Abstract
The tetracyclines function as antibiotics by inhibiting bacterial protein synthesis, but recent work has shown that they are pluripotent drugs that affect many mammalian cell functions including proliferation, migration, apoptosis, and matrix remodeling. Because all of these processes have been implicated in arterial intimal lesion development, the objective of these studies was to examine the effect of doxycycline treatment using a well-characterized model of neointimal thickening, balloon catheter denudation of the rat carotid artery. Rats were treated with 30-mg/kg/day doxycycline. Doxycycline reduced the activity of matrix metalloproteinase (MMP)-2 and MMP-9 in the arterial wall, and inhibited smooth muscle cell migration from media to intima by 77% at 4 days after balloon injury. Replication of smooth muscle cells in the intima at 7 days was reduced from 28.3 ± 2.5% in controls to 17.0 ± 2.8% in doxycycline-treated rats. The synthesis of elastin and collagen was not affected, but accumulation of elastin was blocked in the doxycycline-treated rats. By contrast, collagen accumulation was not affected, which led to the formation of a more collagen-rich intima. At 28 days after injury, the intimal:medial ratio was significantly reduced from 1.67 ± 0.09 in control rats to 1.36 ± 0.06 in the doxycycline-treated rats. This study shows that doxycycline is an effective inhibitor of cell proliferation, migration, and MMP activity in vivo. Further study in more complicated models of atherosclerosis and restenosis iswarranted.
The tetracyclines function as antibiotics by inhibiting bacterial protein synthesis, 1 but recent work has shown that they are pluripotent drugs that affect many cellular functions. Doxycycline, and other derivatives of tetracycline, are potent matrix metalloproteinase (MMP) inhibitors. 2 Studies with chemically modified tetracyclines have shown that the antibiotic and anti-MMP activities lie in different regions of the molecule; with the antibiotic activity residing at the dimethylamino group at the carbon-4 position of the A ring, whereas the anti-MMP activity resides in the carbon-11 carbonyl oxygen and carbon-12 hydroxyl groups. 3 Based on this anti-MMP activity, they have been used to reduce tissue degradation in aortic aneurysms, 4-7 periodontal disease, 3 and arthritis, 8-12 and are used to prevent tumor cell invasion and metastasis, 13,14 and tumor angiogenesis. 15 Clinical studies are in progress using doxycycline treatment for abdominal aortic aneurysm (personal communication, Dr. Robert Thompson, Washington University, St. Louis, MO) and using a chemically modified tetracycline (COL-3) to inhibit tumor angiogenesis. 16
In vitro the tetracyclines influence various cellular functions. They inhibit the proliferation of endothelial and osteosarcoma cells; 13,17,18 induce apoptosis of macrophages, osteosarcoma, and breast carcinoma cells; 19,20 inhibit tumor cell migration; 13,14 and reduce matrix synthesis by chondrocytes. 21,22 Tetracyclines also decrease the expression of and destabilize the mRNA for inducible nitric oxide synthase in macrophages and mesangial cells, 23-25 decrease the production of tumor necrosis factor-α, 2 and scavenge reactive oxygen species. 26 Most of this work has been done in cell culture and little is known about the diverse effects of tetracycline on these cellular processes in vivo.
Currently doxycycline is under investigation in the treatment of atherosclerosis, based on the hypothesis that microorganisms such as Chlamydia pneumoniae are important pathogenic factors. 27 However MMP production, cell proliferation, cell migration, apoptosis, and matrix synthesis have all been implicated in the development of intimal thickening during atherosclerosis and restenosis, and given the evidence cited above it is likely that doxycycline will impact many of these processes, independent of its antibiotic properties. Recent studies have shown that doxycycline inhibits outward vessel remodeling in response to increased blood flow, 28 and improves function in isolated rat hearts injured by ischemia-reperfusion. 29 Intriguingly, a retrospective study of patients presenting with acute myocardial infarction showed decreased incidence of infarction in patients correlated with usage of tetracycline or quinolone antibiotics during the preceding 3 years. 30 This study was far from conclusive, because the time course of antibiotic treatment, and molecular and morphological endpoints were not considered.
Despite the effects on many critical cellular processes and promising results in cardiovascular studies, little is known about the potential for doxycycline in modulating intimal thickening. In the current study we examine the effects of doxycycline treatment after balloon catheter denudation of the rat carotid artery, a model in which intimal thickening occurs in four well-defined sequential phases. The first phase of response, medial smooth muscle cell (SMC) replication, peaks at 2 days after injury and declines thereafter. 31 The second phase, migration of SMCs into the intima, can be measured at 4 days after injury as the first cells arrive in the intima, and before the cells have time to replicate in this layer. 32 We have also measured MMP activity at 4 days, because both MMP-2 and MMP-9 activities are elevated at this time. 32 The third phase, intimal SMC replication peaks at 7 days. 31 The fourth phase, matrix protein synthesis and accumulation, occurs between 1 week and 4 weeks after injury. The mRNAs for tropoelastin and procollagen are increased at 1 week, 33-35 and at 4 weeks maximum neointimal thickening is achieved. 31 Our objectives in this study were two-fold, first to characterize the pluripotent effects of doxycycline on cells of the vessel wall using an in vivo model, and second to elucidate the effects of doxycycline treatment on the phases of neointimal lesion development.
