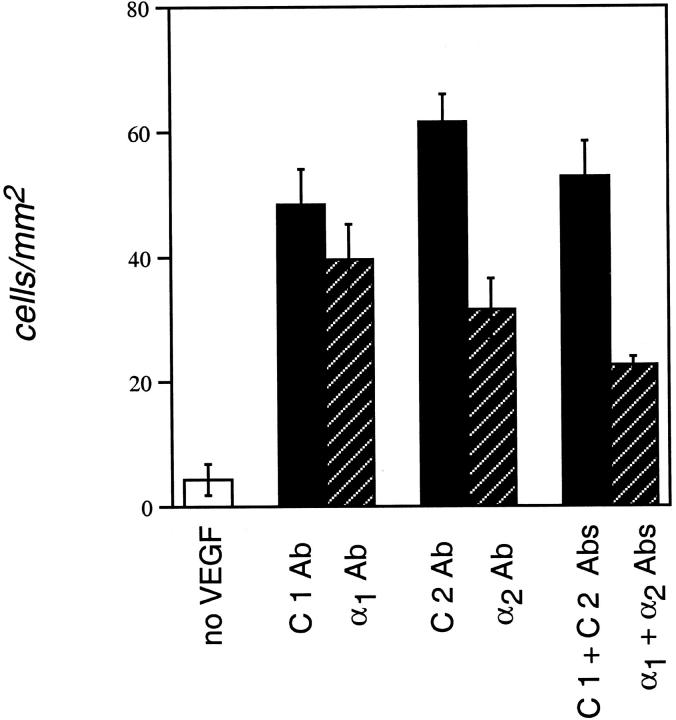
Next, we tested the functions of α1β1 and α2β1 in supporting VEGF-driven chemotaxis, ie, migration in a gradient of soluble VEGF. Filters were coated uniformly with collagen I, soluble Abs were included with the cells in the upper chambers, and VEGF was included in the bottom chambers. As shown in Figure 3 ▶ , antagonism of α2 alone inhibited chemotaxis by ∼45%, whereas antagonism of α1 resulted in only ∼15% inhibition relative to controls. Combined antagonism of α1 and α2 provided the greatest inhibition (∼60%). Thus, we observed greater inhibition of haptotaxis than chemotaxis with combined antagonism of α1 and α2 and this may relate to the fact that haptotaxis is primarily an adhesion-driven phenomenon. Regardless, experiments described here identify important functions for the α1β1 and α2β1 integrins in supporting directed migration of microvascular ECs.
Figure 3.
Antagonism of α1β1 and α2β1 integrins suppresses dermal microvascular EC chemotaxis toward VEGF. Cells were incubated with integrin-blocking or matched isotype control Abs and then placed in Transwell migration chambers containing filters coated uniformly on both sides with collagen I. To stimulate chemotaxis, VEGF was added to the lower chamber; note that migration was relatively insignificant in the absence of VEGF (left, single open column). Error bars indicate standard deviations.
