Abstract
Caveolin-3, a muscle specific caveolin-related protein, is the principal structural protein of caveolar membranes. We have recently identified an autosomal dominant form of limb girdle muscular dystrophy (LGMD-1C) that is due to caveolin-3 deficiency and caveolin-3 gene mutations. Here, we studied by electron microscopy, including freeze-fracture and lanthanum staining, the distribution of caveolae and the organization of the T-tubule system in caveolin-3 deficient human muscle fibers. We found a severe impairment of caveolae formation at the muscle cell surface, demonstrating that caveolin-3 is essential for the formation and organization of caveolae in muscle fibers. In addition, we also detected a striking disorganization of the T-system openings at the sub-sarcolemmal level in LGMD-1C muscle fibers. These observations provide new perspectives in our understanding of the role of caveolin-3 in muscle and of the pathogenesis of muscle weakness in caveolin-3 deficient muscle.
Caveolae are 50 to 100 nm invaginations that represent an appendage or subcompartment of the plasma membrane. They are found in most cell types and are particularly abundant in striated muscle cells. 1-4 Caveolae have been implicated in many cellular functions, particularly in three major processes: endothelial transcytosis, potocytosis, and signal transduction. 1-4
Caveolins are members of a gene family of 21- to 25-kd integral membrane proteins, that are the principal protein components of caveolar membranes. Caveolins play an important structural role in the formation of caveolar membranes, by acting as scaffolding proteins to organize and concentrate specific caveolin-interacting lipids and proteins within caveolae microdomains. 5-9 So far, three different caveolins have been recognized, termed caveolin-1, -2 and -3, which are the products of three different genes. 10-14
Caveolin-3, the most recently recognized member of caveolin gene family, shows a muscle-specific tissue distribution and is the major caveolar protein of differentiated skeletal muscle cells. The expression of caveolin-3 is developmentally regulated, and the protein localizes to the sarcolemma in fully differentiated muscle fibers, where it interacts with dystrophin and with the dystrophin-associated glycoproteins. 12-14
We have recently identified an autosomal dominant form of limb girdle muscular dystrophy (LGMD-1C) that is due to caveolin-3 deficiency and CAV3 mutations. Analysis of genomic DNA revealed two distinct mutations in CAV3: a 9-base pair microdeletion that removes the sequence 63TFT65 from the caveolin scaffolding domain and a missense mutation that changes a proline to a leucine (P105L) in the transmembrane domain. Both mutations leaded to a severe deficiency (95%) of the caveolin-3 protein. 15
It has long been hypothesized that muscle cell caveolae may play a role in the formation or organization of the T-tubule system. In agreement with this concept, recent studies on developing muscle have demonstrated that caveolin-3 associates with vescicles which eventually fuse to form the T-tubule openings at the surface of developing myotubes. 16
To gain further insight into the distribution of caveolae and to evaluate the organization of the T-tubule system in caveolin-3 deficient muscle, we applied electron microscopic techniques, including freeze-fracture and lanthanum staining, to muscle biopsies from four patients with LGMD-1C and caveolin-3 deficiency.
Materials and Methods
Muscle Samples
We studied muscle biopsies (quadriceps) from four patients (case 1, age 45; case 2, age 15; case 3, age 13; case 4, age 10) from two different families with different LGMD-1C mutations. The clinical and molecular genetic aspects of these patients, together with other members of their families, were previously reported. 15 Two different mutations in CAV3 gene were found, a microdeletion in the scaffolding domain (patients 1 and 2) and a missense mutation in the membrane-spanning region (patients 3 and 4), leading to a severe caveolin-3 deficiency (∼ 95%) in muscle fibers. As controls, we used samples of quadriceps muscle from four subjects of comparable age, with normal muscle histology. All samples were frozen in liquid nitrogen-cooled isopentane, sectioned for diagnostic purposes, and stored in liquid nitrogen until this study. A portion of each sample was fixed in glutaraldehyde and processed for ultrastructural examination as described below.