Materials and Methods
Surgery
Male Sprague-Dawley rats (3 to 4 months old) (Charles River, Constant, QB) were used in all experiments. Animal experiments were performed in accordance with the guidelines of the Canada Council on Animal Care. Rats were anesthetized by intraperitoneal injection of xylazine (4.6 mg/kg body weight, Rompum; Bayer Inc., Etobicoke, ON) and ketamine (70 mg/kg body weight, Ketaset; Ayerst Veterinarian Laboratories, Guelph, ON), and balloon catheter injury of the left common carotid artery was performed as previously described. 32 Doxycycline was administered in the drinking water at a dose of 30 mg/kg/day starting 24 hours before surgery. This dose was used previously to inhibit MMPs in studies of abdominal aortic aneurysm formation in the rat. 5 Control rats drank water alone. Rats were sacrificed at various time points after injury, chosen as follows based on previous studies elucidating the kinetics of the injury response. SMC proliferation was measured in the media (2, 4, 7, and 14 days) and intima (7 and 14 days). 31 Migration of cells from media to the intima and MMP activity were measured at 4 days. 32 Development of the neointima was assessed by measuring intimal area and the ratio of intimal:medial area at 14 and 28 days. Collagen and elastin synthesis were measured at 7 days, and collagen and elastin content of the vessels was measured at 7 and 21 days. 31
To label cells entering S phase in the 2, 4, 7, and 14 day groups, a 50-mg pellet of 5-bromo-2′-deoxyuridine (BrdU; Boehringer Mannheim Corp., Montreal, PQ) was implanted subcutaneously at the nape of the neck 24 hours before sacrifice. Rats were killed by an intravenous injection of T-61 (Hoechst Roussel Veterinarian, Regina, SA). Rats were infused with Lactated Ringer’s (Baxter, Toronto, ON) via a catheter placed in the abdominal aorta, followed by perfusion with 0.1 mol/L phosphate-buffered 4% paraformaldehyde at a pressure of 110 mmHg. Vessels were excised and immersed in 4% paraformaldehyde for 1 hour, and then transferred to Ringer’s solution. Samples for histological and morphometric analysis were taken 1 cm and 2 cm downstream of the origin of the common carotid, these were embedded in paraffin blocks and sectioned.
Morphometry
SMC replication rates in the media were measured 2, 4, 7, and 14 days after injury, and replication rates in the intima were measured at 7 and 14 days after injury by immunostaining carotid cross-sections for BrdU and determining the percentage of BrdU-labeled cells present as previously described. 36 Images of the cross-sections were obtained using a Nikon E600 microscope (Nikon, Mississauga, ON), digitized using a digital camera (model C4742-95-12NRB, Hamamatsu, Inc., Nikon) and analyzed using a computer-assisted morphometric analysis system (Simple C Imaging Systems, Mars, PA). Measurements of intimal and medial areas (14 and 28 days after injury) were made as follows. Lumen area was determined by tracing around the inside edge of the vessel and quantitating the area inside. Intimal area was measured as the area encompassed by the internal elastic lamina minus the lumen area. Medial area was measured as the area encompassed by the external elastic lamina minus the area encompassed by the internal elastic lamina (including lumen area). At the 4-day time point, a 1-cm length was excised from the middle of the common carotid artery and used for assay of SMC migration into the intima as previously described. 32 Briefly, intimal cells on the surface of fixed common carotid artery segments were immunostained with an antibody against histone H1. The number of intimal cell nuclei per square mm of surface area was counted by light microscopy. The migration assay takes advantage of the fact that the first SMCs appear in the intima 3 to 4 days after injury; it takes ∼24 hours for the cells to progress through the cell cycle, so the cells are counted before going through a round of replication.
Zymograms
Six rats (n = 3 per group for control and doxycycline) were used to measure MMP-2 and MMP-9 activity by gelatin zymography as previously described. 32 Extracts of individual carotid arteries were subject to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels that contained 0.1% gelatin as a substrate for MMP digestion. After electrophoresis, the gels were incubated 16 hours, then stained with Coomassie Blue, and MMP activity was evident as cleared bands of substrate lysis. The MMPs were identified by their molecular weights and inhibition by ethylenediaminetetraacetic acid or phenanthroline. Activity was quantitated by scanning densitometric analysis using a Bio-Rad gel 700 documentation system and Molecular Analyst software (Bio-Rad Laboratories, Hercules, CA).
Collagen and Elastin Synthesis and Content
Collagen and elastin synthesis was measured at 7 days, and total collagen and elastin content was measured at 7 and 21 days after arterial injury. Briefly, the entire left common carotid artery was excised between its origin at the aorta and the carotid bifurcation, then carefully stripped of adventitia, weighed, cut open, and incubated in Dulbecco’s modified Eagle medium containing 1% fetal calf serum (Invitrogen Canada, Burlington, ON), [14C]-proline (0.5 μCi/ml medium, Amersham Pharmacia Biotech, Baie d’Urfé, PQ), and ascorbic acid (50 μg/ml) for 6 hours. Collagen and elastin content and synthesis were determined according to Strauss and colleagues. 37 Results of collagen and elastin synthesis were expressed as cpm [14C]-proline per mg tissue wet weight. Collagen content was measured as hydroxyproline content, and total collagen was calculated assuming that collagen contains 12.77% hydroxyproline by weight. 38 Total collagen and elastin content were expressed as mg per carotid segment.
Statistical Analysis
Values are expressed as mean ± SEM. Group means were compared by the two-tailed Student’s t-test for independent samples, or by analysis of variance followed by Fisher’s paired least significant difference (PLSD) to determine differences between groups.