Antagonism of Either the α1β1 Integrin or the α2β1 Integrin with Blocking Ab Suppresses VEGF-Driven Angiogenesis in Skin
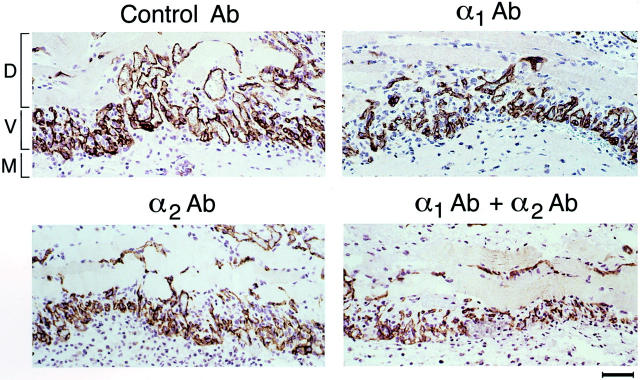
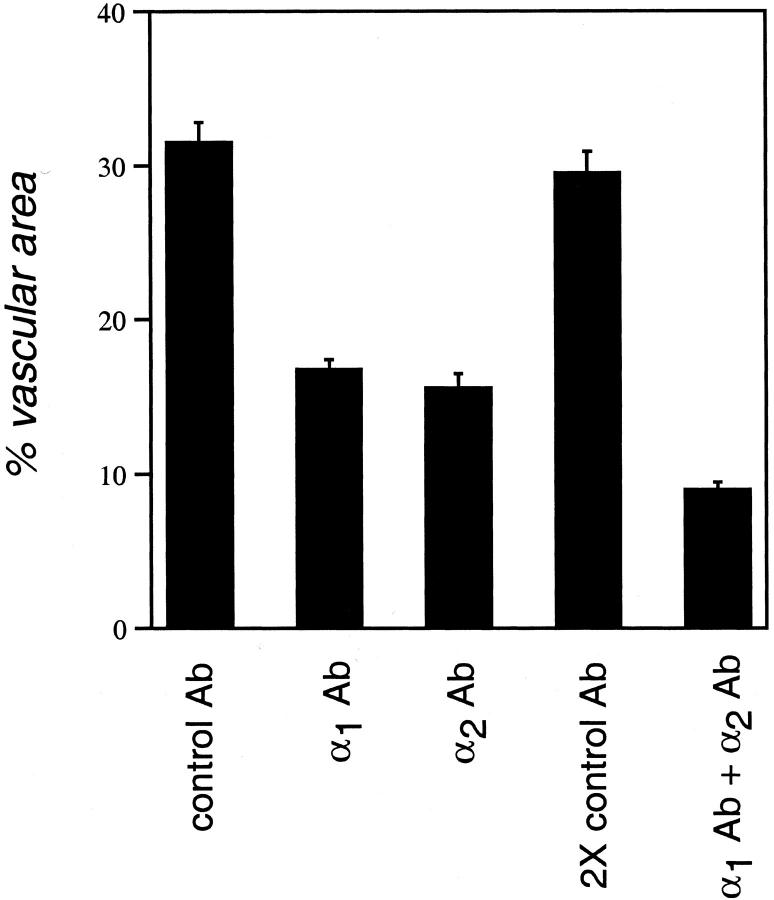
The foregoing observations implicated both the α1β1 and α2β1 integrins in supporting a key VEGF-signaling pathway together with EC migration, raising the possibility that antagonism of either integrin alone might significantly suppress angiogenesis. To test the consequences of individual antagonism of the α1β1 and α2β1 integrins for dermal angiogenesis in vivo, we used blocking Abs in an athymic nude mouse model involving subdermal injection of Matrigel together with immortalized human cells stably transfected for expression of human VEGF165. Neither Matrigel alone nor the untransfected cells in Matrigel provoked angiogenesis in the overlying dermis. In contrast, Matrigel containing VEGF transfectants potently induced neovascularization. Moreover, the hamster monoclonal blocking Abs used in these experiments do not recognize the respective human integrins and therefore did not interact with the transfected cells expressing VEGF. Animals were injected intraperitoneally with integrin-blocking Abs on days 1, 3, and 5. All animals were harvested on day 6, and skin overlying the Matrigel implants was dissected and processed for immunohistochemical analyses. Thus, overlying skin specimens from a total of 10 implants per group were analyzed, and results were highly consistent within each group. Figure 4 ▶ illustrates blood vessels in a cross-section stained with Ab to CD 31, indicating that treatment of animals with either α1 Ab or α2 Ab suppressed angiogenesis, and inhibition was greatest with both Abs in combination. Average vessel diameter (±SE) was reduced with Ab treatment from 9.58 ± 0.51 μm (control Ab) to 5.21 ± 0.24 μm (α1 Ab), 5.23 ± 0.23 μm (α2 Ab), and 3.58 ± 0.22 μm (α1 Ab + α2 Ab). Quantitation of total vascular area as a percentage of total tissue area in cross-section (Figure 5) ▶ established that cross-sectional area of new blood vessels in the α1 Ab and α2 Ab treatment groups were each reduced ∼45% relative to controls (P < 0.001). Administration of α1 Ab together with α2 Ab resulted in further inhibition of neovascularization, yielding an ∼70% reduction in total vascular area in cross-section (P < 0.001).
Figure 4.