Immunohistochemistry
We used a monoclonal antibody directed against caveolin-3 (cl26) that was generated with a synthetic peptide corresponding to amino acids 3 to 24 of the rat caveolin-3 protein sequence. The immunological and immunohistochemical characteristics of this antibody have been previously described in detail. 14 For immunohistochemistry, 4 nm-thick unfixed serial frozen muscle sections were incubated with anti-caveolin-3 mAb diluted 1:1000 in phosphate-buffered saline, and processed for immunofluorescence microscopy as previously described. 15
Ultrastructural Study
For routine electron microscopy, samples were fixed in 2.5% glutaraldheyde, processed and embedded in epon-araldite. Ultrathin sections were cut using a Leica Ultracut UCT ultramicrotome, stained with uranyl acetate and lead citrate, and observed by a Zeiss 110 electron microscope. For lanthanum staining, small biopsy samples were fixed in 2.5% glutaraldheyde-2% paraformaldheyde in 0.1 mol/L cacodylate buffer, pH 7.2 for 30 minutes. Specimens were then reduced to 1 mm × 2 mm pieces, washed three times in buffer, and rinsed overnight at 4°C in 0.5 mol/L cacodylate buffer. The specimens were postfixed at room temperature by vibratory agitation for 2 hours in a medium containing 1.3% osmiun tetroxide in 0.2 s-collidine buffer at pH 7.2 and 2% lanthanum nitrate. They were rapidly dehydrated, washed in propylene oxide 100%, and finally embedded in epon-araldite.
Freeze-Fracture Analysis
The specimens were removed at rest length in a U-shaped muscle clamp or attached to a tick and fixed immediately in 3% glutaraldehyde in 0.1 mol/L phosphate buffer. The samples were cut into small blocks and gradually infiltrated with glycerol up to a concentration of 30% and then frozen in freon-liquid nitrogen. The specimens were fractured at −110°C in a freeze-fracture apparatus at a vacuum of 6 × 10−7 mmHg and immediately replicated with platinum and carbon using electron beam guns. The tissue was digested in sodium hypoclorite. The detached replicas were washed three times in distilled water and finally picked up on Formvar coated grids and examined/photographed using a Zeiss EM-10 transmission electron microscope.
Image Analysis and Quantitation
Tissue sections were examined using a Leitz Diaplan optical microscope equipped with epi-illumination. Images were obtained with an Optronics DEI-750 digital camera connected to a Power Macintosh. Image analysis was performed using Image Pro-Plus software. For electron microscopy and freeze fractures studies, pictures were scanned and analyzed using an Image Pro-Plus program. The density of caveolae was determined by counting the number of caveolae on the P face in at least 30 different fields from different fibers for each sample; 10 different samples were freeze-fractured from each muscle biopsy. The number of caveolae was expressed per square micrometer. Statistical analysis was performed using the two-tailed paired t-test.
Results
Immunohistochemistry
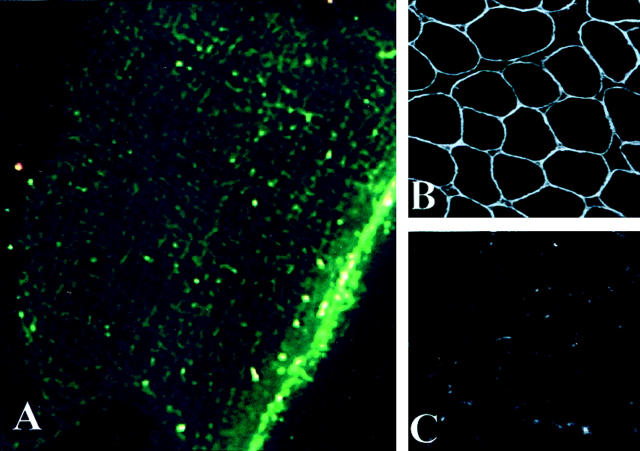
In cross sections, immunofluorescent staining of normal control muscle showed that caveolin-3 is localized at the sarcolemma of human muscle fibers (Figure 1B) ▶ . In longitudinal sections and using high-resolution immunofluorescence, caveolin-3 showed an organized network at the cell surface. This network consisted of a set of major bands running along the longitudinal axis of the fibers and a set of minor interconnecting transverse bands (Figure 1A) ▶ . In contrast, muscle sections prepared from LGMD-1C patients showed a severe reduction in caveolin-3 staining at the sarcolemma (Figure 1C) ▶ . This caveolin-3 deficiency was previously confirmed by immunoblot analysis (data not shown, see Reference 15).
Figure 1.