Results
There were no significant differences in body weight or water consumption between control and doxycycline-treated rats.
SMC Replication and Migration
Medial SMC replication was measured as the percentage of BrdU-labeled cells in the media after balloon injury. In control rats, medial replication peaked at 2 days after injury, then progressively declined between 4 and 14 days (Figure 1A) ▶ . There were no significant differences in medial replication between control and doxycycline-treated rats at any time point examined. Intimal SMC replication in control rats was measured at 7 and 14 days after injury, was highest at 7 days, then declined by 14 days (Figure 1B) ▶ . Doxycycline treatment significantly reduced intimal SMC replication; the replication of SMCs in the intima at 7 days after injury was reduced from 28.3 ± 2.5% in controls to 17.0 ± 2.8% in doxycycline-treated rats. Intimal SMC replication was also decreased at 14 days in the doxycycline-treated rats compared to control rats, but the difference was not statistically significant.
Figure 1.
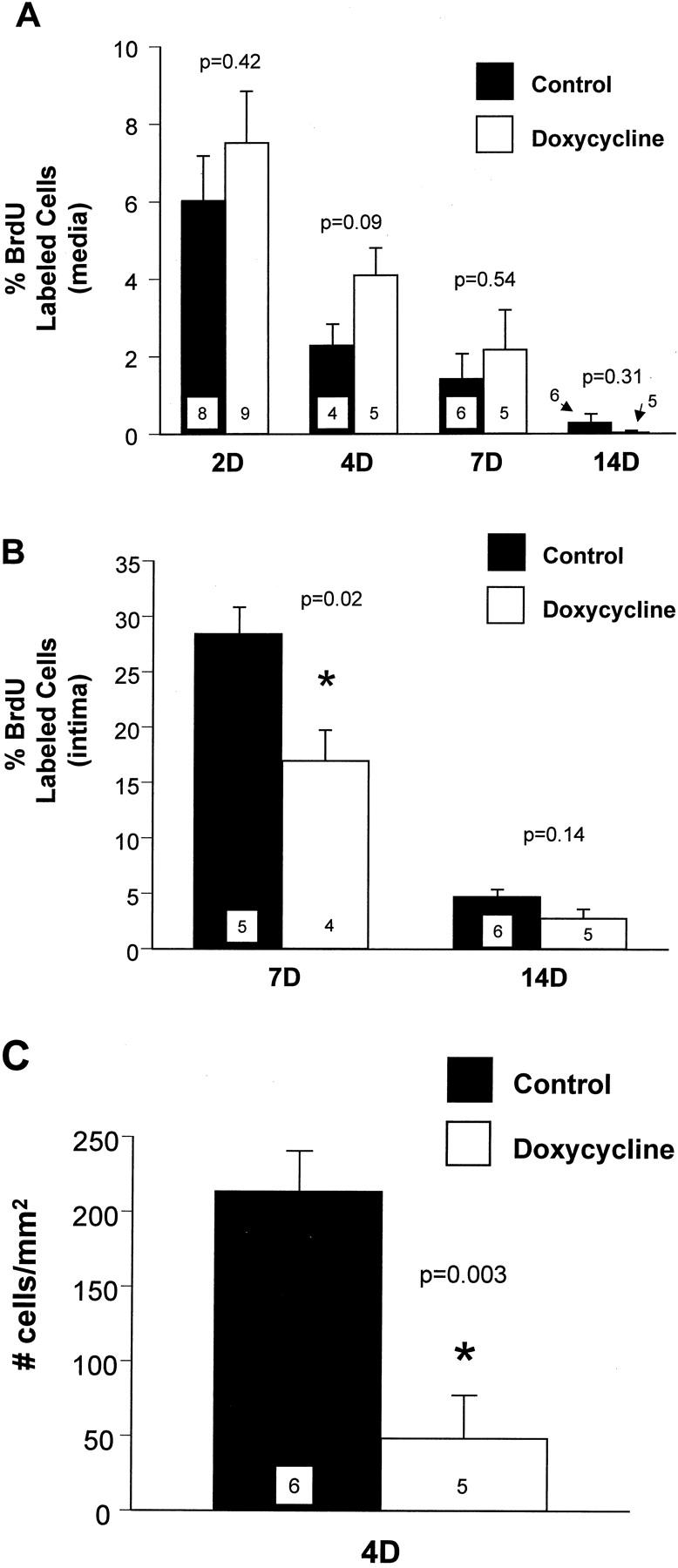
A: SMC replication in the media at various times after carotid artery injury. B: SMC replication in the intima at 7 and 14 days after carotid artery injury. C: SMC migration at 4 days after carotid artery injury. Filled bars represent values from control rats, and open bars values from doxycycline-treated rats. Values are mean ± SEM; the number of rats in each group is indicated at the bottom of the bar. *, The value measured in the doxycycline group was significantly less than the control group.
SMC migration from the media to the intima, measured at 4 days after injury, was reduced by 77% in doxycycline-treated rats compared with control rats (Figure 1C) ▶ . The number of intimal SMCs was 212.6 ± 27.5 cells/mm 2 of intimal surface area in control rats compared to 48.3 ± 28.5 cells/mm 2 in doxycycline-treated rats. In the doxycycline-treated rats there was a slight increase in SMC number in the media; 379 ± 24 cells compared to 367 ± 19 cells in controls (P = 0.7).