Inhibition of VEGF-driven angiogenesis in mouse skin by α1 Ab and α2 Ab, as visualized by CD31 Ab staining of sections cut from paraffin-embedded specimens. New blood vessels (V) at the interface between the Matrigel implant containing the angiogenic stimulus (M) and the overlying dermis and smooth muscle cell layer (D) are stained for CD31 (brown color). Note reduced blood vessel diameters and reduced percentage of vascular cross-sectional area in integrin Ab groups in comparison with control. Scale bar, 50 μm.
Figure 5.
Quantitation of angiogenesis inhibition by α1 Ab and α2 Ab in mouse skin. Vascular cross-sectional area as a percentage of total tissue area was measured at the interface between dermis and the angiogenic stimulus (see Figure 4 ▶ , above) as described in Materials and Methods. Data are presented as the mean ± SEM. Total cross-sectional area of new blood vessels in the α1 Ab and α2 Ab treatment groups were each reduced ∼45% relative to controls (P < 0.001). Administration of α1 Ab together with α2 Ab resulted in further inhibition of neovascularization, yielding an ∼70% reduction (P < 0.001).
Combined Antagonism of the α1β1 and α2β1 Integrins Suppresses Squamous Cell Carcinoma Angiogenesis and Growth
Because there is considerable evidence linking VEGF to tumor angiogenesis, we next investigated whether antagonism of these integrins might also suppress angiogenesis in tumors, using human A431 squamous carcinoma cells implanted orthotopically in nude mice. These cells express VEGF and angiogenesis associated with A431 tumors is at least partially VEGF-dependent. 28 We chose to treat tumor-bearing animals with α1 Ab in combination with α2 Ab because combined antagonism yielded more substantial suppression of VEGF-driven skin angiogenesis than that observed with either Ab alone. Abs were administered every third day beginning with the day of tumor inoculation, and tumors were harvested on day 18. Combined antagonism of α1β1 and α2β1 resulted in reduced tumor vascularity (Figure 6 ▶ ; A to D) with significant reduction both in average vessel density (P < 0.01, Figure 6E ▶ ) and average vessel size (P < 0.05, Figure 6, F and H ▶ ), an ∼60% overall reduction in total vascular area (P < 0.01, Figure 6G ▶ ). Furthermore, average tumor volumes were reduced >40% (Figure 7) ▶ , consistent with suppression of tumor angiogenesis. We observed no adverse effects on animal health resulting from Ab administration in either of these tumor experiments or in the skin angiogenesis experiments described above; α1 Ab and α2 Ab groups were indistinguishable from controls in all aspects other than effects on angiogenesis and tumor growth.
Figure 6.
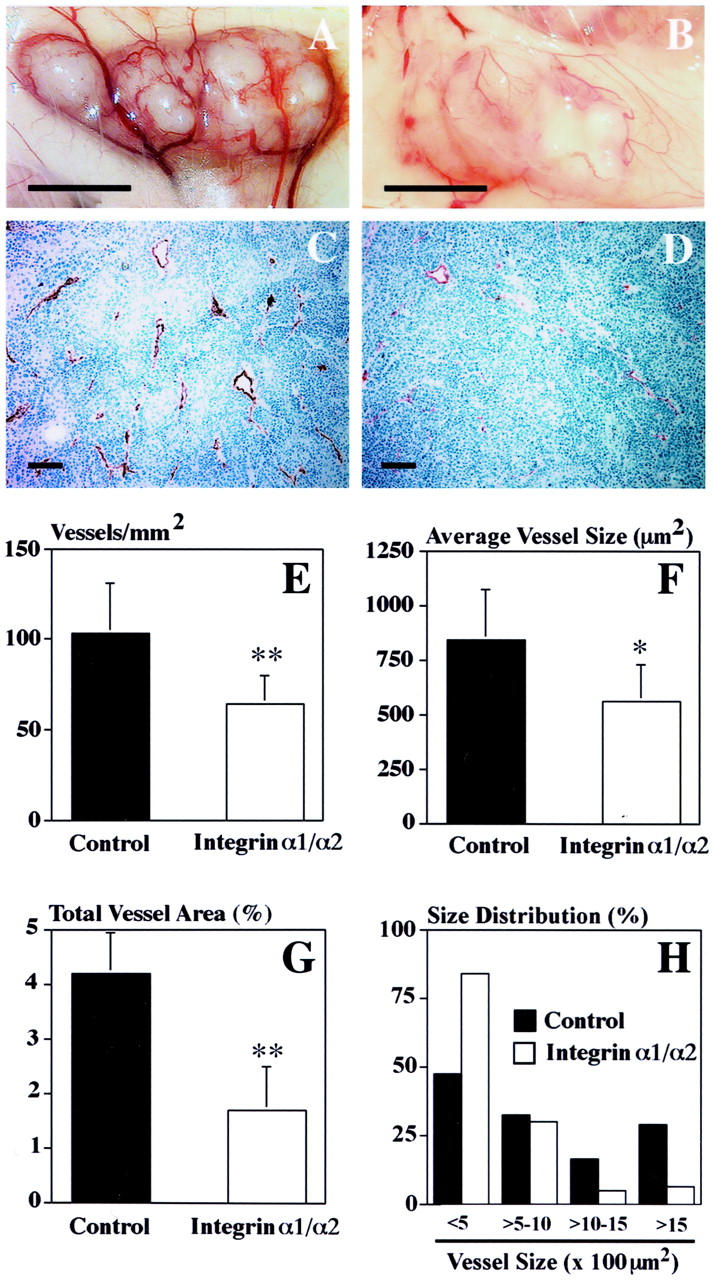
Combined treatment with α1 Ab plus α2 Ab inhibits tumor angiogenesis. Rarefaction of blood vessels in human A431 tumors from nude mice treated with α1 Ab plus α2 Ab (B), as compared with control Ab (A) (scale bar, 5 mm). Immunostaining with an anti-CD31 mAb demonstrated rarefaction and decreased size of tumor blood vessels in the α1 Ab plus α2 Ab treatment group (D) as compared to controls (C) (scale bar, 100 μm). E–H: Quantitative, computer-assisted image analysis revealed a significant inhibition of angiogenesis in A431 tumors from animals treated with α1 Ab plus α2 Ab (P < 0.01), as measured by the number of blood vessels per mm 2 tumor cross-sectional area (E). Furthermore, in A431 tumors from animals treated with α1 Ab plus α2 Ab, there was a significant reduction (P < 0.05) in average vessel size (F) with strong reduction in number of vessels with cross-sectional area >1000 μm 2 (H). G: Overall, blood vessel area in cross-section as a percentage of total tumor area was reduced by ∼60% (P < 0.01) in A431 tumors from animals treated with α1 Ab plus α2 Ab, as compared with control Ab. CD31-stained blood vessels were evaluated in three different ×10 fields in sections obtained from five different tumors for each group.
Figure 7.
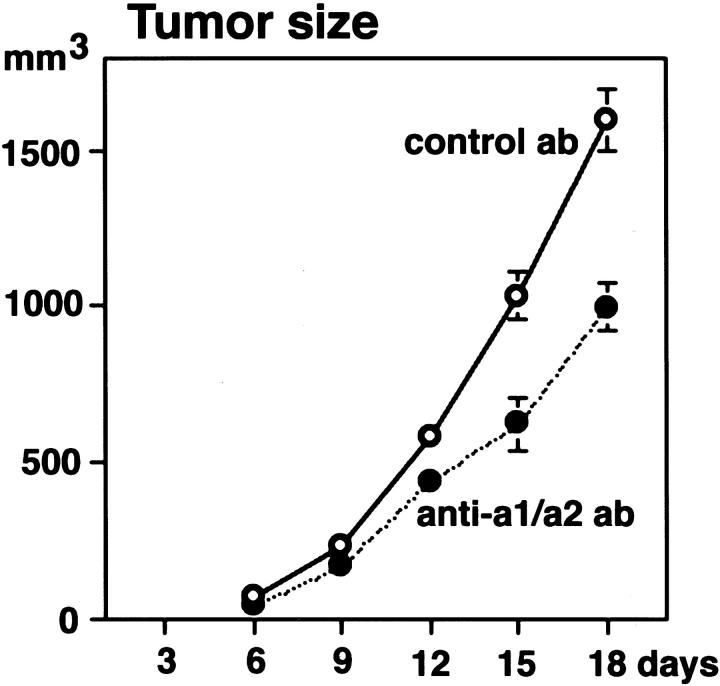
Combined treatment with α1 Ab plus α2 Ab inhibits tumor growth. Administration of α1 Ab plus α2 Ab (closed circles) significantly (P < 0.01) inhibited intradermal tumor growth of human A431 cells, as compared with control Ab (open circles). Values represent mean values ± SEM for 10 tumors for each treatment group and time point. Note that the Abs used recognize mouse but not human integrins, and therefore Ab did not bind A431 tumor cells. Thus, effects of integrin Abs were limited to the mouse host.
