Abstract
Recent studies suggest that apoptosis plays a role in oxygen-induced injury, although the activation pathways and the executioner proteases that lead to cleavage of lung cell proteins and DNA, are not yet identified. We explored previously the tumor necrosis factor/tumor necrosis factor receptor and the Fas/FasL, belonging to the intrinsic pathway, and could not demonstrate any protective effect by interfering with these cell receptors. Lately, it has been shown that interacting with the CD40 system, also known to promote cell death, by administering anti-CD40 ligand (L) antibody was beneficial in several diseases and, in particular, in hyperoxia-induced injury. Using CD40- and CD40L-deficient mice (−/−) as well as administering anti-CD40L antibody, we examined the extent of lung injury in oxygen-breathing mice by several ways (lung weight, histology, inflammatory mediators, and DNA ladder) as well as the mortality. The development of lung injury was similar in wild-type, CD40−/−, CD40L−/−, or in wild-type mice treated with anti-CD40L antibody. Apoptosis was present in all conditions at 72 hours of oxygen exposure. These results show that oxygen-induced injury does not require CD40-CD40L interaction and that apoptosis of lung cells does not involve this pathway.
The CD40-CD40 ligand (CD40L) system has emerged as a critical pathway for the activation of the immune system 1 that can modulate cell proliferation, differentiation, and death of B lymphocytes. 2 In addition, CD40 is also displayed by non–hematopoietic cells such as fibroblasts, and epithelial and endothelial cells, 3-5 whereas the source of CD40L includes B and T lymphocytes, macrophages, and platelets. 6 Mice with perturbation of CD40-CD40L signaling show an attenuated response in several diseases such as encephalitis, arthritis, cerebral malaria, and recently in a model of acute pancreatitis. 5,7-9 As another approach, disruption of the CD40-CD40L by administration of anti-CD40L antibody is very effective in preventing relapse of autoimmune diseases and in reducing atherosclerosis. 10,11
We and other have shown that exposure of rodents to 100% oxygen leads to lung injury characterized by pulmonary cell death and that these epithelial and endothelial cells exhibit apoptotic and necrotic changes. 12,13 Hyperoxic-damaged lung shows a diffuse alveolar damage, leading first to interstitial edema and a moderate influx of inflammatory cells, followed by alveolar edema and animal death. 14 In this regard, it is important to know whether the CD40-CD40L is relevant in the pathogenesis of oxygen-induced injury. It has been reported that administration of anti-CD40L monoclonal antibody (mAb) attenuates radiation-induced pulmonary fibrosis and oxygen-induced lung injury in mice. 15,16 Using genetically deficient CD40 and CD40L mice, we unexpectedly observed that these mice were fully susceptible to the oxygen-induced lung injury, ie, developed pulmonary edema and died at the same time than wild-type. We also treated mice with anti-CD40L mAb without any effect on the course of oxygen-induced lung injury. We found that CD40 mRNA was expressed within the lung but was not changed during hyperoxia, whereas CD40L mRNA was almost undetectable. Lung inflammatory cells and mediators were not modified by CD40-CD40L disruption during hyperoxia. These results therefore suggest that the CD40-CD40L system is not involved in oxygen-induced lung injury in mice.
Materials and Methods
Mice
CD40-deficient mice isolated on C57BL/6 background were obtained from R. Geha, Boston, MA. 17 CD40L-deficient mice (C57BL/6 background) and C57BL/6 used as wild-type (wt) controls were purchased from the Jackson Laboratories (Bar Harbor, ME). CD40- or CD40L-deficient mice do not exhibit gross developmental or health abnormalities, but display selective deficiencies in humoral immunity, such as lower levels of IgG isotypes and undetectable levels of IgE with slight differences in the capacity of B cell proliferation. 17,18 All mice were bred in our animal facilities. Experiments were performed with 2- to 3-month-old mice.
