Abstract
The dramatic opportunities presented by comprehensive gene profiling technologies are curbed by the problem of segregating these large amounts of gene expression data into meaningful categories for study. This is particularly evident in infiltrating carcinomas of the pancreas, in which global gene expression data primarily mirrors the prominent desmoplastic response to the infiltrating neoplasm. In an effort to better characterize the gene expression of invasive pancreatic cancers and their associated desmoplastic response, we performed in situ hybridization on pancreatic cancer tissues to characterize the expression of 12 genes identified by serial analysis of gene expression as highly expressed in invasive pancreatic cancer tissues but not in pancreatic cancer cell lines. In situ hybridization demonstrated that eight genes were expressed within the stromal and/or angioendothelial cells of the desmoplastic response to the invasive tumor, and four of these genes were specifically expressed by the stromal cells immediately adjacent to the invasive neoplastic epithelium, suggesting regional differences in gene expression within the host desmoplastic response. In contrast, four genes were specifically expressed by the invasive neoplastic epithelium, indicating important differences between in vivo and in vitro gene expression of human epithelial neoplasms. We have identified a highly organized structure of gene expression within the host stromal response to invasive pancreatic cancer that may reflect tumor-host communication and serve as a target for therapeutic intervention.
Invasive cancers do not exist in isolation. Rather, they arise from and intimately interact with nonneoplastic host cells. The complexity of this interaction is reflected histologically in the intermingling of different tissue types and is evident as well in comprehensive profiles of gene expression. Indeed, the interpretation of such profiles is impaired by our incomplete understanding of the constituent components that participate in host-tumor interactions. Extreme representations of this process are seen in certain tumor types, such as scirrhous breast cancer, biliary carcinoma, and adenocarcinoma of the pancreas. Typically, these neoplasms are composed of infiltrating adenocarcinoma surrounded by a predominance of dense fibrous (or desmoplastic) stroma, which itself contains proliferating fibroblasts, small endothelial-lined vessels, inflammatory cells, and trapped residual atrophic parenchymal components of the organ invaded. 1 A consistently low ratio of the infiltrating adenocarcinoma component relative to this abundant desmoplastic response is rather unique to duct adenocarcinomas of the pancreas, in contrast to infiltrating carcinomas arising in other organ or tissue types. 2 The availability of extensive gene expression data on pancreatic cancer, combined with the prominent host-tumor interaction in this cancer type, provided a unique model system to define the cellular architecture of gene expression in invasive cancer.
One immediate goal would be a dissection of the nature of the host stromal response to invasive neoplasm. Recently, new insights into the patterns of gene expression associated with the process of tumor invasion were provided by Ryu and colleagues, 1 who demonstrated a cluster of invasion-specific genes in pancreatic cancer using hierarchical clustering and principal component analysis of data from serial analysis of gene expression (SAGE). With this approach, 74 genes were identified that were specifically expressed within tissue specimens of invasive pancreatic cancer, but not within passaged pancreatic cancer cell lines. These invasive tissue-specific genes were thought to primarily reflect the gene expression of the host desmoplastic response within pancreatic cancers that would not be represented in SAGE libraries prepared from cultured pancreatic cell lines. These invasive tissue-specific genes represented many categories of cellular function, including genes associated with extracellular matrix remodeling, angiogenesis, immune responses, and entrapped parenchymal cells. However, because the spatial localization of gene expression was not determined for these tags, their cellular origin within the primary tumor remained unclear (neoplastic epithelium, vasculature, or stromal cells), as did their potential roles within the process of tumor invasion.
We present a set of primary pancreatic cancers studied with in situ hybridization for each of a panel of genes previously found to be highly expressed in invasive pancreatic cancer tissues. 1 First, we sought to determine whether these invasive tissue-specific genes are expressed by the tumor, the host, or both; second, we attempted to determine whether spatially defined regions of invasive tumor or desmoplastic stroma might have unique expression profiles within the invasive process; and third, we sought to determine whether any clues to possible communication between the tumor and host response exist (ie, products produced by one compartment that may bind surface receptors of the other).
Materials and Methods
Tissue Specimens
Samples of bulk tumor (0.5 g) from two pancreaticoduodenectomy specimens were collected from patients undergoing Whipple resections for infiltrating duct adenocarcinoma of the pancreas. In each case, specimens of bulk tumor were harvested within 10 minutes of resection from the patient and snap-frozen in liquid nitrogen before storage at −80°C. Hematoxylin and eosin-stained sections of the frozen tissue were examined to confirm the presence of infiltrating adenocarcinoma within the section. For additional archival studies of gene expression in invasive pancreatic cancer, formalin-fixed paraffin-embedded tissue blocks from pancreaticoduodenectomy specimens (Whipple resections) from six patients with infiltrating duct adenocarcinoma of the pancreas were collected from the files of The Johns Hopkins Hospital. In each case, clinicopathological information was obtained from the surgical pathology files of The Johns Hopkins Hospital, as well as from the patients’ clinical records. The project adhered to a protocol approved by our institutional review board.