Discussion
Activation of the ERK1/ERK2 (p44/42) MAPK signal transduction pathway is essential for VEGF stimulation of EC proliferation 26,27 . Consequently, VEGF activation of this pathway is likely crucial for VEGF-driven angiogenesis in vivo. Integrin ligation is critical for supporting cytokine activation of the Erk1/Erk2 MAP kinases; 15 without cell attachment to extracellular matrix, serum and growth factors activate early components of the MAPK pathway including Ras, but activation of the intermediate effectors Raf and MEK1 is markedly impaired, and consequently the downstream MAP kinases are poorly activated. 29,30 This impaired MAPK signaling has been attributed to deactivation of both focal adhesion kinase and p21-activated kinase that occurs on cell detachment from matrix. 31,32
Our findings reported here indicate that VEGF only marginally stimulated activation of the MAPK pathway in suspended early passage human dermal microvascular ECs, consistent with previous observations made with human umbilical vein ECs and various cell lines. In contrast, in the presence of sufficient ligand, dermal microvascular EC attachment through either α1β1 or α2β1 independently supported robust activation of the Erk1/Erk2 MAP kinases by VEGF. Furthermore, in the presence of low ligand concentrations, ligation of α1β1 and α2β1 together supported VEGF activation of MAPK more potently than ligation of either α1β1 or α2β1 alone. Thus, for dermal microvascular ECs, data presented here clearly implicate the α1β1 and α2β1 integrins as important collaborators with VEGF in MAPK signaling.
Together with EC proliferation, EC migration also plays an important role in angiogenesis, particularly during sprouting of new blood vessels from the existing vasculature. 33 Therefore, we examined the function of the α1β1 and α2β1 integrins in supporting dermal microvascular EC migration in a gradient of immobilized collagen I (haptotaxis) and migration in a uniform collagen I matrix toward VEGF (chemotaxis). During the early stages of dermal neovascularization after dissolution of the basement membrane, haptotaxis toward collagen may function in vascular sprouting by driving EC migration toward the collagen-rich interstitial matrix of skin. In addition, chemotaxis, driven by a gradient of VEGF secreted by tumor cells or stromal cells under hypoxic stress, is also likely important in driving EC motility. We found that haptotaxis toward collagen I was suppressed ∼40% by either α1 Ab or α2 Ab, and a combination of α1 Ab and a α2 Ab blocked haptotaxis >90%. Thus, our findings indicate that both α1β1 and α2β1 are important in supporting EC migration toward collagen I, the principal matrix component of skin. Also, we found that the α1 Ab and especially the α2 Ab inhibited chemotaxis toward VEGF; and, consistent with the haptotaxis migration assays, both Abs in combination provided greatest inhibition. Given that collagens comprise ∼75% of the dry weight of skin and most of it is type I, 34 we conclude from these experiments that the α1β1 and α2β1 integrins likely provide important support for EC migration during dermal angiogenesis.