Hyperoxia Exposure and in Vivo Treatment
Mice were placed in a sealed Plexiglas chamber and exposed to 100% oxygen or to room air in the same conditions as described before. 19 Food and water were available ad libitum. Mice were sacrificed after 72 hours of hyperoxia or, for mortality experiments, when the temperature, measured rectally with a clinical thermometer (Philips, type HP5310) dropped to <32°C, and when their spontaneous movements were almost absent; these events are followed by death within 2 hours. All animals (control or oxygen-exposed) were killed with an intraperitoneal injection of pentobarbital and bled through the abdominal aorta. The thorax was opened, the pulmonary arteries were flushed with phosphate-buffered saline (PBS), and lungs were removed. Lungs were then weighed, subsequently frozen, and prepared for mRNA or DNA extraction. Pulmonary edema was evaluated by measuring the wet weight of the lung as described previously. 19,20 Anti-mouse CD40L mAb was derived from the hybridoma MR1 of hamster origin. 16 mAbs or nonimmune hamster immunoglobulins (NIgs), as a control, were purified by protein A Sepharose and 12.5 mg/kg (250 μg/mouse) (Ig) were injected intraperitoneally on days 0 and 2. 8 The study protocol was approved by the ethical committee on animal experiments (Office Vétérinaire Cantonal of Geneva).
Bronchoalveolar Lavage (BAL) Procedure
In some experiments, after mice were killed and the pulmonary arteries flushed, BAL was performed by instilling through the trachea 2 ml of saline solution under hydrostatic pressure of 20 cmH2O. BAL fluid was recovered immediately on ice under negative hydrostatic pressure. After centrifugation cells were counted and the supernatant was collected. BAL protein concentration was determined by Bradford’s method. 21 BAL concentration of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were determined by DuoSet ELISA (R&D Systems, Minneapolis, MN).
Light Microscopy
In some experiments, lungs were fixed by intratracheal instillation of cold buffered formalin (4%) with a hydrostatic pressure of 20 cm. Transhilar horizontal sections were embedded in paraffin and processed for light microscopy.
RNA Analyses
After removal, lung tissue was immediately frozen in liquid nitrogen and stored at −80°C. Total lung RNA was isolated by Trizol reagent (Gibco). CD40 22,23 and CD40L mRNA expression 24 were evaluated on Northern blots using [32P]-labeled dUTP RNA probes. Although murine CD40 mRNA was first described as a 1579-bp cDNA (accession number, M83312), two mRNA species of ∼1.7 and 1.4 kb resulting from two polyadenylation sites have been observed. 25 Quantification was achieved using the Arcus II system on scanned gels (Agfa, Mortsel, Belgium) with the Image Quant Software (Molecular Dynamics, Inc., Sunnyvale, CA). These blots were analyzed by densitometry, and small differences in loading were normalized by the density of the 18S rRNA bands. Results of mRNA abundance are expressed in arbitrary units as a ratio of the intensity of the signal over 18 S rRNA signal ± SD.
Detection of Internucleosomal DNA Fragmentation
Lungs were homogenized by polytron disruption in PBS and 10 mmol/L of ethylenediaminetetraacetic acid (10 mg of tissue in 0.5 ml). DNA-enriched small fragments were extracted as described previously. 20 The samples were run in 1% agarose gel and DNA fragmentation was revealed with ethidium bromide.
Statistical Analysis
For each parameter measured or calculated, the values for all animals in an experimental group were averaged and the SD of the mean was calculated. The significance of differences between the values of an experimental group and those of the control group was determined with the unpaired Student’s t-test. Where appropriate (LBA results), two-way analysis of variance with multiple comparisons followed by an unpaired t-test were used. Survival parameters were analyzed using the Kaplan-Meier test. Significance levels were set at P < 0.05.
