Abstract
Angiogenesis, the formation of new capillaries from existing vasculature, plays an essential role in tissue repair. The rapid onset and predominance of proangiogenic factors optimizes healing in damaged tissues. One factor that directly mediates wound vessel angiogenesis is vascular endothelial growth factor (VEGF). Although much is known about the biology of VEGF and its cognate receptors, VEGFR1 and VEGFR2, the role of a recently identified co-receptor for VEGF, neuropilin-1, is not well understood. Using a murine model of dermal wound repair, we found that neuropilin-1 was abundantly expressed on new vasculature in healing wounds. Moreover, mice treated with anti-neuropilin-1 antibodies exhibited a significant decrease in vascular density within these wounds (67% decrease, P = 0.0132). In in vitro assays, VEGF induced formation of endothelial cord-like structures on collagen gel and endothelial cell migration toward VEGF was inhibited by antibodies directed against neuropilin-1. These results provide both in vitro and in vivo evidence for a critical role of neuropilin-1 in wound angiogenesis.
Angiogenesis occurs in many biological processes such as embryonic development, reproduction, wound healing, cancer, arthritis, retinopathies, and psoriasis. 1,2 Vascular endothelial growth factor (VEGF), a potent proangiogenic factor, has been extensively studied as a mediator of angiogenesis. VEGF is expressed in high levels and plays a functional role during embryonic development, as the absence of a single VEGF allele in embryos causes embryonic lethality and abnormal vasculogenesis. 3-5 VEGF is also critical in angiogenic processes of the adult, where antibody blockade of VEGF leads to a significant decrease in angiogenic activity in wound fluids and tumors. 6-10 The three soluble isoforms of VEGF, VEGF121, VEGF145, and VEGF165, induce angiogenesis through the binding of cell surface receptors, VEGFR1 and VEGFR2, which are expressed on the endothelial cells of sprouting vessels. VEGFR2 is capable of binding all three isoforms of VEGF, whereas VEGFR1 binds only VEGF121 and VEGF165. 11 Both VEGFR1 and VEGFR2 are functionally active during angiogenesis. 12-19 Recently, a third VEGF receptor, neuropilin-1, has been identified. Neuropilin-1 was originally characterized as a semaphorin receptor that is important for guidance of developing nerves. 20,21 Neuropilin-1 also plays a role in vasculogenesis as neuropilin-1-null mice are embryonic lethal and exhibit cardiovascular defects. 22 Recent studies have shown that expression of neuropilin-1 on endothelial cells enhances the biological activity of VEGFR2 in response to the VEGF165 isoform. 23,24 This finding suggests that neuropilin-1 is a co-receptor for VEGF, and has led to the theory that neuropilin-1 is involved in VEGF-mediated angiogenesis in vivo. More recent studies have suggested a role for neuropilin-1 in tumor growth as neuropilin-1 mRNA expression was observed to be threefold to fivefold higher in prostate tumor tissue compared to normal prostate tissue. 25 Overexpression of neuropilin-1 in AT2.1 prostate carcinoma cells resulted in increased tumor volume, enhanced angiogenesis, and increased proliferation of endothelial cells in comparison to controls. 26 In contrast, overexpression of a soluble form of neuropilin-1 in the AT2.1 prostate carcinoma cells results in increased tumor cell apoptosis. 27 Although these studies indicate that the expression of neuropilin-1 by tumor cells themselves can influence tumor growth and angiogenesis, direct documentation of a role for endothelial-expressed neuropilin-1 in the adult organism is scarce. To date, the strongest evidence for a role of neuropilin-1 in angiogenesis in the adult is the description of its expression on endothelial cells in the adult uterus, a site of physiological angiogenesis. 28
In healing wounds, a period of robust angiogenesis is followed by a period of vascular regression during which capillary density returns to normal levels. 29 Previous investigations have demonstrated that the proangiogenic phase of wound healing is mediated primarily by VEGF. 6,9 The present study examines the expression and functional significance of neuropilin-1 in wound angiogenesis.
Materials and Methods
mRNA Analysis
Total RNA was prepared by homogenization of frozen wound tissue in 4 mol/L guanidine isothiocyanate, pH 6.0, and purified over a 5.7 mol/L CsCl gradient, pH 6.0, by centrifugation at 35,000 × g for 18 hours. For each total RNA preparation, four to five wounds were pooled and yielded 32 to 130 μg of RNA. Northern analysis was performed by electrophoresis of 10 μg of total RNA per lane through 0.8% agarose, 2 mol/L formaldehyde gels in 20 mmol/L MOPS buffer, pH 7.0. Gels were blotted onto Gene Screen Plus (DuPont-NEN, Wilmington, DE) and the membrane hybridized according to the manufacturer’s directions. The following templates were created for probe generation: a 760-bp reverse transcriptase-polymerase chain reaction (RT-PCR) product of the N-terminal a2 domain of neuropilin-1 was cloned into pPCR-Script Amp SK(+) (Stratagene, La Jolla, CA). A 502-bp RT-PCR product of VEGFR-1 was cloned into pPCR-Script Amp SK+ (Stratagene). A 960-bp RT-PCR fragment of VEGFR-2 was cloned into pBluescript KS+ (Stratagene). VEGFR1, VEGFR2, and neuropilin-1-specific probes were made using the RadPrime Labeling System (Life Technologies, Inc., Gaithersburg, MD) to a specific activity of at least 10 8 cpm/μg. GAPDH expression levels were used for normalization.