Previously, we had found that VEGF potently induces expression of the α1β1 and α2β1 integrins in dermal microvascular ECs, suggesting that these two integrins are particularly important to the mechanism by which VEGF promotes neovascularization. 18 We had confirmed this prediction by demonstrating that combined antagonism of these integrins markedly inhibited VEGF-driven angiogenesis in vivo, 18 however the individual contributions of α1β1 and α2β1 remained to be determined. Therefore, we designed experiments with mice to test the importance of α1β1 and α2β1 individually. We used blocking Abs that do not recognize the corresponding human integrins, and therefore Ab effects were limited to corresponding integrins of the murine host. Antagonism of either α1β1 or α2β1 blocked VEGF-driven angiogenesis in skin ∼45% as determined by measuring total vascular area in cross-section, indicating that both of these receptors participated importantly in neovascularization. Moreover, combined antagonism of α1β1 and α2β1 provided ∼70% inhibition of dermal angiogenesis indicating that combined antagonism of α1β1 and α2β1 was more effective than antagonism of either integrin alone. This finding is consistent with our in vitro experiments demonstrating that α1β1 and α2β1 each support a key VEGF-signaling pathway and EC migration. Notably, our observed reductions in total blood vessel cross-sectional areas associated with antagonism of α1β1 and α2β1 was primarily attributable to a reduction in average blood vessel diameters, similar to findings made by others using VEGF-neutralizing Abs. 35
The skin angiogenesis experiments discussed above used VEGF transfectants as the angiogenic stimulus; and therefore they were designed specifically to test α1β1 and α2β1 function in a setting in which neovascularization was driven principally by VEGF. Because there is considerable evidence implicating VEGF in tumor neovascularization, it seemed likely that antagonism of these integrins, particularly in combination, would also suppress tumor angiogenesis and growth. Consistent with this prediction, administration of α1 Ab together with α2 Ab to nude mice bearing human A431 squamous cell carcinoma xenografts suppressed angiogenesis by ∼60% and tumor growth by >40%. Thus, these tumor xenograft experiments demonstrated that combined antagonism of α1β1 and α2β1 also suppressed angiogenesis in a complex setting in which neovascularization was provoked by orthotopic transplantation of a representative human carcinoma.
Collectively, the animal studies presented here underscore the fundamental importance of the α1β1 and α2β1 integrins for angiogenesis in skin, and the in vitro experiments illustrate direct involvement of these integrins in supporting VEGF signaling and EC migration. Studies from other laboratories have provided additional support for the importance of these integrins for angiogenesis and have implicated them functionally in other processes directly relevant to neovascularization. Integrin α2β1 has been implicated in vascular morphogenesis in vitro; 36 and, in particular, in the formation of the vascular lumen, 37 as required for blood vessel maturation. In addition, vascularity of the skin from α1-null mice was found to be reduced in comparison with controls, 38 similarly to findings reported here with animals treated with α1 Ab. Absence of α1 integrin expression in null mice also resulted in elevated expression of matrix metalloproteases and elevated plasma concentrations of angiostatin, an inhibitor of neovascularization, indicating that increased angiostatin was responsible for reduced vascularity. 38 However, antagonism of integrin α1β1 with Ab is functionally different from absence of α1β1 expression, and we did not investigate angiostatin concentrations in mice treated with α1-blocking Ab. Nevertheless, our observations that α1-blocking Ab suppressed VEGF-driven dermal angiogenesis are consistent with observations that α1-null mice exhibit reduced vascularity of the skin.
Although the significance of the α1β1 and α2β1 integrins for angiogenesis in tissues other than skin remains to be determined, our findings predict that these integrins are important in environments where α1β1 and α2β1 ligands, such as collagens, are major components of the extracellular matrix. Given that collagens are abundantly and widely expressed, it seems likely that α1β1 and α2β1 are important for angiogenesis in a variety of tissues. However, it remains to be determined whether the dependence of angiogenesis on particular integrins differs among vascular beds.
Thus far, inhibition of angiogenesis through antagonism of integrins has centered mostly on integrins αvβ3 and αvβ5 that serve as receptors for matrix proteins such as vitronectin, fibrinogen, and fibronectin that contain an arginine-glycine-aspartate (RGD) cell-binding domain. 39-41 Antagonism of α5β1, a fibronectin receptor, was also reported to inhibit angiogenesis induced by basic fibroblast growth factor, but minimal effects on VEGF-stimulated angiogenesis were observed. 42 Surprisingly, and in contrast to angiogenesis assays performed in the presence of αvβ3 and αvβ5 antagonists, β3-null and β5-null mice develop to maturity without apparent vascular defects. 43,44 αv-null mice, which lack all αv integrins including αvβ3 and αvβ5, die at birth with vascular defects in the brain. 45 Thus, experiments with integrin antagonists have not always correlated with the phenotype of corresponding null mice. One possibility relates to observations that some integrins can exert trans-dominant effects over other integrins; 46,47 and therefore, blocking a dominant integrin need not correlate with the phenotype of mice lacking that same integrin. Regardless, as discussed above, our findings with α1-blocking Abs are consistent with the phenotype of α1-null mice that display reduced vascularity in skin. 38 We are unaware of any published analyses of vascular development in α2-null mice, however our Ab experiments suggest that vascular development may be most affected in mice with combined deletions of α1 and α2.