SAGE Analysis of Pancreatic Cancer
The invasion-specific gene cluster identified by SAGE was obtained from previously published work. 1 SAGE was performed as described by Zhang and colleagues 3 and Zhou and colleagues, 4 with principal component analysis and cluster analysis of the data performed by Ryu and colleagues 1 as recently described.
Preparation of Riboprobes
To generate riboprobes for use in in situ hybridization of the genes of interest, DNA templates were generated by polymerase chain reaction with incorporation of a T7 promoter into the antisense or sense primer. 5,6 After phenol:chloroform purification of amplified DNA, 200 ng of the DNA templates were used to generate either antisense or sense riboprobes by in vitro transcription with digoxigenin-labeling reagents and T7 polymerase according to the manufacturer’s protocol (Roche Diagnostics, Indianapolis, IN).
Nonradioactive in Situ Hybridization of Fresh-Frozen and Paraffin Sections
In situ hybridization of fresh-frozen tissues were performed following the methods described by St. Croix and colleagues. 7 Frozen tissues were sectioned at 5-μm thickness at −20°C onto silylated RNase-free microscope slides (CEL Associates, Houston, TX) before fixation in 4% paraformaldehyde/phosphate-buffered saline (PBS) for 1 hour. Next, sections were digested in 0.025% w/v pepsin in 0.2 N HCl for 5 minutes at 37°C, followed by two washes in PBS, and incubation in 5× standard saline citrate (0.75 mol/L sodium chloride and 0.075 mol/L sodium citrate) for 10 minutes. Tissue sections were prehybridized in mRNA hybridization buffer (DAKO, Carpinteria, CA) for 1 hour at 55°C, followed by hybridization overnight at 55°C with 200 ng/ml of antisense or sense riboprobe in mRNA hybridization buffer. The following day, sections were washed in 2× standard saline citrate at 45°C, followed by incubation with a 1/35 dilution of RNase A cocktail (Ambion, Austin, TX) in 10 mmol/L Tris, 500 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, pH 7.5, for 1 hour at 37°C. Next, slides were washed twice in 2× standard saline citrate/50% formamide at 55°C, followed by one wash at 0.08× standard saline citrate also at 55°C. For signal amplification, a horseradish peroxidase-conjugated rabbit anti-digoxigenin antibody (DAKO) was used to catalyze the deposition of biotinyl-tyramide (GenPoint kit, DAKO). Secondary amplification of the signal was achieved by adding horseradish-peroxidase rabbit anti-biotin (DAKO), biotin-tyramide, and then alkaline-phosphatase rabbit anti-biotin (DAKO). Signal was detected with the alkaline-phosphatase substrate Fast Red TR/Napthol AS-MX (Sigma, St. Louis, MO), and the tissue counterstained in hematoxylin for 1 minute.
For formalin-fixed, paraffin-embedded tissues, the above protocol was modified according to the methods described by Kadkol and colleagues 8 to optimize conditions. Briefly, sections were deparaffinized in xylene for 5 minutes, followed by hydration in graded ethanols for 5 minutes each. Next, sections were digested in a 10-μg/ml dilution of Proteinase K at 37°C for 30 minutes, followed by hybridization overnight at 55°C with a 200 ng/ml dilution of antisense or sense riboprobes in mRNA hybridization buffer. The following day, sections were serially washed and incubated with RNase A in the same manner as described for fresh-frozen sections, but signal amplification was achieved by incubation of sections with biotinyl-tyramide, followed by secondary streptavidin complex (GenPoint kit, DAKO). The final signal was developed with diaminobenzidine chromagen (GenPoint kit, DAKO).
Histological Evaluation of Tissue Sections
In situ hybridization labeling of mRNA expression in samples of fresh-frozen or paraffin-embedded pancreatic carcinoma was evaluated by three of the authors (CID, RHH, and SEK) with agreement in all cases examined. For each case of infiltrating duct carcinoma, the labeling pattern obtained after in situ hybridization was evaluated for the presence or absence of gene expression individually within the neoplastic epithelium, tumor stroma, and vasculature. In those cases with positive expression noted of the tumor stroma, gene expression was scored as occurring within the entire stromal region of the tumor, or in the stroma immediately adjacent to tumor epithelium.
