Abstract
Commensal-associated molecular patterns, the major products of nonpathogenic bacteria, are present at high concentrations at the apical surface of the intestinal epithelium. However, the nature of the interaction of commensal-associated molecular patterns with the lumenal surface of the epithelium has not been defined. We have recently demonstrated that intestinal epithelial cells constitutively express several Toll-like receptors (TLRs) in vitro and in vivo that seem to be the key receptors responsible for immune cell activation in response to various bacterial products. In this study we characterize the subcellular distribution of two major TLRs, TLR2 and TLR4, and their ligand-specific dynamic regulation in the model human intestinal epithelial cell line T84. Immunocytochemical studies indicate that TLR2 and TLR4 are constitutively expressed at the apical pole of differentiated T84 cells. After stimulation with lipopolysaccharide or peptidoglycan, TLRs selectively traffic to cytoplasmic compartments near the basolateral membrane. Thus, we demonstrate that TLRs are positioned at the apical pole where they are poised to monitor the sensitive balance of the lumenal microbial array. The results of this dynamic epithelial surveillance can then be conveyed to the underlying cell populations of the lamina propria via these innate immune pattern recognition receptors.
The intestinal epithelium comprises an essential barrier that forms the frontline interface between mucosal surface and constituents of the intestinal lumen. It is, therefore, positioned to play a key role in the detection of the pathogen-associated molecular patterns involving both the complex variety of normal commensal bacteria, as well as those of superimposed pathogenic bacteria. The latter have been designated pathogen-associated molecular patterns (PAMPs) but products of many of these and other constituents also derive from the normal resident microflora, and thus might in many instances be properly designated CAMPs (commensal-associated molecular patterns). Although, intestinal epithelial cells (IECs) are constantly exposed to the broad spectrum of the resident microflora of the gut, little is known about the nature of the interactions between these cells and the high concentrations of lumenal microbial products. 1
Although lumenal bacteria can produce a vast variety of toxic and proinflammatory constituents, lipopolysaccharide (LPS), a glycolipid derived from the outermost membrane of gram-negative bacteria in the gut, is one of the most abundant at the apical IEC surface. In general, lumenal LPS, despite its presence in large amounts, is well tolerated by the mucosal immune system of the healthy intestine in vivo. 1,2 Of interest, enterocytes seem to be especially qualified to clear surplus circulating LPS into the intestinal lumen. 3,4 However, it is conceptually possible that an alteration in lumenal LPS, either in amount or composition, may impair epithelial integrity and elicit several immediate proinflammatory immune responses in the intestinal epithelial mucosal system. 5-7
We and others have recently demonstrated that IECs constitutively express several members of a novel family of transmembrane receptors designated “Toll-like receptors (TLRs)” in vitro and in vivo that may serve as the pattern recognition receptors of the mucosal innate immune system to lumenal CAMPs. 5,8-10 The TLR family is comprised of at least 10 homologues of the Drosophila Toll protein. These receptors seem to function as a major link between innate and adaptive cellular immune gene responses in various mammalian cell systems. 11-13 Recent studies provide compelling evidence that TLR4 serves as the main mediator of innate immune responses to LPS, whereas TLR2 may serve as the dominant cognate receptor for peptidoglycan (PGN). 14-17 It is also presumed that these and other TLR family members that have yet to be fully characterized recognize distinct derived PAMPs/CAMPs. Downstream, LPS-induced signaling through TLR rapidly leads to nuclear factor-κB activation and cytokine expression. 18,19 However, the functional roles of the other TLRs and the possible collaborative interactions between different TLRs and other nonbacterial ligands, as well as the details of the TLR-induced cellular signal transduction pathways have not yet been fully defined.
To further understand the nature of the interaction between the intestinal epithelium and lumenal CAMPs, we characterized the subcellular distribution of TLRs in IECs and their dynamic regulation in response to two major CAMPs, LPS and PGN.
Materials and Methods
Materials and Reagents
LPS (Escherichia coli, 026:B6; product no. L8274, lot 119H4123; protein by Lowry <1.2%; 30,000 endotoxin units per mg), prepared by phenol extraction, was purchased from Sigma Chemical Co. (St. Louis, MO) and prepared as previously described. 5 PGN (Staphylococcus aureus, lot 46242/1) was purchased from Fluka (Buchs, Switzerland) and reconstituted in endotoxin-free water (Sigma Chemical Co.) by thorough vortexing (15 minutes at room temperature). Interleukin (IL)-1β was obtained from Perbio Science, Bonn, Germany. All non-LPS reagents were tested on lack of endotoxin using QCL1000 (Biowhittaker, Walkersville, MD). If not further mentioned in text, all other reagents were purchased from Sigma Chemical Co.. A specific polyclonal antibody to tyrosine-phosphorylated p42/p44 Mapk was purchased from Promega, Madison, WI.
Antibodies
Specific polyclonal, nonpurified anti-TLR2 (HM2076) and anti-TLR4 (HM2077) have been prepared as previously described in detail. 8 Polyclonal anti-pan-akt (lot 5) was purchased from Cell Signaling, Frankfurt, Germany. Normal anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and specific preimmune sera (HM2076 and HM2077) were used as negative controls.
Cell Culture
The human colon cancer cell line T84 (# 54-60) was obtained from the American Type Culture Collection (Manassas, VA) and grown on filters (0.4 μmol/L; Becton Dickinson, Franklin Lanes, NJ). Cell monolayers achieved confluency within 5 to 7 days and a polarized and differentiated state within 30 to 45 days, respectively. 5 Transepithelial resistance (TER) was used to monitor changes in epithelial cell culture integrity and confluency and ranged from >1500 Ωcm 2 for differentiated T84 cells before each experiment (nondifferentiated state was defined as <500 Ωcm2).