MMP Activity
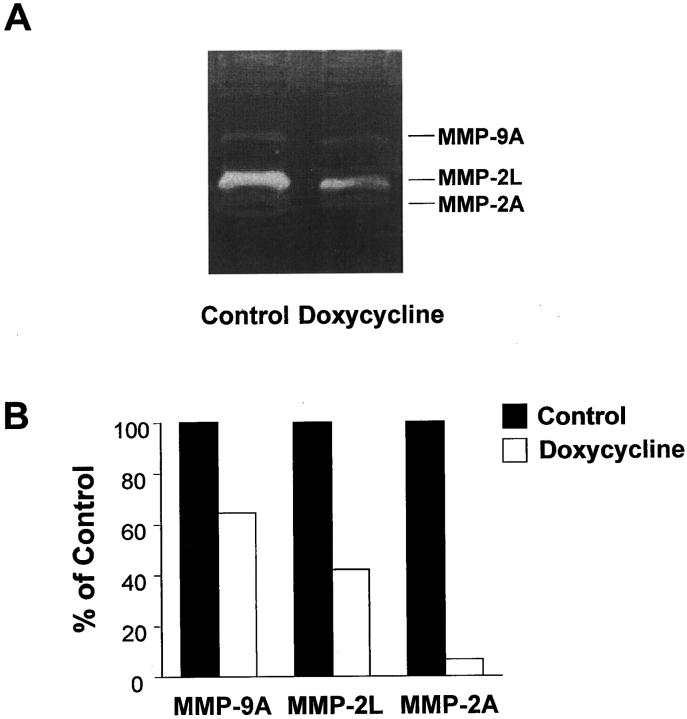
Previous work from our lab and others has shown that MMPs play an important role in regulating SMC migration, therefore gelatin zymograms were used to assess MMP activity in arterial extracts from control and doxycycline-treated rats. Three prominent bands with molecular weights of 88, 70, and 62 kd were noted in extracts from the injured carotids of control rats (Figure 2A) ▶ . We have previously shown that these lytic bands correspond to active MMP-9, latent MMP-2, and active MMP-2, respectively. 32 Activity of all three bands was decreased in arterial extracts from the doxycycline-treated rats (Figure 2A) ▶ . Densitometric analysis of the zymograms revealed that after doxycycline treatment, activity was reduced to 65%, 42%, and 7% of control levels for active MMP-9, latent MMP-2, and active MMP-2, respectively (Figure 2B) ▶ .
Figure 2.
A: Gelatin zymogram showing activity of MMP-9 (88 kd active) and MMP-2 (70 kd latent and 62 kd active) in carotid arteries from control and doxycycline-treated rats 4 days after balloon catheter injury. B: Scanning densitometry was performed on zymogram gels, and the values for each MMP band were expressed as a percentage of the value obtained for control. Individual carotid extracts were prepared and run separately on the gel. Three samples per group were averaged to obtain these measurements.
Matrix Synthesis and Accumulation
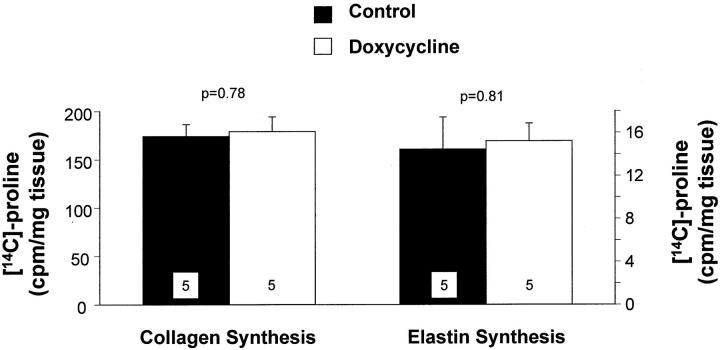
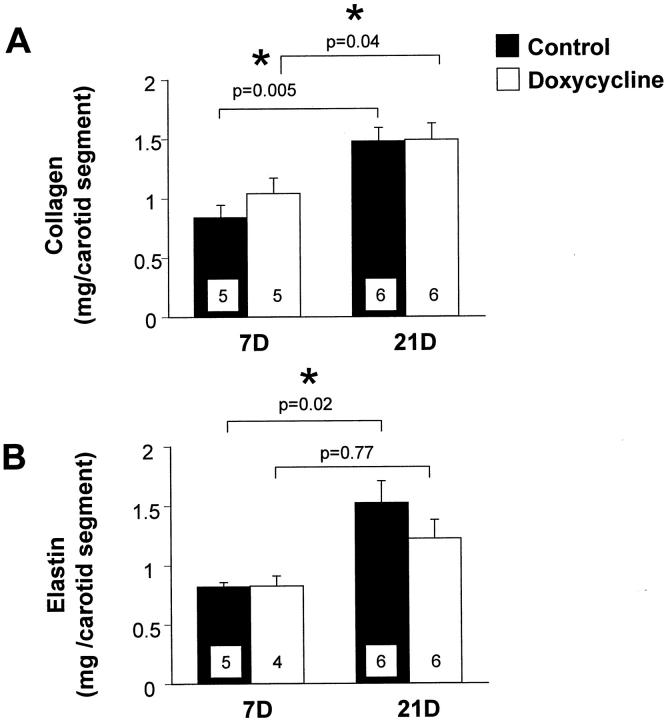
Collagen and elastin are the most abundant matrix proteins in the vessel wall and increased synthesis and accumulation of these molecules contributes to the intimal thickening that follows arterial injury. At 7 days after carotid artery injury there were no significant differences in collagen or elastin synthesis between control and doxycycline-treated rats (Figure 3) ▶ . There were no significant differences in collagen or elastin content between control and doxycycline-treated rats at 7 days after injury (Figure 4, A and B) ▶ . There was no difference in collagen content between the two groups at 21 days (Figure 4A) ▶ . However, there was a decrease in elastin content in the doxycycline-treated rats at 21 days (Figure 4B) ▶ , although this was not statistically significant. We compared the accumulation of matrix proteins between 7 and 21 days, and found that there was a significant increase in collagen content in both control and doxycycline-treated rats throughout this time period (Figure 4A) ▶ . By contrast, there was a significant increase in elastin content in the control carotids, but not in the doxycycline-treated carotids between 7 and 21 days (Figure 4B) ▶ . These data suggest that elastin accumulation was impaired after doxycycline treatment. As a result, the relative proportions of collagen and elastin in the vessel wall were changed after doxycycline treatment. The ratio of collagen:elastin in control vessels at 21 days after injury was 1.0 ± 0.2, whereas the ratio in doxycycline-treated carotids was 1.3 ± 0.1 (P = 0.4).