Results
Identification of Invasive Tissue-Specific Genes
As described by Ryu and colleagues, 1 hierarchical cluster analysis and principal component analysis were applied to SAGE data of both primary invasive tumors and passaged cell lines representing carcinomas of the colon and pancreas. This approach identified a gene cluster specific for invasive pancreatic cancer tissues. 1 A total of 90 tags were identified in this cluster, 74 that matched known transcripts, representing the invasion-specific genes of pancreatic cancer tissues. This cluster is not to be confused with other commonly recognized gene clusters, such as tumor-specific genes (those genes expressed in both neoplastic epithelium derived from invasive cancers and in passaged cancer cell lines), or in tissue-specific genes (those genes expressed in normal tissue, site-matched invasive carcinomas, and passaged cell lines derived from such carcinomas).
Invasive pancreatic cancers represent an aggregate of diverse cell types, such as invasive neoplastic epithelial cells, fibroblasts, inflammatory cells, smooth muscle cells, endothelial cells, and cells of residual nonneoplastic pancreatic parenchyma. Thus, the precise cellular origin of these transcripts cannot be determined without additional study. To define the cellular origin and patterns of expression of these genes associated with the process of tissue invasion, 12 genes were selected for further study of their expression in invasive pancreatic cancer tissues by in situ hybridization (Table 1) ▶ . These gene expression markers were selected to represent different categories of biochemical function, such as cellular growth factors (connective tissue growth factor), signal transduction (β-catenin), cellular adhesion (β-catenin, intercellular adhesion molecule-1), extracellular matrix remodeling (matrix metalloproteinases 2, 11, and 14), and markers of specific cell or tissue types (ie, hevin, endothelium and thrombospondin-1, extracellular matrix). 9-15 In addition, four genes were chosen from the invasion-associated gene cluster whose role is currently unknown in neoplasia (apolipoprotein C-1, apolipoprotein D, α-2 macroglobulin, and α-2 macroglobulin receptor). 16,17 Two classes of genes were excluded from study: those whose expression represented normal parenchymal markers of the pancreas (for example, insulin), and those that were presumed markers of the immune response (immunoglobulin genes).
Table 1.
Markers of Architectural Compartments in Invasive Pancreatic Cancer Tissues
| SAGE tag | Gene | Cellular function/location | Architectural compartment | SAGE tag individual values* | |||
|---|---|---|---|---|---|---|---|
| I1 | I2 | CL1 | CL2 | ||||
| tgcacttcaa | Hevin | Angioendothelial | Angioendothelial | 23 | 19 | 0 | 0 |
| aaatagatcc | Beta-catenin | Signal transduction | Epithelial | 36 | 45 | 0 | 7 |
| gttcactgca | Intercellular adhesion molecule-1 | Adhesion | Epithelial | 20 | 23 | 3 | 7 |
| tttgcacctt | Connective tissue growth factor | Growth factor | Epithelial | 33 | 19 | 3 | 0 |
| aggtcttcaa | Thrombospondin-1 | ECM component | Epithelial, Panstromal | 167 | 29 | 0 | 7 |
| gggaggggtg | MMP14 | Matrix remodeling | Epithelial | 36 | 16 | 3 | 3 |
| caggagaccc | MMP11 | Matrix remodeling | Juxtatumoral stroma | 95 | 45 | 0 | 0 |
| ggaaatgtca | MMP2 | Matrix remodeling | Panstromal, angioendothelial | 62 | 35 | 0 | 0 |
| tcttgattta | Alpha-2 macroglobulin | Secreted protein | Angioendothelial, juxtatumoral stroma | 33 | 29 | 0 | 0 |
| ctcaaccccc | Alpha-2 macroglobulin receptor | Cell-surface receptor | Epithelial, panstromal | 26 | 48 | 3 | 0 |
| tggccccagg | Apolipoprotein C-1 | Secreted lipid carrier | Juxtatumoral stroma | 78 | 42 | 10 | 3 |
| ccctaccctg | Apolipoprotein D | Secreted lipid carrier | Juxtatumoral stroma | 20 | 16 | 0 | 0 |
*Data derived from Ryu and colleagues. 1 I1, invasive pancreatic carcinoma 1; I2, invasive pancreatic carcinoma 2; CL1, pancreatic cancer cell line 1; CL2, pancreatic cancer cell line 2.