Western Blotting
Differentiated T84 cell monolayers, grown on filters in 6-well plates, were placed on ice in lysis buffer [400 μl per well, 1% Triton X-100 (Pierce, Rockford, IL), 150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.5, 2 mmol/L ethylenediaminetetraacetic acid, containing 10 mmol/L sodium fluoride, 10 mmol/L dithiothreitol, 10 mmol/L sodium orthovanadate, complete Mini protease inhibitor cocktail tablet (Roche, Mannheim, Germany), and 2 mmol/L phenylmethyl sulfonyl fluoride plus (Roche)] after stimulation. Lysates were then centrifuged (12,000 × g, 15 minutes at 4°C), and protein concentration in each supernatant was determined by colorimetric Bradford protein assay (Bio-Rad, Hercules, CA). Proteins (per lane 40 μg) from the resulting supernatants were heated in sample buffer (85°C, 3 minutes) after addition of 1 mmol/L of dithiothreitol, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15-well, 4 to 12% Bis-Tris; Invitrogen-Novex, San Diego, CA), and transferred onto a polyvinylidene difluoride membrane (Millipore, Eschborn, Germany) followed by blocking (0.5% Tween-20, 5% nonfat dry milk, 3% goat serum) for 1 hour at room temperature, immunoblotting with primary antibody (1:500; 0.1% Tween-20, 4% nonfat dry milk, 6% sheep serum) for overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibody (1:8,000; 4% nonfat dry milk in TBST) for 1 hour at room temperature (Amersham Pharmacia Biotech, Freiburg, Germany). After washing with phosphate-buffered saline (PBS)/0.1% Tween-20 (1 × 10 minutes) and PBS (3 × 10 minutes each) at room temperature, the membrane was developed with the enhanced chemiluminescence detection kit Renaissance (NEN Life Science, Boston, MA) and then exposed for 10 minutes to Kodak BioMax Light film followed by manual processing (Adefo Chemie, Nürnberg, Germany) in a standardized way (developing, 1 minute; rinsing, 30 seconds; fixation, 5 minutes; washing, 5 minutes). To confirm equal loading, immunoblots were stripped with 62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, containing 100 mmol/L 2-ME at 50°C for 30 minutes and reprobed with anti-pan-Akt (1:1000) as indicated by the manufacturer. Images of Western blots were acquired in a standardized way (800 dpi) using an Epson Perfection 1640SU-Photo scanner.
Blots were also probed with anti-active Mapk (p42/p44) according to the manufacturer’s instructions and recently described elsewhere. 5
Immunofluorescence and Confocal Laser Microscope
Cells were cultured on filters until desired state of differentiation was achieved, then washed with warm Dulbecco’s PBS at 37°C and fixed with fresh, ice-cold 2% or 4% paraformaldehyde (containing 0.1% Triton X-100 or 0.1% glutaraldehyde in 0.1 mol/L sodium cacodylate) for 30 to 60 minutes at 4°C or room temperature. After washing with Dulbecco’s PBS, filters were cut into small pieces with cells attached, then mounted onto glass slides (SuperFrost; Fisher Scientific or Roth, Karlsruhe, Germany). Cells were blocked with normal goat serum (1:100 in PBS; Vector Laboratories, Burlingame, CA) for 60 minutes at room temperature. Samples were incubated with anti-TLR2 (1:100) and anti-TLR4 (1:100) or preimmune serum or normal rabbit IgG (equivalent dilution) for 16 hours at 4°C. Fluorescein-conjugated goat anti-rabbit IgG antibody (Vector Laboratories) was used as secondary antibody (1:250, 60 minutes, room temperature). Samples were mounted (Vectashield Mounting Media, Vector Laboratories) and cells viewed within the next 72 hours using upright immunofluorescence (×100 (oil) objective, model AX70; Olympus, New Hyde Park, NY), or laser-scanning confocal microscopes (×63 (oil) objective; Zeiss Axiovert LSM 510, Germany). Images of 31 to 43 horizontal slices (depth, 1.85 μmol/L) per confluent monolayer were acquired (1024 × 1024 pixels) using standardized settings (software LSM510).
Electron Microscope
Cells were fixed in 2% paraformaldehyde and 0.1% glutaraldehyde in 0.1 mol/L sodium cacodylate, pH 7.4, for 60 minutes at room temperature. After rinsing in sodium cacodylate buffer and then in PBS, cells were incubated with either anti-TLR2 or anti-TLR4 (1:100) in PBS with 1.0% bovine serum albumin for overnight at 4°C. After rinsing in PBS, the cells were incubated in biotinylated goat anti-rabbit IgG (1:100, Vector Laboratories) for 2 hours at room temperature. Cells were then incubated in ABC reagents (Vector Laboratories) for 2 hours at room temperature. After rinsing in PBS, cells were fixed for 30 minutes at room temperature in a solution of 1.0% glutaraldehyde in PBS containing 5.0% sucrose. Cells were then rinsed in 0.05 mol/L Tris buffer, pH 7.6, containing 7.5% sucrose, and incubated for 5 minutes at room temperature in a solution of 0.1% diaminobenzidine in Tris-sucrose. H2O2 was added to a final concentration of 0.01% and cells were incubated for further 10 minutes in the dark. Cells were rinsed in sodium cacodylate buffer and postfixed in 1.0% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA) for 60 minutes at room temperature. After rinsing in sodium cacodylate, cells were dehydrated using a graded series of ethanol to 100% ethanol and then infiltrated in a solution of 1:1, 100% ethanol:EPON overnight. Cells were then placed in fresh EPON for several hours and small pieces of the filters (with attached cells) were embedded in the tips of flat embedding molds. Thin sections were cut onto formvar-coated slot grids using a Reichert Ultracut E ultramicrotome and the sections were poststained with uranyl acetate and lead citrate and examined at 80 kV with a Philips CM 10 transmission electron microscope.