Figure 3.
Collagen and elastin synthesis measured at 7 days after carotid artery injury in control rats (filled bars) or rats treated with doxycycline (open bars). Values are mean ± SEM; the number of rats in each group is indicated at the bottom of the bar.
Figure 4.
Content of collagen per carotid segment (A) and elastin per carotid segment (B) in the injured carotid arteries of control rats (filled bars) and doxycycline-treated rats (open bars) at 7 and 21 days after injury. Values are mean ± SEM; the number of rats in each group is indicated at the bottom of the bar. *, Collagen or elastin content measured at 21 days is significantly greater than content measured at 7 days.
Vessel Wall Morphometry
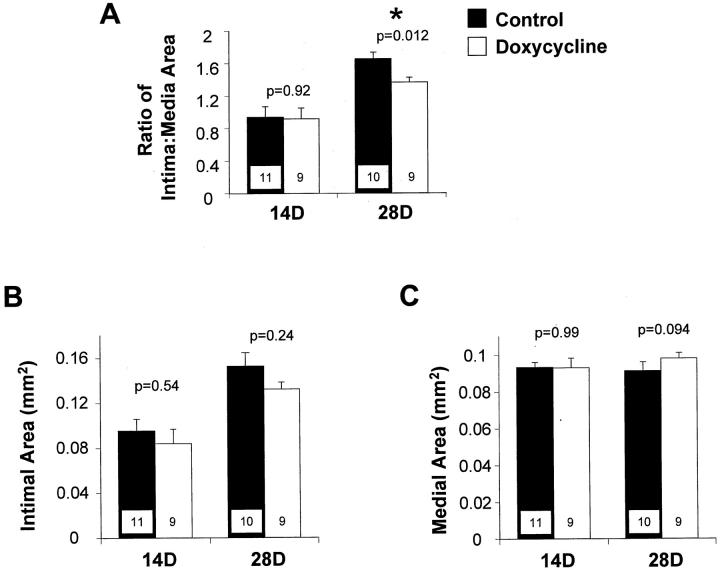
The ratio of intima:media area was significantly decreased at 28 days after injury in the doxycycline-treated rats compared to controls (Figure 5A) ▶ . Intimal area at 14 and 28 days after injury was decreased by doxycycline treatment, however these differences were not statistically significant (Figure 5B) ▶ . There were no significant differences in medial area between the treatment groups at either time point (Figure 5C) ▶ .
Figure 5.
Ratio of intima:media cross-sectional areas (A), intimal cross-sectional area (B), and medial cross-sectional area (C) of carotid arteries from control (filled bars) and doxycycline-treated rats (open bars), measured at 28 days after balloon catheter injury. Values are mean ± SEM; the number of rats in each group is indicated at the bottom of the bar. *, The value in the doxycycline-treated rats is significantly less than the value in controls.
By 28 days after injury, the number of SMCs in the intima and media was not significantly different between control and doxycycline-treated rats (Table 1) ▶ . We also calculated the density of SMC nuclei per unit of cross-sectional area as an index of matrix deposition relative to cell number, and found that SMC density did not differ between the control and doxycycline-treated rats (Table 1) ▶ .
Table 1.
Smooth Muscle Cell Number and Density 28 Days after Injury in the Rat Carotid Artery
| Control | Doxycycline | P value | |
|---|---|---|---|
| Medial SMC no. | 413 ± 30 | 403 ± 29 | =0.81 |
| Intimal SMC no. | 1134 ± 63 | 1098 ± 45 | =0.65 |
| Medial SMC density (cells/mm2) | 4754 ± 381 | 4099 ± 195 | =0.16 |
| Intimal SMC density (cells/mm2) | 7927 ± 545 | 8333 ± 264 | =0.53 |
Discussion
The current study has confirmed many of the pluripotent effects of doxycycline formerly reported only in vitro. Furthermore, our results indicate that doxycycline altered the kinetics of intimal thickening in the rat carotid artery after balloon injury. Doxycycline treatment did not affect medial SMC replication (first phase), but it did reduce SMC migration and MMP activity (second phase) and the proliferation of SMCs in the intima (third phase). Doxycycline treatment did not directly affect matrix synthesis, but there was a decrease in elastin but not collagen accumulation after balloon injury (fourth phase).