Immunofluorescent localization of caveolin-3 in the muscle fibers of normal and LGMD-1C patients. A: By high-resolution immunofluorescence, in longitudinal sections, caveolin-3 shows a network-like organization at the cell surface, with main bands running along the longitudinal axis of the fibers, interconnected by transverse bands. This pattern is reminiscent of the network organization of caveolae at the cell surface, as seen by freeze-fracture in normal muscle fibers (see Figure 3A ▶ ). B: By immunofluorescence in normal muscle, in cross sections, caveolin-3 reveals a uniform staining pattern at the sarcolemma. C: In LGMD-1C patients, note that there is a severe deficiency of caveolin-3 at the cell surface. A: Original magnification, ×1200; B: Original magnification, ×40; C: Original magnification, ×40.
Conventional Electron Microscopy
In normal control muscle fibers, caveolae appeared as flask-shaped profiles located beneath the plasma membrane (Figure 2A) ▶ . Muscle samples from LGMD-1C patients showed that caveolae were virtually absent from most muscle fibers. Only in a few areas we could detect isolated caveolae-like structures adjacent to the muscle plasma membrane. Quantitative analysis of more than 30 different fields revealed that the number of caveolae was less than 5% in muscle fibers from LGMD-1C patients. Furthermore, LGMD-1C fibers contained in several areas large vacuoles close to the cell surface. These vacuolated structures revealed the presence of a membrane at the outskirt and were generally empty, or partially containing amorphous material (Figure 2, B and C) ▶ .
Figure 2.
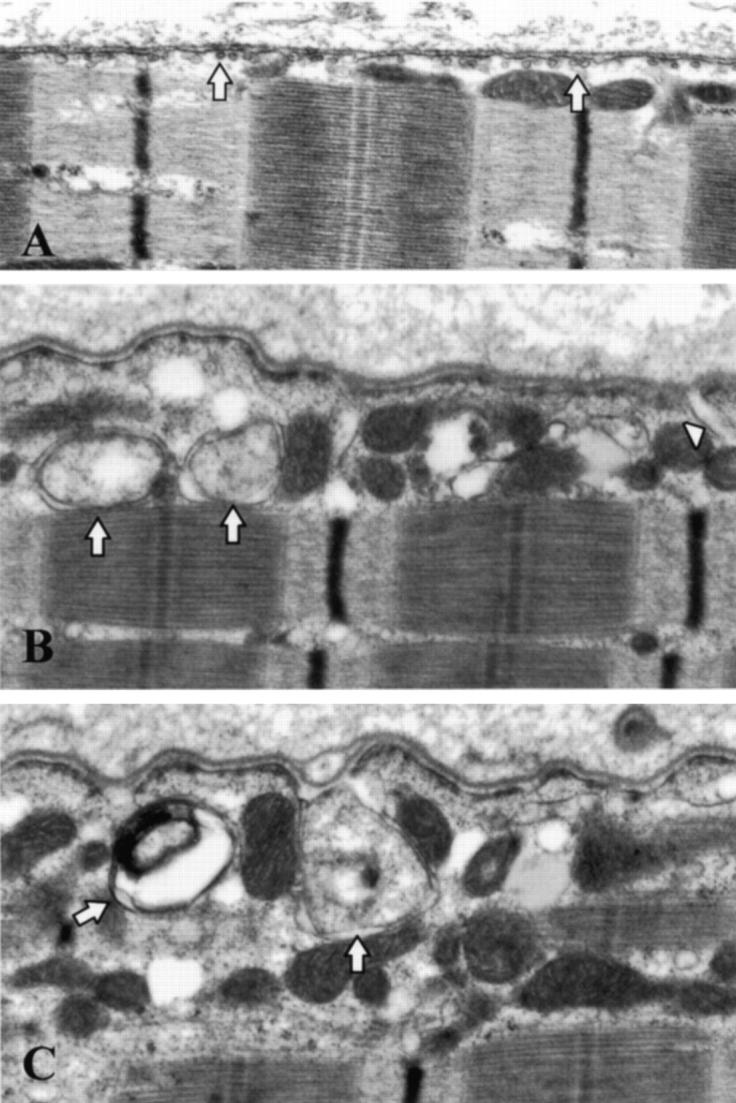
Transmission electron microscopic analysis of muscle fibers from normal and LGMD-1C patients. By routine transmission electron microscopy, caveolae in normal muscle fibers appear as flask shaped vesicles localized subjacent to the subsarcolemmal membrane (A, arrows). In LGMD-1C muscle fibers (patient 1), very few caveolae were found near to the cell surface (B, C). Furthermore, in LGMD-1C fibers, we observed large vacuolated structures close to the cell surface. These vacuolated structures appeared membranous and were generally empty, or containing amorphous material. (B, C, arrows). Some of these structures revealed continuity with the membrane (B, arrowhead). A–C: Original magnification, ×12,500.