Tissue Expression of Invasion-Specific Genes in Pancreatic Cancer
In situ hybridization was performed for each of the 12 invasive tissue-specific genes on two samples of fresh frozen tissue obtained from pancreaticoduodenectomy specimens removed for infiltrating adenocarcinoma of the pancreas. One sample was from a 79-year-old woman with a poorly differentiated infiltrating duct carcinoma, and the other sample was from a 49-year-old woman with a moderate to poorly differentiated infiltrating duct carcinoma. Neither tumor had a medullary histological pattern. 18
Detectable expression of all 12 genes were observed in both neoplasms, localized as precipitated Fast Red chromagen. For each gene, detectable expression was found to localize to one or more of four distinct architectural regions, or gene expression compartments, of the invasive tumors: 1) neoplastic epithelium, 2) angioendothelium and/or vascular smooth muscle, 3) juxtatumoral stroma (ie, only those stromal cells immediately adjacent to the invasive neoplastic epithelium), or to 4) panstromal tissue (ie, all stromal tissue of the invasive tumor) (Figure 1) ▶ .
Figure 1.
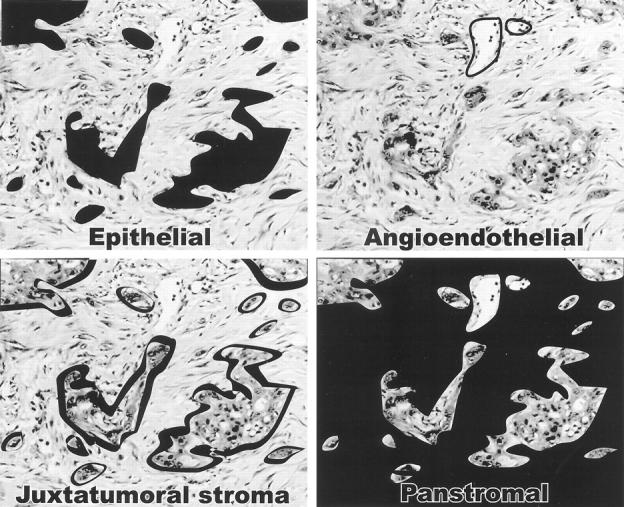
Architectural compartments of gene expression in invasive pancreatic cancer tissues as determined by in situ hybridization. Individual compartments within one histological example of invasive pancreatic carcinoma are highlighted in black to indicate the region of gene expression for each category: epithelial, gene expression detected in neoplastic epithelium; angioendothelial, gene expression detected in endothelial and/or vascular smooth muscle cells; juxtatumoral stroma, gene expression detected in stromal cells immediately adjacent to neoplastic epithelium; panstromal, gene expression detected within all stromal cells of the invasive tumor.
Eight of the 12 genes were expressed within only a single distinct architectural compartment in the two samples of invasive cancer (Figure 2) ▶ . Expression of CTGF, ICAM-1, β-catenin, and MMP14 were all localized to neoplastic epithelium, with no additional expression noted in the surrounding stromal or angioendothelial compartments. In contrast, hevin expression was noted only within endothelial cells of small capillaries and venules, and in scattered smooth muscle cells of small arterioles within the mass of the invasive tumor. No expression of hevin was found within the neoplastic epithelium or in stromal cells of the desmoplastic response. With respect to apolipoprotein C-1, apolipoprotein D, and MMP11, gene expression was observed primarily in the juxtatumoral stroma, whereas the neoplastic epithelial and angioendothelial compartments were negative for expression of these genes.
Figure 2.
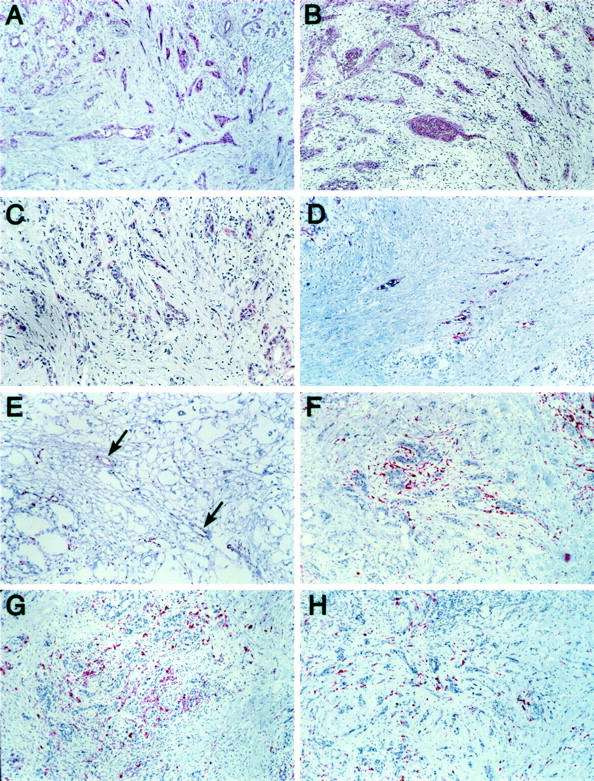
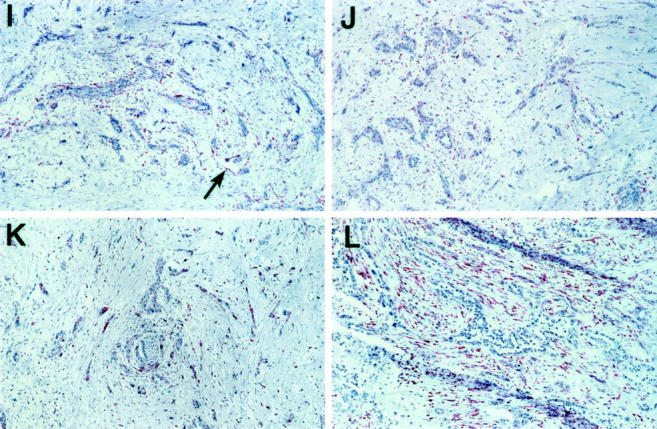
In situ detection of invasive tissue-specific genes in pancreatic cancer. Invasive tissue-specific gene expression was found to be located solely within tumor epithelium (A–D), angioendothelial tissue (E), juxtatumoral stroma (F–H), or simultaneously within several different compartments (I–L). A: Beta-catenin mRNA expression detected within tumor epithelium, but not within adjacent stroma. Similar findings are noted for connective tissue growth factor (B), intercellular adhesion molecule-1 (C), and MMP14 (D). E: Hevin mRNA expression specifically detected within angioendothelium (arrows). Adjacent tumor epithelium and stroma are