Neuropilin-1 Antiserum Production and Purification
Polyclonal anti-neuropilin-1 antibodies were generated by immunizing a rabbit with a histidine-tagged neuropilin-1 protein that was produced in the BL21(DE3)pLysS strain of Escherichia coli and isolated as previously described. 21 Rabbits were immunized with 600 μg of the protein in 0.6 ml of complete Freund’s adjuvant and were boosted every 2 weeks with 300 μg of the protein in 0.5 ml of incomplete Freund’s adjuvant. Serum was collected and purified by protein A-Sepharose chromatography to obtain the IgG fraction. The amount of rabbit IgG was determined by bicinchoninic acid protein assay (Pierce Chemical Company, Rockford, IL). By Western blot analysis, the anti-neuropilin-1 antibodies recognized the MAM fragment of neuropilin-1 as a single 40-kd band (data not shown). The ability of the anti-neuropilin-1 antibodies to block the functional activity of neuropilin-1 receptors on neurons has been previously described. 21
Immunohistochemistry
Ten-μm sections from frozen embedded tissues were prepared for immunohistochemical analysis of PECAM-1 and neuropilin-1 expression. The PECAM-1 analysis detects both progenitor and differentiated endothelial cells, as both populations express CD31. 30,31 All incubations and washes were performed at room temperature. Sections were fixed in acetone for 30 minutes. After three 3-minute washes in phosphate-buffered saline (PBS), pH 7.4, sections were treated with 0.3% H2O2 in methanol to quench endogenous peroxidase activity. The slides were washed in PBS, pH 7.4, and blocked with 1:10 dilution of normal mouse serum (Sigma Chemical Company, St. Louis, MO) in PBS, pH 7.4, for 30 minutes. For PECAM-1 staining, sections were incubated in 1.0 μg/ml of MEC13.3 rat anti-mouse PECAM-1 antibody (anti-CD31; Pharmingen International, San Diego, CA) in PBS, pH 7.4. For the detection of neuropilin-1, sections were incubated in 93 ng/ml of purified rabbit IgG from the anti-neuropilin-1 antiserum. After a 30-minute incubation with either primary antibody, the slides were washed for 3 minutes, three times, in PBS, pH 7.4. Sections were then incubated for 30 minutes with either 13.0 μg/ml of biotinylated mouse anti-rat IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for PECAM-1 detection or 3.0 μg/ml biotinylated goat anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) for neuropilin-1 staining. After three 3-minute washes in PBS, pH 7.4, slides were incubated with avidin-biotin-horseradish peroxidase complex (Vector Laboratories) for 30 minutes. After another set of three washes, slides were incubated in a horseradish peroxidase substrate, 3,3′-diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 10 minutes and then counterstained with Harris hematoxylin (Sigma Chemical Company). Coverslips were mounted with Cytoseal (Stephens Scientific, Kalamazoo, MI).
RT-PCR
For VEGF RT-PCR, sense (5′-CGAGACCCTGGTGGACATCT-3′) and anti-sense (5′-CACCGCCTCGGCTTGTCAC-3′) primers were used. The β-actin control RT-PCR was performed with sense (5′-GTGGGGCGCCCCAGGCACCA-3′) and antisense (5′-CTCCTTAATGTCACGCACGATTTC-3′) primers for β-actin. One μg of total wound RNA was annealed with random hexamers and reverse-transcribed at 42°C for 15 minutes using MuLV reverse transcriptase (Applied Biosystems, Foster City, CA). The concentrations of 10× PCR buffer II, MgCl2 solution, and dNTPs were used in accordance with the manufacturer’s recommendations (Applied Biosystems). The reaction mixture was then heated for 5 minutes at 99°C and chilled on ice. Ampli-Taq Gold was used in the PCR reaction (Applied Biosystems). Primers were added, and the reaction incubated at 95°C for 12 minutes followed by 35 cycles of 1 minute at 95°C, 1 minute at 58°C, 1.5 minutes at 72°C, and a final extension of 7 minutes at 72°C. The PCR product was subjected to electrophoresis in a 2% agarose gel.
Antibody Treatment and Injury Model
Female BALB/c mice aged 8 weeks (Harlan Sprague Dawley, Inc., Indianapolis, IN) were anesthetized by inhalation of methoxyflurane (Schering-Plough Animal Health Corp., Union, NJ). Six full-thickness excisional wounds of 3 mm in diameter were made on the shaved dorsum of the mice with a biopsy punch (Acuderm, Inc., Ft. Lauderdale, FL). At day 5 after wounding, mice (n = 7) were given an intraperitoneal injection of 1 mg of rabbit IgG purified from anti-neuropilin-1 antiserum, PBS, pH 7.4, or 1 mg of purified preimmune rabbit IgG (Pierce Chemical Company, Rockford, IL). At day 7 after injury, the mice were euthanized by halothane inhalation (Halocarbon Laboratories, Riveredge, NJ), and the wound with its surrounding tissue was removed with a 5-mm biopsy punch. Wounds were embedded in TBS Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) for histological analysis. All samples were stored at −80°C until the time of analysis.