Freeze-Fracture Analysis of Caveolae
To evaluate the number and distribution of caveolae in the plasma membrane of the muscle fibers, we examined the two leaflets of the surface membrane, the protoplasmic leaflet (P face) and the external leaflet (E face). In normal muscle plasma membrane, both the P and E faces contained caveolae which appeared relatively uniform in size and shape, and they were characteristically distributed in rows of longitudinal and transverse bands (Figure 3A) ▶ . This highly organized array of caveolae seen in freeze-fracture preparations appeared to correspond to the pattern of longitudinal and transverse bands that we observed by high-resolution immunofluorescence (Figure 1B) ▶ . The mean density of caveolae in normal muscle plasma membrane was 9.68 (SD ± 2.05) per square micron. In sharp contrast, in LGMD-1C muscle plasma membrane we observed very few caveolae in both P and E faces of the membrane. (Figure 3, B and C) ▶ . Quantitative analysis showed that the number of caveolae in LGMD-1C muscle fibers was less than 5%, as compared to normal control muscle.
Figure 3.
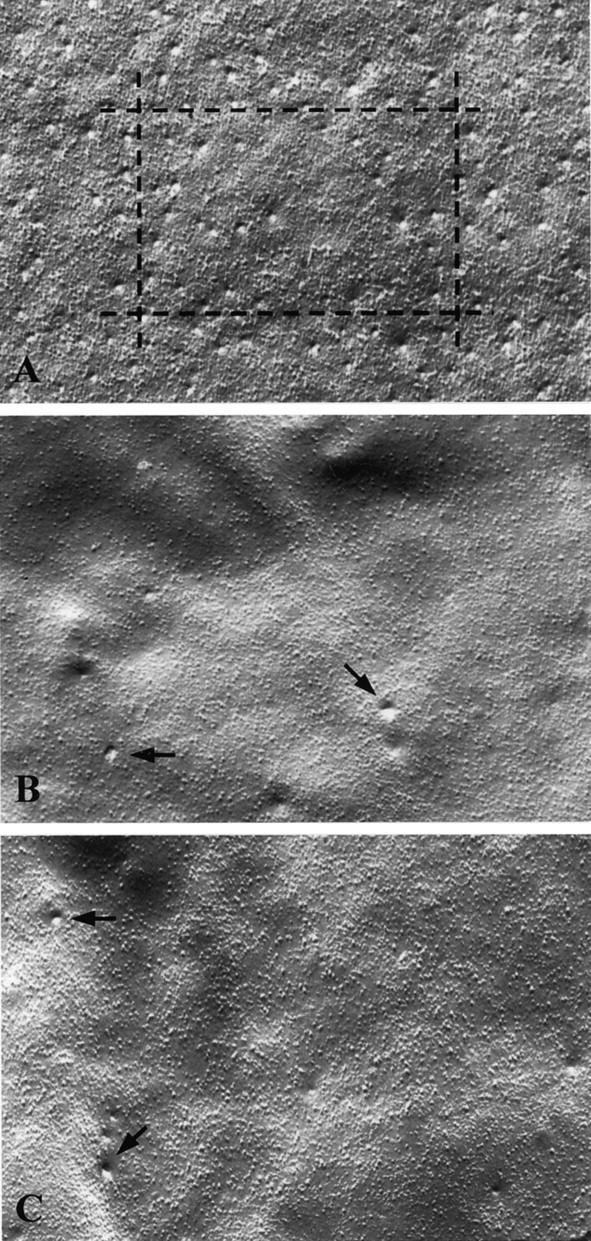
Freeze-fracture analysis of muscle fibers from normal and LGMD-1C patients. In freeze-fracture preparations of normal muscle cell plasma membrane, caveolae appeared as small invaginations or indentations of the plasma membrane (A). The distribution of caveolae at the cell surface was not homogeneous; caveolae were present in ordered arrays or rows that may correspond to the horizontal and vertical band-like structures we observed by immunofluorescence microscopy (see Figure 1A ▶ ). In striking contrast, in LGMD-1C muscle fibers, we observed very few scattered caveolae at the cell surface (B, patient 1 and C, patient 2, arrows). A–C: Original magnification, ×12,500.