Immunofluorescence Microscope on Live Cells
T84 cells were grown on 4-well Permanox slide chambers (NalgeNunc, Naperville, IL) to 70% confluency. Fluorescent (FL) conjugate of LPS (E. coli O55:B5; average labeling efficiency, 1.2 Bodipy-FL molecule per LPS) was obtained from Molecular Probes, Eugene, OR, and prepared as indicated by the manufacturer. As negative control, pure Bodipy-FL (Molecular Probes) was used. Cells were washed twice with warm Dulbecco’s modified Eagle’s medium/F12 without phenol red (Life Technologies, Inc., Grand Island, NY), supplemented with 0.1% fetal calf serum and incubated for 2 hours at 37°C. To avoid attenuation of the fluorescent signals, the usual full serum supplementation of 10% fetal calf serum was avoided. Instead, Bodipy-FL/LPS was mixed with sterile lipofectamine (Life Technologies, Inc.) as substitute of serum at a final concentration of 6 μg/ml and prewarmed for 30 minutes at 37°C. In control experiments, lipofectamine was omitted. LysoTracker Red (Molecular Probes) was prepared as indicated by the manufacturer and then added to this solution in a final concentration of 50 nmol/L. Cells were incubated with this solution (250 μl per well) for 30 to 60 minutes at 37°C in the dark (final concentration of LPS, 10 μg/ml). After washing twice with media, cells were mounted (without fixation) with Vectashield Mounting Media (Vector Laboratories) and glass-coverslipped and immediately viewed using upright immunofluorescence microscopes [×100 objective (oil), model AX70 (Olympus), or ×40 objective (without oil) (model DM LB; Leica, Solms, Germany]. Images were acquired with the software programs Magnafire (Olympus) or DHS Bilddatenbank (Leica, Germany).
Image Analysis
All images were digitized, cropped in Adobe Photoshop LE 5.0 (Adobe Systems, Inc.) and imported to Microsoft PowerPoint 2001 (Macintosh) for assembly and labeling.
Results
TLRs were present at the apical surface of polarized, confluent IEC monolayers (TLR2 and TLR4; Figure 1A ▶ ). Electron microscopy studies demonstrated that TLR4 was localized on the microvilli of apical cell surface of the intestinal epithelium (Figure 1B) ▶ .
Figure 1.
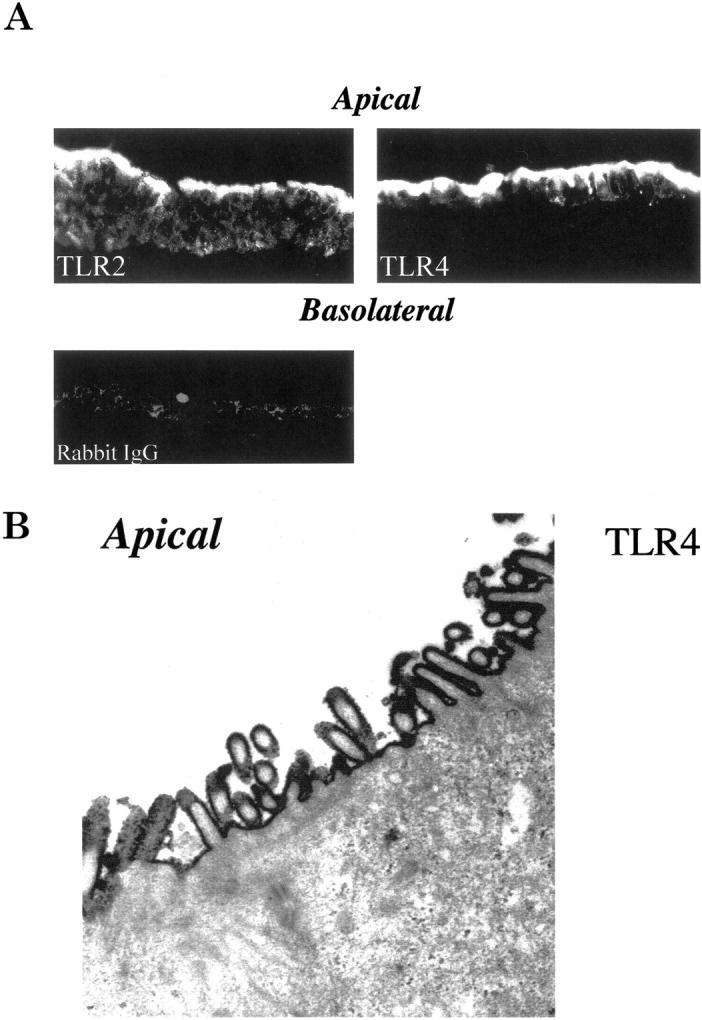
TLR2 and TLR4 are differentially expressed at the apical pole of differentiated IECs. Differentiated T84 cells were fixed on filters with 2% paraformaldehyde containing 0.1% glutaraldehyde and filters were cut into small pieces and incubated with either anti-TLR2, TLR4, or rabbit IgG (negative control) and analyzed by immunofluorescence immunohistochemistry (A; original magnification, ×20, no oil) or electron microscopic analysis (B; original magnification, ×26,700) as further described in Materials and Methods.
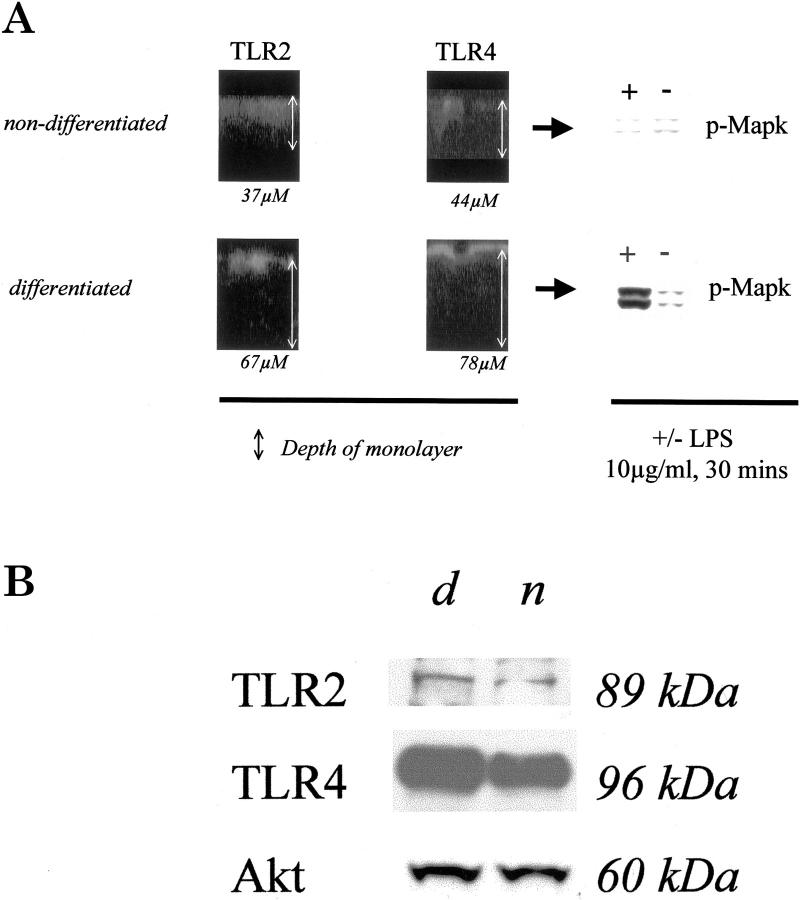
However, expression and localization of TLRs at the apical pole seems to be dependent on the state of IEC differentiation. As shown in Figure 2A ▶ , intense staining of TLR2 and TLR4 was distinctly present at the apical surface of differentiated T84 cells (TER > 1500 Ωcm2). In contrast, in nondifferentiated T84 cells (TER < 500 Ωcm2), TLR2 and TLR4 were mostly present in the cytoplasmic compartment (Figure 2A) ▶ . In contrast to differentiated T84 cells, nondifferentiated T84 cells were unresponsive to LPS. Of note, the overall intensity of the green fluorescent TLR signal was far lower in nondifferentiated versus differentiated cells. Western blotting revealed that total TLR2 and TLR4 protein amounts were slightly less in nondifferentiated when compared to differentiated IECs (Figure 2B) ▶ . Expression of pan-Akt has been shown to be independent of state of cell differentiation in IECs 20 and we therefore reprobed the blots with anti-pan-Akt that confirmed equal loading of total lysate protein (Figure 2B) ▶ .