Analysis of Angiogenesis
Angiogenesis was analyzed by blinded observers as previously described. 29 Briefly, images of PECAM-stained wound sections were captured using Scion Image (Scion Corp., Frederick, MD). The wound bed was outlined using a freehand drawing tool and the area measured. The thickening of the epidermis at the top and muscle edges at the bottom of the section were used to identify the wound edges. The PECAM-positive area within the entire wound bed was measured and the percent vascularization was calculated as:
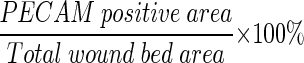 |
In Vitro Cord Formation Assay
One ml of collagen gel containing 4 mg/ml of rat tail collagen (Upstate Biotechnology, Lake Placid, NY) in PBS, pH 7.4, was plated onto each 35-mm dish. Murine endothelial cells, SVEC4-10 (American Type Culture Collection, Rockville, MD), at a concentration of 5 × 10 5 cells per ml, were first incubated for 30 minutes at room temperature in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum and 100 ng/ml of murine VEGF164, a homologue of the human VEGF165 (R&D Systems Inc., Minneapolis, MN). Some cell suspensions also received either 10 μg/ml of anti-neuropilin-1 or 10 μg/ml of rabbit IgG. After this incubation, 1 ml of the cell suspension was then plated on the prepared collagen gel. After 4 hours of incubation on collagen gels, the cells were photographed at five randomly chosen fields on the culture dish. Cord-like structures were counted per field and the average number of cord-like structures per field calculated for experimental and control groups. For each independent experiment, the numbers of endothelial cords formed in the presence of murine VEGF164 were considered as maximal (100%), and experimental values were calculated as a percentage of maximal cord formation.
Endothelial Cell Migration Assay
The cell migration assay was performed as previously described. 32 Briefly, human dermal microvascular endothelial cells (Cell Systems, Kirkland, WA) were starved overnight in media containing 0.1% bovine serum albumin, harvested, resuspended into Dulbecco’s modified Eagle medium (DME) with 0.1% bovine serum albumin, and plated on one side of a modified Boyden chamber (Nucleopore Corporation, Cabin John, MD). Test substances were added to the other side of the well and the cells were allowed to migrate for 4 hours at 37°C. Membranes were recovered, fixed, stained, and the number of cells that had migrated from one side of the semiporous gelatinized membrane per 10 high-power fields counted. Data are reported as the mean number of cells migrated per 10 high-power fields (×400). Each substance was tested in quadruplicate. Human VEGF165 (R&D Systems Inc.) was used at a concentration of 100 pg/ml. The optimal concentration for VEGF was determined by dose-response experiments (data not shown). Anti-neuropilin-1 antibody was tested at the concentrations of 1, 10, 20, 25, and 50 μg/ml. The antibody was tested alone and found to be neutral in that it neither stimulated nor reduced basal levels of migration, indicating that the doses used were neither stimulatory nor toxic (data not shown).
Statistics
Data were analyzed using GraphPad Prism, version 2.01 (GraphPad Software Inc, San Diego, CA). The means and SEM were calculated for each data set. An unpaired t-test was used for comparison of groups. Values of P < 0.05 were considered significant.
Results
VEGF and VEGF Receptor mRNA Expression in Wounds
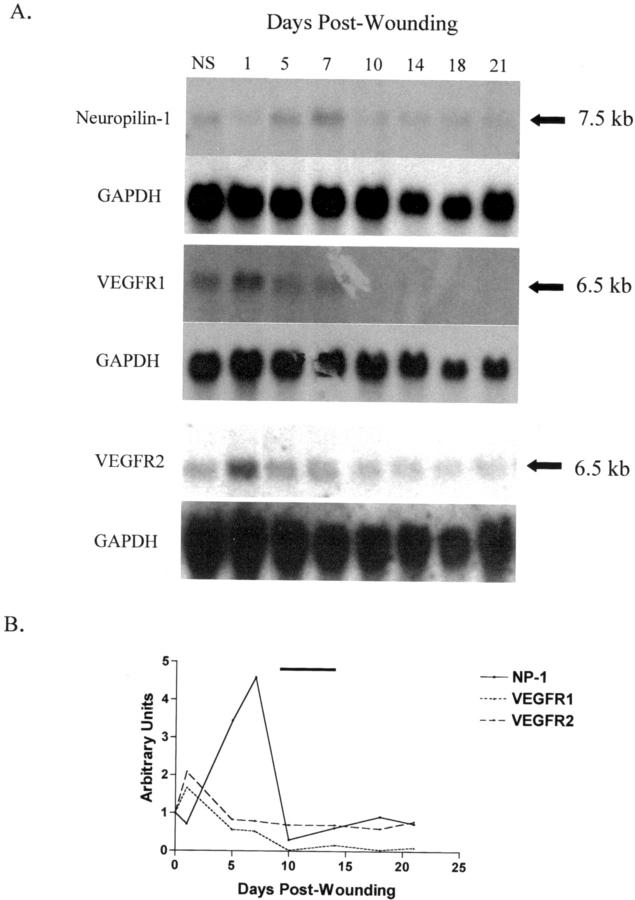
The mRNA expression pattern of VEGFR1, VEGFR2, and neuropilin-1 in wounds was examined by Northern blot analysis of normal skin and wound mRNA from day 1 up to day 21 after wounding (Figure 1) ▶ . Expression for both VEGFR1 and VEGFR2 mRNA was at maximal levels by day 1 after wounding. In contrast, neuropilin-1 mRNA was observed to reach peak expression around day 7 after wounding. The expression of VEGFR1, VEGFR2, and neuropilin-1 mRNA is very low in normal skin suggesting that the effect of remnants of normal skin in wound samples would be minimal. Previous characterization of wound angiogenesis in this model has shown wound vascularity reaches maximal levels from day 10 to 14 (Figure 1B) ▶ . 29 Interestingly, peak neuropilin-1 mRNA expression was seen just before the maximal wound vascularity. The results show that the pattern of neuropilin-1 mRNA expression correlates with wound angiogenesis.