T-System Lanthanum Staining
To study the organization of the T-tubule system in muscle fibers, we used lanthanum nitrate staining combined with transmission electron microscopy. Lanthanum is an electron-dense tracer that, in muscle, stains the extracellular space, and also outlines the caveolae and the T-system for ultrastructural studies. 17,18 Previous reports have shown that lanthanum does not penetrate the sarcoplasmic reticulum, except in specific pathological conditions such as thermal injury. 19
In our experiments, muscle samples from normal controls and from LGMD-1C patients were pre-treated with lanthanum nitrate, and then processed for electron microscopy. In normal muscle, the extracellular space as well as the caveolae were labeled with electron-dense material. The T-tubules were also stained by lanthanum and were observed at the I and A band junction (Figure 4A) ▶ .
Figure 4.
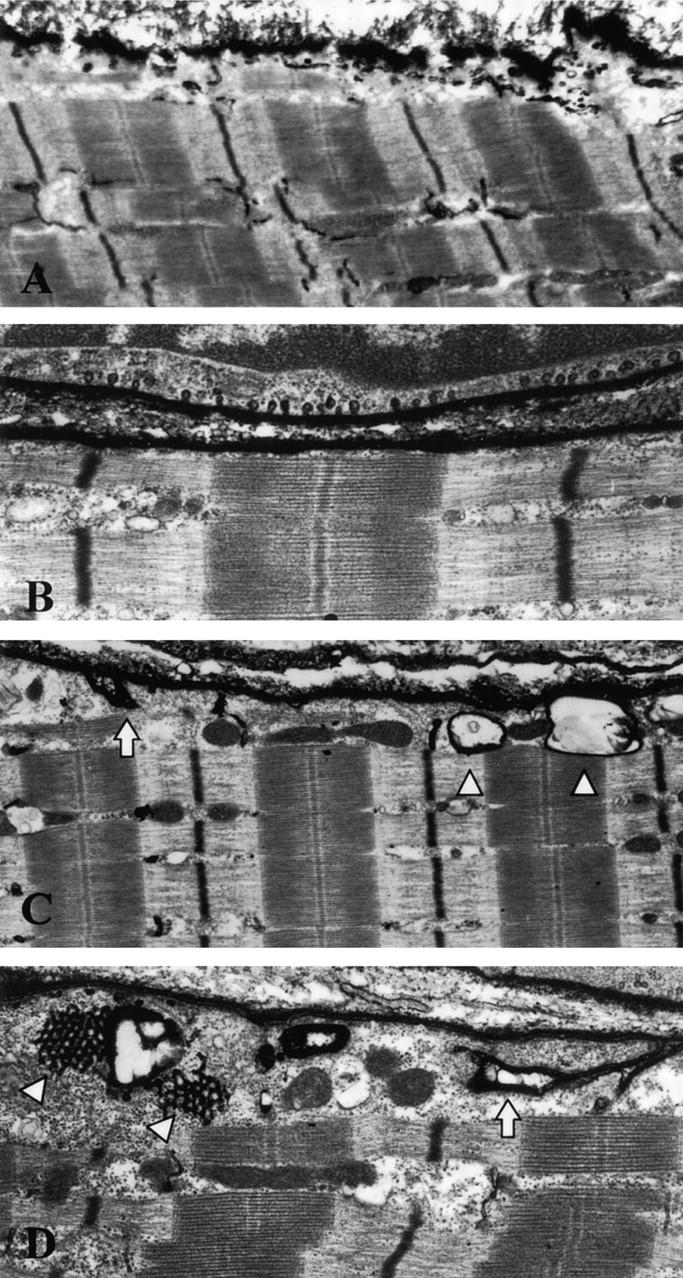
Lanthanum nitrate staining of muscle fibers from normal and LGMD-1C patients. After lanthanum nitrate treatment, normal control muscle samples showed dense staining of the T-tubules, which were generally observed between the I- and A-band. Lanthanum penetration was able to stain the first few sarcomeres close to the membrane (A). In LGMC-1C muscle fibers, we confirmed that only very few caveolae are found near to the cell surface, in contrast with the large number of caveolae that are present in adjacent non-muscle cells (B). In LGMD-1C muscle fibers, note the vacuolated structures that are penetrated by lanthanum and show a lanthanum-positive membrane (C, arrowsheads). Some of these structures revealed continuity with the cell membrane (C, D, arrows). Furthermore, some vacuolated structures were associated with honeycomb structures, typical of abnormal proliferation of the T-tubule system (D, arrowheads). A: Original magnification, ×7,000; B: Original magnification, ×12,500; C: Original magnification, ×7,000; D: Original magnification, ×10,000.