Although our studies and those of others suggest that integrins are attractive targets for inhibition of angiogenesis, it could be argued that integrins are widely expressed, thus predicting adverse side-effects associated with integrin antagonism. Nevertheless, we did not observe detectable toxicity during the maximal experimental interval of 18 days. Explanation for a lack of apparent side-effects associated with integrin antagonism may be drawn from experience with cells in culture. For example, stably adherent cells in culture are remarkably resistant to detachment with integrin antagonists; but conversely, integrin antagonists readily inhibit cell migration and the formation of new adhesive contacts. Consequently, in vitro experiments indicate that integrin antagonism is likely selective toward dynamic cellular processes involving the breaking and re-assembly of adhesive contacts, such as occurs widely during angiogenesis. Such functional selectivity combined with our previous observations that VEGF markedly induces expression of the α1β1 and α2β1 integrins on dermal microvascular ECs provides a dual rationale for a net therapeutic benefit associated with antagonism of these integrins. Importantly, antagonism of α1β1 and α2β1 with Abs also has been shown to suppress leukocyte infiltration and edema in several mouse models of inflammation, thus also supporting the utility of α1β1 and α2β1 antagonism toward suppressing cell invasion and colonization of the interstitium. 48
In summary, studies described here identify important functional cooperativity between the α1β1 and α2β1 integrins and VEGF, a cytokine centrally important for angiogenesis. In particular, they indicate that α1β1 and α2β1 provide critical support not only for EC migration but also for VEGF signal transduction in the collagen-rich matrix of skin. Either antagonism of α1β1 alone or antagonism of α2β1 alone suppressed VEGF-driven dermal angiogenesis; and combined antagonism provided greater inhibition, consistent with the complementary functions of these two integrins identified in vitro. Studies described here also illustrate that antagonism of the α1β1 and α2β1 integrins suppresses the growth and vascularization of human squamous cell carcinoma xenografts without the appearance of adverse consequences for the host. Thus, α1β1 and α2β1 antagonists may prove beneficial in the control of tumor angiogenesis, either alone or in combination with antagonists of other integrins implicated in neovascularization.
Footnotes
Address reprint requests to Donald R. Senger, Department of Pathology, Research North Beth Israel Deaconess Medical Center, 99 Brookline Ave., Boston, MA 02215. E-mail: dsenger@caregroup.harvard.edu.
Supported by grants CA77357 ( to D. R. S.), CA69184 (to M. D.), and CA86410 (to M. D.), awarded by the National Cancer Institute, National Institutes of Health.