Figure 1.
VEGF receptor mRNA expression in wounds. A: Neuropilin-1, VEGFR1, and VEGFR2 mRNA levels were analyzed in normal skin and wound samples isolated at selected times from day 1 to 21 after wounding. Neuropilin-1 mRNA (7.5-kb band) was observed to reach maximal levels at day 7. In contrast, VEGFR1 and VEGFR2 mRNA (each represented by a 6.5-kb band) reached peak expression at day 1. The Northern blot for neuropilin-1 is representative of seven experiments. The Northern blots for VEGFR1 and VEGFR2 were performed in duplicate. GAPDH was used as a control for RNA loading. NS, normal uninjured skin. B: The mRNA expression pattern for each receptor was assessed by densitometry of the Northern blots depicted in (A). For each receptor, mRNA levels were normalized to GAPDH and the values are relative to those of normal skin. The bar above the graph represents the peak of angiogenesis in this model.
VEGF Isoforms mRNA Expression in Wounds
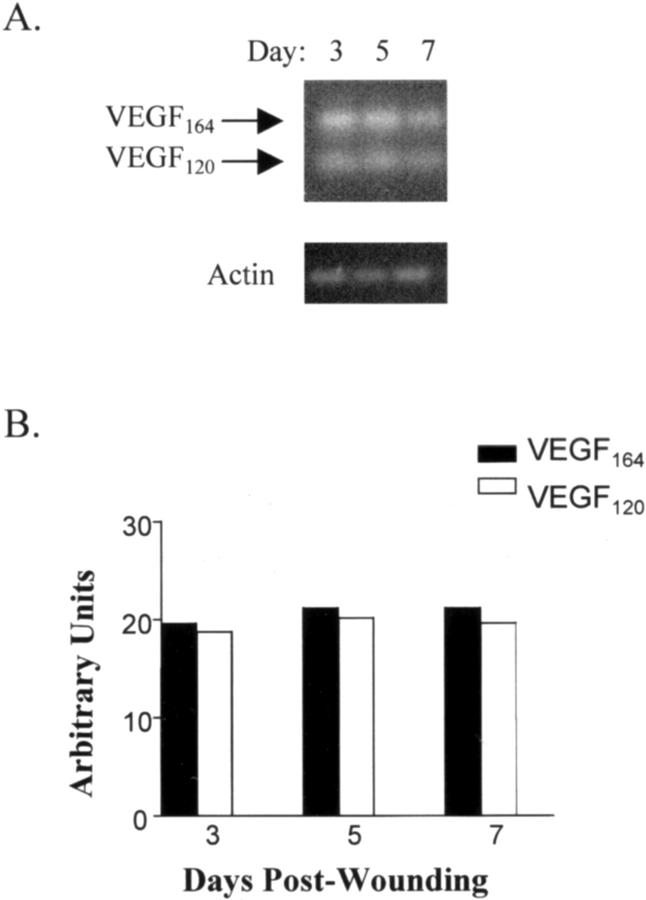
Because neuropilin-1 interacts solely with the VEGF165 isoform, the production of the soluble VEGF isoforms was examined in wounds by RT-PCR. 33 Using a single set of primers, murine VEGF120 and VEGF164 isoforms could be detected as differentially sized products of 279 and 411 bp, respectively. Both murine VEGF120 and VEGF164 were detected by RT-PCR at all time points examined (Figure 2) ▶ . Densitometry of RT-PCR products revealed that the levels of the murine VEGF120 and VEGF164 isoforms were nearly equal in the wound, suggesting that both isoforms may contribute to wound angiogenesis.
Figure 2.
Expression of VEGF isoforms in wounds. A: VEGF isoform mRNA expression was examined by RT-PCR analysis of wound mRNA from day 3, day 5, and day 7 wounds. The murine VEGF120 and VEGF164 isoforms correspond to 279- and 411-bp bands, respectively. B: Densitometric analysis of the RT-PCR results demonstrated that VEGF120 and VEGF164 levels were approximately equivalent in wounds. The mRNA levels of each isoform were normalized to β-actin.
Immunohistochemical Localization of Neuropilin-1 in Wounds
Neuropilin-1 protein expression in the wound bed was examined by immunohistochemistry of day 5, 7, 10, and 14 wounds (Figure 3A) ▶ . In early wounds (day 5) few vessels and little neuropilin-1 were observed in the wound bed. However, developing vasculature was noted immediately adjacent to the wound bed at this time point. By day 7 after wounding, both the infiltration of vessels into the wound bed and robust expression of neuropilin-1 were evident. The immunostaining patterns for PECAM-1, an endothelial cell marker, and neuropilin-1 were very similar and examination of neuropilin-1 staining at high magnification (×1000) showed neuropilin-1 localized to endothelial cells (Figure 3B) ▶ . Neuropilin-1 seemed to localize to the developing vasculature in the wound bed at days 7, 10, and 14 after wounding.
Figure 3.