Figure 2.
Expression and localization of TLRs depends on state of intestinal epithelial differentiation. A: Confocal microscopic analysis of vertical sections (standard, 1.85 μmol/L; original magnification, ×63, oil) of differentiated (TER > 1500 Ωcm2) or nondifferentiated (TER < 500 Ωcm2) T84 cells grown on transwells. Filters were fixed with ice-cold 4% paraformaldehyde containing 0.1% Triton X-100, blocked with goat serum, and incubated with specific primary antisera (TLR2 or TLR4) or corresponding preimmune sera at 4°C overnight. Fluorescein-conjugated secondary anti-rabbit antiserum was added subsequently for 1 hour at room temperature. Samples were viewed within 72 hours after fixation. Western blot analysis of tyrosine phosphorylation of p42/p44 Mapk revealed that only differentiated T84 cells, expressing TLR4 at the apical pole, responded to LPS instead of nondifferentiated T84 cells that expressed TLR4 very weakly in a distal cytoplasmic compartment. B: Western blot analysis of TLR2 and TLR4 protein expression before (n) and after (d) differentiation of nonstimulated T84 cells. TLR2 (89 kd) and TLR4 (96 kd) were identified using specific antisera as detailed in Materials and Methods. Individual blots were reprobed with anti-pan-Akt (60 kd), which confirmed equal loading of total protein cell lysate.
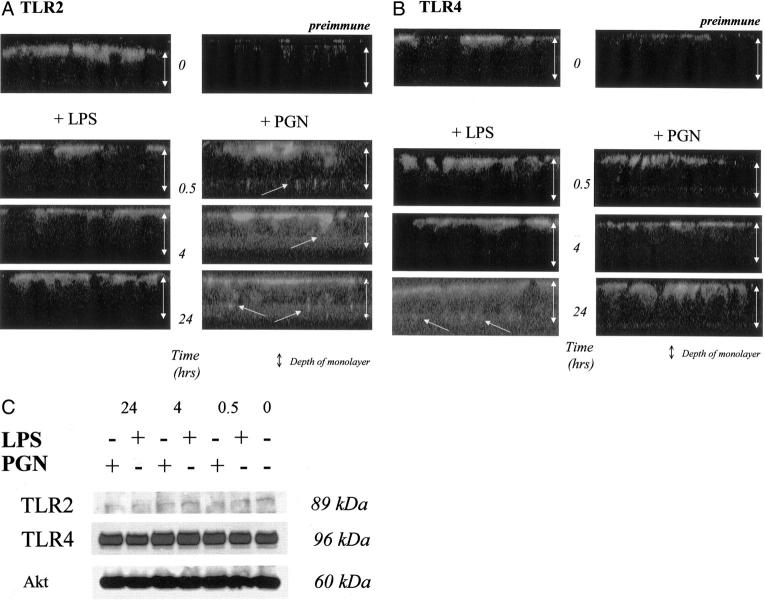
To analyze the subcellular distribution of TLRs in IECs when exposed to CAMPs, either phenol-extracted LPS (E. coli O26:B6) or purified PGN (S. aureus), each at a concentration of 10 μg/ml, were added separately to the apical and basolateral poles of differentiated T84 cells for different time periods (0, 0.5, 4, and 24 hours) at 37°C in fresh serum-supplemented media (10% fetal calf serum). As shown by confocal laser microscopy studies (Figure 3A) ▶ , TLR2 specifically scattered to cytoplasmic compartments near the basolateral membrane of differentiated IECs in response to a 30-minute PGN stimulation. This effect was observed up to 24 hours after start of stimulation. However, no redistribution of TLR2 to the basolateral side was observed in differentiated IECs after exposure to LPS at any time point. In contrast, TLR4 specifically redistributed from apical to basolateral after addition of LPS, but not PGN (Figure 3B) ▶ . However, in comparison with PGN-induced trafficking of TLR2, LPS-induced redistribution of TLR4 to cytoplasmic compartments near the basolateral membrane was significantly delayed (24 hours after stimulation) (this observation was confirmed by electron microscopy, data not presented). Western blotting demonstrated that total TLR2 and TLR4 protein expression was not significantly modulated by LPS or PGN (Figure 3C) ▶ . We confirmed equal loading of total lysate protein by reprobing the individual blots with anti-pan-Akt, because a recent study has shown that total protein expression of Akt remains stable in response to long-term stimulation with LPS. 21 These findings suggest that the described effect of TLR redistribution in response to CAMPs is not related to altered protein expression but might rather reflect ligand-specific-induced TLR trafficking.
Figure 3.