negative in expression. F: Apolipoprotein D mRNA expression detected within stromal cells immediately adjacent to tumor epithelium (juxtatumoral stroma), but not in stromal cells away from the invasive tumor glands. Apolipoprotein C-1 (G) and MMP11 (H) show a similar pattern of mRNA expression. I: Alpha-2 macroglobulin mRNA expression detected within juxtatumoral stroma, as well as within angioendothelium of small vessels within the tumor mass (arrow). J: Alpha-2 macroglobulin receptor mRNA expression detected within the tumor epithelium, as well as scattered throughout the stromal response. K: Thrombospondin-1 mRNA expression detectable within tumor epithelium as well as the stromal response. L: MMP2 mRNA expression detectable within the entire stromal response. Focal mRNA expression was also detected within angioendothelial tissue (not shown in section).
Four genes demonstrated expression within two or more gene expression compartments. For example, α-2 macroglobulin expression was localized to endothelial cells and juxtatumoral stroma. In contrast, α-2 macroglobulin receptor and thrombospondin-1 both demonstrated gene expression within both the neoplastic epithelium and the panstromal compartments. Only endothelial cells and vascular structures were negative for expression of α-2 macroglobulin receptor and thrombospondin-1. MMP2 gene expression was localized to the panstromal compartment of the tumors, as well as to angioendothelium and vascular smooth muscle of small arterioles. Neoplastic epithelium was negative for expression of MMP2.
Focal expression of some genes was also noted in atrophic pancreatic parenchyma adjacent to the tumor mass, suggestive of epithelial-stromal interactions that also exist within the nonneoplastic pancreas. For example, CTGF, β-catenin, and ICAM-1 were detected in scattered atrophic pancreatic ducts, whereas apolipoprotein D, MMP2, and α-2 macroglobulin expression was observed in stromal cells within lobules of acinar cells undergoing atrophy adjacent to the invasive tumor. Alpha-2 macroglobulin receptor expression was detected in both atrophic ducts and stroma within areas of lobular atrophy. As expected, hevin and thrombospondin-1 were expressed in angioendothelial tissues in nonneoplastic pancreas.
Expression of Apolipoprotein D and MMP2 in Archival Samples of Pancreatic Carcinoma
To confirm our observations of distinct compartments of gene expression, we studied a separate set of invasive ductal adenocarcinomas of the pancreas, a series of paraffin-embedded specimens obtained from the files of The Johns Hopkins Hospital. The genes chosen for this confirmation were apolipoprotein D and MMP2, which were found in our initial observations to be expressed in different architectural compartments of gene expression within the host stromal response to invasive pancreatic carcinoma.
In situ hybridization was performed for both genes on six samples of paraffin-embedded invasive pancreatic carcinoma (Figure 3) ▶ . In situ hybridization of apolipoprotein D demonstrated tissue labeling in four of six cases (66%). In all four positive cases, mRNA expression of apolipoprotein D was localized to the juxtatumoral stroma, as well as to small nerves within the pancreatic parenchyma. MMP2 mRNA expression was also observed in four of six cases (66%). In contrast to apolipoprotein D, MMP2 expression was localized to the entire host stromal response, including the endothelium of small arterioles.