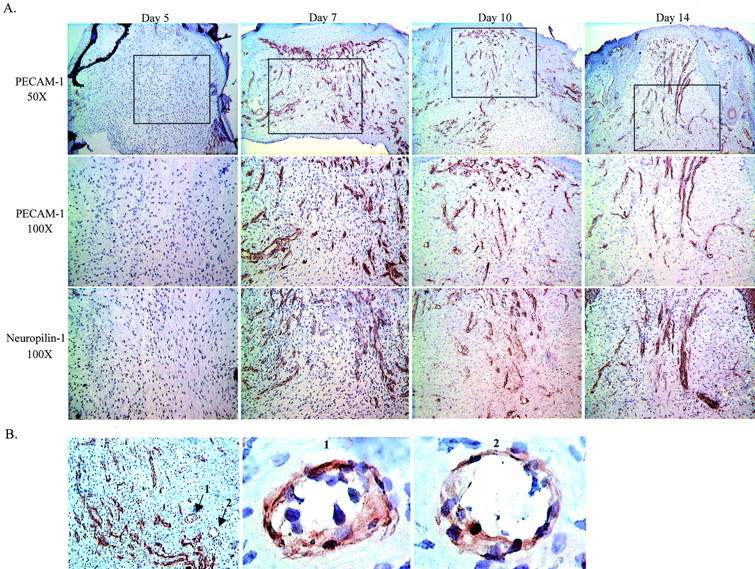
PECAM-1 and neuropilin-1 localization in wounds by immunohistochemistry. A: Histological sections from days 5, 7, 10, and 14 after wounding were stained with anti-neuropilin-1 or anti-PECAM-1 antibodies. Top: PECAM-1 staining at a ×50 original magnification. Capillary growth was minimal at day 5, with increasing numbers of capillaries observed at days 7, 10, and 14. The boxed region represents the area depicted at an original magnification of ×100 in the middle and bottom panels. Serial sections stained for PECAM-1 (middle) and neuropilin-1 (bottom) demonstrate coincident patterns of expression on the developing capillaries in the wound bed. B: Neuropilin-1 staining of a day 7 wound is shown at the left at an original magnification of ×100. Two vessels in cross-section are indicated by the arrows numbered 1 and 2. Vessel 1 and 2 are depicted at an original magnification of ×1000 in the panels labeled 1 and 2.
Functional Analysis of Neuropilin-1 in Wound Angiogenesis
To examine the functional activity of neuropilin-1 during wound angiogenesis, mice were treated with anti-neuropilin-1 antibodies. In these experiments, mice were injected with a single dose of either rabbit IgG purified from anti-neuropilin-1 antiserum or control preimmune rabbit IgG at day 5 after injury, a time point that precedes maximal neuropilin-1 mRNA expression. In this model, complete re-epithelialization of the wound occurs before day 5, so anti-neuropilin-1 antibody treatment had no effect on wound closure. After antibody treatment, the degree of wound angiogenesis was examined at day 7, a time point when robust angiogenesis occurs in this model. As compared to control, the wounds of mice treated with anti-neuropilin-1 exhibited a 67% decrease in wound angiogenesis (P = 0.0132) (Figure 4) ▶ . These results indicate that antibody treatment of neuropilin-1 has a negative impact on wound angiogenesis, and strongly suggest that neuropilin-1 plays a role in the angiogenesis of healing wounds.
Figure 4.
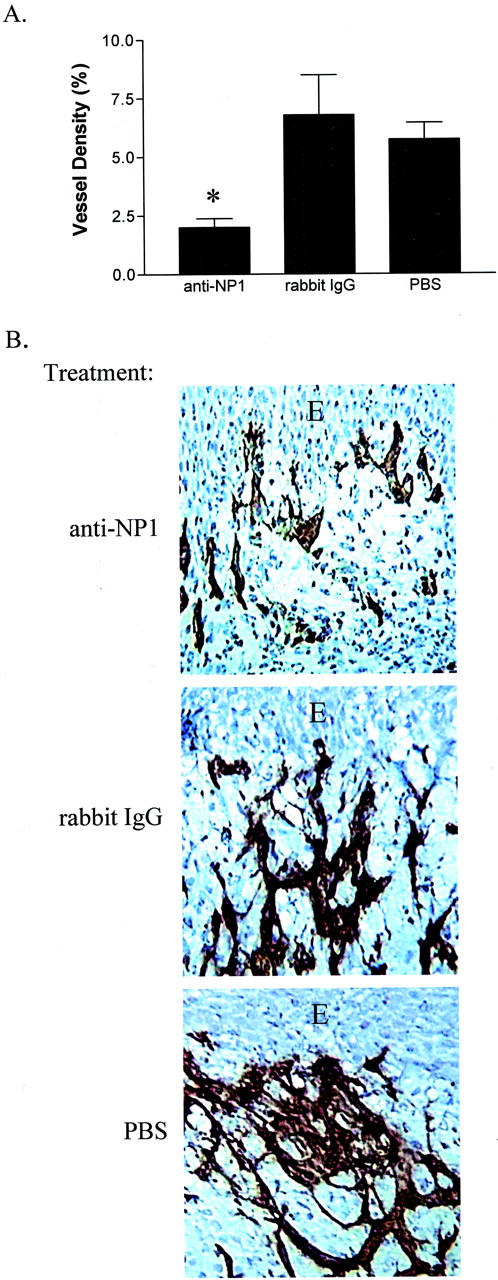
Effect of neutralization of neuropilin-1 on in vivo wound angiogenesis. A: The vascular density of the wounds of mice receiving an intraperitoneal injection of 1 mg of anti-neuropilin-1 (anti-NP1), rabbit IgG, or PBS was determined by image analysis. Whereas control mice injected with rabbit IgG or PBS exhibited a vessel density of 6.7 ± 4.1% and 5.7 ± 1.9%, respectfully, mice injected with anti-neuropilin-1 exhibited a vessel density of just 2 0 ± 12 1.0% (*, P = 0.0132 compared to rabbit IgG; *, P = 0.0005 compared to PBS; n = 7). B: Immunohistochemical localization of vessels in the wound bed was performed using anti-PECAM-1. The photographs depict stained sections of wounds from mice treated with anti-neuropilin-1 antibodies (anti-NP1), rabbit IgG, and PBS. The wounds of mice injected with anti-neuropilin-1 exhibited greatly reduced wound vascularity. Original magnification, ×200. E, epithelium.
