To the Editor-in-Chief:
Recently, we read with special interest the paper published by Graubert et al 1 , concerning the modulation of vascular endothelial growth factor (VEGF) during the menstrual phases. The authors made several statements regarding the VEGF mRNA levels in endometrial stromal cell cultures submitted to different estrogen and progesterone in vitro treatments. They concluded that hypoxia induced a 2.4-fold increase in VEGF mRNA levels by 48 hours of exposure, estrogen and progesterone stimuli slightly raised the VEGF mRNA levels, and no decrease in VEGF mRNA was observed after withdrawal of the estrogen and progesterone. 1 In addition, the authors also infer that it is unlikely that steroids play a direct role on VEGF regulation, a view that is controversial, and in contrast with our findings in breast epithelial cells. 2,3
We have been studying the effects of estrogen and progesterone in the expression of VEGF mRNA and protein using a human breast cancer cell line, MCF-7 (ATCC). Briefly, after culturing MCF-7 breast cancer cell line for 48 hours with 17β-estradiol 1 × 10−9 mol/L (Sigma) or progesterone 1 × 10−8 mol/L (Sigma), we evaluated the expression of mRNA and protein levels of angiogenic factors, namely VEGF, by RT-PCR and Western blotting, respectively. Whereas Graubert et al 1 observed only a slight increase in VEGF transcript after estrogen stimulation, our preliminary results demonstrate that, somehow, estrogen induces VEGF overexpression, both in mRNA and protein levels. In accordance with our results, a recent report showed the presence of estrogen response elements in VEGF gene promotor region, 4 indicating that estrogens are, in fact, involved in VEGF up-regulation.
In the last two years, several papers concerning the different patterns of estrogen receptor (ER) α and β expression by epithelial, stromal, and vascular endothelial endometrial cells have been published. 5–8 Mueller et al l5 and Lecce et al 6 showed a highly complex pattern of α and β receptor distribution during the menstrual cycle. It has been shown that ER-α and ER-β mRNA levels in the eutopic endometrium were affected by a cycle change in ovarian hormones. 7 We would be interested in knowing the estrogen receptor profile of those stromal endometrial cells during the cell culture, since no basal tonus hormonal stimulation was maintained during the experiment, or at least it is not shown. This might lead to a down-regulation of the estrogen receptors, since their expression is transient during the menstrual cycle and is highly dependent on the estrogen, progesterone, luteinizing hormone, and follicle stimulating hormone levels. 7,8 A distinct pattern of ER among breast and endometrium tissues would also explain the discrepancy between the Graubert et al 1 results and ours. It is also known that different ER modulators (both ER coactivators and corepressors) are differentially expressed within different organs, which would lead to different responses after estrogen stimulus.
Conversely, the authors showed a moderate increase in VEGF mRNA levels after progesterone treatment; 1 these findings are very similar to what we observed in MCF7 in vitro experiments. VEGF is involved in proliferation and migration of vascular endothelial cells. Since progesterone is mainly synthesized during endometrial secretory phase, this steroid hormone is likely to mediate the growth and maintenance of stable coiled arterioles that characterize this phase, through the activation of growth factors other than VEGF.
Moreover, these authors did not find any increase in mRNA levels of TGF-α and IL-1β when the endometrial cells were submitted to hypoxic stimulus. 1 We also evaluated the hypoxic effects in MCF-7 cultures using a different model (hypoxia-like effect induced by CoCl2 added to culture medium). Despite the differences in our model and the one used by the authors, 1 our results were very similar concerning the TGF-α mRNA and protein levels. In fact, TGF-α expression was not induced by hypoxic conditions in MCF-7 cells.
Our group has previously reported that TGF-α, a growth factor activated by estrogen, 2,3 associated with higher angiogenic rates in a series of 86 invasive breast cancer cases. 2,3 Since ER-α is the predominant activated isotype in breast tissue, and in agreement with TGF-α-driven VEGF up-regulation reported by Graubert et al 1 in stromal endometrial cells, we can hypothesize that ER-α activated on estrogen stimulus might promote TGF-α expression, which up-regulates VEGF. This putative mechanism defines a relevant role of estrogen in angiogenic switch. However, further studies are needed to reach a conclusive model of ER-α, ER-β, TGF-α, and VEGF cross-talk.
Vascular Endothelial Growth Factor, Transforming Growth Factor-α, and Estrogen Receptors: Possible Cross-Talks and Interactions
Luisa Iruela-Arispa, Michael D. Graubert
Authors’ Reply:
We thank the group at the University of Porto (Portugal) for their interest in our work and for bringing to discussion some important aspects related to regulation of VEGF by steroids, a much-debated issue.
In our recently published manuscript 1 we described consistent, but modest, increases in VEGF mRNA levels under culture conditions when exposed to steroids. This was contrasted by the effect of hypoxia and other cytokines (IL-1 and TGF-α), which elevated VEGF mRNA nearly 10-fold. Results from Northern blots of total endometrial tissue using human subjects with hormonal determination of cycle stage revealed that overall VEGF mRNA is significantly increased upon menstruation, a time when both estrogen and progesterone levels are lowest and hypoxia is highest. The combination of these results and much validation from in vitro experiments supported our general conclusion that “it is unlikely that sex steroids play a significant role on VEGF regulation during postmenstrual repair as circulating estrogen and progesterone levels are physiologically low at this point in the cycle” (Am J Pathol 158:1408). If one is to focus attention on the proliferative and secretory phases, results from our Northern analysis support that VEGF is increased by 1.6- and 1.8-fold, respectively, considering 1-fold levels in early proliferative phase. Whether steroids alone are responsible for these increases requires further investigation.