A and B: Stimulation with CAMPs induces selective trafficking of TLR2 or TLR4 from apical to basolateral in IECs. Confocal microscopic analysis of vertical sections (standard, 1.85 μmol/L; original magnification, ×63, oil) of differentiated (TER > 1500 Ωcm2) T84 cells after stimulation with either LPS or PGN (10 μg/ml) from apical and basolateral in full serum for different time periods (0, 0.5, 4, 24 hours). TLR2 (A) and TLR4 (B) were identified using specific antisera as detailed in Materials and Methods. Preimmmune serum was used as negative control in an equivalent dilution as primary antibody and confirmed specificity of individual TLR staining. C: Stimulation with CAMPs does not alter total protein amount of TLRs in IECs. Western blot analysis of TLR2 and TLR4 protein expression after stimulation with either LPS or PGN (10 μg/ml) for 0, 0.5, 4, or 24 hours. Blots were reprobed with anti-pan-Akt to confirm equal protein loading in individual samples. TLR2 (89 kd), TLR4 (96 kd), and Akt (60 kd) were identified using specific antisera as detailed in Materials and Methods.
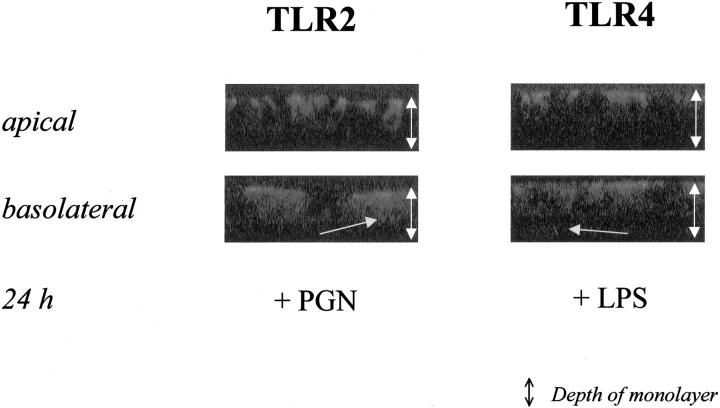
We then analyzed whether there would be a polarized difference in ligand-specific-induced subcellular redistribution of TLRs in IECs when preferentially exposed either to the apical or basolateral pole of differentiated T84 cells for 24 hours. As shown by confocal laser microscopy studies (Figure 4) ▶ , TLR2 specifically scattered to cytoplasmic compartments of differentiated IECs in response to either apical or basolateral PGN stimulation. However, this effect was far more impressive after basolateral than apical stimulation. Similarly, only very minimal redistribution of TLR4 was detected after apical stimulation with LPS. In contrast, significant amounts of TLR4 scattered to cytoplasmic compartments when LPS was only applied to the basolateral chamber.
Figure 4.
Basolateral stimulation with CAMPs preferentially leads to TLR trafficking from apical to basolateral. Confocal microscopic analysis of vertical sections (standard, 1.85 μmol/L; original magnification, ×63, oil) of differentiated (TER > 1500 Ωcm2) T84 cells after stimulation with either LPS or PGN (10 μg/ml) in fresh full media (10% fetal calf serum) from either apical or basolateral for 24 hours. TLR2 and TLR4 were identified using specific antisera as detailed in Materials and Methods.
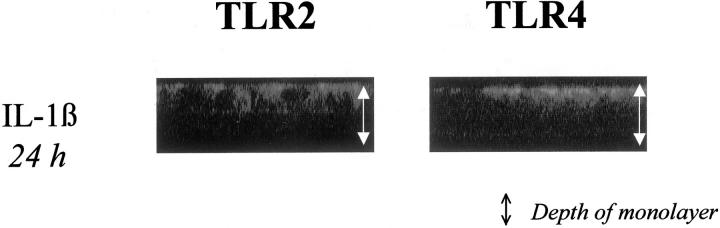
Furthermore, we added IL-1β (25 ng/ml, 24 hours) that did not induce trafficking of either TLR, regardless whether applied from apical or basolateral or both (Figure 5) ▶ , suggesting that the observed effect of ligand-induced redistribution of TLRs was CAMP-specific.
Figure 5.
As a non-CAMP, IL-β does not induce trafficking of either TLR. Confocal microscopic analysis of vertical sections (standard, 1.85 μmol/L; original magnification, ×63, oil) of differentiated (TER > 1500 Ωcm2) T84 cells after stimulation with endotoxin-free IL-1β (25 ng/ml, 24 hours) from either apical or basolateral or both (data shown) in fresh serum-supplemented Dulbecco’s modified Eagle’s medium/F12. TLR2 and TLR4 were identified using specific antisera as detailed in Materials and Methods.
Finally, live IECs grown on chamber-slides were incubated with fluorescein isothiocyanate-LPS and LysoTracker Red for 30 to 60 minutes at 37°C. Analysis of LPS association with single IECs (Figure 6A) ▶ revealed LPS-coated vesicular structures beneath the cell surface that co-localized with lysosomes (LysoTracker Red, merged), suggesting that LPS may be present in lysosomes in IECs. However, intestinal epithelial endocytosis of E. coli-LPS required addition of lipofectamine as substitute of serum (Figure 6B) ▶ . No lysosomes were detectable when only fluorescein isothiocyanate or lipofectamine were added without LPS (Figure 6C) ▶ , suggesting that detection of lysosomes was a specific effect of LPS-related endocytosis in IECs and not induced by fluorescein isothiocyanate or other fluorescein isothiocyanate-related contaminants.
Figure 6.