Figure 3.
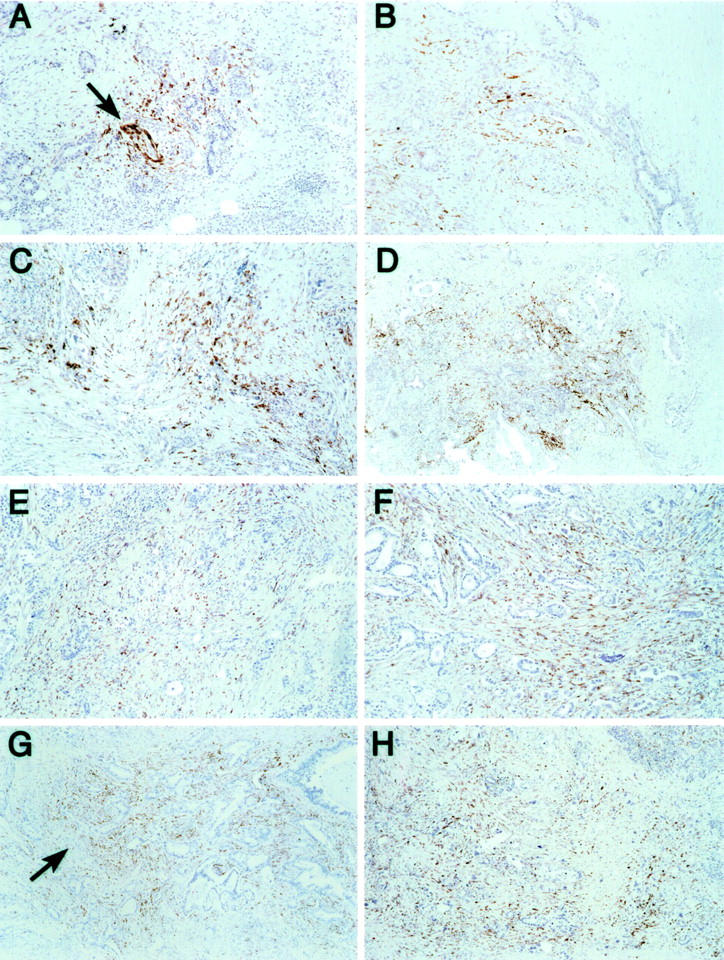
In situ detection of apolipoprotein D and MMP2 in paraffin-embedded tissues. A–D: Apolipoprotein D mRNA expression. Expression is primarily localized to the stromal cells adjacent to the tumor epithelium, as well as to small nerves within the pancreatic parenchyma (A, arrow) (original magnification, ×100). E–H: MMP2 mRNA expression. Expression is seen throughout the host stromal response, as well as within lining endothelium of small arterioles (G, arrow) (original magnification, ×100).
Discussion
Since the initial description of the SAGE methodology, 19 various reports have validated the utility of this technique to expand our understanding of neoplasia. 6,20-24 Recently, the application of multidimensional relational tools such as principal component analysis and hierarchical clustering have allowed a more categorical evaluation of the data generated by SAGE. 1 In particular, the identification of a cluster of genes highly expressed in invasive pancreatic cancer tissues, but not in pancreatic cancer cell lines derived from invasive pancreatic cancers, emphasized the importance of the host desmoplastic response, as well as host-tumor interactions in tumor invasion, and highlighted several new genes that may offer diagnostic or therapeutic targets. We can now explore additional utilities in the dissection of these host-tumor interactions.
Our findings demonstrate that the genes found to be overexpressed in SAGE libraries are indeed overexpressed in tissues. Of the 12 genes selected from 74 invasion-specific genes identified, all were found to be highly expressed in samples of invasive carcinoma. Unexpectedly, four (CTGF, ICAM-1, β-catenin, and MMP14) showed expression localized to the neoplastic epithelium in all cases wherein the transcripts were detectable. The expression of these four genes in the epithelium of invasive pancreatic cancers, but not in passaged cancer cell lines derived from pancreas cancer, may indicate that important differences exist in the gene expression profiles of in vivo and in vitro systems of carcinoma cells. Of the remaining eight genes studied, none were expressed by neoplastic epithelium, but rather were localized to either angioendothelial cells within the tumor mass, or instead to the stromal elements of the tumor. The expression of these genes in samples of primary pancreatic carcinoma, but not in passaged cell lines of pancreatic carcinoma, is now rationalized as reflecting the presence of stromal elements in the primary specimens. Thus, these genes represent markers of the host reaction to the neoplasm.