More revealing and pertinent to the discussion at hand are results from in situ hybridization. Evaluation of VEGF transcripts in the endometrium of women during the proliferative phase showed low levels in the glands (Figure 1 ▶ A and B, open arrows) and higher expression in the stroma (closed arrows). In contrast, secretory endometrium showed strong transcript levels in the glands (open arrows) with light expression in the stroma (C and D, closed arrows). These results were not included in our manuscript because the major conclusions have been previously published by another group using immunocytochemistry, but with identical results. 2 The take-home message is that different cellular compartments respond differently to the same hormonal levels. Naturally it is the combination of multiple signaling pathways and their integration that results in variations of transcript levels. We feel that keeping this in mind is essential for interpretation of in vitro data.
Figure 1.
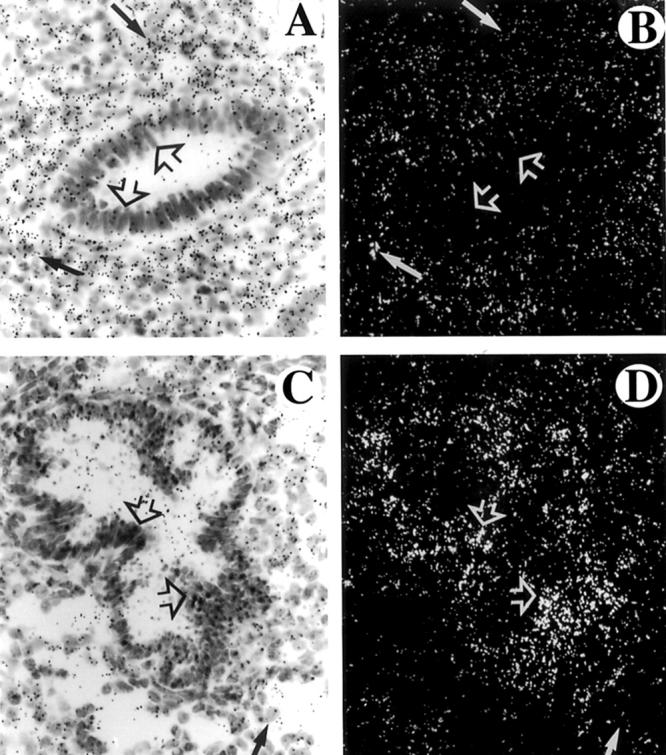
Localization of VEGF mRNA in human endometrium during late proliferative (A and B) and late secretory (C and D) stages. Shown are paired bright-field and dark-field photomicrographs of the same microscopic field. Epithelial cells from glands are indicated with open arrows. Stromal endometrial cells are indicated with closed arrows.
The group of Porto reports unpublished information using MCF-7 cells. We do not argue with their findings. In fact, increases of VEGF by estradiol has been reported by several groups (a brief evaluation shows 12 published papers centered on this subject alone). Our overall assessment from working on this problem and closely following the literature is that different cells will respond differently to similar signals and this appears to be the case with VEGF. In addition, in vivo validation is essential to ascertain the biological relevance of in vitro findings. In this light, in a recent publication in AJP, using the VEGF promoter linked to GFP might prove to be of extreme value. 3 Not to be repetitive, we would like to direct interested readers on the subject to a well put together commentary by Drs. Sengers and Van De Water 4 on that same issue.
Finally, thanks to the editorial panel of The American Journal of Pathology for providing us with the opportunity to present our response and to Dr. Larry Brown (Department of Pathology, BIDMC, Boston, MA) for allowing to use his in situ data as part of this response.
- 1.Graubert MD, Ortega MA, Kessel B, Mortola JF, Iruela-Arispe ML: Vascular repair after menstruation involves regulation of vascular endothelial growth factor receptor. Am J Pathol 2001, 158:1399-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Scott PA, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, Mackenzie IZ, Rees MC, Bicknell R: Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol 1998, 185:402-408 [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto J, Ehama R, Ge Y, Kobayashi T, Nishiyama T, Detmar M, Bugerson RE: In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am J Pathol 2000, 157:103-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senger DR, Van De Water L: VEGF expression by epithelial and stromal cell compartments: resolving a controversy. Am J Pathol 2000, 157:1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Graubert MD, Ortega MA, Kessel B, Mortola JF, Iruela-Arispe ML: Vascular repair after menstruation involves regulation of vascular endothelial growth factor-receptor phosphorylation by sFLT-1. Am J Pathol 2001, 158:1399-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt FC, Soares R: TGF-α and angiogenesis. Am J Surg Pathol 1999, 23:358-359 [DOI] [PubMed] [Google Scholar]
- 3.Schmitt FC, Soares R: Hormonal control of angiogenesis in breast cancer: TGF-α, a missed link? The Breast 1999, 8:154-156 [Google Scholar]
- 4.Hyder S, Nawaz Z, Chiappetta C, Stancel GM: Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor VEGF. Cancer Res 2000, 60:3183-3190 [PubMed] [Google Scholar]
- 5.Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN: Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA 2000, 97:10972-10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M: Presence of estrogen receptor beta in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab 2001, 86:1379-1386 [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki S, Uehara S, Murakami T, Fujiwara J, Funato T, Okamura K: Quantitative analysis of estrogen receptor alpha and beta messenger ribonucleic acid levels in normal endometrium and ovarian endometriotic cysts using a real-time reverse transcription-polymerase chain reaction assay. Fertil Steril 2000, 74:753-759 [DOI] [PubMed] [Google Scholar]
- 8.Critchley HO, Brenner RM, Henderson TA, Williams K, Nayak NR, Slayden OD, Millar MR, Saunders PT: Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and non-human primate endometrium. J Clin Endocrinol Metab 2001, 86:1370-1378 [DOI] [PubMed] [Google Scholar]