Results
Expression of CD40 and CD40L mRNA within the Lung during Hyperoxia
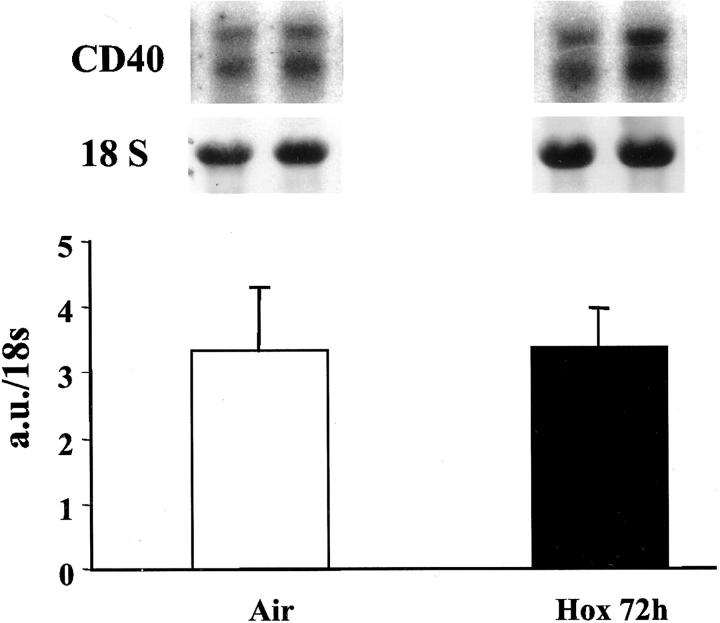
CD40 mRNA was expressed in normal lungs and was not up-regulated at any time during hyperoxia (Figure 1) ▶ , whereas CD40L was barely detectable in normal and oxygen-exposed lungs (not shown). We used spleen mRNA as a positive control for CD40L, which was strongly expressed in this organ.
Figure 1.
Northern blot of lung mRNA for CD40. Mice were exposed to air or to hyperoxia for 72 hours. Total lung RNA was isolated and 10 μg were electrophoresed and transferred onto a nylon membrane and subsequently hybridized with 32P-labeled RNA probe. Quantification of the signals was performed by scanning photodensitometry as described in Materials and Methods. Values represent the means ± SD of five animals and are expressed in arbitrary units (a.u.) over 18S rRNA ratio. No change was observed at any time of hyperoxia (24, 48, or 72 hours). Top: CD40 mRNA representative blots of two different mice.
CD40-CD40L Disruption Does Not Protect from Hyperoxia
As lung wet weight reflects alveolar damage, 20 we measured lung weight at 72 hours of exposure. CD40−/− and wt mice exposed to 100% oxygen showed the same amount of pulmonary edema at 72 hours of exposure (Figure 2A) ▶ (P = NS, n = 5 in each group). Interestingly, the lung weight of oxygen-breathing CD40L−/− mice was higher compared to wt (P = 0.002) or CD40−/− mice (P = 0.008; n = 5 in each group). Administration of anti-CD40L antibody to wt animals during hyperoxia did not change alveolar edema compared to NIg treatment as measured by lung weight (Figure 2B) ▶ .
Figure 2.