Medial SMC replication was not affected by doxycycline treatment. However, doxycycline treatment did attenuate SMC migration from the media to the intima. Furthermore, MMP-2 and MMP-9 activities were reduced in the injured carotid arteries of rats treated with doxycycline. This effect on cell migration was consistent with data we obtained using a different MMP inhibitor, the peptide hydroxamic acid GM 6001. 32 We infer therefore that the decrease in migration after doxycycline treatment was secondary to the MMP inhibition. Our finding of decreased MMP activity was in agreement with previous studies showing decreased MMP activity after doxycycline treatment in a rat model of abdominal aortic aneurysm, 4,5 and in tissue samples taken from patients treated with doxycycline before aneurysm repair. 6
The mechanisms by which doxycycline inhibits the MMPs are not completely understood. Doxycycline binds directly to Zn2+ or Ca2+ associated with the enzyme, blocking the active site or inducing conformational changes that render the proenzyme susceptible to fragmentation during activation. 2,39 In our experiments, it is likely that doxycycline dissociated from the MMP enzyme during gel electrophoresis, therefore a reduction in activity on the zymogram gel could indicate that less enzyme was actually present in the arterial extract. This strongly suggests that the mechanisms leading to lowered MMP activity may be related to inhibition of the transcription of MMP mRNAs, 40-44 or degradation of the pro-MMP zymogen during extracellular activation. 39 We saw decreases in the amount of the active forms of MMP-2 and MMP-9, therefore it is also possible that doxycycline prevented the in vivo activation of pro-MMP zymogens by reactive oxygen species, 26 or by the MT1-MMP. 45
We found that the third phase of intimal thickening, intimal SMC replication, was reduced after doxycycline treatment. We do not know why doxycycline selectively inhibited the proliferation of intimal and not medial SMCs, however there are important phenotypic differences between these cell types. 46 Interestingly, in tissue culture we found that doxycycline inhibited the growth of SMCs derived from the neointima more effectively than SMCs derived from the media (unpublished data), suggesting that there were stable phenotypic differences in doxycycline responsiveness between the SMCs in these two layers of the vessel wall. Another possible explanation for the differential effect on growth is that intimal SMCs are not firmly attached to the matrix because a new immature matrix is being deposited and remodeled around the cells. Reductions in matrix attachment leave cells more susceptible to cell death by apoptosis, and there is in vitro evidence that doxycycline may induce apoptosis. 19,20
In our previous work, the MMP inhibitor GM 6001 did not reduce cell proliferation in the vessel wall after injury, and in fact high rates of intimal SMC replication were prolonged from 7 to 10 days after injury in the GM 6001-treated rats compared to controls. 47 Similar results were reported by Zempo and colleagues 48 using another MMP inhibitor BB-94 (batimastat). Thus the effects of doxycycline seemed to differ from these MMP inhibitors, suggesting that the cell growth inhibition by doxycycline was independent of the anti-MMP activity. In contrast to the in vivo results, other investigators have reported that GM6001 and BB-94 inhibit the proliferation of SMCs in tissue culture. 49,50 Tissue culture studies aimed at distinguishing whether the effects of tetracyclines on cell growth are dependent on anti-MMP activity are contradictory. Gilbertson-Beadling and colleagues 18 showed that doxycycline inhibited endothelial cell sprouting in an angiogenesis assay whereas GM 6001 did not. Another study comparing several chemically modified tetracyclines either with or without anti-MMP activity showed that the ability of these compounds to inhibit the proliferation of endothelial cells was linked to the anti-MMP activity. 17 It seems that the mechanism of the doxycycline effect on cell growth many be different in vivo versus in vitro, and further studies to elucidate the mechanisms of growth control in vivo are needed.
We studied the fourth phase, matrix protein synthesis and accumulation in the injured arterial wall, and found that doxycycline did not inhibit matrix synthesis rates measured at 1 week after injury. However doxycycline did inhibit the accumulation of elastin, but not collagen, in the injured vessel between 1 and 3 weeks. MMPs and serine elastases are both up-regulated after injury; doxycycline inhibits MMP but not serine elastase activity. Therefore, it seems that doxycycline selectively spared the degradation of collagen but not elastin after injury, resulting in an increase in the proportion of collagen to elastin in the artery. This is important because it indicates that doxycycline may have a selective collagen-sparing effect, and therefore it may be a useful treatment to prevent plaque rupture.
The intima:media area ratio was reduced in the doxycycline-treated rats, however there was only a small reduction in intimal size in the doxycycline-treated rats. The most likely explanation for this catch-up effect is that SMC migration was prolonged in the doxycycline-treated rats longer than controls, with SMCs from the media eventually replenishing the intima throughout a longer time course. Migration can only be directly measured at the 4-day time point before cells undergo the first round of replication in the intima, so we cannot measure the full time course of migration. Decreased cell turnover could also result in intimal cell accumulation, and we cannot rule out the possibility that doxycycline enhances the survival of SMCs in the injured vessel wall. However, we think this unlikely because the literature suggests that doxycycline is proapoptotic. 19,20
Taken together, our data shows that doxycycline inhibits several phases of the intimal thickening response, and alters the matrix composition of the neointima. One limitation of the rat carotid injury model, as reflected in this study and our previous work, 47 is that there is a catch-up phenomenon, which resulted in little change in intimal area after doxycycline treatment. However, our findings of decreased intimal:medial ratio, and the protection against collagen degradation suggested some beneficial long-term effects of doxycycline. This should be pursued in larger animal models of restenosis that do not demonstrate this catch-up phenomenon, and are suitable for the study of plaque rupture.