Functional Analysis of Neuropilin-1 in Vitro
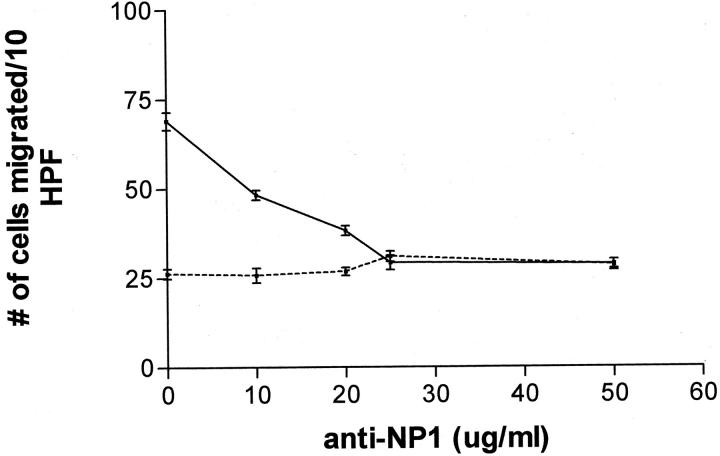
To provide further support for the role of neuropilin-1 in VEGF-mediated angiogenesis, in vitro cord formation and endothelial cell migration assays were used. When cultured in the presence of murine VEGF164, endothelial cells form cord-like structures on collagen gels. When cells stimulated with murine VEGF164 were treated with anti-neuropilin-1 antibodies, the formation of cord-like structures was completely inhibited (Figure 5) ▶ . The effect of anti-neuropilin-1 on endothelial cell chemotaxis was also examined. In the presence of the antibodies, the migration of human microvascular endothelial cells to human VEGF165 was severely inhibited (Figure 6) ▶ . This inhibition was dose-dependent, because the ability of endothelial cells to migrate toward human VEGF165 continued to decrease as the concentration of antibodies was increased. Together, these findings confirm that neuropilin-1 plays a critical role in VEGF-mediated angiogenesis and support the in vivo observation that anti-neuropilin-1 treatment leads to decreased wound vascularity.
Figure 5.
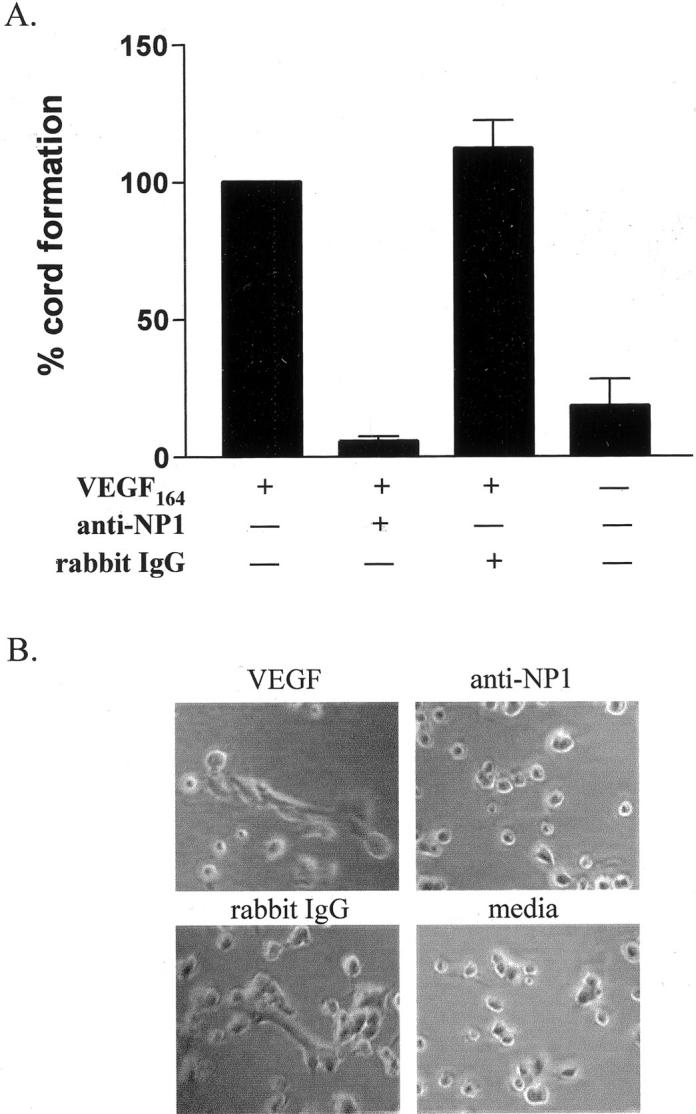
Effect of anti-neuropilin-1 endothelial cell cord formation in vitro. A: Endothelial cells were cultured on collagen matrix, photographed 4 hours after plating, and counted for cord-like structures. For each individual experiment, cord formation in cultures with VEGF was set at 100%. The percentage of cord formation in cultures with VEGF and anti-neuropilin-1 (anti-NP1) was 5.6 ± 2 3.2%. Control cultures with VEGF and the control antibody (rabbit IgG) or media alone showed 112.0 ± 18.0% and 18.0 ± 17.0% cord formation, respectively. B: The photographs of cell cultures are shown for VEGF, anti-NP1, rabbit IgG, and media groups at ×100 original magnification.