The use of 12 different markers of invasive tissue-specific gene expression helped define a transcriptomic architecture of an invasive cancer. Indeed, the presence of a marked desmoplastic host response within invasive pancreatic cancer allows for a facile determination and mapping of these various architectural compartments that might be more difficult to discern in other tumor types. Four different compartments were identified based on the regions of positive gene expression, which we describe as neoplastic epithelium, angioendothelial, panstromal, and juxtatumoral stroma—a specialized region of the stroma immediately adjacent to the invasive tumor epithelium. These distinct and reproducible compartments of gene expression of the invasive tissue-specific genes indicate a highly organized, structured, and coordinated process of tumor invasion in the pancreas.
Our data also indicate that these compartments are not segregated from each other; rather, the data suggest potential lines of communication between different compartments of the invasive tumor. For example, we found α-2 macroglobulin to be expressed by juxtatumoral stroma, whereas the receptor for this gene product, α-2 macroglobulin receptor, is expressed by the neoplastic epithelium. Alpha-2 macroglobulin has been suggested to act as a growth factor in malignant cells expressing α-2 macroglobulin receptor. 17 Thus, the juxtatumoral stroma (to at least some extent in that it involves highly expressed genes) may represent an active participant in the invasive process that may signal to the invading neoplastic epithelium. Such models have been suggested in the past, but had not been derived from unbiased surveys of gene expression. 25,26 Conversely, we have shown that MMP14, a membrane-bound metalloproteinase, is expressed by neoplastic epithelium, whereas MMP2, a known substrate of MMP14, is expressed by the surrounding stroma. If MMP14 were to proteolytically cleave proMMP2, MMP2 would be activated in sites within the stroma to promote neoplastic cell invasion.
The identification of several lipocalins as invasive tissue-specific gene markers in invasive pancreatic cancer sheds new light on this gene family with a potentially important and unrecognized role in the process of tumor invasion. Lipocalins have been identified in other studies of gene expression as determined by SAGE. 24 The function of lipocalins is not well understood, but they seem to play a role in lipid homeostasis. 16 Thus, their expression by juxtatumoral stroma in invasive pancreatic cancer, to provide one suggestion, may indicate a role for lipid metabolism that could serve to supplement and provide a nutritive role by the juxtatumoral stroma for the tumor epithelium. At the least, the analysis of architectural compartments serves well to generate hypotheses that would be otherwise not considered.
In summary, our finding of distinct architectural compartments of gene expression in invasive cancer, and particularly within the desmoplastic response, provides new insight into the host response to invasive cancer. The regional gene expression of the host response, together with the finding of possible communication between the stroma and invasive neoplastic epithelium, suggests that the host response plays an active role in promoting invasiveness of the neoplastic epithelium. The biological nature of juxtatumoral stroma needs exploration, separate from the panstromal compartment. Further studies to understand the biology of this desmoplastic response to invasive neoplasms may aid in identifying new targets for clinical imaging, serological diagnosis, drug development, and delivery.
Note added in Proof
The receptor for connective tissue growth factor has recently been identified as the alpha-2 macroglobulin receptor. 27
Footnotes
Address reprint requests to Scott E. Kern, Department of Oncology, 451 Cancer Research Building, 1650 Orleans St., The Johns Hopkins University School of Medicine, Baltimore, MD 21231. Email: sk@jhmi.edu.
Supported by the National Institutes of Health Specialized Programs of Research Excellence in Gastrointestinal Cancer grant CA62924.