Finally, it must be remembered that the nonantibiotic effects of doxycycline on arterial remodeling, as shown in this study, may complicate the mechanistic interpretation of studies in which doxycycline is used for the treatment of vascular diseases based on its antibiotic activity. Thus the MMP inhibiting effects of tetracyclines need to be taken into account in the design of future investigations.
Acknowledgments
We thank Dr. David Courtman for helpful discussions regarding these studies and critical review of the manuscript.
Footnotes
Address reprint requests to Dr. Michelle P. Bendeck, Ph.D., Department of Laboratory Medicine and Pathobiology, University of Toronto, Medical Sciences Bldg., Room 6217A, 1 King’s College Circle, Toronto, ON M5S 1A8. E-mail: michelle.bendeck@utoronto.ca.
Supported by the Heart and Stroke Foundation of Ontario (grant NA4800 to M. B.) and a Premier’s Research Excellence Award (to M. B. and S. F.). M. B. was a Research Scholar of the Heart and Stroke Foundation of Ontario.
References
- 1.Nelson MW: Chemical and biological dynamics of tetracyclines. Adv Dent Res 1998, 12:5-11 [DOI] [PubMed] [Google Scholar]
- 2.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T: Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 1998, 12:12-26 [DOI] [PubMed] [Google Scholar]
- 3.Ryan ME, Ramamurthy S, Golub LM: Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol 1996, 3:85-96 [PubMed] [Google Scholar]
- 4.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW: Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg 1996, 23:336-346 [DOI] [PubMed] [Google Scholar]
- 5.Curci JA, Petrinec D, Liao S, Golub LM, Thompson RW: Pharmacologic suppression of experimental abdominal aortic aneurysms: a comparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg 1998, 28:1082-1093 [DOI] [PubMed] [Google Scholar]
- 6.Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW: Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg 2000, 31:325-342 [DOI] [PubMed] [Google Scholar]
- 7.Boyle JR, McDermott E, Crowther M, Wills AD, Bell PR, Thompson MM: Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg 1998, 27:354-361 [DOI] [PubMed] [Google Scholar]
- 8.Steinmeyer J, Daufeldt S, Taiwo YO: Pharmacological effect of tetracyclines on proteoglycanases from interleukin-1-treated articular cartilage. Biochem Pharmacol 1998, 55:93-100 [DOI] [PubMed] [Google Scholar]
- 9.Greenwald RA, Golub LM, Lavietes B, Ramamurthy NS, Gruber B, Laskin RS, McNamara TF: Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol 1987, 14:28-32 [PubMed] [Google Scholar]
- 10.Greenwald RA, Moak SA, Ramamurthy N, Golub LM: Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J Rheumatol 1992, 19:927-938 [PubMed] [Google Scholar]
- 11.Brandt KD: Modification by oral doxycycline administration of articular cartilage breakdown in osteoarthritis. J Rheumatol 1995, 43:S149-S151 [PubMed] [Google Scholar]
- 12.Greenwald RA, Golub LM, Ramamurthy NS, Chowdhury M, Moak SA, Sorsa T: In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone 1998, 22:33-38 [DOI] [PubMed] [Google Scholar]
- 13.Fife RS, Sledge Jr GW: Effects of doxycycline on in vitro growth, migration, and gelatinase activity of breast carcinoma cells. J Lab Clin Med 1995, 125:407–411 [PubMed]
- 14.Seftor RE, Seftor EA, De LJ, Kleiner DE, Leferson J, Stetler-Stevenson WG, McNamara TF, Golub LM, Hendrix MJ: Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin Exp Metastasis 1998, 16:217–225 [DOI] [PubMed]
- 15.Tamargo RJ, Bok RA, Brem H: Angiogenesis inhibition by minocycline. Cancer Res 1991, 51:672-675 [PubMed] [Google Scholar]
- 16.Rudek MA, Figg WD, Dyer V, Dahut W, Turner ML, Steinberg SM, Liewehr DJ, Kohler DR, Pluda JM, Reed E: Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J Clin Oncol 2001, 19:584-592 [DOI] [PubMed] [Google Scholar]
- 17.Guerin C, Laterra J, Masnyk T, Golub LM, Brem H: Selective endothelial growth inhibition by tetracyclines that inhibit collagenase. Biochem Biophys Res Commun 1992, 188:740-745 [DOI] [PubMed] [Google Scholar]
- 18.Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, Petzold G, Mitchell M, Ledbetter S, Poorman R: The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol 1995, 36:418-424 [DOI] [PubMed] [Google Scholar]
- 19.Fife RS, Rougraff BT, Proctor C, Sledge Jr GW: Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med 1997, 130:530–534 [DOI] [PubMed]
- 20.Bettany JT, Wolowacz RG: Tetracycline derivatives induce apoptosis selectively in cultured monocytes and macrophages but not in mesenchymal cells. Adv Dent Res 1998, 12:136-143 [DOI] [PubMed] [Google Scholar]
- 21.Davies SR, Cole AA, Schmid TM: Doxycycline inhibits type X collagen synthesis in avian hypertrophic chondrocyte cultures. J Biol Chem 1996, 271:25966-25970 [DOI] [PubMed] [Google Scholar]
- 22.TeKoppele JM, Beekman B, Verzijl N, Koopman JL, DeGroot J, Bank RA: Doxycycline inhibits collagen synthesis by differentiated articular chondrocytes. Adv Dent Res 1998, 12:63-67 [DOI] [PubMed] [Google Scholar]