In LGMD-1C muscle samples, most of the fibers did not show caveolae, while lanthanum-stained caveolae were seen in adjacent fibroblasts or endothelial cells. (Figure 4B) ▶ . This may be explained by the observation that non-muscle cells predominantly express caveolin-1, a more ubiquitously expressed product of the caveolin gene family. 8-11 Interestingly, in LGMD-1C muscle fibers, we also observed beneath the plasma membrane several large vacuolated structures which were labeled by electron-dense lanthanum (Figure 4C) ▶ . These structures may correspond to the large vacuoles seen in subsarcolemmal regions by conventional electron microscopy in LGMD-1C muscle fibers (Figure 2, B and C) ▶ . In addition, some of the large vacuolated structures showed continuity with the plasma membrane, possibly representing abnormal caveolae-like membrane invaginations or disrupted T-tubule openings. Furthermore, some of the vacuolated structures were also associated with lanthanum positive honeycomb structures, a known indicator of abnormal proliferation of the T-tubule system (Figure 4D) ▶ . 17,18 The described abnormalities were seen in all LGMD-1C muscle samples.
For comparative purposes and to exclude the possibility that our observations may be due to technical artifacts, we also examined by lanthanum staining muscle biopsies from two patients affected with dermatomyositis. It has been reported that in this disorder, the T-system is generally abnormal and that it proliferates in affected muscle fibers. 20 In these biopsies, we found disorganization and proliferation of the T-tubules, but we did not detect lanthanum-positive large vacuoles beneath the plasma membrane, similar to the structures we noted in LGMD-1C muscle fibers (data not shown).
Discussion
This is the first demonstration that caveolin-3 deficiency, due to mutations in the caveolin-3 gene, leads in human muscle fibers to a severe impairment of caveolae formation at the cell surface. The immediate consequence of this observation is the conclusion that caveolin-3 is essential for the formation and organization of caveolae at the cell surface in human muscle.
During the process of caveolae formation, caveolin undergoes two stages of self-association or oligomerization. First, shortly after caveolin synthesis, caveolin oligomerizes in the endoplasmic reticulum to form homo-oligomers of 300 to 350 kd, each containing approximately 14 to 16 individual caveolin monomers. Second, at a later stage, these caveolin homo-oligomers can interact with each other to form clusters of caveolin oligomers that are approximately 25 to 50 nm in diameter. Thus, through the interaction of caveolin with itself and the caveolin-mediated selection of endogenous lipid components, a caveolae-sized vesicle is generated. 6-8
We demonstrated that heterozygous mutations in CAV3 gene are associated with a severe caveolin-3 deficiency at the cell surface. 15 The molecular mechanisms underlying this deficiency have not yet been elucidated. A possible mechanism is that the heterozygous mutations exert a dominant-negative effect that induces rapid degradation of both the wild-type and mutant caveolin-3 proteins. Consistent with this hypothesis, we investigated the phenotypic behavior of these caveolin-3 mutations using heterologous expression in NIH 3T3 cells. The results show that LGMD-1C mutants of caveolin-3 form unstable high molecular mass aggregates of caveolin-3, which are retained within the Golgi complex and are not targeted to the plasma membrane. 21 These data provide a molecular explanation for why caveolin-3 levels are down-regulated in patients with LGMD-1C.
Caveolin-3 deficient mutant mice, designed to disrupt exon 2 of the caveolin-3 gene, show a reduction of caveolae at the plasma membrane. 22 Interestingly, the pathogenetic mechanisms leading to the impairment of caveolae formation at the cell surface seem to be different in the mutant mice as compared with humans. In fact, heterozygous mutant mice do not behave in a dominant negative fashion and only homozygous mutants reveal a severe impairment of caveolae formation. Furthermore, no apparent muscle degeneration was observed in these mutants. 22 In contrast, here we demonstrate that heterozygous mutations of the CAV-3 gene, involving the caveolin scaffolding and membrane spanning domains, exert in a significant down-regulation of caveolin-3 protein, leading to a severe impairment of the formation of caveolae at the cell membrane of muscle fibers from LGMD-1C patients.