References
- 1.Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF: Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993, 12:303-324 [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N: The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 1995, 36:127-137 [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 4.Mustonen T, Alitalo K: Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995, 129:895-898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N, Henzel WJ: Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989, 161:851-858 [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF: Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res 1990, 50:1774-1778 [PubMed] [Google Scholar]
- 8.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N: Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998, 273:30336-30343 [DOI] [PubMed] [Google Scholar]
- 9.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995, 1:1024-1028 [DOI] [PubMed] [Google Scholar]
- 10.Pierce EA, Foley ED, Smith LE: Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 1996, 114:1219-1228 [DOI] [PubMed] [Google Scholar]
- 11.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF: A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 2000, 6:851-860 [PubMed] [Google Scholar]
- 12.Senger DR: Vascular endothelial growth factor/vascular permeability factor: multiple biological activities for promoting angiogenesis. Voest EE D’Amore PA eds. Tumor Angiogenesis and Microcirculation. 2001, :pp 167-184 Marcel Dekker, Inc., New York [Google Scholar]
- 13.Meredith JE, Schwartz MA: Integrins, adhesion and apoptosis. Trends Cell Biol 1997, 7:146-150 [DOI] [PubMed] [Google Scholar]
- 14.Giancotti FG, Ruoslahti E: Integrin signaling. Science 1999, 285:1028-1032 [DOI] [PubMed] [Google Scholar]
- 15.Aplin AE, Short SM, Juliano RL: Anchorage-dependent regulation of the mitogen-activated protein kinase cascade by growth factors is supported by a variety of integrin alpha chains. J Biol Chem 1999, 274:31223-31228 [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 17.Ruoslahti E, Noble NA, Kagami S, Border WA: Integrins. Kidney Int 1994, 44(Suppl):S17-S22 [PubMed] [Google Scholar]
- 18.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M: Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci USA 1997, 94:13612-13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M: Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol 1996, 149:293-305 [PMC free article] [PubMed] [Google Scholar]
- 20.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR: A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992, 67:519-528 [PubMed] [Google Scholar]
- 21.Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ: Glomerular epithelial and mesangial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab Invest 1995, 72:367-375 [PubMed] [Google Scholar]
- 22.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M: Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 1999, 96:14888-14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Detmar M, Velasco P, Richard L, Claffey KP, Streit M, Riccardi L, Skobe M, Brown LF: Expression of vascular endothelial growth factor induces an invasive phenotype in human squamous cell carcinomas. Am J Pathol 2000, 156:159-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seger R, Krebs EG: The MAPK signaling cascade. FASEB J 1995, 9:726-735 [PubMed] [Google Scholar]
- 25.Vinals F, Pouyssegur J: Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol Cell Biol 1999, 19:2763-2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M: Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem 1998, 273:4220-4226 [DOI] [PubMed] [Google Scholar]
- 27.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR: Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999, 5:1390-1395 [DOI] [PubMed] [Google Scholar]
- 28.Melnyk O, Shuman MA, Kim KJ: Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res 1996, 56:921-924 [PubMed] [Google Scholar]
- 29.Lin TH, Chen Q, Howe A, Juliano RL: Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J Biol Chem 1997, 272:8849-8852 [PubMed] [Google Scholar]
- 30.Renshaw MW, Ren XD, Schwartz MA: Growth factor activation of MAP kinase requires cell adhesion. EMBO J 1997, 16:5592-5599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renshaw MW, Price LS, Schwartz MA: Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J Cell Biol 1999, 147:611-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe AK, Juliano RL: Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol 2000, 2:593-600 [DOI] [PubMed] [Google Scholar]
- 33.Ausprunk DH, Folkman J: Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 1977, 14:53-65 [DOI] [PubMed] [Google Scholar]
- 34.Weinstein GD, Boucek RJ: Collagen and elastin of human dermis. J Invest Dermatol 1960, 35:227-229 [PubMed] [Google Scholar]
- 35.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK: Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 1996, 93:14765-14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson CJ, Knop A, Giles I, Jenkins K, Schrieber L: VLA-2 mediates the interaction of collagen with endothelium during in vitro vascular tube formation. Cell Biol Int 1994, 18:859-867 [DOI] [PubMed] [Google Scholar]
- 37.Davis GE, Camarillo CW: An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res 1996, 224:39-51 [DOI] [PubMed] [Google Scholar]
- 38.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA: Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 2000, 97:2202-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 40.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA: Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995, 270:1500-1502 [DOI] [PubMed] [Google Scholar]
- 41.Drake CJ, Cheresh DA, Little CD: An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 1995, 108:2655-2661 [DOI] [PubMed] [Google Scholar]
- 42.Kim S, Bell K, Mousa SA, Varner JA: Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol 2000, 156:1345-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO: Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 1999, 103:229-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hynes RO, Bader BL: Targeted mutations in integrins and their ligands: their implications for vascular biology. Thromb Haemost 1997, 78:83-87 [PubMed] [Google Scholar]
- 45.Bader BL, Rayburn H, Crowley D, Hynes RO: Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 1998, 95:507-519 [DOI] [PubMed] [Google Scholar]
- 46.Yang JT, Hynes RO: Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell 1996, 7:1737-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH: Trans-dominant inhibition of integrin function. Mol Biol Cell 1996, 7:1939-1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE: Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest 2000, 105:721-729 [DOI] [PMC free article] [PubMed] [Google Scholar]