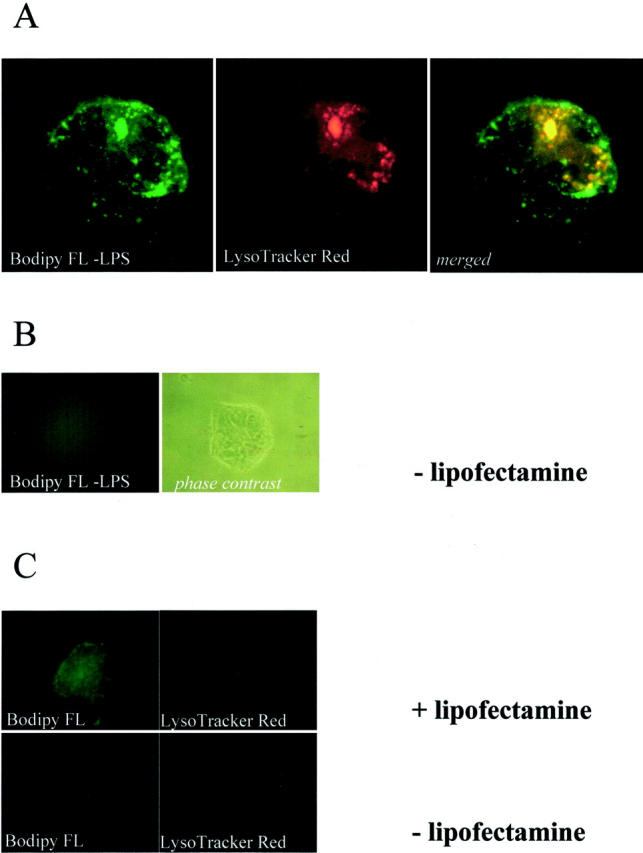
Endocytosed LPS is organized in multivesicular, lysosomal bodies in IECs. T84 cells were grown on slide chambers to 70% confluency. Cells were washed and incubated for 2 hours with fresh Dulbecco’s modified Eagle’s medium/F12 (0.1% fetal calf serum) without phenol red. Cells were then incubated with a mixture of Bodipy FL-LPS and lipofectamine for 30 to 60 minutes at 37°C. After washing, cells were mounted and immediately viewed using an upright immunofluorescence microscope. Immunofluorescence microscopy showed vesicular structures in live single T84 cells after staining with Bodipy FL-LPS (E. coli 055:B5) that co-localized with LysoTracker Red (A; original magnification, ×100, oil). Intestinal epithelial endocytosis of Bodipy FL-LPS was dependent on supplementation of serum, ie, lipofectamine (B; original magnification, ×40, no oil). The negative controls, endotoxin-free Bodipy FL or lipofectamine, were not endocytosed, because lysosomes were not detectable with LysoTracker Red (C; original magnification, ×40, no oil).
Electron microscopy showed that TLR4 was localized in vesicular structures in the apical cytoplasm of LPS-stimulated (10 μg/ml, 24 hours) IECs. Some of these structures resembled multivesicular lysosomes whereas others were smooth, membrane-bound vesicles resembling endosomes (Figure 7) ▶ . The apical vesicular structures containing TLR4 were not visible without LPS stimulation.
Figure 7.
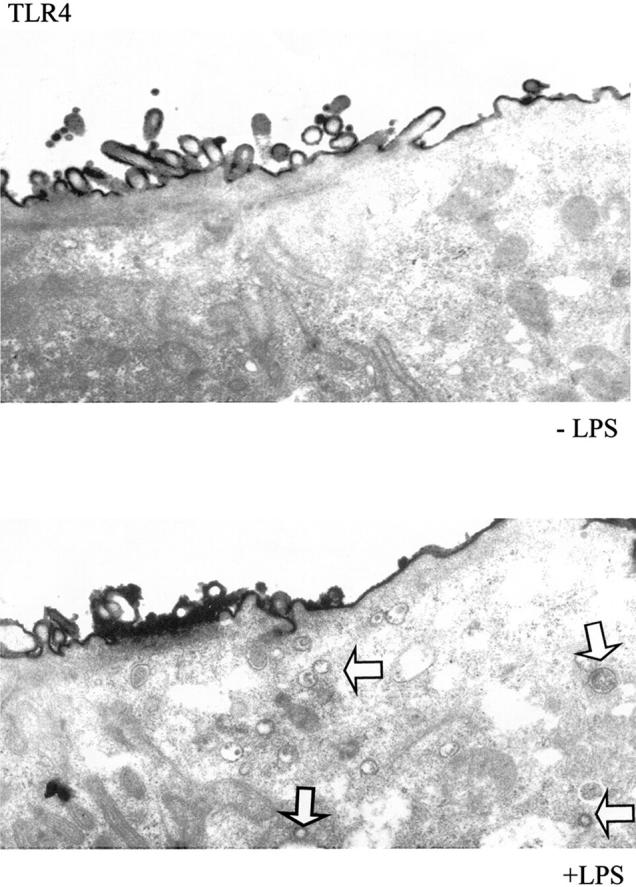
TLR4 coats vesicular structures in the apical cytoplasm of differentiated IECs in response to LPS stimulation. Electron microscopic analysis demonstrated that TLR4 was located in vesicular structures in the apical cytoplasm of differentiated T84 cells, but which were only detectable after LPS stimulation (10 μg/ml, 16 hours) (white arrow: TLR4-positive vesicles).
Discussion
The intestinal epithelium is highly polarized with two distinct membrane compartments: the apical surface facing the broad spectrum of lumenal microbes and the basolateral surface linking innate and adaptive immune responses with underlying lamina propria cells. 1 The present study demonstrates that the pattern recognition receptors TLR2 and TLR4 are present at the apical pole of differentiated IECs in culture and thus are optimally positioned to monitor the lumenal milieu of CAMPs. However, apical polarization does not seem to be an unique feature for all individual TLRs in the intestinal epithelium, as we and others have recently demonstrated that, in contrast, TLR5 seems to be preferentially expressed at the basolateral pole in vitro 10 and in vivo. 8
In contrast to fully differentiated cells, immature IECs lacked a stringent apical polarization of TLR. Interestingly, immature IECs do not respond adequately to LPS in vitro, whereas differentiated IECs exhibit cellular immune responses to LPS via activation of p42/p44 Mapk and nuclear factor-κB in a serum-dependent manner. 5 This phenomenon of unresponsiveness of immature IECs to bacterial products could be illustrated by the assumption that LPS may not access cytoplasmic TLR. 22 On the other hand, one may hypothesize that cytoplasmic TLR may be dysfunctional. This observation of decreased level of expression and altered localization of TLRs in nondifferentiated IECs could possibly explain why others have recently failed to demonstrate any responsiveness of IECs to LPS. 23 Of interest, microbes, which preferentially adhere to differentiated, rather than immature, IECs in vivo, 24 may be easily recognized by apical TLR4 of differentiated IECs, rather than cytoplasmic TLR4 of nondifferentiated IECs, thus rapidly initiating immediate immune responses without spatial delays. Of note, it has recently been demonstrated that the largest amount of LPS taken up from enterocytes was indeed found in more differentiated IECs near the villus tip rather than in crypts. 4
We have recently demonstrated that TLR2 and TLR4 are normally present in only small amounts on IECs in vivo, thus minimizing lumenal CAMP recognition in the healthy intestine. 8 However, active inflammation in inflammatory bowel disease may be accompanied by an earlier result from broken host tolerance to lumenal LPS because of altered pattern recognition via up-regulated TLR4 in IECs. 8 In this study we show that TLR polarization may be dynamically influenced by their specific ligands. TLR4 redistribution was significantly induced by LPS in vitro. In response to simultaneous apical and basolateral LPS stimulation in fresh serum-supplemented media, TLR4 was not only present in apical, but also in distal, cytoplasmic compartments near to the basolateral membrane. After preferential basolateral stimulation with LPS, TLR4 underwent transcytotic redistribution from the apical to the basolateral cytoplasmic domain of differentiated T84 cells, directly trafficking toward to the additional basolateral stimulus. Of note, TLR4 did not traffic in response to non-CAMPs, such as IL-1β.