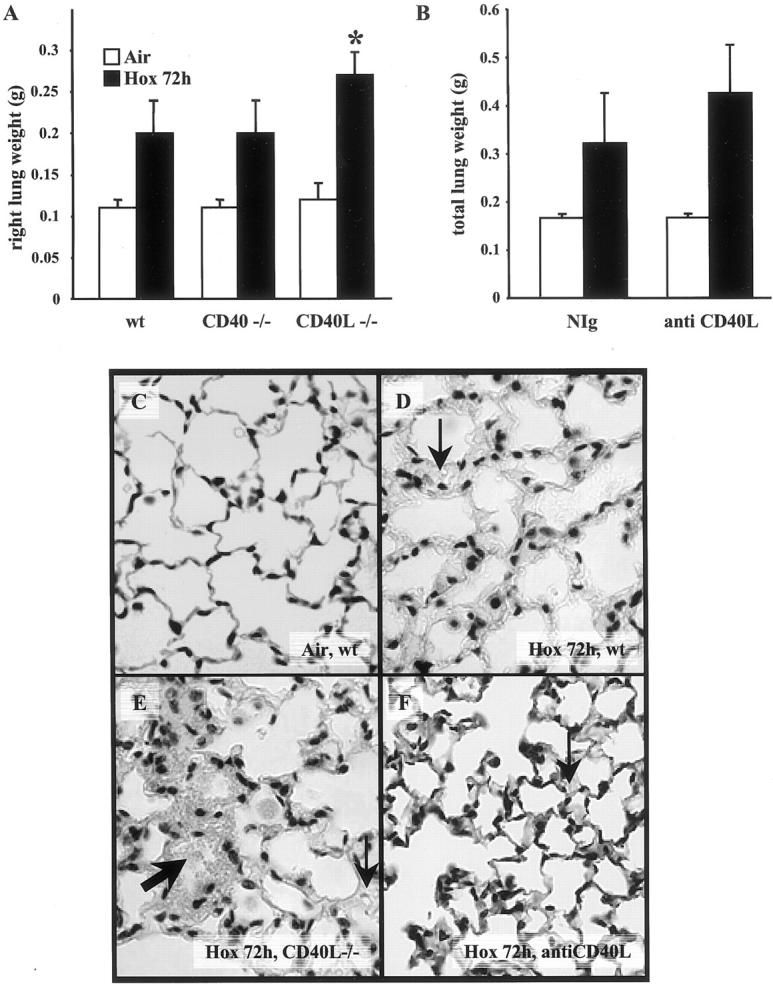
A: Lung weight (right) from air-breathing or hyperoxia-exposed (hox 72 hours) mice: wild-type (wt), CD40−/−, and CD40L−/−. Lung weight of CD40L−/− hyperoxia-exposed animals was significantly higher compared to CD40−/− or wt (*, P < 0.05). The bar graph represents the mean of seven animals ± SD. B: Total lung weight (right and left) from air-breathing and from hyperoxia-exposed wt mice treated with nonimmune hamster immunoglobulins (NIg) or with 250 μg/day of anti-CD40L antibody on day 0 and day 2, n = 9. No statistical difference was observed with anti-CD40L antibody treatment. C: Representative lung histology of a control mouse (air-breathing wt). D and E: Lungs of wt and CD40L−/− mice exposed to hyperoxia for 72 hours. F: Lung of a wt mouse treated with anti-CD40L antibody. The thick arrow points to alveolar hemorrhages and the thin arrow to interstitial edema. Original magnifications, × 40.
Lung damage detected by histology was very similar in CD40L−/−, CD40−/− (not shown), and in wt mice treated with anti-CD40L antibody (Figure 2 ▶ ; C, D, E, and F). Lungs exposed to hyperoxia showed patchy areas of increased interstitial cell number, thickening of interstitial space, and alveolar edema.
To determine precisely the time course of oxygen-induced injury, we assessed the mortality and did not find any significant difference between the three strains: survival time was 78.8 ± 1.3 (mean ± SD) for wt, 77.2 ± 8.4 for CD40−/−, and 82.3 ± 6.3 for CD40L−/− mice (P = NS).
Inflammatory Cells and Cytokines in BAL
As CD40 is a member of the TNF family and its blockade is known to decrease TNF production by macrophages, 26 we measured cell number, protein content, and two proinflammatory cytokines in BAL: IL-6 and TNF (Table 1) ▶ . The number of cells in BAL increased significantly (threefold to fivefold) in wt, CD40−/−, and CD40L−/− mice exposed to hyperoxia compared to air-breathing mice as well as wt mice treated with NIg. The cell number recovered by BAL from mice treated with anti-CD40L mAb was also enhanced (although not significantly, P = 0.08) compared to air-breathing controls. Protein content increased also by 5- to 10-fold in BAL of hyperoxia-exposed mice, and was not affected by anti-CD40L antibody treatment. IL-6 levels were significantly higher in all groups of mice exposed to hyperoxia compared to air-breathing mice, whereas no significant TNF increase was observed.