Electron microscopic studies have shown that caveolae-like structures are associated with forming T-tubules at an early developmental stage. 23 Several morphological studies have demonstrated that caveolae initially form tubular structures that later associate to develop into a larger three-dimensional tubular network. 23,24 Furthermore, it has been recently reported that caveolin-3 transiently associates with T-tubules during development and may be involved in the early development of the T-tubule system in muscle. 16,25,26 In mature muscle fibers, however, caveolin-3 is no longer detectable within the T-tubule system, but is highly concentrated in sarcolemmal caveolae. 25 These results suggest that a functional relationship may exist between caveolin-3 expression, caveolae formation, and T-tubule biogenesis.
We have recently reported that caveolin-3 null mice, lacking caveolin-3 protein expression and sarcolemmal caveolae membranes, show T-tubule abnormalities in muscle fibers. 27 Thus, we examined here the possible consequences of caveolin-3 deficiency on the formation of T-tubule domains in caveolin-3 deficient muscle fibers of LGMD-1C patients. We found the presence of abnormal large vacuolated and membranous structures in the subsarcolemmal area, associated with an abnormal proliferation of T-tubule like structures. These alterations were independent from the age of the patients, varying from 10 to 45 years, and were more severe than those we observed in muscle from caveolin-3 null mice.
In conclusion, our data suggest that LGMD-1C muscle fibers with severe caveolin-3 deficiency show an impairment of caveolae formation at the cell surface and a disorganization of the T-system openings at the subsarcolemmal level. Because the T-system plays an important role in muscle contraction and relaxation by regulating the distribution of the Ca2+ ions, this alteration may explain, at least in part, the pathogenesis of muscle weakness in LGMD-1C patients.
It has been recently reported that mutations in the CAV3 gene may cause the mechanical hyperirritability of skeletal muscle seen in rippling muscle disease. 28 However, the pathogenetic mechanism(s) underlying this disorder still remain to be elucidated. Further studies will be necessary to evaluate if the alterations we find in LGMD1C muscle fibers are also present in other clinical phenotypes due to CAV3 mutations, such as isolated hyperCKemia and rippling muscle disease.
Table 1.
Morphological Alterations in Caveolin-3-Deficient Muscle
| Method | Observations |
|---|---|
| Optical microscopy | Mild myopathic pattern (15) : scattered necrotic fibers, increased connective tissue |
| Conventional electron microscopy | Lack of caveolae at the muscle plasma membrane in most fibers |
| Large membranous vacuoles close to the cell surface, generally empty or containing amorphous material | |
| Freeze-fracture analysis of caveolae | Severe reduction (<5%) of caveolae at the cell surface |
| T-system lanthanum staining | Lack of caveolae at the muscle plasma membrane in most fibers |
| Staining of large membranous vacuolated structures beneath the plasma membrane | |
| Staining of some vacuolated structures in continuity with plasma membrane | |
| Proliferation of the T-tubule system (honeycomb structures) at the subsarcolemmal level |
Footnotes
Address reprint requests to Dr. Carlo Minetti, Dipartimento di Pediatria, Università di Genova, Istituto G.Gaslini, Largo Gaslini 5, 16147 Genova, Italy. E-mail: minettic@unige.it.
Supported by grants from Telethon-Italy (GP0271/01), and the Italian Ministry of Health (G. Gaslini Institute, Ricerca Finalizzata). F.S. was supported by a fellowship from Telethon-Italy. M.P.L. was supported by grants from the National Institutes of Health, the Komen Breast Cancer Foundation, the American Heart Association, and The Muscular Dystrophy Association. M.P.L. is a the recipient of a Scholar award from the Irma T. Hirschl/Monique Weil-Caulier Trust.