However, it remains to be determined what functional impact this observation of redistribution may have in vivo. Interestingly, TLR4 is mostly present at the basolateral compartment of IECs in active ulcerative colitis, 8 which may suggest that intestinal epithelial TLR4 may redistribute in response to constant exposure to high amounts of lumenal CAMPs. Furthermore, LPS stimulation may induce impaired intestinal epithelial integrity, 25-27 which could facilitate paracellular trafficking of LPS across the epithelium in vitro and in vivo. Thus, direct physical access of lumenal LPS to the basolateral side of the epithelium may induce TLR4 trafficking from the apical to basolateral compartment in vivo. However, to prove a direct causal effect in vivo, future studies in gnotobiotic mice, both before and after colonization with normal microbial flora, would be more elucidating. Furthermore, at this point of our investigation, we cannot exclude the possibility that other bacterial products that can be present as contaminants in commercially available LPS may also affect TLR4 trafficking. 28
Interestingly, TLR2 also trafficked from apical to cytoplasmic compartments in response to its major ligand, PGN, 17,29 a major cell wall component of pathogenic (S. aureus) and nonpathogenic (B. subtilis) gram-positive bacteria. It has recently been demonstrated that LPS is not a ligand of TLR2. 14,30 Our study shows that TLR2 does not traffic in response to LPS, or TLR4 in response to PGN, respectively, suggesting that the observed effects of induced redistribution must be ligand-specific. Recent studies have suggested that TLR2 expression is not significantly altered in the intestinal epithelium of patients with Crohn’s disease or ulcerative colitis, 8 suggesting that TLR2 and its ligands may not play a significant role in the pathogenesis of inflammatory bowel disease.
Taken together, the findings of our study imply that TLR2 and TLR4 trafficking may reflect polarized cell-specific responses to their respective ligands. Furthermore, this study suggests that despite rigid cell polarity, the IEC is adapted to dynamically monitor its entire perimeter (ie, apical and basolateral) lumenal agents, if required.
As previously shown by this laboratory, 5 LPS-induced innate immune responses in IECs require serum, presumably as a source of various signaling mediators, which may complex with TLR4 to ultimately activate nuclear factor-κB. It has recently been demonstrated that as a tripartite receptor complex, LPS binds directly to TLR4 and MD-2, only when CD14 is also present. 31 This interaction may lead to cell activation, but it may also promote internalization of both LPS and CD14. 32 The findings of our study suggest that intestinal-epithelial LPS uptake depends on serum, ie, the presence of sCD14 and other mediators, which may then induce various intracellular stress responses.
After binding to the cell surface and internalization, LPS is routed to lysosomes in macrophages. 33 Our study suggests that endocytosed LPS is at least partially organized in multivesicular, lysosomal bodies in IECs, too. TLR4 appears in vesicular structures in the apical cytoplasm of IECs after exposure to LPS. Using immunofluorescent imaging, these TLR4-related vesicular structures appear similar to those observed with Bodipy-FL LPS. These TLR4-positive vesicles may also carry endocytosed LPS as cargo. It is well known that macrophages eliminate pathogens by phagocytosis 34 and that TLR2 is specifically recruited to these macrophage phagosomes in response to yeast. 35,36 It has been suggested that there are two distinct fates for internalized LPS in epithelial cells: monomeric LPS may be delivered to the Golgi apparatus, whereas LPS aggregates may move to lysosomes. 3,37 In future studies it will be essential to determine what functional role these TLR-coated vesicles may have: are they, eg, specifically equipped to sample and detoxify LPS in IECs, possibly acting as IEC-specific phagosomes? Are cytoplasmic TLRs still capable to specifically recognize endocytosed LPS and induce downstream signaling effects? Preliminary data from this laboratory demonstrate that long-term stimulation with LPS of IECs leads to immune hyporesponsiveness to CAMPs when re-exposed suggesting that cytoplasmic TLRs may indeed render dysfunctional after redistribution ( E Cario, DK Podolsky, unpublished observation), presumably being uncoupled from downstream effects and thus simply reflecting ligand-specific receptor internalization. In this context it will be crucial to clarify in further studies which signaling mechanism may regulate dynamic TLR redistribution, switching on and off immune responses to CAMPs in IECs.
Based on the results of this morphological study, we note that, in contrast to conventional primary effector cells of the immune system, IECs have established distinctive defensive features at the polarized apex via dynamic regulation of diverse pattern recognition receptors, thus defining them as unique frontline innate immune cells at the mucosal surface of the gastrointestinal tract. Clearly, further studies will be needed to determine which signaling mechanism may modulate dynamic ligand-specific TLR redistribution and what functional consequence of signaling this novel phenomenon may imply in IECs.
Acknowledgments
We thank Mr. Peter Babioch (confocal laser microscope) from the Institute of Anatomy and Mrs. Ina Konietzka (immunofluorescence microscope—live cells) from the Division of Pathophysiology, University of Essen, Germany, for superb technical support; and Drs. Emiko and Atsushi Mizoguchi, Division of Immunopathology, Department of Pathology, Massachusetts General Hospital, Boston, MA, for helpful discussions.