Table 1.
Cell Number, Protein Content, and Cytokines in BAL
| Cells × 103/ml | Proteins (mg/ml) | IL-6 (pg/ml) | TNF (pg/ml) | |||||
|---|---|---|---|---|---|---|---|---|
| Air | Hox | Air | Hox | Air | Hox | Air | Hox | |
| wt | 17.0±12.2 | 45.1 ±18.9* | 0.28±0.10 | 1.17±0.50* | 1.9 ±0.9 | 5.1±1.0† | 5.4±1.9 | 9.1±4.7 |
| CD40−/− | 10.9±3.7 | 55.6 ±17.3† | 0.28±0.10 | 2.10 ±0.82† | 1.5±0.4 | 10.0 ±3.3‡ | 5.0±1.0 | 7.9±4.9 |
| CD40L −/− | 16.0±7.4 | 50.7±11.8‡ | 0.23 ±0.17 | 2.78±0.91† | 2.5±1.3 | 14.9 ±6.9* | 12.8±9.3 | 3.8±1.1 |
| wt+anti-CD40L | 20.8 ±9.5 | 36.4±14.3 | 0.13±0.09 | 2.05 ±0.70‡ | 1.5±0.2 | 5.7 ±0.9‡ | 6.3±1.8 | 8.0±2.5 |
| wt +NIg | 20.8±6.8 | 38.7±6.3† | 0.10 ±0.06 | 2.75±0.77‡ | 2.0±0.6 | 4.1 ±1.2† | 6.5±1.3 | 4.9±1.4 |
CD40−/−, CD40L −/−, and wt (C57BL/6) mice (n = 5) were exposed to air to or to hyperoxia for 72 hours. BAL was performed by instilling 2 ml of PBS intratracheally and recovered by hydrostatic pressure. Cells were counted and proteins and cytokines were measured in the supernatant. The same procedure was applied in wt mice injected with anti-CD40L or with nonimmune hamster Ig (NIg). Results represent the mean value ± SD for each group.
*P < 0.05, †P < 0.01, ‡P < 0.001 hyperoxia compared to air-breathing mice. Within air-breathing mice, no significant difference was seen between the different conditions.
Apoptosis Is Present at 72 Hours of Exposure in All Strains
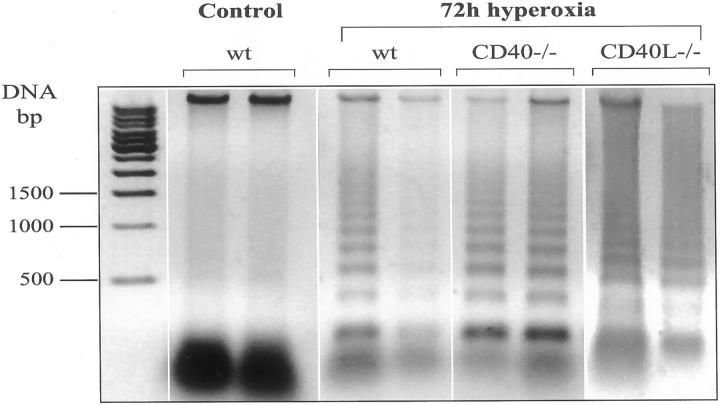
Because CD40-CD40L is able to promote apoptosis in certain cells, we explored whether DNA fragmentation was present in the lungs during hyperoxia. Presence of DNA nucleosomal degradation was assessed on DNA agarose gel. DNA ladder was present in CD40−/−, CD40L−/−, as well as in wt mice at 72 hours of exposure (Figure 3) ▶ . In air-breathing mice, DNA ladder was neither detectable in wt nor in CD40−/− or CD40L−/− mice (data not shown).
Figure 3.