References
- 1.Couet J, Li S, Okamoto T, Scherer PS, Lisanti MP: Molecular and cellular biology of caveolae: paradoxes and plasticities. Trends Cardiovasc Med 1997, 7:103-110 [DOI] [PubMed] [Google Scholar]
- 2.Parton RG: Caveolae and caveolins. Curr Opin Cell Biol 1996, 8:542-548 [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Zhang XL, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP: Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer’s disease, and muscular dystrophy. Am J Hum Genet 1998, 63:1578-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razani B, Schlegel A, Lisanti MP: Caveolin proteins in signaling, oncogenic transformation, and muscular dystrophy. J Cell Sci 2000, 113:2103-2109 [DOI] [PubMed] [Google Scholar]
- 5.Rothberg KG, Heuser JE, Donzell WC, Ying Y, Glenney JR, Anderson RGW: Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68:673-682 [DOI] [PubMed] [Google Scholar]
- 6.Sargiacomo M, Scherer PE, Tang ZL, Kubler E, Song KS, Sanders MC, Lisanti MP: Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA 1995, 92:9407-9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP: Identification of peptide and protein ligands for the caveolin-scaffolding domain: implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 1997, 272:6525-6533 [DOI] [PubMed] [Google Scholar]
- 8.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP: Cell-type and tissue-specific expression of caveolin-2: caveolins-1 and -2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 1997, 272:29337-29346 [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T, Schlegel A, Scherer PE, Lisanti MP: Caveolins, a family of scaffolding proteins for organizing “pre-assembled signaling complexes” at the plasma membrane. J Biol Chem 1998, 273:5419-5422 [DOI] [PubMed] [Google Scholar]
- 10.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP: Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 1996, 93:131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Z, Okamoto T, Boontrakulpoontawee P, Katada T, Otsuka AJ, Lisanti MP: Identification, sequence and expression of an invertebrate caveolin gene family from the nematode Caenorhabditis elegans: implications for the molecular evolution of mammalian caveolin genes. J Biol Chem 1997, 272:2437-2445 [DOI] [PubMed] [Google Scholar]
- 12.Way M, Parton RG: M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett 1995, 376:108-112 [DOI] [PubMed] [Google Scholar]
- 13.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP: Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 1996, 271:2255-2261 [DOI] [PubMed] [Google Scholar]
- 14.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP: Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 1996, 271:15160-15165 [DOI] [PubMed] [Google Scholar]
- 15.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Cordone G, Dagna Bricarelli F, Lisanti MP, Zara F: Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998, 18:365-368 [DOI] [PubMed] [Google Scholar]
- 16.Parton RG, Way M, Zorzi N, Stang E: Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 1997, 136:137-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguchi K, Tsukagoshi H: An electronmicroscopic study of the T-system in progressive muscular dystrophy (Duchenne) using lanthanum. J Neurol Sci 1980, 44:161-168 [DOI] [PubMed] [Google Scholar]
- 18.Oguchi K, Yanagisawa N, Tsukagoshi H: The structure of the T-system in human muscular dystrophy. J Neurol Sci 1982, 57:333-341 [DOI] [PubMed] [Google Scholar]
- 19.Fahimi HD, Cotran RS: Permeability studies in heat-induced injury of skeletal muscle using lanthanum as fine structural tracer. Am J Pathol 1971, 62:143-157 [PMC free article] [PubMed] [Google Scholar]
- 20.Chou SM, Nonaka I, Voice GF: Anastomoses of transverse tubules with terminal cisternae in polymyositis. Arch Neurol 1980, 37:257-266 [DOI] [PubMed] [Google Scholar]
- 21.Galbiati F, Volonté D, Minetti C, Chu JB, Lisanti MP: Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C): retention of LGMD-1C caveolin-3 mutants within the Golgi complex. J Biol Chem 1999, 274:25632-25641 [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T: Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet 2000, 9:3047-3054 [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa H: Formation of elaborate network of T-system tubules in cultured skeletal muscle with special reference to the T-system formation. J Cell Biol 1968, 38:51-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzini-Amstrong C: Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev Biol 1991, 146:353-362 [DOI] [PubMed] [Google Scholar]
- 25.Ralston E, Ploug T: Caveolin-3 is associated with the T-tubules of mature skeletal muscle fibers. Exp Cell Res 1999, 246:510-515 [DOI] [PubMed] [Google Scholar]
- 26.Carozzi AJ, Ikonen E, Lindsay MR, Parton RG: Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic 2000, 1:326-341 [DOI] [PubMed] [Google Scholar]
- 27.Galbiati F, Engelman JA, Volontè D, Zhang XL, Minetti C, Li M, Hou H, Kneitz B, Edelmann W, Lisanti MP: Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and T-tubule abnormalities. J Biol Chem 2001, 276:21425-21433 [DOI] [PubMed] [Google Scholar]
- 28.Betz RG, Schoser BGH, Kasper D, Ricker K, Ramirez A, Valentin S, Torbergsen T, Lee YA, Nothen MM, Wienker TF, Malin JP, Propping P, Reis A, Mortier W, Jentsch TJ, Vorgerd M, Kubisch C: Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat Genet 2001, 28:218-219 [DOI] [PubMed] [Google Scholar]