Footnotes
Address reprint requests to Dr. Daniel K. Podolsky, MD; Massachusetts General Hospital; Gastrointestinal Unit–GRJ 719, 55 Fruit St., Boston, MA 02114. E-mail: dpodolsky@partners.org.
Supported by the National Institutes of Health [grants DK 41557, DK 43351 (to D. K. P.), and DK 38452 (to D. B.)]; the Deutsche Forschungsgemeinschaft [grants Ca 226/2-1, Ca 226/4-1 (to E. C.)], and the Research Funding Program IFORES (to E. C.) from the Medical Faculty at the University of Essen.
References
- 1.Xavier RJ, Podolsky DK: Microbiology. How to get along—friendly microbes in a hostile world. Science 2000, 289:1483-1484 [DOI] [PubMed] [Google Scholar]
- 2.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH: Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995, 102:448-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty WL, Meresse S, Gounon P, Davoust J, Mounier J, Sansonetti PJ, Garvel JP: Trafficking of: Shigella lipopolysaccharide in polarized intestinal epithelial cells. J Cell Biol 1999, 145:689-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y, Ezzell RM, Warren HS: Localization of endotoxin in the rat intestinal epithelium. J Infect Dis 2000, 182:873-881 [DOI] [PubMed] [Google Scholar]
- 5.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK: Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000, 164:966-972 [DOI] [PubMed] [Google Scholar]
- 6.Lahde M, Korhonen R, Moilanen E: Regulation of nitric oxide production in cultured human T84 intestinal epithelial cells by nuclear factor-kappa B-dependent induction of inducible nitric oxide synthase after exposure to bacterial endotoxin. Aliment Pharmacol Ther 2000, 14:945-954 [DOI] [PubMed] [Google Scholar]
- 7.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF: A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995, 95:55-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cario E, Podolsky DK: Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 2000, 68:7010-7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA: Evidence for an innate immune response in the immature human intestine: Toll-like receptors on fetal enterocytes. Pediatr Res 2001, 49:589-593 [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL: Cutting edge: bacterial flagellin activates basolaterally expressed tlr5 to induce epithelial proinflammatory gene expression. J Immunol 2001, 167:1882-1885 [DOI] [PubMed] [Google Scholar]
- 11.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF: A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA 1998, 95:588-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzhitov R, Janeway Jr C: Innate immunity. N Engl J Med 2000, 343:338–344 [DOI] [PubMed]
- 13.O’Neill LA, Dinarello CA: The IL-1 receptor/Toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today 2000, 21:206-209 [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi O, Hoshino K, Kawai T, Sanja H, Takada H, Ogawa T, Takeda K, Akira S: Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11:443-451 [DOI] [PubMed] [Google Scholar]
- 15.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ: Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem 1999, 274:17406-17409 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi ST, Gros P, Malo D: The Lps locus: genetic regulation of host responses to bacterial lipopolysaccharide. Inflamm Res 1999, 48:613-620 [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Hoshino K, Akira S: Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to staphylococcus aureus infection. J Immunol 2000, 165:5392-5396 [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S: Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol 2000, 12:113-117 [DOI] [PubMed] [Google Scholar]
- 19.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F: Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 1999, 274:10689-10692 [DOI] [PubMed] [Google Scholar]
- 20.Gauthier R, Harnois C, Drolet JF, Reed JC, Vezina A, Vachon PH: Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol 2001, 280:C1540-C1554 [DOI] [PubMed] [Google Scholar]
- 21.Monick MM, Carter AB, Robeff PK, Flaherty DM, Peterson MW, Hunninghake GW: Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J Immunol 2001, 166:4713-4720 [DOI] [PubMed] [Google Scholar]
- 22.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B: Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA 2000, 97:2163-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M: Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 2001, 167:1609-1616 [DOI] [PubMed] [Google Scholar]
- 24.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF: Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 1998, 115:1405-1413 [DOI] [PubMed] [Google Scholar]
- 25.Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA: Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med 2000, 161:1705-1712 [DOI] [PubMed] [Google Scholar]
- 26.Chakravortty D, Kumar KS: Modulation of barrier function of small intestinal epithelial cells by lamina propria fibroblasts in response to lipopolysaccharide: possible role in TNFalpha in inducing barrier dysfunction. Microbiol Immunol 1999, 43:527-533 [DOI] [PubMed] [Google Scholar]
- 27.Kimura H, Sawada N, Tobioka H, Isomura H, Kokai Y, Hirata K, Mori M: Bacterial lipopolysaccharide reduced intestinal barrier function and altered localization of 7H6 antigen in IEC-6 rat intestinal crypt cells. J Cell Physiol 1997, 171:284-290 [DOI] [PubMed] [Google Scholar]
- 28.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ: Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol 2000, 165:618-622 [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D: Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol 1999, 163:1-5 [PubMed] [Google Scholar]
- 30.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS: Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol 2000, 165:5780-5787 [DOI] [PubMed] [Google Scholar]
- 31.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ: Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2. J Biol Chem 2001, 26:26. [DOI] [PubMed] [Google Scholar]
- 32.Poussin C, Foti M, Carpentier JL, Pugin J: CD14-dependent endotoxin internalization via a macropinocytic pathway. J Biol Chem 1998, 273:20285-20291 [DOI] [PubMed] [Google Scholar]
- 33.Forestier C, Moreno E, Pizarro-Cerda J, Gorvel JP: Lysosomal accumulation and recycling of lipopolysaccharide to the cell surface of murine macrophages, an in vitro and in vivo study. J Immunol 1999, 162:6784-6791 [PubMed] [Google Scholar]
- 34.Aderem A, Underhill DM: Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 1999, 17:593-623 [DOI] [PubMed] [Google Scholar]
- 35.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A: The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999, 401:811-815 [DOI] [PubMed] [Google Scholar]
- 36.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A: The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA 2000, 97:13766-13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thieblemont N, Wright SD: Transport of bacterial lipopolysaccharide to the Golgi apparatus. J Exp Med 1999, 190:523-534 [DOI] [PMC free article] [PubMed] [Google Scholar]