Figure 6.
Effect of increasing concentrations of anti-neuropilin-1 on endothelial cell migration. Human endothelial microvascular cells were examined for their ability to migrate in the absence (dashed line) or presence (solid line) of VEGF in a standard chemotaxis assay. The addition of increasing concentrations of anti-neuropilin-1 antibodies abrogated chemotaxis of endothelial cells toward VEGF in a dose-dependent manner.
Discussion
Since its initial discovery nearly 2 decades ago, a growing body of evidence has demonstrated the importance of vascular endothelial growth factor in angiogenesis. Within the healing wound, VEGF has been well-described as a critical proangiogenic growth factor. 6,9 In addition, many other conditions, including tumor growth, are dependent on VEGF-mediated angiogenesis. 34 The emergence of VEGF as a central regulator of angiogenesis has led to widespread interest in its production and functional interactions. These intensive investigations have documented that VEGF is a potent inducer of both mitogenic and chemotactic responses in endothelial cells, and that both the regulation of the production of VEGF and its receptor interactions are complex. The exceptional complexity of VEGF function has been increased with the description of a role for neuropilin-1 in VEGF-mediated angiogenesis. 23,24,35
Our studies provide three pieces of evidence to support a functional role for neuropilin-1 in wound angiogenesis. First, the protein expression pattern of neuropilin-1 coincides with the observed pattern of wound angiogenesis, as neuropilin-1 protein can be immunohistochemically localized to the developing vasculature. Secondly, in vitro introduction of anti-neuropilin-1 antibodies blunts the endothelial cell chemotactic response to human VEGF165 and inhibits VEGF-induced cord formation. Finally, and perhaps most significantly, in vivo neutralization of neuropilin-1 causes a significant decrease in capillary growth in healing wounds. Taken together, the evidence strongly supports the contention that neuropilin-1 plays a functional role in VEGF-mediated wound angiogenesis.
Results from the RT-PCR experiments show that mRNA of both the 120- and the 164-amino acid isoforms of murine VEGF (VEGF120, VEGF164) are present in healing dermal wounds. Although both are proangiogenic, neuropilin-1 binds only to human VEGF165 . 33 If anti-neuropilin-1 antibody treatment does abrogate only murine VEGF164-mediated angiogenesis, then VEGF164 may account for the majority of the soluble proangiogenic activity in our experiments. The presence of murine VEGF120 may account for the residual angiogenic response seen in mice treated with anti-neuropilin-1 antibodies. Overall, these observations suggest that the complex of VEGF165-VEGFR2-neuropilin plays an essential role in wound angiogenesis.
Although our findings provide supporting evidence for a functional role for neuropilin-1 in angiogenesis, the mechanism by which neuropilin-1 transduces a cellular signal on ligation with VEGF165 is not yet clear. 36,37 Neuropilin-1 is thought to act as a co-receptor for VEGFR2, as neuropilin-1 co-immunoprecipitates with VEGFR2 and increases the signaling potency of VEGF165 as compared to VEGF121. 23,24 Although many studies have established the signaling pathway for VEGFR2, the signal transduction events of VEGFR2 in coordination with neuropilin-1 remains to be elucidated. 38-43
Neuropilin-1 may have many other functional roles beyond mediating VEGF165-induced angiogenesis. Expression of neuropilin-1 has been detected in the epicardium, myocardium, and endocardium of the human fetal heart. 44 Hematopoietic cells have also been shown to express the neuropilin-1 receptor. 45 An incidental finding in our own experiments was the immunohistochemical identification of neuropilin-1 on epidermal keratinocytes (data not shown). Although the role of neuropilin-1 on keratinocytes remains to be examined, the identification of neuropilin-1 on a diverse number of cell types suggests a multifunctional role for this receptor.
Our studies provide good evidence for a functional role for neuropilin-1 in VEGF-mediated wound angiogenesis. As such, the current findings are complementary to previous studies that have suggested a role for neuropilin-1 in the angiogenesis of solid tumors, rheumatoid arthritis, and diabetic retinopathy. 25,46,47 To our knowledge, the current study is the first to correlate the production of neuropilin-1 with active angiogenesis and the first to use anti-neuropilin-1 antibodies to block angiogenesis in vivo. Additional studies are needed to more fully dissect the mechanism of neuropilin-1 activity in both pathological and nonpathological angiogenesis.
Acknowledgments
We thank Dr. Alex Kolodkin for his generous assistance with the production of the anti-neuropilin-1 antiserum.
Footnotes
Address reprint requests to Luisa A. DiPietro, Loyola University Medical Center, Burn and Shock Trauma Institute, 2160 S. First Ave., Maywood, IL 60153. E-mail: 1dipiet@lumc.edu.