Lung DNA ladder during hyperoxia. Lung DNA from air-breathing wt mice (control) and hyperoxia-exposed mice (wt, CD40−/−, and CD40L−/−) for 72 hours. Enriched fragments of DNA extracts were electrophoresed on 1% agarose gel. Note the presence of DNA ladder indicating apoptosis in all strains of mice exposed to hyperoxia for 72 hours.
Discussion
In the present study, we explored the role of CD40-CD40L in oxygen-induced alveolar injury. CD40- and CD40L-deficient mice developed lung injury, as did wt mice, indicating that the CD40-CD40L system is not a prerequisite for the development of oxygen-induced pulmonary lesions. Results obtained with blocking antibody and genetic deficiencies are therefore consistent. This is not always the case because this has been reported with Fas-FasL disruption in mice with bleomycin-induced fibrosis. 27,28
Our findings do not support the results of Adawi and co-workers 16 who recently reported that oxygen-induced injury was attenuated in mice by treatment with anti-CD40L antibody. The difference between their results and ours cannot be because of the experimental protocol, since we used the same strain of mice (C57BL/6) and the same mAb antibody at the same dosage that was effective in our hands in preventing severe malaria damage. 8 This difference could more likely be explained by the methods of evaluation of lung lesions. Adawi and colleagues 16 used only histology and indeed, pulmonary edema, which results from endothelial and epithelial damage, is difficult to quantify by light microscopy. In contrast, the measurement of lung weight is objective and provides a straightforward evaluation of the exudate. Mortality is generally believed to be a consequence of the alveolar damage, and Adawi and colleagues 16 did not provide data concerning the mortality. Under our conditions, CD40, and CD40L deficiency or the treatment with the anti-CD40L antibody did not influence mice survival, also indicating that the alveolar damage was proceeding at the same speed in absence of CD40-CD40L signaling.
Adawi and colleagues 16 also showed a great influx of inflammatory cells, mainly leukocytes, at 65 hours of exposure in their sections that was apparently decreased by the anti-CD40L mAb treatment. In our experiments, the aspect of the alveolar septa in oxygen-breathing mice was similar to what they observed and also showed an increased number of inflammatory cells. A more precise quantification of inflammatory cells by counting the cell number in BAL was performed, which confirms that there was no difference between the groups of knock-out and wt mice. Anti-CD40L mAb treatment had a moderate effect on the number of cells recovered by BAL during hyperoxia-compared NIg, suggesting that this antibody might influence cell trafficking. Because it has been shown that disruption of CD40-CD40L could affect TNF-α production either by macrophages in vitro or by several organs in vivo, 8,26 we therefore measured the levels of two proinflammatory cytokines produced mainly by alveolar macrophages, ie, TNF and IL-6 in BAL. We already reported that IL-6 plasma levels increased and TNF levels did not change during hyperoxia. 29 Hyperoxia increased BAL levels of IL-6 similarly in wt, CD40−/−, CD40L−/−, and in mice treated with anti-CD40L antibody, whereas it did not change significantly the levels of TNF. These results suggest that CD40-CD40L disruption has no effect on lung inflammatory mediators.
We and other have shown that apoptosis is an essential feature of oxygen-induced pulmonary injury. Furthermore overexpression of IL-11 or IL-6 in Clara cells conferred protection against oxygen-induced apoptosis and increased the survival of the mice. 30 We chose to evaluate apoptosis by examining the presence of DNA ladder. DNA ladder is regarded as a qualitative and specific rather than a quantitative method to measure apoptosis. Although this method does not allow the identification of the cell type or the number of cells undergoing apoptosis. Indeed, neutrophils and macrophages present in the alveolar space might also contribute to DNA degradation observed during hyperoxia. However, we showed previously on electronic microscopy that apoptosis occurred mainly in epithelial and endothelial cells. 12,20 Accordingly, DNA ladder was similar in CD40- and CD40L-deficient mice also supporting the concept that CD40-CD40L does not contribute to the alveolar damage.