References
- 1.Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE: Invasion-specific genes in malignancy: SAGE comparisons of primary and passaged cancers. Cancer Res 2001, 61:1833-1838 [PubMed] [Google Scholar]
- 2.Seymour AB, Hruban RH, Redston M, Caldas C, Powell SM, Kinzler KW, Yeo CI, Kern SE: Allelotype of pancreatic adenocarcinoma. Cancer Res 1994, 54:2761-2764 [PubMed] [Google Scholar]
- 3.Zhang L, Zhou W, Velculescu VF, Kern SE, Hruban RH, Hamilton SR, Kinzler KW, Vogelstein B: Gene expression profiles in normal and cancer cells. Science 1997, 276:1268-1272 [DOI] [PubMed] [Google Scholar]
- 4.Zhou W, Sokol LJ, Bruzek DJ, Zhang L, Velculescu VE, Kinzler KW, Goldin SB, Hruban RH, Kern SE, Hamilton SR, Chan DW, Vogelstein B: Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev 1997, 7:109-112 [PubMed] [Google Scholar]
- 5.Baklanov MM, Golikova LN, Malygin EG: Effect of DNA transcription of nucleotide sequences upstream to T7 promoter. Nucleic Acids Res 2001, 24:3659-3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RF, Marra MA, Riggins GJ, Polyak K, Papdopoulos N, Vogelstein B, Kinzler KW, Strausberg RL, Prange C, Marin PJ: A public database for gene expression in human cancers. Cancer Res 1999, 59:5403-5407 [PubMed] [Google Scholar]
- 7.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 [DOI] [PubMed] [Google Scholar]
- 8.Kadkol S, Gage W, Pasternak G: In situ hybridization—theory and practice. Mol Diagn 2001, 4:169-183 [DOI] [PubMed] [Google Scholar]
- 9.Hubbard AK, Rothlein R: Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med 2000, 28:1379-1386 [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ze’ev A, Shtutman M, Zhurinsky J: The integration of cell adhesion with gene expression: the role of B-catechin. Exp Cell Res 2000, 261:75-82 [DOI] [PubMed] [Google Scholar]
- 11.Volpert OV: Modulation of endothelial cell survival by an inhibitor of angiogenesis thrombospondin-1: a dynamic balance. Cancer Metastasis Rev 2000, 19:87-92 [DOI] [PubMed] [Google Scholar]
- 12.Girard JP, Baekkevold ES, Yamanaka T, Haraldsen G, Brandtzaeg P, Amairic F: Heterogeneity of endothelial cells. The specialized phenotype of human high endothelial venules characterized by suppression subtractive hybridization. Am J Pathol 1999, 155:2043-2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G, Gress TM: Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene 1999, 18:1073-1080 [DOI] [PubMed] [Google Scholar]
- 14.Maatta M, Soini Y, Kiakka A, Autio-Harmaine H: Differential Expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res 2000, 6:2726-2734 [PubMed] [Google Scholar]
- 15.Bramhall SR, Neoptolemon JP, Stamp GWH, Lemoine NR: Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol 1997, 182:347-355 [DOI] [PubMed] [Google Scholar]
- 16.Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirosis J, Terrisse L, Milne R: Apolipoprotein D. Biochem Biophys Acta 2000, 1482:185-198 [DOI] [PubMed] [Google Scholar]
- 17.Asplin IR, Misra UK, Gawki G, Gonzalez-Gronow M, Pizzo SV: Selective upregulated expression of the alpha2-macroglobulin signaling in highly metastatic 1-LN prostate carcinoma cells. Arch Biochem Biophys 2000, 383:135-141 [DOI] [PubMed] [Google Scholar]
- 18.Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJ, Hruban RH, Kern SE: Genetic, immunohistochemical and clinical features of medullary carcinoma of the pancreas: a newly described and characterized entity. Am J Pathol 2000, 156:1641-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velculescu VE, Zhang L, Vogelstein B, Kinzler KW: Serial analysis of gene expression. Science 1995, 270:484-487 [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Zhou G, Clark T, Chen J, Rowley JD, Wang SM: The pattern of gene expression in human CD15+ myeloid progenitor cells. Proc Natl Acad Sci USA 2001, 98:3340-3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charpentier AH, Bednarek AK, Daniel RL, Hawkins KA, Laflin KJ, Gaddis S, MacLeod MC, Aldaz CM: Effects of estrogen on global gene expression: identification of novel targets of estrogen action. Cancer Res 2000, 60:5977-5983 [PubMed] [Google Scholar]
- 22.Ferguson AT, Evron E, Umbricht CB, Pandita TK, Chan TA, Hermeking H, Marks JR, Lambers AR, Futreal PA, Stampfer MR, Sukumar S: High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer. Proc Natl Acad Sci USA 2000, 97:6049-6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibi K, Liu Q, Beaudry GA, Madden SL, Westra WH, Wehage SL, Yangg SC, Heitmiller RF, Bertelsen AH, Sidransky D, Jen J: Serial analysis of gene expression in non-small cell lung cancer. Cancer Res 1998, 58:5690-5694 [PubMed] [Google Scholar]
- 24.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ: Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000, 60:6281-6287 [PubMed] [Google Scholar]
- 25.Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R: Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol 1993, 120:253-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebeau A, Nerlich AG, Sauer U, Lichtinghagen R, Lohrs U: Tissue distribution of major matrix metalloproteinases and their transcripts in human breast carcinomas. Anticancer Res 1999, 19:4257-4264 [PubMed] [Google Scholar]
- 27.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, 3rd, Carmichael DF: The low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem 2001, 276:40659-40667 [DOI] [PubMed] [Google Scholar]