- 23.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB: A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA 1996, 93:14014-14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trachtman H, Futterweit S, Greenwald R, Moak S, Singhal P, Franki N, Amin AR: Chemically modified tetracyclines inhibit inducible nitric oxide synthase expression and nitric oxide production in cultured rat mesangial cells. Biochem Biophys Res Commun 1996, 229:243-248 [DOI] [PubMed] [Google Scholar]
- 25.Amin AR, Patel RN, Thakker GD, Lowenstein CJ, Attur MG, Abramson SB: Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett 1997, 410:259-264 [DOI] [PubMed] [Google Scholar]
- 26.Ramamurthy NS, Vernillo AT, Greenwald RA, Lee HM, Sorsa T, Golub LM, Rifkin BR: Reactive oxygen species activate and tetracyclines inhibit rat osteoblast collagenase. J Bone Miner Res 1993, 8:1247-1253 [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Egan D, Skarlatos S: Roles of infectious agents in atherosclerosis and restenosis. Circulation 1997, 96:4095-4103 [DOI] [PubMed] [Google Scholar]
- 28.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A: Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol 2000, 20:120-126 [DOI] [PubMed] [Google Scholar]
- 29.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R: Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 2000, 101:1833-1839 [DOI] [PubMed] [Google Scholar]
- 30.Meier CR, Derby LE, Jick SS, Vasilakis C, Jick H: Antibiotics and risk of subsequent first time acute myocardial infarction. J Am Med Assoc 1999, 281:427-431 [DOI] [PubMed] [Google Scholar]
- 31.Clowes AW, Reidy MA, Clowes MM: Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 1983, 49:327-333 [PubMed] [Google Scholar]
- 32.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA: Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res 1994, 75:539-545 [DOI] [PubMed] [Google Scholar]
- 33.Majesky MW, Giachelli CM, Reidy MA, Schwartz SM: Rat carotid neointimal smooth muscle cells re-express a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res 1992, 71:759-768 [DOI] [PubMed] [Google Scholar]
- 34.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA: Production of transforming growth factor β1 during repair of arterial injury. J Clin Invest 1991, 88:904-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikkari ST, Jarvelainen HT, Wight TN, Ferguson M, Clowes AW: Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol 1994, 144:1348-1356 [PMC free article] [PubMed] [Google Scholar]
- 36.Lindner V, Olson NE, Clowes AW, Reidy MA: Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest 1992, 90:2044-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strauss BH, Chisholm RJ, Keeley F, Gotlieb AI, Logan RA, Armstrong PW: Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res 1994, 75:650-658 [DOI] [PubMed] [Google Scholar]
- 38.Keeley FW, Morin JD, Vesely S: Characterization of collagen from normal human sclera. Exp Eye Res 1984, 39:535-542 [DOI] [PubMed] [Google Scholar]
- 39.Smith GNJ, Brandt KD, Hasty KA: Activation of recombinant human neutrophil procollagenase in the presence of doxycycline results in fragmentation of the enzyme and loss of enzyme activity. Arthritis Rheum 1996, 39:235-244 [DOI] [PubMed] [Google Scholar]
- 40.Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, van HV, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T: Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem 1997, 272:31504-31509 [DOI] [PubMed] [Google Scholar]
- 41.Uitto VJ, Firth J, Nip L, Golub L: Doxycycline and chemically modified tetracyclines inhibit gelatinase A (MMP-2) gene expression in human skin keratinocytes. Ann N Y Acad Sci 1994, 732:140-151 [DOI] [PubMed] [Google Scholar]
- 42.Nip L, Uitto VJ, Golub LM: Inhibition of epithelial cell matrix metalloproteinases by tetracyclines. J Periodontal Res 1993, 28:379-385 [DOI] [PubMed] [Google Scholar]
- 43.Hanemaaijer R, Visser H, Koolwijk P, Sorsa T, Salo T, Golub LM, van Hinsbergh VW: Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res 1998, 12:114-118 [DOI] [PubMed] [Google Scholar]
- 44.Jonat C, Chung FZ, Baragi VM: Transcriptional downregulation of stromelysin by tetracycline. J Cell Biochem 1996, 60:341-347 [DOI] [PubMed] [Google Scholar]
- 45.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI: Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloproteinase. J Biol Chem 1995, 270:5331-5338 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz SM, deBlois D, O’Brien ER: The intima. Soil for atherosclerosis and restenosis. Circ Res 1995, 77:445-465 [DOI] [PubMed] [Google Scholar]
- 47.Bendeck MP, Irvin C, Reidy MA: Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res 1996, 78:38-43 [DOI] [PubMed] [Google Scholar]
- 48.Zempo N, Koyama N, Kenagy RD, Lea HJ, Clowes A: Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol 1996, 16:28-33 [DOI] [PubMed] [Google Scholar]
- 49.Lovdahl C, Thyberg J, Hultgardh-Nilsson A: The synthetic metalloproteinase inhibitor batimastat suppresses injury-induced phosphorylation of MAP kinase ERK1/ERK2 and phenotypic modification of arterial smooth muscle cells in vitro. J Vasc Res 2000, 37:345-354 [DOI] [PubMed] [Google Scholar]
- 50.Southgate KM, Davies M, Booth RFG, Newby AC: Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem J 1992, 288:93-99 [DOI] [PMC free article] [PubMed] [Google Scholar]