Another factor, which is also arguing against an important role of CD40-CD40L, is the absence of CD40 or CD40L mRNA up-regulation in the lungs of oxygen-breathing mice. Up-regulation of CD40 has been reported in several tumor cell lines and in activated T lymphocytes, and precisely in these cells the treatment with anti-CD40L results in significant inhibition of proliferation and apoptosis. 31 Thus, the present results indicate that CD40-CD40L does not play a significant role in the oxygen-induced alveolar damage. Together with previous published results (anti-TNF administration and Fas pathway blockade 12,19 ), it is likely that oxygen-induced alveolar cell death is not mediated through the membrane receptors (intrinsic pathway) but rather through the increased presence of reactive oxygen species known to activate directly the release of proapoptotic mitochondrial products (extrinsic pathway).
Acknowledgments
We thank Dominique Belin for his helpful scientific advice.
Footnotes
Address reprint requests to Dr. Constance Barazzone Argiroffo, Department of Pathology, Centre Médical Universitaire, 1211 Geneva 4, Switzerland. E-mail: constance.barazzone@medecine.unige.ch.
Supported by the Fonds National Suisse Recherche Scientifique (Swiss National Science Foundation) (grant no. 3200-056949.99 to C. B. A. and grant no. 31-56839.99 to P. F. P.) and the Wolfermann-Nägele Foundation.
References
- 1.Noelle RJ: CD40 and its ligand in host defense. Immunity 1996, 4:415-419 [DOI] [PubMed] [Google Scholar]
- 2.Grell M, Zimmermann G, Gottfried E, Chen CM, Grunwald U, Huang DCS, Lee YHW, Durkop H, Engelmann H, Scheurich P: Induction of cell death by tumor necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J 1999, 18:3034-3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fries K, Gaspari A, Blieden T, Looney RJ, Phipps RP: CD40 expression by human fibroblasts. Clin Immunol Immunopathol 1995, 77:42-51 [DOI] [PubMed] [Google Scholar]
- 4.Gaspari A, Sempowski G, Chess P, Gish J, Phipps RP: Human epidermal keratinocytes are induced to secrete IL-6 and co-stimulate T cell proliferation by a CD40-dependent mechanism. Eur J Immunol 1996, 26:1371-1377 [DOI] [PubMed] [Google Scholar]
- 5.Durie F, Fava R, Foy A, Aruffo A, Ledbetter J, Noelle R: Prevention of collagen induced arthritis with an antibody to gp39, the ligand for CD40. Science 1993, 261:1328-1330 [DOI] [PubMed] [Google Scholar]
- 6.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Mueller-Berghaus G, Kroczek R: CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391:591-594 [DOI] [PubMed] [Google Scholar]
- 7.Gerritse K, Laman J, Noelle R, Aruffo A, Ledbetter J, Boersma W, Classen E: CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 1996, 93:2499-2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piguet PF, Kan CD, Vesin C, Rochat A, Donati Y, Barazzone C: Role of CD40-CD40L in mouse severe malaria. Am J Pathol 2001, 159:733-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frossard JL, Kwak B, Chanson M, Morel P, Hadengue A, Mach F: CD40 Ligand-deficient mice are protected against cerulein-induced pancreatitis and pancreatitis-associated lung injury. Gastroenterology 2001, 121:184-194 [DOI] [PubMed] [Google Scholar]
- 10.Howard LM, Miga AL, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, Miller SD: Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Invest 1999, 103:281-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P: Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 1998, 394:200-203 [DOI] [PubMed] [Google Scholar]
- 12.Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF: Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 1998, 19:573-581 [DOI] [PubMed] [Google Scholar]