Supported by grant GM50875 from the National Institutes of Health (to L. A. D.).
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA: Cutaneous wound healing. N Engl J Med 1999, 34:738-746 [DOI] [PubMed] [Google Scholar]
- 3.Breier G, Albrecht U, Sterrer S, Risau W: Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114:521-532 [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380:439-442 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A: Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380:435-439 [DOI] [PubMed] [Google Scholar]
- 6.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 8.Senger DR, Perruzzi CA, Feder J, Dvorak HF: A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res 1986, 46:5629-5632 [PubMed] [Google Scholar]
- 9.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA: Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998, 152:1445-1452 [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 11.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 12.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993, 53:4727-4735 [PubMed] [Google Scholar]
- 13.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC: Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376:62-66 [DOI] [PubMed] [Google Scholar]
- 14.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A: Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 1994, 367:576-579 [DOI] [PubMed] [Google Scholar]
- 15.Fong GH, Rossant J, Gertsenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M: VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol 1996, 270:H1803-H1811 [DOI] [PubMed] [Google Scholar]
- 17.Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, Soriano JV, Montesano R, Iruela-Arispe ML: Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn 2000, 218:507-524 [DOI] [PubMed] [Google Scholar]
- 18.Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Muller M, Wood J, Martiny-Baron G, Unger C, Marme D: Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res 2000, 60:4819-4824 [PubMed] [Google Scholar]
- 19.Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE: Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res 2000, 60:5117-5124 [PubMed] [Google Scholar]
- 20.He Z, Tessier-Lavigne M: Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90:739-751 [DOI] [PubMed] [Google Scholar]
- 21.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD: Neuropilin is a semaphorin III receptor. Cell 1997, 90:753-762 [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H: A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126:4895-4902 [DOI] [PubMed] [Google Scholar]
- 23.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92:735-745 [DOI] [PubMed] [Google Scholar]
- 24.Whitaker GB, Limberg BJ, Rosenbaum JS: Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of vegf165 and vegf121. J Biol Chem 2001, 276:25520-25531 [DOI] [PubMed] [Google Scholar]
- 25.Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O, Lidereau R: VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer 2000, 89:167-171 [DOI] [PubMed] [Google Scholar]
- 26.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M: Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J 2000, 14:2532-2539 [DOI] [PubMed] [Google Scholar]
- 27.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M: Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and antitumor activity. Proc Natl Acad Sci USA 2000, 97:2573-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavelock K, Braas K, Ouafik L, Osol G, May V: Differential expression and regulation of the vascular endothelial growth factor receptors neuropilin-1 and neuropilin-2 in rat uterus. Endocrinology 2001, 142:613-622 [DOI] [PubMed] [Google Scholar]
- 29.Swift ME, Kleinman HK, DiPietro LA: Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 1999, 79:1479-1487 [PubMed] [Google Scholar]
- 30.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275:964-967 [DOI] [PubMed] [Google Scholar]
- 31.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E: Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol 1994, 63:247-254 [PubMed] [Google Scholar]
- 32.Lingen MW, Polverini PJ, Bouck NP: Inhibition of squamous cell carcinoma angiogenesis by direct interaction of retinoic acid with endothelial cells. Lab Invest 1996, 74:476-483 [PubMed] [Google Scholar]
- 33.Soker S, Gollamudi-Payne S, Fidder H, Charmahelli H, Klagsbrun M: Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165. J Biol Chem 1997, 272:31582-31588 [DOI] [PubMed] [Google Scholar]
- 34.Plate KH, Breier G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359:845-848 [DOI] [PubMed] [Google Scholar]
- 35.Fuh G, Garcia KC, de Vos AM: The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem 2000, 275:26690-26695 [DOI] [PubMed] [Google Scholar]
- 36.Petrova TV, Makinen T, Alitalo K: Signaling via vascular endothelial growth factor receptors. Exp Cell Res 1999, 253:117-130 [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N: Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol 2001, 280:C1358-C1366 [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Shibuya M: The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene 1997, 14:2079-2089 [DOI] [PubMed] [Google Scholar]
- 39.Kroll J, Waltenberger J: The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem 1997, 272:32521-32527 [DOI] [PubMed] [Google Scholar]
- 40.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N: Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996, 271:5638-5646 [DOI] [PubMed] [Google Scholar]
- 41.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH: Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1997, 269:26988-26995 [PubMed] [Google Scholar]
- 42.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N: Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998, 273:30336-30343 [DOI] [PubMed] [Google Scholar]
- 43.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N: Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 2001, 276:3222-3230 [DOI] [PubMed] [Google Scholar]
- 44.Partanen TA, Makinen T, Arola J, Suda T, Weich HA, Alitalo K: Endothelial growth factor receptors in human fetal heart. Circulation 1999, 100:583-586 [DOI] [PubMed] [Google Scholar]
- 45.Tordjman R, Ortega N, Coulombel L, Plouet J, Romeo PH, Lemarchandel V: Neuropilin-1 is expressed on bone marrow stromal cells: a novel interaction with hematopoietic cells? Blood 1999, 94:2301-2309 [PubMed] [Google Scholar]
- 46.Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E: Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol 2000, 191:426-433 [DOI] [PubMed] [Google Scholar]
- 47.Ishida S, Shinoda K, Kawashima S, Oguchi Y, Okada Y, Ikeda E: Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2000, 41:1649-1656 [PubMed] [Google Scholar]