- 13.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S: Cellular oxygen toxicity. Oxidant injury without apoptosis. J Biol Chem 1996, 271:15182-15186 [DOI] [PubMed] [Google Scholar]
- 14.Crapo JD: Morphologic changes in oxygen toxicity. Ann Rev Physiol 1986, 48:721-731 [DOI] [PubMed] [Google Scholar]
- 15.Adawi A, Zhang Y, Baggs R, Rubin P, Williams J, Finkelstein J, Phipps RP: Blockade of CD40-CD40 Ligand interaction protects against radiation-induced pulmonary inflammation and fibrosis. Clin Immunol Immunopathol 1998, 89:222-230 [DOI] [PubMed] [Google Scholar]
- 16.Adawi A, Zhang Y, Baggs R, Finkelstein J, Phipps RP: Disruption of the CD40-CD40 ligand prevents an oxygen-induced respiratory distress syndrome. Am J Pathol 1998, 152:651-657 [PMC free article] [PubMed] [Google Scholar]
- 17.Castigli E, Alt FW, Davidson L, Bottaro A, Mizogushi E, Bhan A, Geha RS: CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci USA 1994, 91:12135-12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Ligitt D, Wright B, Davison BL, Maliszewski CR: Humoral responses in CD40 ligand-deficient mice. J Exp Med 1994, 180:1889-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barazzone C, Tacchini-Cottier F, Vesin C, Rochat AF, Piguet PF: Hyperoxia induces platelet activation and lung sequestration: an event dependent on tumor necrosis factor-alpha and CD11a. Am J Respir Cell Mol Biol 1996, 15:107-114 [DOI] [PubMed] [Google Scholar]
- 20.Barazzone C, Donati YR, Rochat AF, Vesin C, Laperrousaz CD, Pache JC, Piguet PF: Keratinocyte growth factor protects both alveolar epithelium and endothelium from oxygen-induced injury in mice. Am J Pathol 1999, 154:1479-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 22.Castigli E, Young F, Carossino AM, Alt FW, Geha RS: CD40 expression and function in murine B cell ontogeny. Int Immunol 1996, 8:405-411 [DOI] [PubMed] [Google Scholar]
- 23.Grimaldi JC, Torres R, Kozak CA, Chang R, Clark EA, Howard M, Cockayne DA: Genomic structure and chromosomal mapping of the murine CD40 gene. J Immunol 1992, 149:3921-3926 [PubMed] [Google Scholar]
- 24.Mendoza RB, Cantwell MJ, Kipps TJ: Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J Immunol 1997, 159:5777-5781 [PubMed] [Google Scholar]
- 25.Torres RM, Clark EA: Differential increase of an alternatively polyadenylated mRNA species in murine CD40 upon B lymphocyte activation. J Immunol 1992, 148:620-626 [PubMed] [Google Scholar]
- 26.Imaizumi K, Kawabe T, Ichiyama S, Kikutani H, Yagita H, Shimokata K, Hasegawa Y: Enhancement of tumoricidal activity of alveolar macrophages via CD40-CD40 ligand interaction. Am J Physiol 1999, 277:L49-L57 [DOI] [PubMed] [Google Scholar]
- 27.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N: Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997, 17:272-278 [DOI] [PubMed] [Google Scholar]
- 28.Aoshiba K, Yasui S, Tamaoki J, Nagai A: The Fas/Fas-ligand system is not required for bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 2000, 162:695-700 [DOI] [PubMed] [Google Scholar]
- 29.Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF: Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol 2001, 281:L1150-L1156 [DOI] [PubMed] [Google Scholar]
- 30.Barazzone C, White C: Mechanisms of cell injury and death in hyperoxia: role of cytokines and Bcl-2 family proteins. Am J Respir Cell Mol Biol 2000, 22:517-519 [DOI] [PubMed] [Google Scholar]
- 31.Alexandroff AB, Jackson AM, Paterson T, Haley JL, Ross JA, Longo DL, Murohy WJ, James K, Taub DD: Role of CD40-CD40 ligand interactions in the immune response to solid tumors. Mol Immunol 2000, 37:515-526 [DOI] [PubMed] [Google Scholar]